Abstract
The antiapoptotic properties of the inhibitor of apoptosis (IAP) family of proteins have been linked to caspase inhibition. We have previously described an alternative mechanism of XIAP inhibition of apoptosis that depends on the selective activation of JNK1. Here we report that two other members of the IAP family, NAIP and ML-IAP, both activate JNK1. Expression of catalytically inactive JNK1 blocks NAIP and ML-IAP protection against ICE- and TNF-α-induced apoptosis, indicating that JNK1 activation is necessary for the antiapoptotic effect of these proteins. The MAP3 kinase, TAK1, appears to be an essential component of this antiapoptotic pathway since IAP-mediated activation of JNK1, as well as protection against TNF-α- and ICE-induced apoptosis, is inhibited when catalytically inactive TAK1 is expressed. In addition, XIAP, NAIP, and JNK1 bind to TAK1. Importantly, expression of catalytically inactive TAK1 did not affect XIAP inhibition of caspase activity. These data suggest that XIAP's antiapoptotic activity is achieved by two separate mechanisms: one requiring TAK1-dependent JNK1 activation and the second involving caspase inhibition.
Apoptosis, or programmed cell death (PCD), is an active process in which an individual cell responds to internal and/or external cues by dying. PCD is involved in many homeostatic processes in multicellular organisms, both during development and in the mature organism. Too much cell death can lead to impaired development and degenerative diseases, whereas too little cell death can lead to diseases such as cancer and persistent viral infections (23, 47, 62). Apoptosis is controlled by several pro- and antiapoptotic families of genes that are conserved from nematodes through mammals and viruses (46).
The inhibitor of apoptosis (IAP) family of proteins was first discovered in baculovirus, where IAPs were shown to substitute for the viral inhibitor p35 in suppressing the host cell death response to viral infection (4, 38). IAP homologues were subsequently isolated from Drosophila, Caenorhabditis elegans, yeast, and mammalian cells. To date, seven members of the IAP family in mammalian cells have been identified (8, 13, 38): XIAP (32, 12, 66), c-IAP1 and c-IAP2 (49), NAIP (51, 32), Survivin (1), Bruce (16), and the most recent member, ML-IAP (also known as Livin and KIAP) (25, 31, 67). When compared with other antiapoptotic proteins, such as p35 or CrmA, IAPs are found to protect against the broadest spectrum of apoptotic signals.
A suggested mechanism of IAP apoptotic suppression appears to be through direct caspase inhibition. Several of the human IAP family proteins have been reported to directly bind and inhibit specific members of the caspase family. For example, XIAP, c-IAP1, c-IAP2, and Survivin directly bind and inhibit caspases 3, 7, and 9 but not caspase 1, 6, 8, or 10 (9, 10, 26, 50, 56, 61). In contrast, NAIP does not seem to bind caspases (50), even though inhibition of caspase 3-like caspases (3 and 7) has been reported (48, 57). In fact, recent studies indicate that NAIP acts by both caspase-dependent and -independent pathways (35).
Other intracellular components, such as the NF-κB pathway and JNK1, reportedly play a role in the antiapoptotic activity of IAPs (19, 53, 58). Transcription of c-IAP1, c-IAP2, and XIAP genes was found to be strongly up regulated upon treatment of cells with tumor necrosis factor alpha (TNF-α), interleukin 1β, or lipopolysaccharide (LPS). c-IAP1 and c-IAP2 have been shown to activate NF-κB (58). XIAP also strongly stimulates NF-κB via the TAK1 signaling pathway (19).
We found previously that selective activation of the mitogen-activated protein (MAP) kinase JNK1 is necessary for the antiapoptotic activity of XIAP but not that of c-IAP1 and c-IAP2 (53). These findings lead us to investigate whether other members of the IAP family would depend on a similar MAP kinase-dependent mechanism to exert their antiapoptotic effect. Therefore, we compared the effects of other components of the IAP family, NAIP, Survivin, and ML-IAP on the activation of MAP kinase pathways, we identified additional components of this antiapoptotic pathway, and we investigated the mechanism by which activation of MAP kinase inhibits apoptosis.
MATERIALS AND METHODS
Plasmids.
Plasmids encoding JNK1, JNK2, p38, ERK2, MKK4, β-galactosidase (β-Gal)-ICE, XIAP, and the catalytically inactive mutants MKK4 (AA), JNK1 (AF), and p38 (AF) used in this study have been previously described (53). JNK3, MKK7, catalytically inactive mutant MKK7 (KM), Survivin, NAIP-BIR1-3, ML-IAP, TAB1, and ASK1 (KM) were expressed from pcDNA3 (Invitrogen); TAK1 and TAK1 (KW) were expressed in pCMV6. Human Survivin was subcloned into a pcDNA3 vector containing an HA tag. JNK3-FLAG was also subcloned into pcDNA3. We, as well as others (35, 50), have not been able to express the full-length NAIP protein in 293T cells. For this reason, we used a truncated form of the NAIP protein that contains the first 367 amino acids (aa), inclusive of the three baculovirus IAP repeats (BIR domains), which has been described to be a functional protein since it is able to protect against apoptosis (35, 48, 50).
The ability of the MKK4 (AA) and MKK7 (KM) mutants to act as dominant negatives was determined by their ability to block at least 50% of MEKK1-mediated JNK1 activation (data not shown). The capacity of TAK1 (KW) to act as dominant negative was determined previously (28). The ability of JNK1 (AF) to act as a dominant-negative mutant and therefore to inhibit JNK1 activation was assessed as previously described (53). In addition, JNK1 (AF) was also able to block ∼60% of TAK1/TAB1 mediated c-Jun phosphorylation (data not shown). The same effect was also observed in 293T cells transfected with ICE- and TNF-α-treated MCF7-Fas cells. The expression of JNK1 (AF) did not affect expression of XIAP, NAIP-BIR1-3, or ML-IAP in any of the in vivo and in vitro experiments, as assessed by Western blotting.
Transfection and cell culture.
Human embryonic kidney cells (293T) were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. For transfection, each well of a six-well plate was seeded with 7 × 105 cells. Cells were transfected 18 h later using Lipofectamine Plus reagent (Gibco) for 3 h and incubated for 18 h before lysis. MCF7-Fas cells were grown in RPMI 1640 containing 10% FBS, 200 μg of G418/ml, and 100 μg of hygromycin/ml and were grown at 37°C in 5% CO2. For transfection, each well of a six-well plate was seeded with 2.5 × 105 cells, and 24 h after plating, wells were transfected using Lipofectamine Plus reagent (Gibco). Twenty-four hours after transfection, cells were treated with TNF-α (100 ng/ml). After 16 h, cells were fixed and stained as described below.
Establishment of stable transfectants were obtained as follows: human embryonic kidney cells (293T) were transfected with pBMN-Z-I-Blasto, pBMN-TAK1(KW)-I-Blasto, or pBMN-ASK1(KW)-I-Blasto by the calcium phosphate precipitation method and selected in medium containing blasticidine S (10 μg/ml) (28).
Cell lysis and kinase assay.
Cell lysis was performed for 30 min at 4°C with lysis buffer (25 mM HEPES [pH 7.6], 1% Triton X-100, 137 mM NaCl, 3 mM β-glycerophosphate, 3 mM ethylendiaminetetraacetic acid, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF]). Expression of MAP kinase proteins was quantified by densitometry after Western blot analysis, and equivalent amounts were immunoprecipitated at 4°C for 2 h. The immunoprecipitates were washed twice with lysis buffer and twice with kinase buffer (see below) before performing the kinase assay. Hemagglutinin (HA)- and Myc-tagged proteins were immunoprecipitated using 20 μl of agarose-protein A (Pierce) pre-incubated with anti-HA antibody or anti-Myc antibody (5 μg; Boehringer Mannheim and Upstate, respectively) and FLAG-tagged proteins with 20 μl of agarose conjugated with the M2 anti-FLAG monoclonal antibody (Sigma).
In vitro kinase assays were performed as previously described (53) with the difference that a 30-min incubation time was used for the detection of JNK2 and JNK3 kinase activity. Activation of JNK1 was carried out in 293T cells. As previously reported (53), ICE expression did not inhibit XIAP-mediated JNK1 activation. We performed similar experiments with MCF7-Fas cells. We found that XIAP, NAIP, or ML-IAP activates JNK1 in MCF7-Fas cells and that neither expression of ICE in 293 cells or treatment with TNF-α in MCF7-Fas cells affected XIAP-, NAIP-, or ML-IAP-mediated activation of JNK1.
Detection of apoptotic cells. (i) β-Gal staining.
Cells were transfected with the indicated plasmids together with β-Gal-expressing vector and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (reagent for β-Gal expression) to allow the visualization of transfected cells and morphology observation. Quantification of apoptotic cells was determined at the microscope by counting over five fields for each sample. Apoptotic cells appear to be smaller and rounder and show condensed and misshapen nuclei compared to viable cells, which are flat and well spread and have easily discernible nuclei. Protein expression for all the transfected constructs was assessed by Western blotting on duplicate lysates of original transfections used for the apoptosis assays.
(ii) AnnexinV-PE/FACS analysis.
Cell were transfected with the indicated plasmids together with green fluorescent protein (GFP) vector (Clontech Laboratories) to allow quantitation of transfection efficiency. AnnexinV-phycoerythrine (PE) staining was performed as suggested by the manufacturer (PharMingen). Briefly, adherent cells were detached from the plates and centrifuged for 5 min at 65 × g. After removing the supernatant, cells were washed with AnnexinV binding buffer and centrifuged again, and the supernatant decanted by inversion of the tube. Cells were resuspended, and AnnexinV-PE conjugate (5 μl) was added to each sample, incubated for 10 min in the dark, and then analyzed by fluorescence-activated cell sorting (FACS) within 1 h.
Death by apoptosis was quantified both by X-Gal staining of cells and AnnexinV-PE-FACS analysis for each experiments. The results obtained using the two different techniques were comparable, and therefore, the data show representative experiments.
Coimmunoprecipitations and immunoblot assays.
