Abstract
Alteration of the control of DNA replication and mitosis is considered to be a major cause of genome instability. To investigate the mechanism that controls DNA replication and genome stability, we used the RNA silencing-interference technique (RNAi) to eliminate the Drosophila geminin homologue from Schneider D2 (SD2) cells. Silencing of geminin by RNAi in SD2 cells leads to the cessation of mitosis and asynchronous overreplication of the genome, with cells containing single giant nuclei and partial ploidy between 4N and 8N DNA content. The effect of geminin deficiency is completely suppressed by cosilencing of Double parked (Dup), the Drosophila homologue of Cdt1, a replication factor to which geminin binds. The geminin deficiency-induced phenotype is also partially suppressed by coablation of Chk1/Grapes, indicating the involvement of Chk1/Grapes in the checkpoint control in response to overreplication. We found that the silencing of cyclin A, but not of cyclin B, also promotes the formation of a giant nucleus and overreplication. However, in contrast to the effect of geminin knockout, cyclin A deficiency leads to the complete duplication of the genome from 4N to 8N. We observed that the silencing of geminin causes rapid downregulation of Cdt1/Dup, which may contribute to the observed partial overreplication in geminin-deficient cells. Analysis of cyclin A and geminin double knockout suggests that the effect of cyclin A deficiency is dominant over that of geminin deficiency for cell cycle arrest and overreplication. Together, our studies indicate that both cyclin A and geminin are required for the suppression of overreplication and for genome stability in Drosophila cells.
The cell cycle is tightly regulated to ensure the proper duplication and segregation of chromosomes into daughter cells (17). Alteration of cell cycle regulation leads to genome instability and promotes chromosome polyploidy and/or aneuploidy. One of the critical controls of the cell cycle is to ensure that DNA replication is followed by mitosis and occurs only once per cell cycle. For Saccharomyces cerevisiae, such regulation has been shown to be associated with the activity of cyclin-dependent kinases (Cdks) (5). Several levels of control exist. Cdks phosphorylate and regulate the protein stability of Cdc6, an essential replication initiation factor. The phosphorylated Cdc6 is targeted for ubiquitination by the SCFCDC4 ubiquitin E3 ligase, and the polyubiquitinated Cdc6 is subsequently degraded by the 26S proteasome (2, 7, 10). Cdks also promote the export of Mcm (2-7) proteins, which likely function as a DNA helicase, from the nucleus (20, 29). In higher eukaryotic cells, the control of DNA rereplication by Cdks is less well defined. It appears that Cdc6 is primarily regulated by nuclear export in response to the activation of Cdks during the transition of G1 and S phases (18, 33, 36). Genetic studies of Drosophila suggest that cyclin E is required for endoreplication, in which multiple rounds of DNA replication occur without intervening mitosis in certain tissues during development (8, 19, 35, 38). Although cyclin E is required for S-phase entry, biochemical evidence from Xenopus egg extracts suggests that sustained high levels of cyclin E in the nucleus are inhibitory for replication initiation (16). In addition, in Drosophila and Xenopus embryos, both cyclin A and cyclin B are required for chromosome condensation and segregation during mitosis (21, 22, 28). However, recent studies of Drosophila suggest that cyclin A and cyclin B may play distinct roles in the cell cycle. Overexpression of cyclin A, but not of cyclin B, can activate S phase (6, 23, 42). Furthermore, mutations in cyclin A have been shown to be associated with endoreduplication in certain cells, such as thoracic cells between S phases 16 and 17 (38). These studies suggest that cyclin A may play a regulatory role in S phase for Drosophila.
Recent works on Xenopus egg extracts suggest the existence of an additional control mechanism for DNA replication by geminin, a replication inhibitor initially identified as a substrate for the anaphase-promoting complex/cyclosome ubiquitin E3 ligase (27). It has been shown that G2 nuclei are refractory to rereplicating its chromatins. Mitosis allows the nuclei to be relicensed to become competent for DNA replication. Recent evidence suggests that geminin inhibits replication relicensing during mitosis by binding to Cdt1/Double parked (Cdt1/Dup) and consequently preventing the loading of the Mcm proteins onto the prereplication complex (43, 49). Cdt1 has been shown to be associated with the Orc-Cdc6 prereplication complex and is an essential component of the replication licensing factor RLF-B in Xenopus egg extracts (15, 25, 30, 43, 49). However, direct depletion of geminin from Xenopus egg extracts does not appear to support an extra round of DNA synthesis after the completion of a normal round of DNA replication (27).
