Abstract
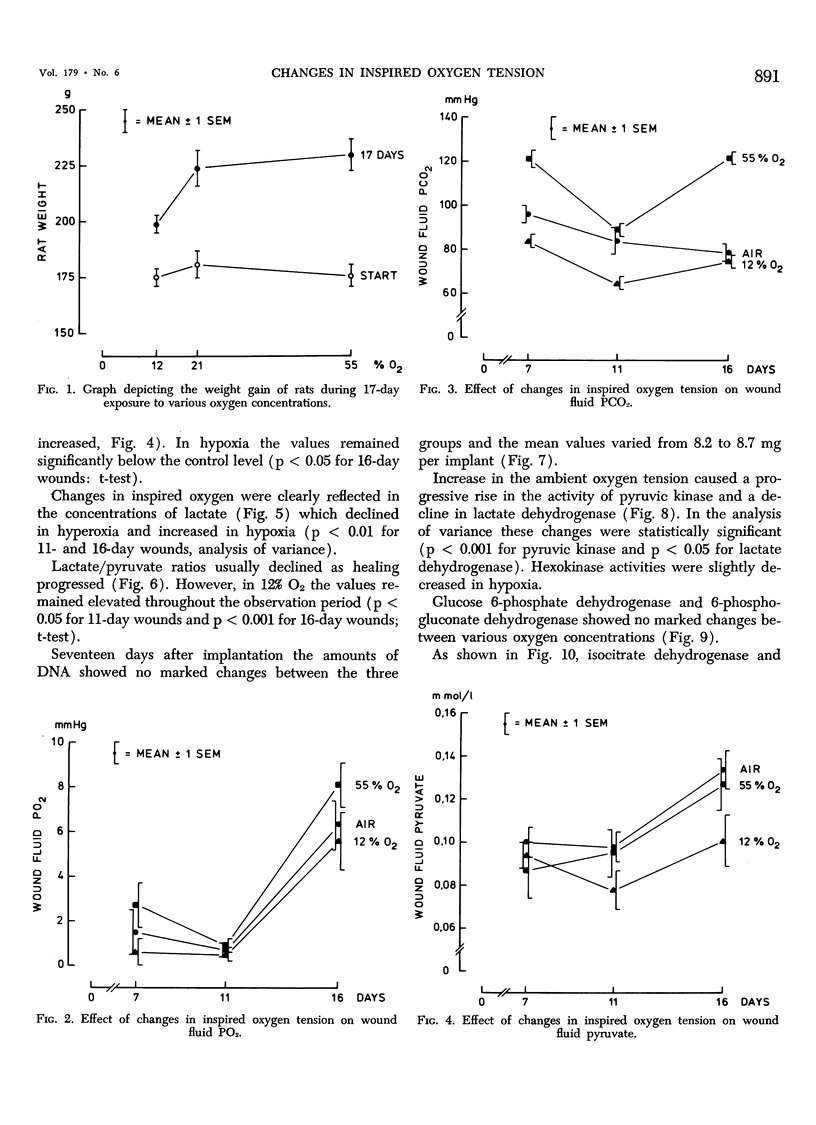
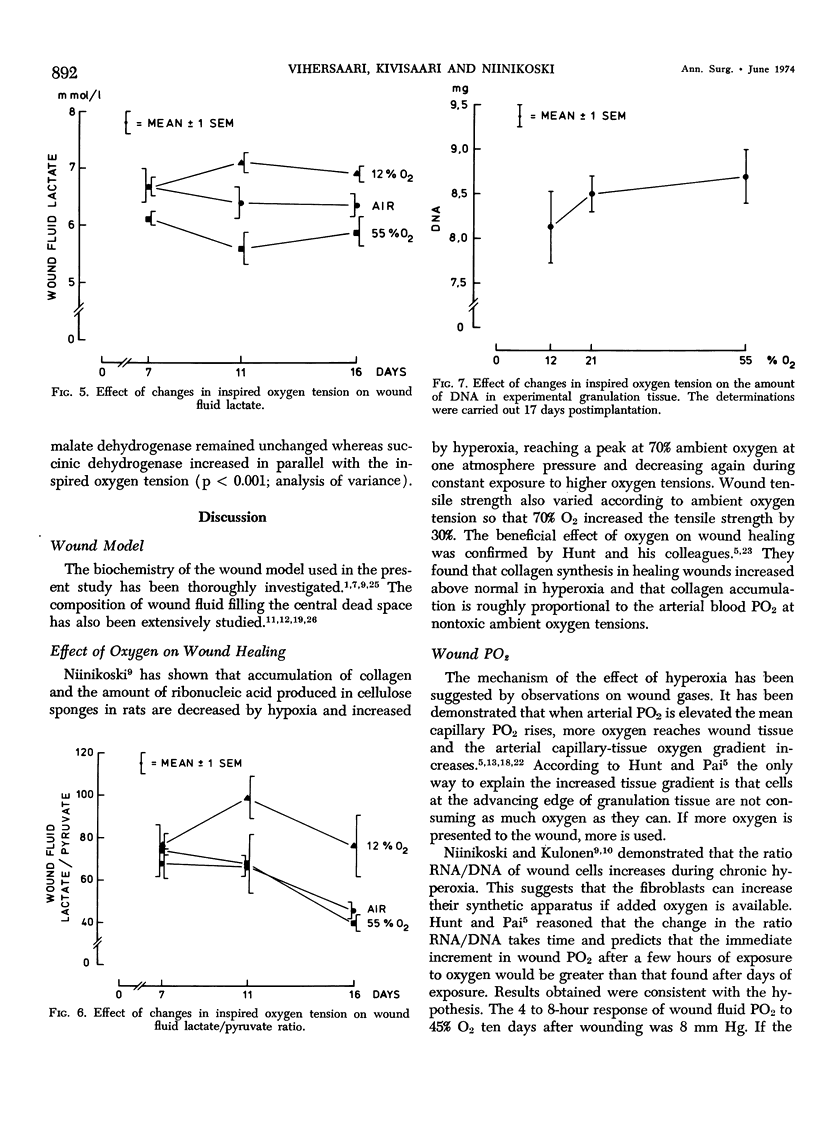
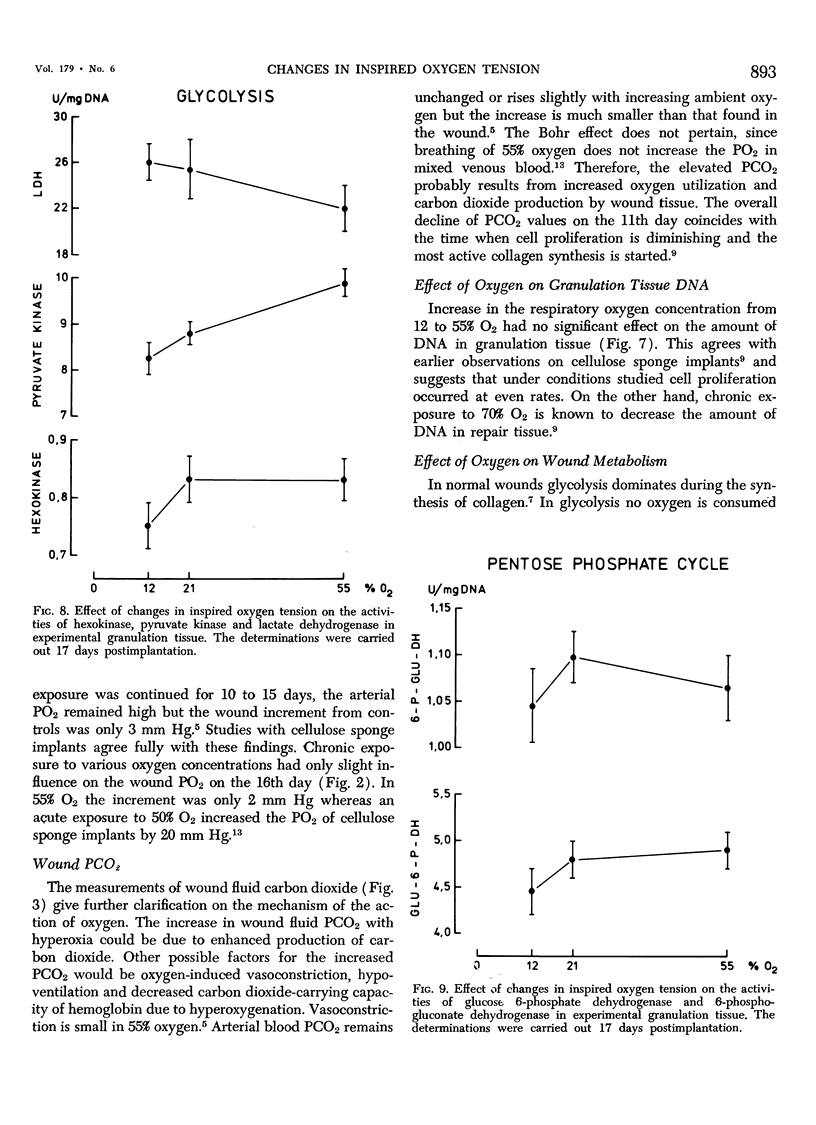
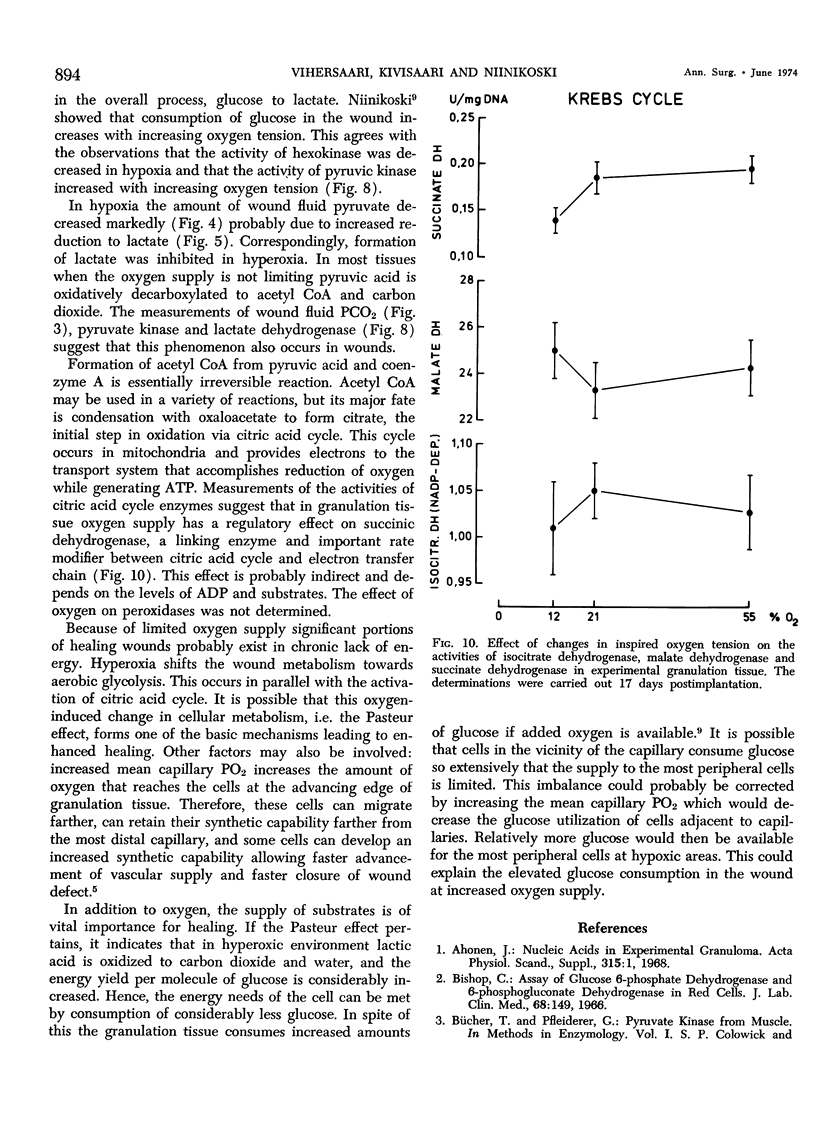
This work was prompted by earlier findings of the beneficial effect of increased oxygen supply on wound healing. Enzyme activities in the limiting step of glycolysis, citric acid cycle and pentose phosphate cycle were determined in cellulose sponge implants of rats chronically, breathing 12% O2, air or 55% O2. Respiratory gas tensions and concentrations of pyruvate and lactate were measured in wound fluid aspirated from the implants. Significant portions of repair tissue exist in conditions of extremely low oxygen tension. Probably because all added oxygen is readily consumed, the wound fluid PO2 increased only slightly in hyperoxic environment. The wound PCO2 increased in parallel with the inspired PO2, probably due to enhanced production of carbon dioxide. Hyperoxia shifted the wound metabolism from anaerobic towards aerobic glycolysis. This occurred concurrently with activation of citric acid cycle. Succinic dehydrogenase, a linking enzyme between citric acid cycle and electron transfer chain, also increased with increasing oxygen tension. This oxygen-induced metabolical change has been previously observed in many other tissues.