Cells were washed extensively and lysed in 200 μl of lysis buffer containing 50 mM HEPES, 100 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Nonidet P-40, 14 mM pepstatin A, 100 mM leupeptin, 3 mM benzamidine, 1 mM PMSF, 1 mM sodium pyrophosphate, 10 mM sodium orthovanadate, 100 U of aprotinin/ml, and 100 mM sodium fluoride. After incubation for 30 min on ice, cell lysates were centrifuged (13,000 × g, 10 min, 4°C) and the supernatants were recovered. Cell lysates were precleared three times for 20 min at 4°C with 20 μl of protein A-Sepharose beads and were mixed with specified antibodies for 3 h at 4°C under constant agitation. Immune complexes were allowed to bind to 20 μl of protein A-Sepharose beads overnight, beads were washed three times with lysis buffer, and the washed beads resuspended in 30 μl of Laemmli buffer and boiled for 10 min. Immunoprecipitates were separated on sodium dodecyl sulfate (SDS)-12% polyacrylamide gels and transferred to nitrocellulose membranes. Filters were blocked with 5% nonfat milk in blocking buffer (Tris-buffered saline [TBS], 50 mM Tris-Cl [pH 7.5], 150 mM NaCl, 0.1% Tween 20) and incubated with specified antibody for 2 h and with peroxidase-conjugated secondary antibody for 1 h at ambient temperature. Specific bands were revealed using the ECL Plus system (Amersham).
In vitro binding assays.
In vitro translation of TAK1 was performed using standard procedures (Promega). XIAP-GST protein was expressed from a pGEX vector (Pharmacia) and purified as suggested by the manufacturer, and JNK1-HIS protein was purchased from Santa Cruz. Gluthatione- or Ni-nitrilotriacetic acid (NTA)-conjugated beads (from Sigma and Qiagen, respectively) were used to precipitate XIAP-GST and JNK1-HIS. Binding assays were performed in lysis buffer, and TAK1 interaction with XIAP or JNK1 was detected by Western blotting using an anti-TAK1 antibody (Santa Cruz).
Caspase activation in cytosolic extracts.
Cytosolic extracts from transfected 293T (100-mm-diameter dishes) were prepared essentially as previously described (33), with several modifications (11). Briefly, cells were washed once with ice-cold buffer A and pelleted by centrifugation. Packed cell pellets were suspended in 1 or 2 volumes of buffer A, incubated on ice for 20 min, and then disrupted by 15 to 30 passages through a 26-gauge needle. Cell extracts were clarified by centrifugation at 16,000 × g for 10 min, and the resulting supernatants were used for cell-free assays. For initiating caspase activation, 10 μM horse heart cytochrome c (Sigma) together with 1 mM dATP was added, and the assays were incubated at 30°C for 10 min. One microliter (10 μg of total protein) was measured for caspase activity by monitoring the release of 7-amino-4-trifluoromethyl coumarin (AFC) DEVD-containing synthetic peptides using continuously reading instruments as previously described (10). Fluorogenic AFC caspase substrate (Ac-DEVD-AFC) was purchased from Sigma.
RESULTS
XIAP, NAIP, and ML-IAP selectively activate JNK kinases.
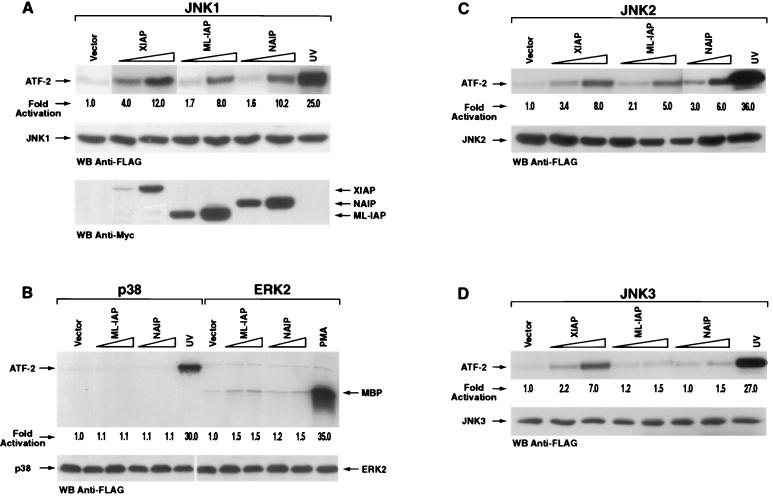
The ability of the IAP family members NAIP and ML-IAP to induce MAP kinase activation was assessed. 293T cells were transfected with plasmids encoding XIAP, NAIP-BIR1-3, or ML-IAP and MAP kinase JNK1, p38, or ERK2. After immunoprecipitation, an in vitro kinase assay was performed using ATF-2 or MBP as substrates. As previously reported (53), XIAP activates JNK1 (Fig. 1A). Expression of NAIP-BIR1-3 or ML-IAP together with JNK1 resulted in 10- and 8-fold increases in the phosphorylation of ATF-2 substrate, respectively (Fig. 1A). In contrast, neither p38 nor ERK2 activity was increased by coexpression with XIAP, NAIP-BIR1-3, or ML-IAP (Fig. 1B). We also observed activation of JNK2 and JNK3 when XIAP was coexpressed; however, detection required prolonged incubation with the ATF-2 substrate, suggesting that the extent of activation of these isoforms was markedly less than that observed with JNK1 (Fig. 1C and D). NAIP-BIR1-3 and ML-IAP activated JNK1 and JNK2 but not JNK3 (Fig. 1A, C, and D). However, NAIP and ML-IAP activation of JNK2 was weaker than that observed for JNK1 (Fig. 1A and C). Therefore, NAIP-BIR1-3 and ML-IAP activate both JNK1 and JNK2 although to a lesser extent than XIAP. JNK1 activation mediated by XIAP, NAIP-BIR1-3, and ML-IAP was also observed in MCF7-Fas cells (data not shown). These data support the contention that the IAP family members have a selective effect on activation of the JNK pathway with the predominant effect being on JNK1.
FIG. 1.
XIAP, NAIP, and ML-IAP selectively activate JNK kinases. 293T cells were transfected with vectors encoding JNK1 (A), p38 or ERK2 (B), JNK2 (C), or JNK3 (D) (200 ng each) in the absence or presence of increasing concentrations of XIAP, NAIP-BIR1-3, or ML-IAP (200 or 800 ng). The amount of transfected cDNA was kept constant in each sample by adding control pcDNA3 vector. An in vitro kinase assay was performed using ATF-2 or MBP as substrate. Kinase activity was quantitated by PhosphorImager and is expressed as fold induction relative to the basal level of phosphorylation of each MAP kinase. UV and phorbol myristate acetate were used as positive controls. Western blottings showing equal expression levels of JNK1 are reported for each experiment. Expression levels of XIAP, NAIP-BIR1-3, or ML-IAP were comparable in each experiment and therefore are shown only in panel A.
Survivin was also assayed for MAP kinase activation. Expression vectors encoding for mouse or human Survivin were transiently transfected, together with JNK1, p38, and ERK2, into 293T cells and activation of the MAP kinases measured. Neither murine nor human Survivin was able to activate JNK1, p38, or ERK2 (data not shown).
XIAP, NAIP, and ML-IAP require JNK1 for protection against TNF-α- and ICE-induced apoptosis.
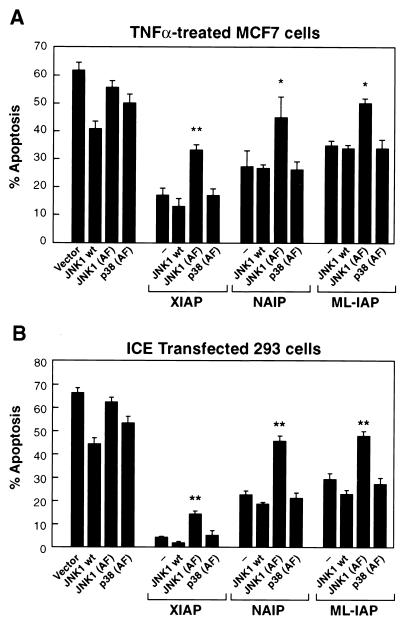
Previous reports demonstrated the ability of XIAP and NAIP to protect against TNF-induced apoptosis (12, 14, 32, 59, 61). In order to investigate whether activation of JNK1 is also important for the protective activity of the IAPs against TNF-α-induced apoptosis, MCF7 cells were transiently transfected with plasmids expressing XIAP, NAIP-BIR1-3, or ML-IAP together with either empty vector, wild-type (wt) JNK1, or catalytically inactive JNK1 [JNK1 (AF)]. The ability of JNK1 (AF) to act as a dominant-negative mutant and therefore inhibit activation of endogenous JNK1 has been previously assessed (see Materials and Methods). Cells were then treated with TNF-α, and the effects of coexpression of wt JNK1, JNK1 (AF), or a catalytically inactive form of p38 [p38 (AF)] used as a control on XIAP, NAIP-BIR1-3, or ML-IAP protection against apoptosis were assessed. Each apoptotic assay was quantified both with X-Gal staining of cells and AnnexinV-PE-FACS analysis. The results obtained using the two different techniques were comparable, and therefore, the data show representative experiments performed with X-Gal. Interestingly, in the presence of JNK1 (AF), the protective effect of XIAP against TNF-α-induced apoptosis was markedly reduced (Fig. 2A). Moreover, the ability of NAIP-BIR1-3 and ML-IAP to protect against TNF-α-induced apoptosis also was impaired when the catalytically inactive form of JNK1 was coexpressed (Fig 2A). On the contrary, expression of p38 (AF) did not inhibit XIAP, NAIP-BIR1-3, and ML-IAP protection against TNF-α-induced apoptosis, therefore showing the specificity of the JNK1 pathway. In addition, whereas expression of p38 (AF) or JNK1 (AF) alone did not seem to significantly affect TNF-α−induced apoptosis, some degree of protection was observed in the presence of wt JNK1. These results further support our contention that a functional JNK1 is important for the full inhibition of TNF-α-mediated apoptosis by XIAP, NAIP-BIR1-3, and ML-IAP.