The cell cycle is regulated by various checkpoint controls, which respond to such stresses as DNA damage, replication arrest, and defects in the mitotic spindle assembly (9, 52). Many lines of evidence indicate that DNA damage activates a signaling pathway from ataxia telangiectasia-mutated protein (ATM) (or ATR- and Rad3-related protein [ATR]) to downstream protein kinases such as Chk2/Cds1 and Chk1 (52). One of the critical targets of Chk2/Cds1 and Chk1 is Cdc25, a phosphatase that activates Cdks by removing the inhibitory phosphates from threonine14 and tyrosine15 on Cdks (46, 52). It has been shown that both Chk2/Cds1 and Chk1 can phosphorylate Cdc25 (1, 3, 13, 32, 37). Phosphorylated Cdc25 is then associated with 14-3-3 and localized in cytoplasm. Such modification of Cdc25 leads to the inactivation of Cdks and causes cell cycle arrest. These observations are supported by genetic studies using the mouse knockout technique. It has been shown that Chk2−/− mouse embryonic stem cells fail to arrest in G2 during gamma irradiation (14). The Chk1-deficient mice die during early development, with massive cell death (24, 44). At the cellular level, the Chk1−/− cells contain aberrant, highly condensed chromosomes, and Chk1 deficiency abolishes the G2/M cell cycle checkpoints during DNA replication blockage and DNA damage response.
We have attempted to isolate proteins that are associated with human Chk2. One of the proteins we identified was human geminin. We report here that in contrast to the effect of geminin depletion on Xenopus egg extract, the silencing of geminin expression in Drosophila Schneider D2 (SD2) cells causes overreplication. However, such geminin deficiency-induced overreplication is insufficient for the duplication of the entire genome. This is in sharp contrast to the elimination of cyclin A, which results in full duplication of the genome. We found that geminin deficiency causes the rapid downregulation of Cdt1/Dup, suggesting that loss of Cdt1 expression may limit the overreplication caused by geminin silencing.
MATERIALS AND METHODS
Cells.
The Drosophila SD2 cells were obtained from the American Type Culture Collection and were grown at 26°C in Schneider's Drosophila medium (Life Technologies) supplemented with 10% fetal bovine serum and antibiotics.
In vitro transcription.
The DNA templates were generated as DNA fragments of about 700 to 1,000 bp in length, corresponding to the 5′ coding sequences of each gene. The Drosophila expressed-sequence tag cDNA clones were obtained from Research Genetics and subcloned for sense and antisense orientations. Some of the genes were directly amplified by PCR from SD2 genomic DNA by using their coding sequences. The PCR fragments were confirmed by internal restriction digestion. For direct transcription of the PCR product, we added the T7 polymerase recognition site (GAATTAATACGACTCACTATAGGGAGA) to the 5′ regions of PCR oligonucleotides. The PCR fragments or linearized plasmid constructs were isolated after electrophoresis and extracted with phenol-chloroform. The purified DNAs were used as the templates for in vitro transcription with T7 RNA polymerase and the MEGASCRIPT T7 transcription kit (Ambion). Equal amounts of sense and antisense RNAs were mixed, denatured, and annealed to form double-stranded RNA (dsRNA). The formation of dsRNA was confirmed by agarose gel electrophoresis and quantified by spectrophotometry.
RNAi and silencing in SD2 cells.
The RNA interference (RNAi) was conducted according to the method of Clemens et al. (4) with slight modifications. Healthy and growing SD2 cells were harvested and diluted to a final concentration of 106 cells/ml in Schneider's Drosophila medium without fetal bovine serum. The dsRNAs (15 to 20 μg) were added directly to 1 ml of cells and mixed gently. After 30 min of incubation with constant mixing, 2 ml of Schneider's medium containing fetal bovine serum was added, and cells were cultured at 26°C before being harvested. For morphological studies, the cells were seeded onto coverslips. All other dsRNA-treated cells were cultured in six-well culture plates.
Western blotting, immunoprecipitation, kinase assays, and Northern blotting.
The antigeminin rabbit polyclonal antibodies were raised against the fusion protein between glutathione S-transferase and full-length Drosophila geminin. The anti-Double parked guinea pig antibody was kindly provided by Terry Orr-Weaver (Massachusetts Institute of Technology). The monoclonal antibodies against cyclin A (A12) and cyclin B (F2F4) were obtained from Developmental Studies Hybridoma Bank. Western blotting and immunoprecipitation were conducted as described previously, with equal amounts of proteins in the cell lysates (45). Northern blotting and the histone H1 kinase assay were performed as described previously (51). The Drosophila embryos were collected and stained according to the method of Fogarty et al. (12).
Flow cytometry and DNA staining of cells.
Flow cytometry was conducted as described previously (26). For DNA staining, the SD2 cells were fixed with 4% formaldehyde and then stained with 4′,6′-diamidine-2-phenylindole-dihydrochloride (DAPI).
RESULTS
Identification of a Drosophila geminin homologue.