Full text
PDF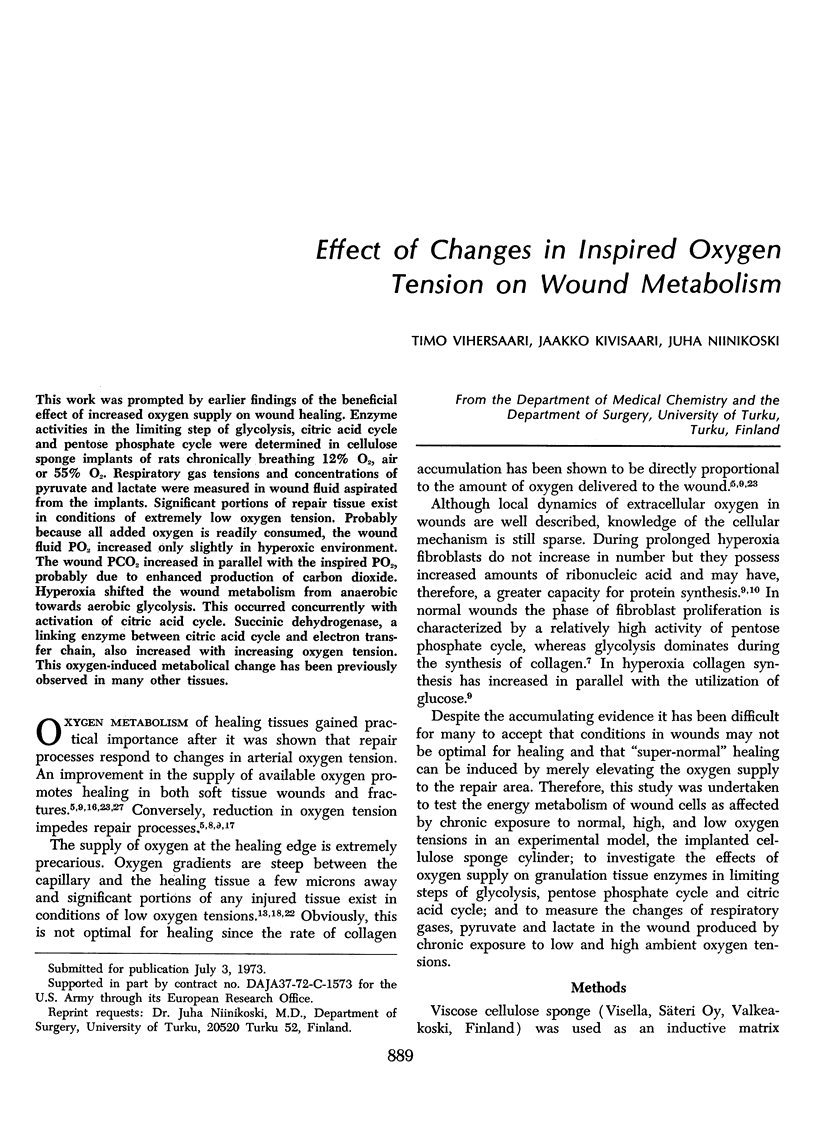


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop C. Assay of glucose-6-phosphate dehydrogenase (E.C. 1.1.1.49) and 6-phosphogluconate dehydrogenase (E.C. 1.1.1.43) in red cells. J Lab Clin Med. 1966 Jul;68(1):149–155. [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. K., Pai M. P. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972 Oct;135(4):561–567. [PubMed] [Google Scholar]
- Lampiaho K., Kulonen E. Metabolic phases during the development of granulation tissue. Biochem J. 1967 Oct;105(1):333–341. doi: 10.1042/bj1050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren C. E., Zederfeldt B. H. Influence of low oxygen pressure on wound healing. Acta Chir Scand. 1969;135(7):555–558. [PubMed] [Google Scholar]
- Niinikoski J. Effect of oxygen supply on wound healing and formation of experimental granulation tissue. Acta Physiol Scand Suppl. 1969;334:1–72. [PubMed] [Google Scholar]
- Niinikoski J., Grislis G., Hunt T. K. Respiratory gas tensions and collagen in infected wounds. Ann Surg. 1972 Apr;175(4):588–593. doi: 10.1097/00000658-197204000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinikoski J., Hunt T. K., Dunphy J. E. Oxygen supply in healing tissue. Am J Surg. 1972 Mar;123(3):247–252. doi: 10.1016/0002-9610(72)90277-2. [DOI] [PubMed] [Google Scholar]
- Niinikoski J., Kulonen E. Reparation at increased oxygen supply. Experientia. 1970 Mar 15;26(3):247–248. doi: 10.1007/BF01900072. [DOI] [PubMed] [Google Scholar]
- Ninikoski J., Heughan C., Hunt T. K. Oxygen and carbon dioxide tensions in experimental wounds. Surg Gynecol Obstet. 1971 Dec;133(6):1003–1007. [PubMed] [Google Scholar]