FIG. 2.
XIAP, NAIP, and ML-IAP require JNK1 for protection against apoptosis. (A) Effect of wt JNK1, JNK1 (AF), or p38 (AF) on XIAP, NAIP, or ML-IAP protection against TNF-α-induced apoptosis. MCF7 cells were transfected with control vector pcDNA3 alone or plasmids encoding XIAP, NAIP-BIR1-3, or ML-IAP (200 ng each), together with control vector, wt JNK1, JNK1 (AF), or p38 (AF) as a control (800 ng each). Transfected cells were treated with TNF-α for 12 h (100 ng/ml) and processed as described in Materials and Methods. Effects on cell viability were determined using X-Gal staining and AnnexinV-PE/FACS analysis. % apoptosis, incidence of apoptotic cells among the β-Gal-positive cells (transfected cells). Data represent the mean ± standard error of at least three experiments, each run in duplicate and scored blind. ∗, P < 0.05 (determined by an unpaired t test); ∗∗, P < 0.001. Values were calculated compared to the control samples expressing only pcDNA3. (B) Effect of wt JNK1 or JNK1 (AF) on XIAP, NAIP, or ML-IAP protection against ICE-induced apoptosis. 293T cells were transfected with plasmids encoding ICE-β-Gal alone (200 ng) or together with wt JNK1, JNK1 (AF), or p38 (AF) alone or in the presence of pcDNA3, XIAP, NAIP-BIR1-3, or ML-IAP (200 ng each). Parental pcDNA3 vector was added to normalize the amount of transfected DNA.
To address the role of IAP-mediated activation of JNK1 in other apoptosis pathways, 293T cells were transfected with an expression vector encoding for β-Gal-ICE (caspase 1) fusion protein plus a control vector, XIAP, NAIP-BIR1-3, or ML-IAP in the absence or presence of JNK1 or JNK1 (AF). Samples were stained for β-Gal and cell mortality was assessed (39). Each of the IAP proteins protected against ICE-induced apoptosis. A remarkable decrease in their antiapoptotic activity was observed when the catalytically inactive JNK1, but not wt JNK1, was coexpressed (Fig 2B). Wt JNK1 or JNK1 (AF) expression vectors were also transfected with β-Gal-ICE in the absence of XIAP, NAIP-BIR1-3, or ML-IAP to determine the effect of their expression on apoptosis. These results suggest that activation of JNK1 contributes to XIAP, NAIP-BIR1-3, or ML-IAP protection against ICE-induced apoptosis.
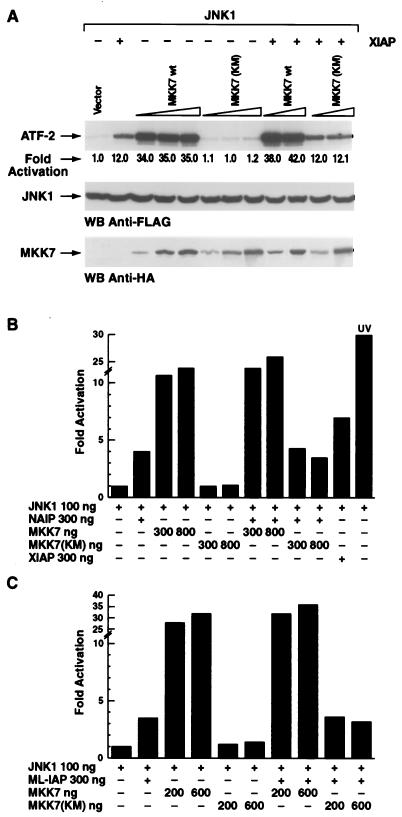
XIAP, NAIP, and ML-IAP activate JNK1 independently of MKK7/MKK4 signaling cascade.
JNK proteins are directly activated by the MAP kinase kinase family member MKK4 (6, 40), which also activates p38, and by MKK7, which selectively activates the components of the JNK family (20, 63). We have previously reported that XIAP-induced activation of JNK1 is independent of the MKK4 signaling cascade (53). Catalytically inactive mutants of both MKK7 [MKK7 (KM)] and MKK4 [MKK4 (AA)] were used to determine whether NAIP-BIR1-3 or ML-IAP acts through MKK7 or MKK4 to activate JNK1. The ability of MKK7 (KM) and MKK4 (AA) to behave as dominant negatives was established in control experiments (see Materials and Methods). Expression of MKK7 (KM) did not decrease XIAP-mediated activation of JNK1 (Fig. 3A). Moreover, transfection of increasing amounts of MKK7 (KM) in 293T cells did not significantly affect NAIP-BIR1-3- or ML-IAP-dependent JNK1 activation (Fig. 3B and C). Similar results were observed when using MKK4 (AA) (data not shown). XIAP, NAIP, and ML-IAP expression were not altered by the expression of the MKK7 wild type or MKK7 (KM) (data not shown).
FIG. 3.
XIAP, NAIP, and ML-IAP activate JNK1 independently of MKK7/MKK4. Increasing amounts (from 200 to 800 ng) of wt MKK7 or MKK7 (KM), were cotransfected together with JNK1 (100 ng) in the presence or absence of XIAP (A), NAIP (B), or ML-IAP (C) (200 ng each). In vitro kinase assays were performed on immunoprecipitated JNK1 using ATF-2 as substrate, and kinase activity was quantitated by PhosphorImager. Western blottings show consistent expression of JNK1 and wt MKK7 or MKK7 (KM) using anti-FLAG and anti-HA antibody, respectively. UV activation is shown as a positive control of JNK1 activation. The standard deviation was always <6%.
To exclude the possibility that MKK4 and MKK7 function in the IAP-JNK1 pathway redundantly, increasing concentrations of MKK7 (KM) and MKK4 (AA) were coexpressed together with XIAP and the effects on JNK1 activation were determined. XIAP-mediated activation of JNK1 was not altered by simultaneous expression of catalytically inactive MKK7 and MKK4 (data not shown). These data suggest that XIAP, NAIP-BIR1-3, and ML-IAP activate JNK1 by a mechanism that is independent of the MKK7 and MKK4 signaling cascade.
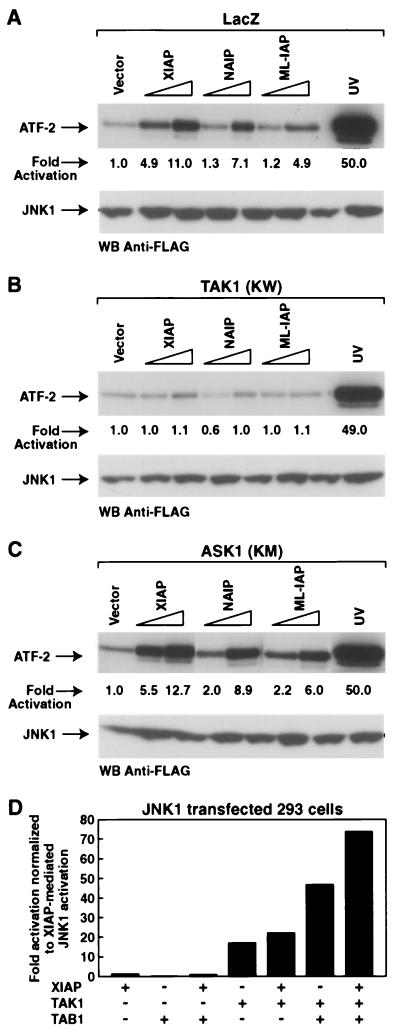
XIAP, NAIP, and ML-IAP activate JNK1 through TAK1.
Since XIAP-, NAIP-, and ML-IAP-mediated activation of JNK1 occurs independently of the MKK4/MKK7 pathway, we searched for other molecules that could be responsible for the transmission of the signal. XIAP has been reported to functionally interact with both the BMP receptor and the adapter molecule TAB1, which is a coactivator of TAK1 (70). TAK1 is an upstream MAP3 kinase that activates JNK1 and p38 in response to transforming growth factor β1 (TGF-β1) stimulation (17, 18, 41, 68). In addition, XIAP-mediated stimulation of NF-κB is inhibited in the presence of a catalytically inactive mutant of TAK1 [TAK1 (KW)] (19). Therefore, we investigated whether TAK1 might be involved in the IAP-mediated activation of JNK1. We also used a catalytically inactive mutant of ASK1 [ASK1 (KM)] as a control of specificity. For this purpose, 293T stably transfected cells expressing either LacZ control vector, TAK1 (KW), or ASK1 (KM) were transfected with vectors encoding XIAP, NAIP-BIR1-3, or ML-IAP and JNK1 activation was determined. XIAP-, NAIP-BIR1-3-, or ML-IAP-mediated JNK1 activation was inhibited in the presence of TAK1 (KW) (Fig 4B), whereas LacZ or ASK1 (KM) had no effect (Fig. 4A and C). These data suggest that activation of JNK1 by XIAP, NAIP, and ML-IAP is dependent upon TAK1.
FIG. 4.
XIAP, NAIP, and ML-IAP activate JNK1 through TAK1. (A, B, C) Effects of LacZ, TAK1 (KW), or ASK1 (KM) on XIAP-, NAIP-, and ML-IAP-dependent JNK1 activation. Plasmids encoding wild-type JNK1 (100 ng) and increasing amounts of XIAP, NAIP-BIR1-3, or ML-IAP (200 and 600 ng) were transfected in 293T cells stably expressing LacZ control gene (A), TAK1 (KW) (B), or ASK1 (KM) (C). An in vitro kinase assay was performed on immunoprecipitated JNK1 using ATF-2 as substrate, and kinase activity was quantitated by PhosphorImager. UV stimulation is also shown. Western blottings show equal expression of JNK1. Expression of XIAP, NAIP, or ML-IAP was not altered by the expression of TAK1 (KW) (data not shown). (D) Effects of XIAP, TAK1, and TAB1 on JNK1 activation. 293T cells were transfected with vectors encoding JNK1 (100 ng) plus XIAP (600 ng), TAK1 (15 ng), or TAB1 (5 ng) alone or in combination. In vitro kinase assay was performed using ATF-2 as a substrate. The levels of kinase activity are expressed as fold induction normalized to the XIAP-mediated activation of JNK1.