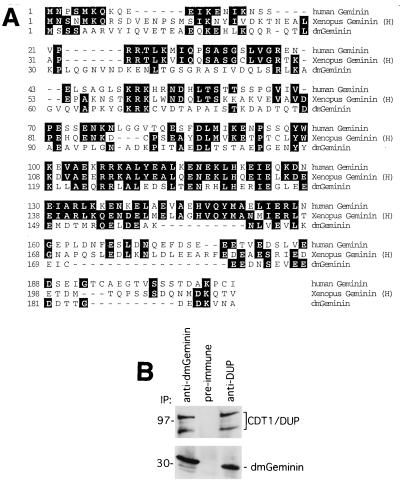
In the process of purifying a 32-kDa protein that is associated with human Chk2 in HeLa cells, a peptide was obtained that corresponded to the amino acid sequence of human geminin (T. Kondo and H. Zhang, unpublished data). To investigate the potential function and regulation of geminin in the cell, we took a genetic approach, using the RNAi technique developed for Drosophila SD2 cells (4). A sequence homology search of the Drosophila genome database identified a Drosophila geminin homologue (CG3183) which shares limited but significant homology with the human (18.8% identity) and Xenopus (23.4% identity) geminins at the amino acid level (Fig. 1A). To determine whether this is indeed a geminin homologue, an antibody was raised against the full-length geminin. Immunoprecipitation followed by Western blotting revealed that this antibody recognized a 32-kDa protein from the SD2 cell lysates (Fig. 1B) that was similar in size to the reported human and Xenopus geminins. In addition, we found that the Double parked protein (48), the Drosophila homologue of human, Xenopus, and Schizosaccharomyces pombe Cdt1, was associated with geminin (Fig. 1B).
FIG. 1.
Identification of a Drosophila geminin homologue. (A) Homology between human (Homo sapiens), Xenopus (Xenopus laevis), and Drosophila (Drosophila melanogaster) geminin proteins. Xenopus has two cDNAs encoding geminin H and L. Only geminin H is shown. (B) Association between geminin and Dup. The geminin and Cdt1/Dup proteins were immunoprecipitated from SD2 cell lysates with antigeminin, preimmune serum, and anti-Cdt1/Dup antibodies. Western blotting was performed with anti-Cdt1/Dup (top) and antigeminin (bottom) antibodies. The molecular mass standards are indicated on the left (in kilodaltons). Cdt1/Dup migrates at about 98 kDa, and the faster migrating band is likely a proteolytic fragment.
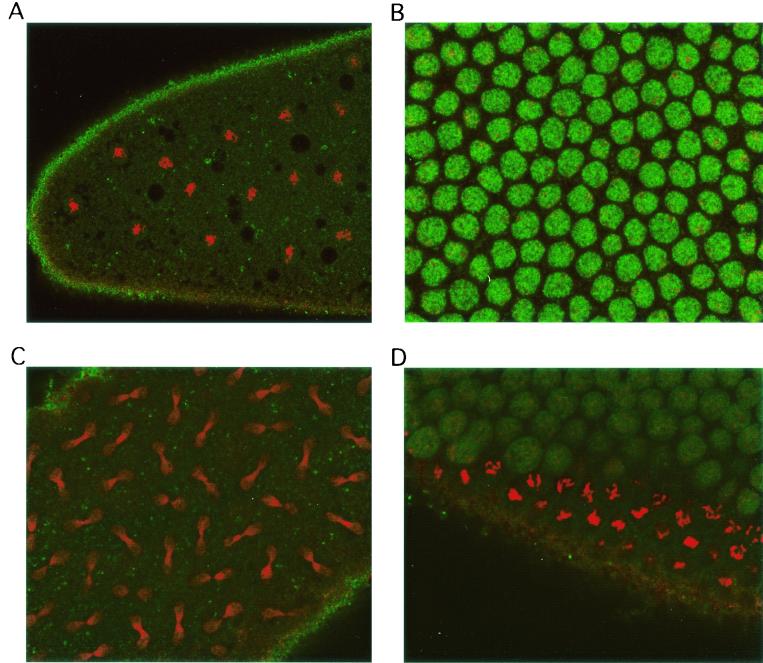
Geminin was originally identified as a substrate of anaphase-promoting complex/cyclosome that was degraded in Xenopus mitotic extract (27). To determine whether Drosophila geminin behaves similarly to the Xenopus homologue, we immunostained early-stage Drosophila embryos, which undergo alternating S phase and mitosis. The geminin protein accumulated in the nuclei of S-phase embryos and disappeared during mitosis (Fig. 2). These results suggest that we have identified the Drosophila homologue of geminin.
FIG. 2.
Drosophila geminin accumulates in S phase and disappears in mitosis during embryonic divisions. Drosophila embryos were collected and stained with antigeminin antibody and then with fluorescein isothiocyanate-conjugated secondary antibody (green). The nuclei were counterstained with propidium iodide (red). (A) Mitotic embryos in late prophase-metaphase; (B) S-phase embryos; (C) anaphase-telophase embryos; (D) an embryo undergoing S phase (upper right) and mitosis (lower left).
Silencing of geminin induces a polyploid and giant nucleus in SD2 cells.
To investigate the function of geminin in cells, we tried to use the RNAi technique to silence the expression of geminin and examine its effect on the cell cycle (4). The geminin dsRNA and a control dsRNA from the Neo resistance gene were synthesized in vitro and incubated with SD2 cells. At various time points after dsRNA addition, the cells were collected and analyzed for the efficiency of knockout, DNA content, bromodeoxyuridine (BrdU) incorporation, and morphological changes of individual cells.