- Penttinen R., Niinikoski J., Kulonen E. Hyperbaric oxygenation and fracture healing. A biochemical study with rats. Acta Chir Scand. 1972;138(1):39–44. [PubMed] [Google Scholar]
- Penttinen R., Rantanen J., Kulonen E. Fracture healing at reduced atmospheric pressure. A biochemical study with rats. Acta Chir Scand. 1972;138(2):147–151. [PubMed] [Google Scholar]
- Remensnyder J. P., Majno G. Oxygen gradients in healing wounds. Am J Pathol. 1968 Feb;52(2):301–323. [PMC free article] [PubMed] [Google Scholar]
- SCHILLING J. A., MILCH L. E. Fractional analysis of experimental wound fluid. Proc Soc Exp Biol Med. 1955 Jun;89(2):189–192. doi: 10.3181/00379727-89-21753. [DOI] [PubMed] [Google Scholar]
- Silberberg R., Stamp W. G., Lesker P. A., Hasler M. Aging changes in ultrastructure and enzymatic activity of articular cartilage of guinea pigs. J Gerontol. 1970 Jul;25(3):184–198. doi: 10.1093/geronj/25.3.184. [DOI] [PubMed] [Google Scholar]
- Stephens F. O., Hunt T. K. Effect of changes in inspired oxygen and carbon dioxide tensions on wound tensile strength: an experimental study. Ann Surg. 1971 Apr;173(4):515–519. doi: 10.1097/00000658-197104000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE B. N., SHETLAR M. R., SHURLEY H. M., SCHILLING J. A. Wound healing: investigation of proteins, glycoproteins, and lipids of experimental wound fluid in the dog. Proc Soc Exp Biol Med. 1959 Jun;101(2):353–356. doi: 10.3181/00379727-101-24937. [DOI] [PubMed] [Google Scholar]
- Yablon I. G., Cruess R. L. The effect of hyperbaric oxygen on fracture healing in rats. J Trauma. 1968 Mar;8(2):186–202. doi: 10.1097/00005373-196803000-00007. [DOI] [PubMed] [Google Scholar]