In order to investigate the functional interactions between XIAP, TAK1, and TAB1, 293T cells were transfected with plasmids encoding JNK1 plus XIAP, TAK1, or TAB1 alone or in combination, and the effects on JNK1 activation were determined by in vitro kinase assay. TAB1 expression had no effect on JNK1 activation and did not increase XIAP-mediated activation of JNK1 (Fig. 4D). As expected, TAK1 alone activated JNK1, and a strong activation of JNK1 was observed when TAK1 and TAB1 were coexpressed. Interestingly, a significantly enhanced activation of JNK1 was observed when XIAP was coexpressed with TAK1; however, the most dramatic increase of JNK1 activation was observed when XIAP was coexpressed with TAK1/TAB1, indicating that XIAP/TAK1/TAB1 synergistically activate JNK1 and suggesting that TAB1 is a required element for the XIAP/TAK1-mediated activation of JNK1.
XIAP, NAIP, and JNK1 interact with TAK1.
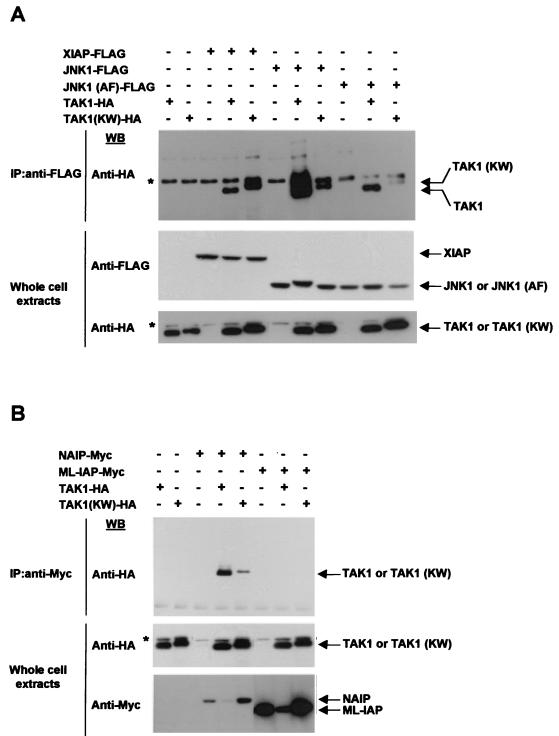
Since TAK1 and TAB1 appear to transmit the signal between the IAPs and JNK1, we investigated the possibility that the IAPs and JNK1 would physically interact with TAK1 and TAB1. In in vivo binding assays, vectors encoding XIAP-FLAG, NAIP-BIR1-3-Myc, ML-IAP-Myc, JNK1-FLAG, or JNK1 (AF)-FLAG were cotransfected with wt TAK1-HA or TAK1 (KW)-HA in 293T cells. Cell extracts were immunoprecipitated using anti-FLAG or anti-Myc antibody and analyzed by Western blotting with an anti-HA antibody. Cell extracts were also directly subjected to immunoblot analysis to check for protein expression (Fig. 5A, B). XIAP, NAIP, JNK1, and JNK1 (AF), but not ML-IAP, were found to coprecipitate with TAK1 and to a lesser extent with TAK1 (KW), thereby demonstrating that an interaction exists between XIAP, NAIP, JNK1, and TAK1 (Fig. 5A and B).
FIG. 5.
XIAP, NAIP, and JNK1 interact with TAK1 in vivo. (A) In vivo interaction of XIAP, JNK1, or JNK1 (AF) with TAK1 or TAK1 (KW). Vectors encoding XIAP-FLAG, JNK1-FLAG, or JNK1 (AF)-FLAG were cotransfected with wt TAK1-HA or TAK1 (KW)-HA in 293T cells. Cell extracts were immunoprecipitated (IP) using anti-FLAG antibody-conjugated beads. Coprecipitated TAK1 or TAK1 (KW) was detected by Western blot analysis with an anti-HA antibody. Cell extracts were also directly subjected to immunoblot analysis (IB) to check for protein expression. Asterisks indicate the presence of an unspecific band that appears when the anti-HA antibody is used. (B) Interaction of NAIP or ML-IAP with TAK1 or TAK1 (KW). Vectors encoding NAIP-BIR1-3-Myc or ML-IAP-Myc were cotransfected with TAK1-HA or TAK1 (KW)-HA in 293T cells. Cell extracts were immunoprecipitated using anti-Myc antibody. Coprecipitated TAK1 or TAK1 (KW) was detected by Western blot analysis with an anti-HA antibody. Cells extracts were also directly subjected to immunoblot analysis to check for protein expression.
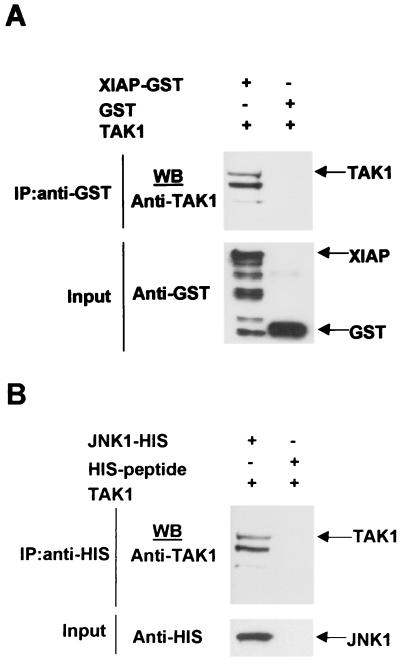
In order to confirm the in vivo binding assays, in vitro binding assays were performed using in vitro-translated TAK1 protein and recombinant GST-XIAP and HIS-JNK1. Glutathione S-transferase (GST) protein and a HIS-peptide were used as negative controls. XIAP-GST, JNK1-HIS, or their respective negative control proteins were incubated with glutathione- or Ni-NTA-conjugated beads, respectively. TAK1 was then added to the reaction mixtures, and products were detected by Western blotting using an anti-TAK1 antibody. Input proteins were also directly subjected to immunoblot analysis (Fig. 6A and B). TAK1 was found to coprecipitate with XIAP or JNK1 but not with GST or HIS-peptide, thereby confirming the in vivo interactions and suggesting that XIAP and JNK1 interact directly with TAK1 (Fig. 6A and B). These results support the idea that XIAP, TAK1, and JNK1 coexist in a complex.
FIG. 6.
XIAP, NAIP, and JNK1 directly interact with TAK1. (A and B) In vitro interaction of XIAP or JNK1 with TAK1. GST-XIAP or HIS-JNK1 or the respective negative controls GST and HIS-peptide recombinant proteins were incubated with gluthatione or Ni-NTA-conjugated beads and in vitro-translated TAK1 protein was added. Coprecipitation of TAK1 was detected by Western blotting using an anti-TAK1 antibody. Input proteins were detected by Western blot using anti-GST and anti-HIS antibodies.
XIAP, NAIP, and ML-IAP interact with TAB1.
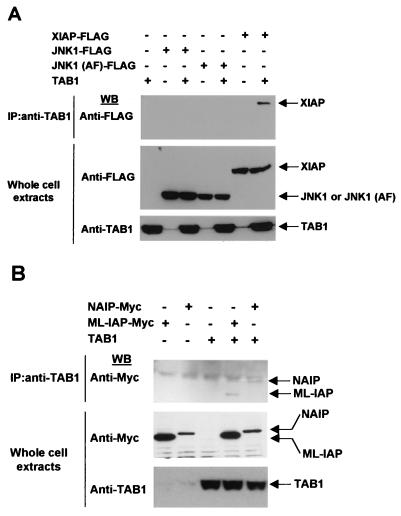
XIAP reportedly recruits TAB1 to the BMP/TAK1 receptor complex which results in TAK1 activation (55, 71). Thus, we investigated the possibility that NAIP, ML-IAP or JNK1 also would coprecipitate with TAB1. Vectors encoding XIAP-FLAG, JNK1-FLAG, JNK1 (AF)-FLAG, NAIP-BIR1-3-Myc, or ML-IAP-Myc were cotransfected with plasmid encoding TAB1 in 293T cells. Cell extracts were subjected to immunoprecipitation with anti-TAB1 antibody, and XIAP, JNK1, JNK1 (AF), NAIP, and ML-IAP were detected by Western blotting using an anti-FLAG or anti-Myc antibody. Cell extracts were also directly subjected to immunoblot analysis to check for protein expression. All analyzed IAPs did bind to TAB1 (Fig. 7A and B).
FIG. 7.
XIAP, NAIP, and ML-IAP interact with TAB1. (A) Interaction of XIAP, JNK1, or JNK1 (AF) with TAB1. Vectors encoding XIAP-FLAG, JNK1-FLAG, or JNK1 (AF)-FLAG were cotransfected with TAB1 in 293T cells. Cell extracts were subjected to immunoprecipitation with anti-TAB1 antibody, and coprecipitated XIAP, JNK1, or JNK1 (AF) was detected by Western blot analysis using anti-FLAG antibody. Cells extracts were subjected to immunoblot analysis to check protein expression. (B) Interaction of NAIP or ML-IAP with TAB1. Vectors encoding NAIP-BIR1-3-Myc or ML-IAP-Myc were cotransfected with TAB1 in 293T cells. Cell extracts were subjected to immunoprecipitation with anti-TAB1 antibody and precipitated NAIP or ML-IAP were detected by Western blot analysis with anti-Myc antibody. Cells extracts were subjected to immunoblot analysis to check protein expression.
We also investigated whether the IAPs would interact with JNK1. XIAP-Myc, NAIP-BIR1-3-Myc, and ML-IAP-Myc were cotransfected together with JNK1-FLAG or JNK1 (AF)-FLAG, and cell lysates were immunoprecipitated with anti-Myc antibody. JNK1-FLAG or JNK1 (AF)-FLAG were detected by Western blot analysis with an anti-FLAG antibody. We were not able to detect significant interactions between XIAP, NAIP, or ML-IAP and JNK1 (data not shown), therefore suggesting that TAK1 and TAB1 may mediate the functional interaction between XIAP and JNK1.