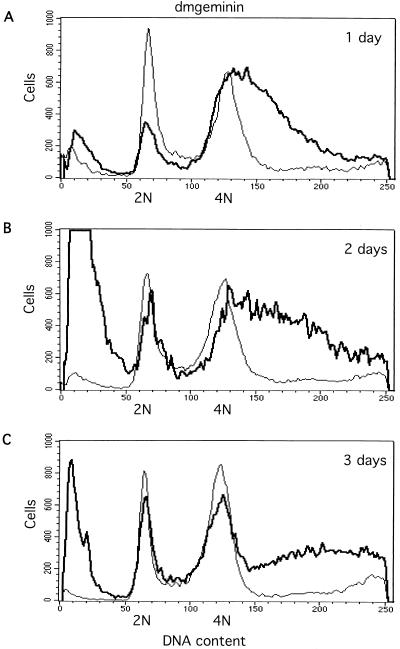
To determine the cellular effects of geminin deficiency, we used flow cytometry to measure DNA content. We found that within 24 h (1 day), a large fraction of cells (50 to 60%, with variability between experiments) contained more than 4N DNA content, with a broad and continuous peak extending from 4N to 8N (Fig. 3A). At the 2- and 3-day time points, the high ploidy peak between 4N and 8N became broader (Fig. 3A to C). This effect was observed repeatedly. The distribution of the partial polyploidy cells suggests that these cells only partially overreplicated their genome, with overreplication being asynchronous between cells and the extent of overreplication being predetermined at the time of silencing. This is in sharp contrast to the effect of the silencing of cyclin A on SD2 cells (see below). BrdU incorporation experiments indicated that the geminin-deficient cells continued to incorporate BrdU, suggesting that they were actively synthesizing DNA (data not shown). We also observed that the silencing of geminin caused substantial apoptotic cell death, as judged by the decrease in cell number and the presence of dead cell debris in the flow cytometry analysis (Fig. 3A to C, far left).
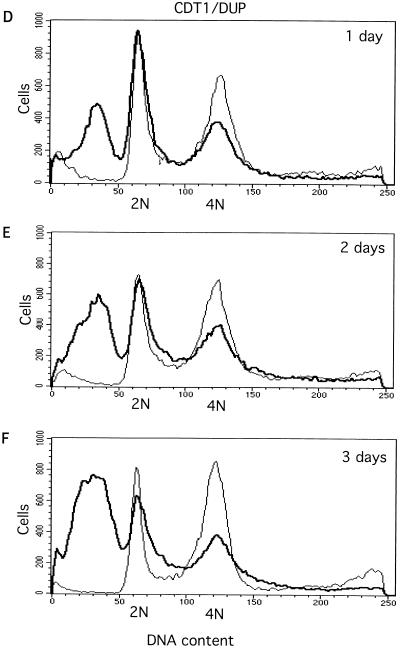
FIG. 3.
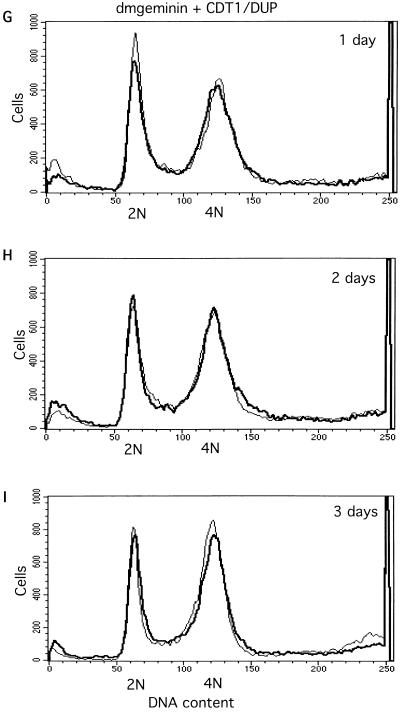
Effects of silencing geminin or Cdt1/Dup and cosilencing geminin and Cdt1/Dup or Cdc6, measured by flow cytometry analysis. (A to C) Geminin knockout induces asynchronous overreplication and partial polyploidy. 2N and 4N represent the cells containing 2N and 4N DNA content, respectively. (D to F) Cdt1/Dup silencing promotes accumulation of the sub-G1 population. (G to I) Cdt1/Dup knockout suppresses geminin deficiency-induced polyploidy and overreplication. For all profiles, the control (Neo) is indicated by a thin line while the geminin, Cdt1/Dup, and geminin plus Cdt1/Dup knockouts are indicated by thick lines. (J and K) Cdc6 knockout partially suppresses geminin deficiency-induced polyploidy and overreplication. The geminin single knockout is indicated by a thin line, while the geminin plus Cdc6 double knockout is indicated by a thick line.