IAP protection against TNF-α- and ICE- or BAX-induced apoptosis occurs via distinct mechanisms.
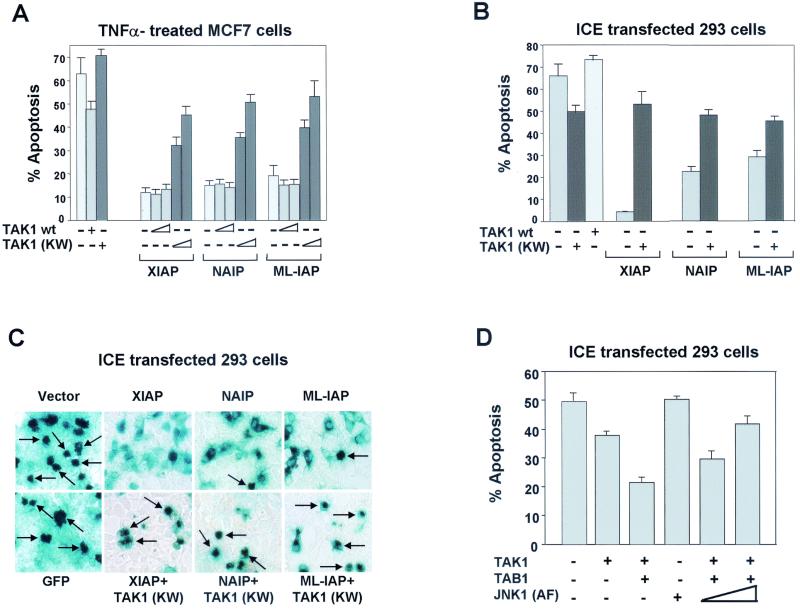
Expression of TAK1 (KW) blocked the XIAP-, NAIP-, and ML-IAP-mediated activation of JNK1. In addition, JNK1 activation appears necessary for the suppression of TNF-α- and ICE-induced apoptosis by these proteins. Thus, we next evaluated the ability of TAK1 (KW) to inhibit the anti-apoptotic effect of XIAP, NAIP-BIR1-3-, or ML-IAP on TNF-α-, ICE-, or BAX-induced cell death. MCF7 cells were transiently transfected with plasmids expressing XIAP, NAIP-BIR1-3, or ML-IAP together with either empty vector, TAK1, or TAK1 (KW). A β-Gal-expressing plasmid was also transfected to allow cell morphology observation and quantitation of the transfection efficiency. Cells were then treated with TNF-α, and the effects of TAK1 or TAK1 (KW) on XIAP, NAIP-BIR1-3 or ML-IAP protection against apoptosis were assessed by β-Gal staining. Similar experiments were performed using AnnexinV-PE-FACS analysis to quantify cell death. Expression of TAK1 did not affect the ability of XIAP, NAIP-BIR1-3, or ML-IAP to protect against TNF-α-induced apoptosis. In contrast, the presence of TAK1 (KW) remarkably reduced the protective effect of XIAP, NAIP-BIR1-3, or ML-IAP to protect against TNF-α-induced apoptosis (Fig. 8A). Expression of wt TAK1 partially inhibited the ability of TNF-α to induce apoptosis, whereas TAK1 (KW) partially enhanced TNF-α-induced apoptosis (Fig. 8A). As a control, catalytically inactive ASK1 (ASK1 (KM)) was transfected together with XIAP, NAIP-BIR1-3, or ML-IAP and had no effect on their ability to protect against TNF-α-induced apoptosis (data not shown). Thus, XIAP, NAIP, and ML-IAP antiapoptotic activity against TNF-α-induced apoptosis is inhibited by JNK1 (AF) and TAK1 (KW).
FIG. 8.
TAK1 (KW) inhibits XIAP, NAIP, and ML-IAP protection against TNF-α- and ICE-induced apoptosis. (A) Effect of TAK1 or TAK1 (KW) on XIAP, NAIP, or ML-IAP protection against TNF-α-induced apoptosis. MCF7-Fas cells were transfected with plasmids encoding XIAP, NAIP-BIR1-3, or ML-IAP (200 ng each), together with control vector or increasing concentrations of wt TAK1 or TAK1 (KM) (200 and 800 ng). Plasmid expressing β-Gal (200 ng) was also transfected to allow quantitation of apoptotic cells. The effect of expression of empty vector, TAK1, or TAK1 (KW) (800 ng each) without IAPs is also shown. Cells were treated with TNF-α (100 ng/ml) and processed as described in Materials and Methods. Effects on cell viability were determined by X-Gal staining (shown) and AnnexinV-PE/FACS analysis (not shown) and produced similar results. % apoptosis, incidence of apoptotic cells among the β-Gal positive (transfected cells). Data represent the mean ± standard error of at least three experiments, each run in duplicate and scored blind. (B and C) Effect of TAK1 (KW) on XIAP, NAIP, or ML-IAP protection against ICE-induced apoptosis. 293T cells were transfected with plasmids encoding ICE-β-Gal (200 ng) in the absence or presence of wt TAK1, TAK1 (KW), XIAP, NAIP-BIR1-3, or ML-IAP (300 ng each). Parental pcDNA3 vector was added to normalize the amount of transfected DNA. Cell viability was determined by X-Gal staining and AnnexinV-PE/FACS analysis. Arrows indicate examples of apoptotic cells. (D) Effect of activated TAK1 on apoptosis. 293T cells were transfected with plasmids encoding ICE-β-Gal (100 ng) in the presence or absence wt TAK1 or TAB1 alone (50 ng each) or in combination with increasing concentrations of JNK1 (AF) (200 and 800 ng). Cell viability was determined by X-Gal staining and AnnexinV-PE/FACS analysis and produced similar results.
We also assessed the effects of expression of TAK1 (KW) on XIAP, NAIP, and ML-IAP protection against ICE-induced apoptosis. 293T cells were transfected with expression vector encoding a β-Gal-ICE plus control vector or XIAP, NAIP-BIR1-3, or ML-IAP in the absence or presence of TAK1 (KW) mutant and cell death was evaluated by X-Gal staining (Fig. 8C). A significant increase in ICE-induced apoptosis was observed when TAK1 (KW) was coexpressed together with XIAP, NAIP-BIR1-3, or ML-IAP (Fig. 8B and C). As a control, ASK1 (KM) was transfected together with XIAP, NAIP-BIR1-3, or ML-IAP and had no effect on their ability to protect against ICE-induced apoptosis (data not shown). Expression of TAK1 (KW) alone slightly increase the ability of ICE to induce apoptosis whereas TAK1 wt partially reduced ICE apoptotic effect (Fig. 8B). Thus, XIAP, NAIP, and ML-IAP antiapoptotic activity against ICE-induced apoptosis is inhibited by JNK1 (AF) and TAK1 (KW).
Since XIAP protection from ICE- and TNF-α- mediated apoptosis involves TAK1-mediated activation of JNK1, suggesting that an active TAK1 molecule is needed for protection, the effects of the expression of active TAK1 on ICE- and TNF-α-induced apoptosis were investigated. 293T cells were transfected with expression vector encoding for β-Gal-ICE or GFP plus control vector or TAK1, TAB1 alone, or in combination, and cell death was evaluated by X-Gal staining and AnnexinV-PE staining (Fig. 8D). Similar experiments were performed using MCF7-Fas cells and TNF-α to induce apoptosis, and comparable results were obtained (data not shown). TAB1 itself had no effect on ICE- or TNF-α-induced apoptosis (data not shown), whereas some degree of protection was observed in the presence of TAK1 alone, possibly due to TAK1 activation by endogenous components. However, higher protection was obtained when TAK1 and TAB1 were coexpressed, suggesting that activation of TAK1 is important for protection against ICE- and TNF-α-induced apoptosis. Interestingly, coexpression of increasing concentrations of JNK1 (AF) inhibited the protective effect of TAB1-activated TAK1, therefore supporting the idea that protection against apoptosis by the XIAP/TAK1 pathway involves activation of JNK1.
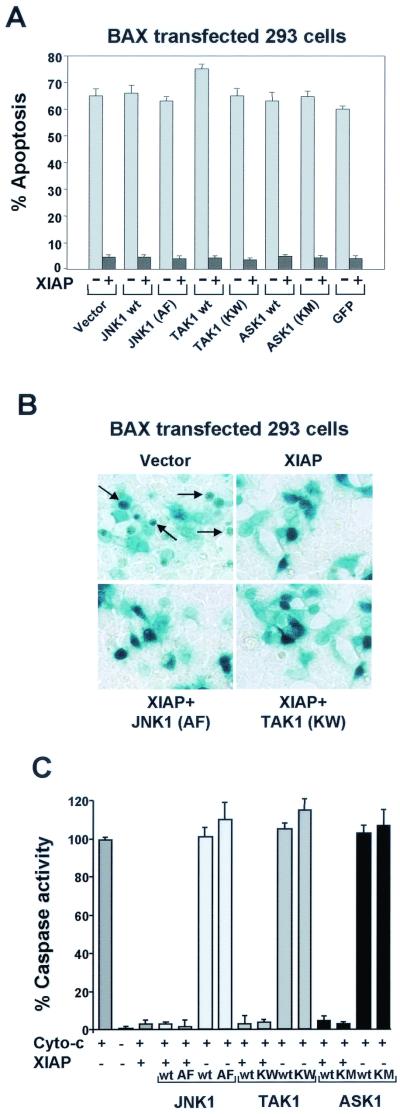
The proapoptotic activity of BAX is correlated with its ability to induce the release of cytochrome c, normally secluded in the mitochondria, in the cytoplasm, thereby leading to caspase activation (2, 5, 64). XIAP as been shown to inhibit BAX-induced apoptosis by binding and directly inhibiting caspase 9 (9, 10). In order to investigate if JNK1 (AF) or TAK1 (KM) would also block XIAP inhibition of caspase activity, we transfected 293T cells with BAX alone or BAX plus XIAP in the presence of control vector, JNK1 (AF), or TAK1 (KW). Transfection efficiency and morphological observation of the cells was evaluated cotransfecting a cDNA expressing β-Gal (Fig. 9B). Expression of neither JNK1 (AF) nor TAK1 (KW) inhibited the antiapoptotic activity of XIAP against BAX-induced apoptosis (Fig. 9A and B). Wild-type JNK1, TAK1, ASK1, ASK1 (KM), or GFP was also coexpressed with XIAP as controls, and no effect on BAX-induced apoptosis was observed (Fig. 9A and B). Therefore, JNK1 (AF) and TAK1 (KW) did not inhibit the XIAP antiapoptotic effect against BAX-induced apoptosis. These observations suggest that XIAP protection against ICE- or BAX-induced apoptosis is achieved by two separate mechanisms: one requiring JNK1 activation and passing through TAK1 and a second involving caspase inhibition.
FIG. 9.
JNK1 (AF) and TAK1 (KW) do not inhibit XIAP, NAIP, and ML-IAP protection against BAX- or caspase-induced apoptosis. (A and B) Effect of JNK1 (AF), TAK1 (KW), or ASK1 (KM) on XIAP protection against BAX-induced apoptosis. 293T cells were transfected with plasmids encoding BAX (100 ng) or XIAP together with one of the following: wt JNK1, JNK1 (AF), TAK1, TAK1 (KW), ASK1, or ASK1 (KM) (600 ng each). Plasmid encoding GFP was also transfected with BAX alone or BAX plus XIAP as a control. Expression vector encoding β-Gal (50 ng) was transfected together with the different plasmids to allow morphological observation of the cells. Effects on cell viability were determined as described above. Arrows indicate examples of apoptotic cells. (C) Effect of JNK1 (AF), TAK1 (KW), or ASK1 (KM) on XIAP inhibition of caspase activity. 293T cells were seeded in 100-mm-diameter dishes and transfected with plasmids encoding XIAP alone (3 μg) or with one of the following: wt JNK1, TAK1, ASK1, JNK1 (AF), TAK1 (KW), or ASK1 (KM) (3 μg). Empty vector was also transfected alone as a control (6 μg). Cell extracts were prepared and cytochrome c was added to induce proteolytic processing of pro-caspase 3. Caspase activity was measured by monitoring the release of AFC DEVD-containing synthetic peptides.
To further investigate this possibility, we used a cell-free system where exogenously added cytochrome c was used to induce proteolytic activation of caspase 9 and subsequently caspase 3 in cytosolic extracts, which can be measured using short fluorogenic peptides (DEVD-AFC [10, 33]). In these cell-free assays, IAPs, and in particular XIAP, have been reported to be strong inhibitors of caspase activity (10, 50). Addition of cytochrome c to extracts prepared from 293T cells transfected with control vector or vectors encoding JNK1, TAK1, wt ASK1, or catalytically inactive ASK1 caused a rapid accumulation of DEVD-specific protease activity (Fig. 9C). Cotransfection of XIAP with the above-mentioned encoding vectors markedly reduced cytochrome c-induced generation of caspase activity similar to control extract expressing only XIAP. Thus, expression of JNK1, TAK1, wt ASK1, or catalytically inactive ASK1 does not appear to affect XIAP inhibition of caspase activity, suggesting that XIAP protection against ICE- or BAX-induced apoptosis is achieved by two separate mechanisms (Fig. 10).
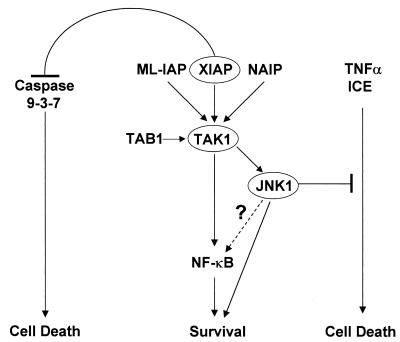
FIG. 10.
Model for the activation of JNK1 by IAPs. IAP-mediated activation of JNK1 passes through TAK1 and promotes cell survival by inhibiting TNF-α- and ICE-induced apoptosis. This event is separate from XIAP inhibition of caspases (see text for details).
DISCUSSION
We previously described another feature of the antiapoptotic mechanism of XIAP which involves the activation of the MAP kinase JNK1 (53). Herein, we extend our initial findings by showing that two other members of the IAP family, NAIP and the newly discovered ML-IAP, are also able to activate JNK1 and that this activation is necessary for protection against TNF-α- and ICE-induced apoptosis. XIAP-, NAIP-, and ML-IAP-mediated activation of JNK1 requires the TAB1/TAK1 but not the MKK4/MKK7 signaling cascade. Expression of TAK1 (KW) inhibited IAPs-mediated activation of JNK1, suggesting that this activation is dependent upon TAK1. Moreover, a dramatic increase of JNK1 activation was observed when XIAP was coexpressed with TAK1/TAB1, indicating that XIAP/TAK1/TAB1 synergistically activate JNK1. In addition, we demonstrate that XIAP, NAIP, and JNK1 associate with TAK1, whereas JNK1 does not seem to coprecipitate with the IAPs, suggesting that TAK1 may mediate the functional interactions between the IAPs and JNK1. Expression of TAK1 or JNK1 inhibitory mutants interfered with the ability of XIAP, NAIP, or ML-IAP to suppress TNF-α- and ICE-induced apoptosis, suggesting that the antiapoptotic function of these IAPs depends upon the TAK1 and JNK1 signaling transduction pathway. However, TAK1 (KW) or JNK1 (AF) did not block XIAP's ability to inhibit BAX or cytochrome c activation of caspases nor did they interfere with the ability of XIAP to directly inhibit caspase 9 or caspase 3. These data suggest that the antiapoptotic effect of XIAP and possibly of other IAPs is achieved by at least two mechanisms: one requiring JNK1 activation and passing through TAK1 and the second involving caspase inhibition (Fig. 10).
Certainly, the ability of some IAP family members to activate MAP kinases is intriguing, as we found that only XIAP, NAIP, and ML-IAP activated JNK1 whereas c-IAP1, c-IAP2 (53), and Survivin failed to do so. We also investigated whether JNK2 and JNK3 were activated by coexpression of XIAP, NAIP, and ML-IAP. XIAP, NAIP, and ML-IAP activated JNK2, although to a lesser extent than JNK1. XIAP was also able to activate JNK3, but only a very weak activation was observed in the presence of NAIP and ML-IAP. However, we cannot rule out the possibility that full-length NAIP would be able to activate all three JNK proteins as we observed with XIAP. The diverse activation of JNKs is interesting and may reflect the distinct tissue expression/function patterns. In contrast, none of the IAP family was able to activate p38 or ERK2.
XIAP-, NAIP-, and ML-IAP-mediated activation of JNK1 occurs independently of the MKK4/MKK7 pathway. XIAP has been shown to participate in the BMP signaling pathway by binding with both the BMP receptor and the adapter molecule TAB1, which is a coactivator of TAK1, thus linking the BMP receptors to TAB1-TAK1 (70). Therefore, we investigated the possibility of a functional interaction between XIAP, NAIP, ML-IAP, and TAK1. We found that a catalytically inactive form of TAK1 blocks XIAP, NAIP, and ML-IAP activation of JNK1. Using in vivo binding assays, we were able to show that TAK1 binds to XIAP and NAIP and also to JNK1. In addition, in vitro binding experiments determined that TAK1 and XIAP or JNK1 bind directly. Surprisingly, we were not able to detect any interaction between ML-IAP and TAK1. However, the possibility that ML-IAP and TAK1 do interact in a physiological relevant manner cannot be totally ruled out since some protein-protein interactions are transient and unstable, making these associations difficult to detect by coprecipitation. Certainly the ability of ML-IAP to bind to TAB1 could support the idea that a binding with TAK1 exists, although this difficult to detect by in vivo coprecipitation. In this scenario it could be envisioned that ML-IAP activation of JNK1 passes through TAK1.
JNK proteins are directly activated by the MAP kinase kinase family members MKK4 and MKK7. The findings showing that IAP-mediated activation of JNK1 occurs independently of MKK4/MKK7, XIAP/TAK1/TAB1 synergistically activate JNK1, and IAPs coprecipitate with TAK1 and TAB1 and that direct binding occurs between XIAP or JNK1 and TAK1 suggest the possibility that a mechanism of JNK1 activation alternative to the TAK1/TAB1, MKK4/7 might exist. For instance, TAK1/TAB1 could activate JNK1 directly. Preliminary results suggest that this may be the case, and further work is in progress.
Activation of JNK1, and therefore subsequent protection against apoptosis, does not seem to correlate with the ability of antiapoptotic proteins to inhibit a specific class of caspases. In fact, XIAP, c-IAP1, and c-IAP2 are all capable of inhibiting caspases 3, 7, and 9; nevertheless, c-IAP1 and c-IAP2 do not activate JNK1. In addition, NAIP and ML-IAP are remarkable activators of JNK1, even though it is still not clear if they are direct caspase inhibitors (48, 50, 57; K. Nomoto and Q. L. Deveraux, unpublished data), suggesting that inhibition of caspases by the IAP family members does not correlate with activation of JNK1. Consistent with this idea, we found that expression of catalytically inactive mutants of TAK1 (KW) or JNK1 (AF) inhibited XIAP, NAIP, and ML-IAP protection against TNF-α- and ICE-induced apoptosis. In contrast, TAK1 (KW) or JNK1 (AF) had no effect on BAX-induced apoptosis or on XIAP inhibition of caspase activity. These observations strongly suggest that XIAP protection against ICE- or BAX-induced apoptosis is achieved by two separate mechanisms.