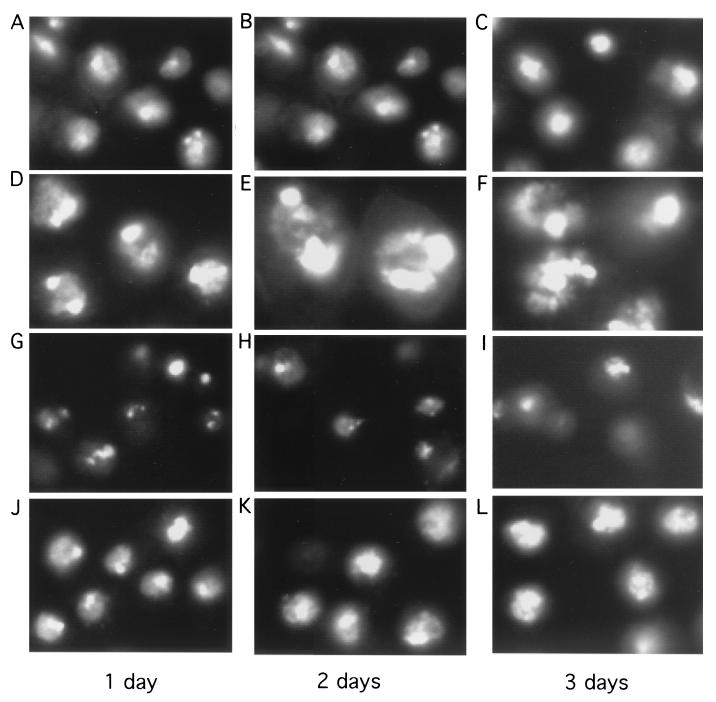
Nuclear staining revealed that many geminin knockout cells contained single giant nuclei (Fig. 4D to F). The DAPI staining of geminin knockout nuclei usually was not homogeneous in intensity and was quite irregular in shape, with more compact masses attached to diffusely stained regions. We often observed that some cells had two giant and intensely stained half masses within their nuclei, which appeared to be connected by more diffusely stained areas and surrounded by a nuclear envelope. It is possible that these cells might have attempted aberrant nuclear division but that cytokinesis was inhibited in the absence of geminin. Our attempt to pulse-label the nuclei with BrdU did not allow us to determine whether the differentially stained regions in the nucleus represent various degrees of DNA synthesis (data not shown).
FIG. 4.
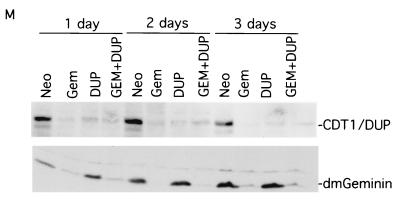
Morphological analysis of silencing geminin or Cdt1/Dup and cosilencing geminin and Cdt1/Dup. The cells were incubated with dsRNAs. At various times, the cells were fixed and the nuclei were stained with DAPI. The nuclear stainings were examined under the same magnification. (A to C) The control (Neo) knockout. (D to F) The geminin knockout. Note the induction of giant nuclei containing densely and diffusely stained regions in geminin-deficient cells. (G to I) The Cdt1/Dup knockout. Note the accumulation of unusual, small cells after the silencing of Cdt1/Dup. (J to L) The geminin and Cdt1/Dup double knockout. The morphological suppression of geminin deficiency by the cosilencing of Cdt1/Dup is shown. (M) Western blot analysis of knockout samples for control (Neo), geminin (Gem), Cdt1/Dup (DUP), and geminin plus Cdt1/Dup (GEM + DUP) knockouts, as shown in panels A to L and in Fig. 3.
Cosilencing of Cdt1/Dup suppresses the effect of geminin deficiency.
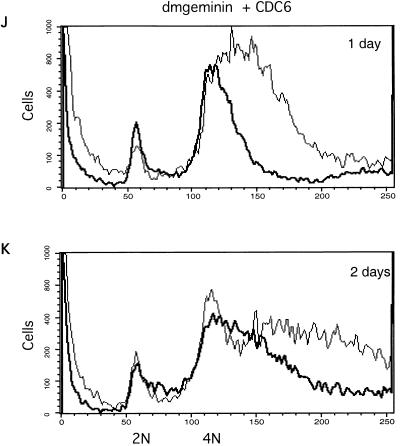
During our analysis of the geminin knockout effect in SD2 cells, it was reported that Xenopus and human geminins bind to Cdt1 (43, 49). Geminin binding appears to prevent Cdt1-mediated loading of Mcm proteins onto the prereplication complex on the origin of replication, leading to the inhibition of DNA synthesis. To test whether the giant nucleus and ploidy changes observed in geminin knockout cells are dependent on Cdt1, we cosilenced Double parked, the Drosophila Cdt1 homologue, and geminin. We found that cosilencing of Dup completely suppressed the effects of geminin deficiency by flow cytometry and morphological analyses. This result suggests that the overreplication and partial polyploidy induced in the absence of geminin are dependent on the presence of Cdt1/Dup in the cell (Fig. 3G to I and 4J to L). Since Cdt1/Dup is a replication initiation factor for the prereplication complex, we also silenced other components of the prereplication complex, such as the Drosophila Cdc6 homologue (CG5971). Our data show that the silencing of Cdc6 caused partial rescue of the overreplication phenotype in geminin-deficient cells (Fig. 3J to K). The elimination of Cdt1, therefore, may affect both geminin binding and Cdt1/Dup-mediated DNA replication.