Although knockout studies of mice suggest that caspase 1 does not play a significant role in developmental PCD (27), these studies failed to address the role of caspase 1 in apoptotic pathways related to other aspects of mammalian cell biology and disease development. For example, recent studies suggest that silencing or loss of caspase 1 is a key mediator of the oncogenic process and survival of renal cells (65) and prostate carcinomas (69) and increased caspase 1 expression may be a factor in Huntington-related neuronal cell death (30). Interestingly, although caspase 1 was the first human caspase identified, its mechanism for inducing apoptosis is still unclear. In this regard, our data suggest that caspase 1-induced apoptosis does not necessarily depend upon caspase 9 or 3 since JNK1 (AF) or TAK1 (KW) block the ability of XIAP to inhibit ICE-induced apoptosis without affecting XIAP ability to inhibit caspase 3 or 9.
Notably the JNK1 and TAK1 dependent antiapoptotic function of XIAP, NAIP, and ML-IAP appears confined to pathways that regulate a balance between apoptotic and pro-inflammatory pathways (e.g., TNF and ICE). In contrast, IAP protection against overexpression of proapoptotic BAX and Fas pathways (data not shown) appears solely dependent upon IAP interaction with caspases. In this regard, NF-κB is a key regulator of the balance between apoptosis and inflammatory responses, and NF-κB activation has been linked to protection against apoptosis (24, 34, 43-45). Interestingly, XIAP-mediated stimulation of NF-κB is inhibited in the presence of catalytically inactive TAK1 (19). Thus, we cannot rule out the possibility that changes in NF-κB activation in the presence of TAK1 (KW) will act downstream or synergistically with the inhibition of JNK1 activation to prevent XIAP protection against PCD. Ongoing studies will aim to elucidate the relationship between XIAP activation of TAK1, JNK1, and NF-κB.
IAP family proteins are characterized by a domain of ∼70 amino acids termed the baculovirus IAP repeat (BIR). Three tandem copies of the BIR domain are present within the known IAP family proteins. In addition to BIR motifs, some IAPs also contain C-terminal RING domains, which have been recently described to be responsible for the autoubiquitination and degradation of the IAPs during the apoptotic process (72, 73). Several studies have suggested that the presence of at least one BIR domain (BIR2) is necessary to inhibit the activity of caspases 3 and 7 and BIR3 for caspase 9 and is therefore essential for the antiapoptotic activity of several IAPs (10, 50, 60). Caspase inhibition by BIR domains appears to be conserved from insects to humans (21). Therefore, it is generally agreed that BIRs are essential domains for the IAPs' antiapoptotic activity. BIR domains also appear to be important for MAP kinase activation. We have previously shown that the XIAP deletion mutant expressing only the three BIR domains is still able to activate JNK1 and to protect against ICE-induced apoptosis (53). Here we show that a construct expressing only the three BIR domains of NAIP is also able to activate JNK1 and to protect against various stimuli such as TNF-α- or ICE-induced apoptosis. Therefore, our results support the idea that the BIR domains are important and sufficient for the antiapoptotic activity of the IAPs.
The fact that the deletion mutant of NAIP carrying only the three BIR domains is able to bind TAK1 suggests the possibility that XIAP would also interact with TAK1 through its BIR domains. Interestingly, we could not demonstrate binding between JNK1 and XIAP, NAIP, or ML-IAP, which suggests that TAK1 is connecting XIAP and NAIP with JNK1. In addition, XIAP, NAIP, and ML-IAP also bind to TAB1; therefore, we cannot exclude the possibility that the interaction between XIAP, NAIP, and TAK1 is mediated by TAB1.
Reportedly, c-IAP1 and c-IAP2 were shown to associate with TRAF1 and TRAF2, whereas XIAP and NAIP do not (49, 50). This association occurs through their BIR domains, which we have also shown to be important for XIAP- and NAIP-mediated JNK1 activation (53), XIAP binding to TAB1 (70), and caspase inhibition (7, 50, 60). Thus, it could be envisioned that the BIR domains have different functions among the IAP family members, which allows them to selectively bind to or activate different signaling molecules.
Taken together, our results describe a TAK1/JNK1-dependent mechanism of IAP protection against apoptosis that appears distinct from caspase inhibition. Our data support the contention that MAP kinase activation, specifically of JNK family members, is related to protection against apoptosis (3, 29, 37, 42, 54). There is a growing consensus that if any correlation exists between activation of MAP kinases and protection or induction of apoptosis, it is stimuli and/or cell type dependent (15, 22, 36, 52). Thus, IAPs evolved as multifunctional proteins that employ at least MAP kinase signal transduction and caspase inhibition as mechanisms to regulate diverse cell death pathways.
Acknowledgments
We thank C. Duckett for providing mouse Survivin and the MCF7-Fas cells, D. Altieri for the human Survivin, N. Roy and J. Reed for the NAIP-BIR1-3-expressing vector, and F. Mercurio for the JNK3 construct. We also thank C. Fearns and A. Ghetti for critical reading of the manuscript and for helpful discussions.
Nicolas Schrantz is a recipient of a fellowship from The Association pour la Recherche Contre le Cancer. This work was supported by grants GM36796, GM28485, and AI15136.
REFERENCES
- 1.Ambrosini, G., C. Adida, and D. C. Altieri. 1997. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3:917-921. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. 1999. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264:687-701. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y. R., and T. H. Tan. 2000. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int. J. Oncol. 16:651-662. [DOI] [PubMed] [Google Scholar]
- 4.Clem, R. J., and L. K. Miller. 1994. Control of programmed cell death by the baculovirus genes p35 and iap. Mol. Cell. Biol. 14:5212-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decaudin, D., I. Marzo, C. Brenner, and G. Kroemer. 1998. Mitochondria in chemotherapy-induced apoptosis: a prospective novel target of cancer therapy. Int. J. Oncol. 12:141-152. [PubMed] [Google Scholar]
- 6.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 7.Deveraux, Q. L., E. Leo, H. R. Stennicke, K. Welsh, G. S. Salvesen, and J. C. Reed. 1999. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 18:5242-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins—suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 9.Deveraux, Q. L., N. Roy, H. R. Stennicke, T. Van Arsdale, Q. Zhou, S. M. Srinivasula, E. S. Alnemri, G. S. Salvesen, and J. C. Reed. 1998. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux, Q. L., R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300-304. [DOI] [PubMed] [Google Scholar]
- 11.Deveraux, Q. L., K. Welsh, and J. C. Reed. 2000. Purification and use of recombinant inhibitor of apoptosis proteins as caspase inhibitors. Methods Enzymol. 322:154-161. [DOI] [PubMed] [Google Scholar]
- 12.Duckett, C. S., F. Li, Y. Wang, K. J. Tomaselli, C. B. Thompson, and R. C. Armstrong. 1998. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol. Cell. Biol. 18:608-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duckett, C. S., V. E. Nava, R. W. Gedrich, R. J. Clem, J. L. Van Dongen, M. C. Gilfillan, H. Shiels, J. M. Hardwick, and C. B. Thompson. 1996. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 15:2685-2694. [PMC free article] [PubMed] [Google Scholar]
- 14.Gotz, R., C. Karch, M. R. Digby, J. Troppmair, U. R. Rapp, and M. Sendtner. 2000. The neuronal apoptosis inhibitory protein suppresses neuronal differentiation and apoptosis in PC12 cells. Hum. Mol. Genet. 9:2479-2489. [DOI] [PubMed] [Google Scholar]
- 15.Ham, J., A. Eilers, J. Whitfield, S. J. Neame, and B. Shah. 2000. c-Jun and the transcriptional control of neuronal apoptosis. Biochem. Pharmacol. 60:1015-1021. [DOI] [PubMed] [Google Scholar]
- 16.Hauser, H. P., M. Bardroff, G. Pyrowolakis, and S. Jentsch. 1998. A giant ubiquitin-conjugating enzyme related to IAP apoptosis inhibitors. J. Cell Biol. 141:1415-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirose, T., W. Fujimoto, T. Tamaai, K. H. Kim, H. Matsuura, and A. M. Jetten. 1994. TAK1: molecular cloning and characterization of a new member of the nuclear receptor superfamily. Mol. Endocrinol. 8:1667-1680. [DOI] [PubMed] [Google Scholar]
- 18.Hirose, T., D. A. O'Brien, and A. M. Jetten. 1995. Cloning of the gene encoding the murine orphan receptor TAK1 and cell- type-specific expression in testis. Gene 163:239-242. [DOI] [PubMed] [Google Scholar]
- 19.Hofer-Warbinek, R., J. A. Schmid, C. Stehlik, B. R. Binder, J. Lipp, and R. de Martin. 2000. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J. Biol. Chem. 275:22064-22068. [DOI] [PubMed] [Google Scholar]
- 20.Holland, P. M., M. Suzanne, J. S. Campbell, S. Noselli, and J. A. Cooper. 1997. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J. Biol. Chem. 272:24994-22998. [DOI] [PubMed] [Google Scholar]
- 21.Huang, Q., Q. L. Deveraux, S. Maeda, G. S. Salvesen, H. R. Stennicke, B. D. Hammock, and J. C. Reed. 2000. Evolutionary conservation of apoptosis mechanisms: lepidopteran and baculoviral inhibitor of apoptosis proteins are inhibitors of mammalian caspase-9. Proc. Natl. Acad. Sci. USA 97:1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ip, Y. T., and R. J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol. 10:205-219. [DOI] [PubMed] [Google Scholar]
- 23.Jaattela, M. 1999. Escaping cell death: survival proteins in cancer. Exp. Cell Res. 248:30-43. [DOI] [PubMed] [Google Scholar]