Cdt1/Dup deficiency causes accumulation of unusual, small cells with sub-G1 DNA content.
We observed that in the Dup single-knockout experiments, there was a marked accumulation of small cells containing pieces of DNA fragments (Fig. 4G to I). Flow cytometry analysis revealed that Cdt1/Dup deficiency caused a significant fraction of cells (25 to 35%, with variability between experiments) to contain sub-G1 DNA content (Fig. 3D to F). With Drosophila dup mutants, it was previously observed that aberrant mitosis occurred in certain tissues (48). Our results confirm these observations and suggest that Dup may couple DNA replication to mitosis. In the absence of Dup, mitosis is uncoupled and aberrant cell divisions might occur even in G1 cells (2N DNA content), resulting in the accumulation of unusual, small cells containing sub-G1 DNA content. To confirm that we have silenced the expression of geminin and Cdt1/Dup, we examined the effect of silencing on these proteins by Western blot analysis (Fig. 4M). Our data suggest that these proteins were effectively downregulated by these RNAi experiments. Interestingly, we also found that the silencing of geminin leads to the downregulation of Cdt1/Dup (Fig. 4M), suggesting that the expression of Cdt1/Dup is affected by geminin deficiency (see below).
Deficiency of cyclin A, but not of cyclin B, causes polyploidy and overreplication.
We wondered whether the overreplication induced by geminin deficiency undergoes an aberrant mitosis between the first and second rounds of DNA synthesis. To examine this possibility, we silenced either cyclin A, cyclin B, or both in the presence or absence of geminin. We were surprised to find that cyclin A knockout alone can induce polyploidy and a giant nucleus on the second day, which was preceded by a short G2 arrest on the first day (Fig. 5A and B). However, the silencing of cyclin B did not have such a marked effect on SD2 cells (Fig. 5C and D). Cyclin A deficiency-induced distribution of DNA content (Fig. 5A and B) was quite different from that caused by geminin deficiency, which induced partial overreplication of the genome with DNA content distributed over a broad region between 4N and 8N (Fig. 3A to C). Cyclin A knockout promoted a more complete duplication of the genome, with total ploidy of a fraction of cells moving from 4N to 8N on the second day (Fig. 5A and B). The differential effects of cyclin A and cyclin B could be repeatedly observed at either low or high cell seeding densities after incubation with the specific dsRNAs.
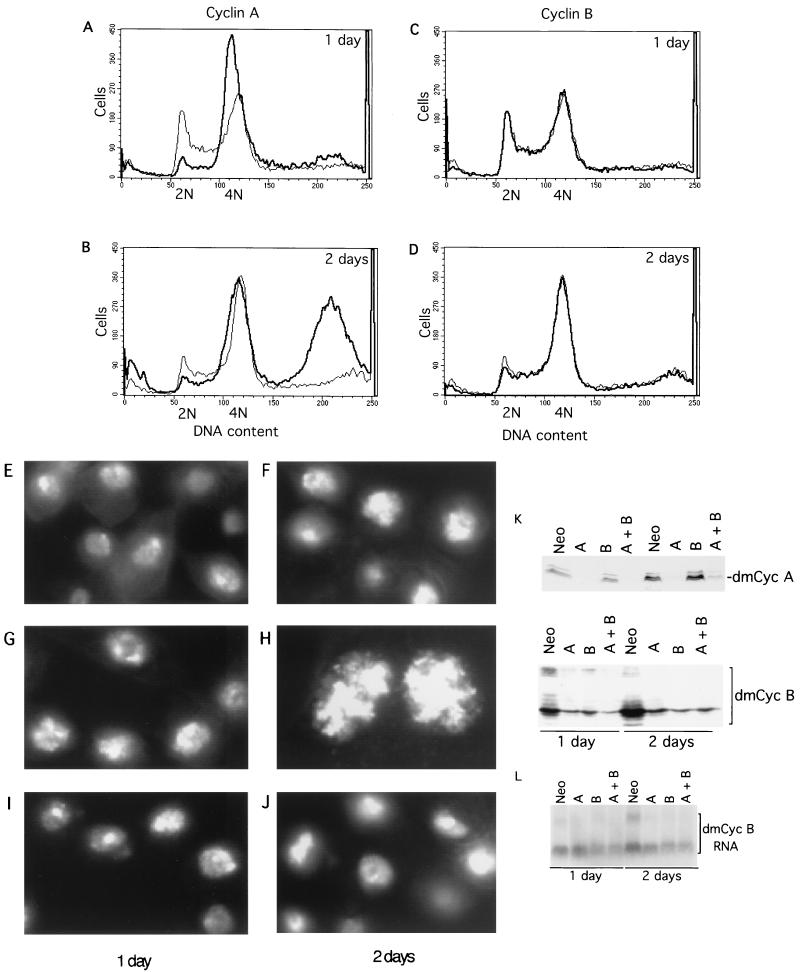
FIG. 5.
Silencing cyclin A induces the accumulation of 8N cells and the formation of a single giant nucleus within a cell. (A to D) Flow cytometry analysis of cyclin A silencing (A and B) and cyclin B knockout (C and D) compared with the control knockout (Neo). The cyclin A and B knockouts are indicated by thick lines, and the control is indicated by a thin line. (E to J) Nuclear morphology analysis of control (Neo) (E and F), cyclin A silencing (G and H), and cyclin B knockout (I and J). The same magnification was used for all panels. (K) Cyclin A knockout suppresses cyclin B expression. Western blot analysis of equal amounts of cell lysates from cyclin A (upper) and cyclin B (lower) levels in the control (Neo), cyclin A (A), cyclin B (B), and cyclin A plus cyclin B (A + B) knockout samples is shown. (L) Northern blot analysis of cyclin B RNA levels in the knockout samples. Abbreviations are the same as those for panel K.
Nuclear staining of cyclin A-deficient cells also reveals a difference (Fig. 5G and H). While both geminin and cyclin A knockouts induced the formation of a giant nucleus within a cell (Fig. 4D to F and 5H), the nuclear staining pattern of geminin-deficient cells was quite heterogeneous and diffuse (Fig. 4D to F). In comparison, the nuclei of cyclin A-deficient cells were much sharper and more defined. They were also more uniform in size (Fig. 5H).
Silencing of cyclin A causes downregulation of cyclin B.
Since both cyclin A and cyclin B are implicated in the mitotic functions of Drosophila (21, 22), one possible explanation for the difference between the cyclin A and cyclin B knockouts could be differences in silencing efficiency in our experiments. To examine this possibility, we analyzed the levels of cyclin A and cyclin B after silencing by Western blot analysis (Fig. 5K). We found that the silencing of cyclin A or cyclin B greatly reduced its respective protein level (Fig. 5K). Surprisingly, while cyclin B knockout did not appear to affect cyclin A levels, cyclin A deficiency caused marked downregulation of the cyclin B protein (Fig. 5K). Northern blotting indicated that cyclin A deficiency modestly downregulated cyclin B RNA levels on the second day (Fig. 5L), suggesting that cyclin A deficiency affects cyclin B at both the RNA and protein levels. This might provide an explanation for how cyclin A deficiency alone can cause G2 cell cycle arrest, overreplication, and polyploidy, while cyclin B silencing appears to cause no detectable polyploidy or overreplication (Fig. 5). However, we could not completely rule out the possibility that cyclin B knockout was incomplete and leaky. Residual cyclin B and the presence of cyclin A might be sufficient to suppress overreplication. We found that the effects of cyclin A and B double knockout resemble the overreplication and polyploidy phenotypes of cyclin A single knockout (data not shown), suggesting that cyclin B does not significantly contribute to overreplication control in SD2 cells.
Chk1/Grapes silencing partially rescues the effect of geminin deficiency.
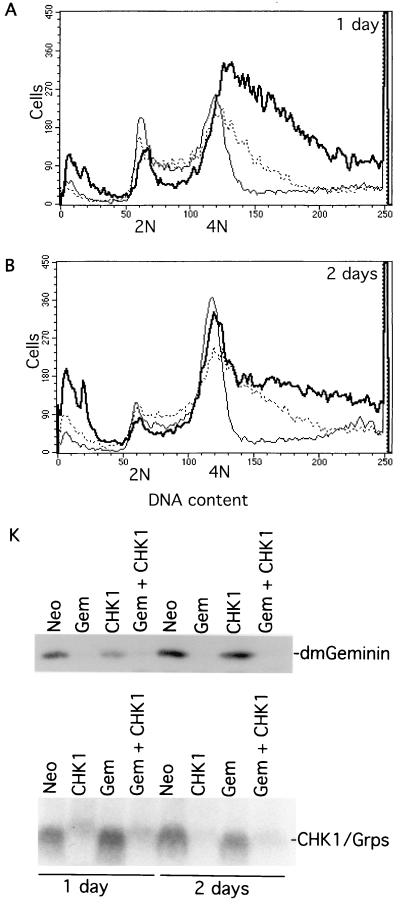
Since we originally isolated geminin during the analysis of Chk2-associated proteins, we tried to determine whether geminin deficiency-induced overreplication activates a checkpoint. If a cell cycle checkpoint is activated, the cosilencing of checkpoint control genes should rescue overreplication in geminin-deficient cells. To test this idea, we tried to silence several candidate checkpoint genes in geminin-deficient cells. We found that while the coelimination of Chk2 did not have any detectable effect (data not shown), the cosilencing of Chk1/Grapes significantly rescued overreplication and polyploidy caused by geminin deficiency (Fig. 6A and B). Chk1/Grapes knockout also suppressed the formation of giant nuclei and cell death in geminin-deficient cells (Fig. 6E, F, I, and J). Our studies also show that silencing Chk1/Grapes alone did not significantly affect the levels of geminin or Cdt1/Dup or the geminin/Dup complex formation (Fig. 6 and 7 and data not shown). These data suggest that Chk1/Grapes is part of the checkpoint control mechanism activated in response to geminin deficiency-induced overreplication.