- 24.Jobin, C., and R. B. Sartor. 2000. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am. J. Physiol. Cell. Physiol. 278:C451-462. [DOI] [PubMed] [Google Scholar]
- 25.Kasof, G. M., and B. C. Gomes. 2001. Livin, a novel inhibitor of apoptosis protein family member. J. Biol. Chem. 276:3238-3246. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, K., M. Hatano, M. Otaki, T. Ogasawara, and T. Tokuhisa. 1999. Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc. Natl. Acad. Sci. USA 96:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuida, K., J. A. Lippke, G. Ku, M. W. Harding, D. J. Livingston, M. S. Su, and R. A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000-2003. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J., L. Mira-Arbibe, and R. J. Ulevitch. 2000. TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J. Leukoc. Biol. 68:909-915. [PubMed] [Google Scholar]
- 29.Leppa, S., and D. Bohmann. 1999. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18:6158-6162. [DOI] [PubMed] [Google Scholar]
- 30.Li, S. H., S. Lam, A. L. Cheng, and X. J. Li. 2000. Intranuclear huntingtin increases the expression of caspase-1 and induces apoptosis. Hum. Mol. Genet. 9:2859-2867. [DOI] [PubMed] [Google Scholar]
- 31.Lin, J. H., G. Deng, Q. Huang, and J. Morser. 2000. KIAP, a novel member of the inhibitor of apoptosis protein family. Biochem. Biophys. Res. Commun. 279:820-831. [DOI] [PubMed] [Google Scholar]
- 32.Liston, P., N. Roy, K. Tamai, C. Lefebvre, S. Baird, G. Cherton-Horvat, R. Farahani, M. McLean, J. E. Ikeda, A. MacKenzie, and R. G. Korneluk. 1996. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349-353. [DOI] [PubMed] [Google Scholar]
- 33.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 34.Mattson, M. P., C. Culmsee, Z. Yu, and S. Camandola. 2000. Roles of nuclear factor kappaB in neuronal survival and plasticity. J. Neurochem. 74:443-456. [DOI] [PubMed] [Google Scholar]
- 35.Mercer, E. A., L. Korhonen, Y. Skoglosa, P. A. Olsson, J. P. Kukkonen, and D. Lindholm. 2000. NAIP interacts with hippocalcin and protects neurons against calcium- induced cell death through caspase-3-dependent and-independent pathways. EMBO J. 19:3597-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mielke, K., A. Damm, D. D. Yang, and T. Herdegen. 2000. Selective expression of JNK isoforms and stress-specific JNK activity in different neural cell lines. Brain Res. Mol. Brain Res. 75:128-137. [DOI] [PubMed] [Google Scholar]
- 37.Mielke, K., and T. Herdegen. 2000. JNK and p38 stresskinases—degenerative effectors of signal-transduction-cascades in the nervous system. Prog. Neurobiol. 61:45-60. [DOI] [PubMed] [Google Scholar]
- 38.Miller, L. K. 1999. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell. Biol. 9:323-328. [DOI] [PubMed] [Google Scholar]
- 39.Miura, M., H. Zhu, R. Rotello, E. A. Hartwieg, and J. Yuan. 1993. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75:653-660. [DOI] [PubMed] [Google Scholar]
- 40.Moriguchi, T., H. Kawasaki, S. Matsuda, Y. Gotoh, and E. Nishida. 1995. Evidence for multiple activators for stress-activated protein kinase/c-Jun amino-terminal kinases. Existence of novel activators. J. Biol. Chem. 270:12969-12972. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi, T., N. Kuroyanagi, K. Yamaguchi, Y. Gotoh, K. Irie, T. Kano, K. Shirakabe, Y. Muro, H. Shibuya, K. Matsumoto, E. Nishida, and M. Hagiwara. 1996. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J. Biol. Chem. 271:13675-13679. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura, S., H. Takahashi, M. Kinouchi, A. Manabe, A. Ishida-Yamamoto, Y. Hashimoto, and H. Iizuka. 2001. Differential phosphorylation of mitogen-activated protein kinase families by epidermal growth factor and ultraviolet B irradiation in SV40-transformed human keratinocytes. J. Dermatol. Sci. 25:139-149. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor, L., D. C. Huang, L. A. O'Reilly, and A. Strasser. 2000. Apoptosis and cell division. Curr. Opin. Cell Biol. 12:257-263. [DOI] [PubMed] [Google Scholar]
- 44.Perkins, N. D. 2000. The Rel/NF-kappa B family: friend and foe. Trends Biochem. Sci. 25:434-440. [DOI] [PubMed] [Google Scholar]
- 45.Rayet, B., and C. Gelinas. 1999. Aberrant rel/NFkB genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 46.Reed, J. C. 1999. Dysregulation of apoptosis in cancer. J. Clin. Oncol. 17:2941-2953. [DOI] [PubMed] [Google Scholar]
- 47.Reed, J. C. 1999. Mechanisms of apoptosis avoidance in cancer. Curr. Opin. Oncol. 11:68-75. [DOI] [PubMed] [Google Scholar]
- 48.Robertson, J. D., S. Orrenius, and B. Zhivotovsky. 2000. Review: nuclear events in apoptosis. J. Struct. Biol. 129:346-358. [DOI] [PubMed] [Google Scholar]
- 49.Rothe, M., M. G. Pan, W. J. Henzel, T. M. Ayres, and D. V. Goeddel. 1995. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83:1243-1252. [DOI] [PubMed] [Google Scholar]
- 50.Roy, N., Q. L. Deveraux, R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16:6914-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy, N., M. S. Mahadevan, M. McLean, G. Shutler, Z. Yaraghi, R. Farahani, S. Baird, A. Besner-Johnston, C. Lefebvre, X. Kang, et al. 1995. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 80:167-178. [DOI] [PubMed] [Google Scholar]
- 52.Sabapathy, K., W. Jochum, K. Hochedlinger, L. Chang, M. Karin, and E. F. Wagner. 1999. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev. 89:115-124. [DOI] [PubMed] [Google Scholar]
- 53.Sanna, M. G., C. S. Duckett, B. W. Richter, C. B. Thompson, and R. J. Ulevitch. 1998. Selective activation of JNK1 is necessary for the anti-apoptotic activity of hILP. Proc. Natl. Acad. Sci. USA 95:6015-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulze-Osthoff, K., D. Ferrari, M. Los, S. Wesselborg, and M. E. Peter. 1998. Apoptosis signaling by death receptors. Eur. J. Biochem. 254:439-459. [DOI] [PubMed] [Google Scholar]
- 55.Shibuya, H., K. Yamaguchi, K. Shirakabe, A. Tonegawa, Y. Gotoh, N. Ueno, K. Irie, E. Nishida, and K. Matsumoto. 1996. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science 272:1179-1182. [DOI] [PubMed] [Google Scholar]
- 56.Shin, S., B. J. Sung, Y. S. Cho, H. J. Kim, N. C. Ha, J. I. Hwang, C. W. Chung, Y. K. Jung, and B. H. Oh. 2001. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40:1117-1123. [DOI] [PubMed] [Google Scholar]
- 57.Simons, M., S. Beinroth, M. Gleichmann, P. Liston, R. G. Korneluk, A. E. MacKenzie, M. Bahr, T. Klockgether, G. S. Robertson, M. Weller, and J. B. Schulz. 1999. Adenovirus-mediated gene transfer of inhibitors of apoptosis protein delays apoptosis in cerebellar granule neurons. J. Neurochem. 72:292-301. [DOI] [PubMed] [Google Scholar]
- 58.Stehlik, C., R. de Martin, I. Kumabashiri, J. A. Schmid, B. R. Binder, and J. Lipp. 1998. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha- induced apoptosis. J. Exp. Med. 188:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki, A., T. Ito, H. Kawano, M. Hayashida, Y. Hayasaki, Y. Tsutomi, K. Akahane, T. Nakano, M. Miura, and K. Shiraki. 2000. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene 19:1346-1353. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi, R., Q. Deveraux, I. Tamm, K. Welsh, N. Assa-Munt, G. S. Salvesen, and J. C. Reed. 1998. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 273:7787-7790. [DOI] [PubMed] [Google Scholar]
- 61.Tamm, I., Y. Wang, E. Sausville, D. A. Scudiero, N. Vigna, T. Oltersdorf, and J. C. Reed. 1998. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58:5315-5320. [PubMed] [Google Scholar]
- 62.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 63.Tournier, C., A. J. Whitmarsh, J. Cavanagh, T. Barrett, and R. J. Davis. 1997. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 94:7337-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsujimoto, Y. 1998. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3:697-707. [DOI] [PubMed] [Google Scholar]
- 65.Ueki, T., T. Takeuchi, H. Nishimatsu, T. Kajiwara, N. Moriyama, Y. Narita, K. Kawabe, K. Ueki, and T. Kitamura. 2001. Silencing of the caspase-1 gene occurs in murine and human renal cancer cells and causes solid tumor growth in vivo. Int. J. Cancer 91:673-679. [DOI] [PubMed] [Google Scholar]
- 66.Uren, A. G., M. Pakusch, C. J. Hawkins, K. L. Puls, and D. L. Vaux. 1996. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl. Acad. Sci. USA 93:4974-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vucic, D., H. R. Stennicke, M. T. Pisabarro, G. S. Salvesen, and V. M. Dixit. 2000. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 10:1359-1366. [DOI] [PubMed] [Google Scholar]
- 68.Wang, W., G. Zhou, M. C. T. Hu, Z. Yao, and T. H. Tan. 1997. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-beta)-activated kinase (TAK1), a kinase mediator of TGF beta signal transduction. J. Biol. Chem. 272:22771-22775. [DOI] [PubMed] [Google Scholar]
- 69.Winter, R. N., A. Kramer, A. Borkowski, and N. Kyprianou. 2001. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 61:1227-1232. [PubMed] [Google Scholar]
- 70.Yamaguchi, K., S. Nagai, J. Ninomiya-Tsuji, M. Nishita, K. Tamai, K. Irie, N. Ueno, E. Nishida, H. Shibuya, and K. Matsumoto. 1999. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 18:179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamaguchi, K., K. Shirakabe, H. Shibuya, K. Irie, I. Oishi, N. Ueno, T. Taniguchi, E. Nishida, and K. Matsumoto. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270:2008-2011. [DOI] [PubMed] [Google Scholar]
- 72.Yang, Y., S. Fang, J. P. Jensen, A. M. Weissman, and J. D. Ashwell. 2000. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288:874-877. [DOI] [PubMed] [Google Scholar]
- 73.Yang, Y. L., and X. M. Li. 2000. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 10:169-177. [DOI] [PubMed] [Google Scholar]