FIG. 6.
Drosophila Chk1/Grapes silencing suppresses geminin deficiency-induced polyploidy and giant nucleus formation. (A and B) Flow cytometry analysis. Control (Neo) is indicated by a thin line; geminin silencing is indicated by a thick line; Chk1/Grapes and geminin double knockout is indicated by a dotted thin line. (C to J) Nuclear morphological analysis of control (Neo) knockout (C and D), geminin knockout (E and F), Chk1/Grapes knockout (G and H), and geminin and Chk1/Grapes double knockout (I and J). The same magnification was used for all panels. (K) Western blot of geminin (top) and Northern blot of Chk1/Grapes (bottom) in the knockout samples. Abbeviations are the same as those for Fig. 4M.
FIG. 7.
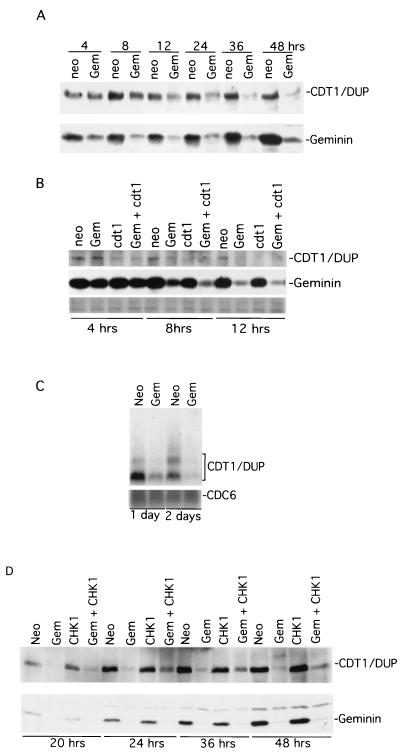
Drosophila geminin silencing induces rapid downregulation of Cdt1/Dup. (A) Time course of Cdt1/Dup in control and geminin knockout samples. Proteins from equal amounts of cell lysates were detected by anti-Cdt1/Dup (top) and antigeminin (bottom) Western blotting. (B) The kinetics of loss of Cdt1/Dup in control, geminin, Cdt1/Dup, and geminin plus Cdt1/Dup knockout cells. The levels of geminin are also shown. The bottom row shows a Ponceau S staining of the protein gel to demonstrate equal protein loading. (C) Northern blots of Cdt1/Dup and Cdc6 in control and geminin knockout samples. (D) The effect of Chk1 cosilencing on the geminin deficiency-induced Cdt1/Dup downregulation. Neo, control; Gem, geminin; cdt1, Cdt1/Dup; Gem + cdt1, geminin plus Cdt1/Dup.
Geminin silencing downregulates Cdt1/Dup expression.
The partial and asynchronous overreplication in geminin-silenced cells suggests that in the absence of geminin, additional factors limit the duplication of the entire genome. One possible factor could be Cdt1/Dup. As we have observed previously, the silencing of geminin causes marked downregulation of Cdt1/Dup (Fig. 4M). To further analyze the effect of geminin deficiency, we examined geminin and Cdt1/Dup levels shortly after the addition of dsRNA. A time course experiment using control and geminin knockout cells indicated that while geminin expression was silenced within 4 h, the level of Cdt1 was also downregulated within 12 h in parallel in the geminin knockout cells (Fig. 7A). There was a lag of about 8 h between the decreases in geminin and Cdt1/Dup levels. This delayed decrease of Cdt1/Dup might explain why the geminin-deficient cells were still capable of overreplicating to various degrees to produce the partial polyploidy (Fig. 3). To determine whether geminin knockout affects the level of Cdt1/Dup RNA, Northern blotting was conducted. Our data indicate that geminin knockout caused the reduction of Cdt1/Dup RNA levels (Fig. 7C). This geminin effect appears to be specific for Cdt1, since the RNA level of Cdc6, another component of the prereplication complex, was not significantly affected by geminin deficiency (Fig. 7C).
Since the cosilencing of Cdt1/Dup by dsRNA completely suppressed the overreplication caused by geminin deficiency (Fig. 3), we wondered whether the rate of decrease of Cdt1/Dup in geminin-deficient cells (Fig. 7A) might be lower than that caused by RNA silencing. To examine this possibility, we compared the kinetics of the loss of Cdt1/Dup between geminin and Cdt1/Dup knockout cells by Western blotting. Our data indicate that Cdt1/Dup indeed disappeared faster in the Cdt1/Dup knockout cells than in those affected by geminin deficiency (Fig. 7B).
We considered the possibility that downregulation of Cdt1/Dup might be a consequence of activating a checkpoint in response to geminin deficiency-induced overreplication. Since Chk1/Grapes knockout partially suppressed geminin deficiency-induced morphological and ploidy changes (Fig. 6), we tried to determine whether Chk1/Grapes cosilencing could rescue the level of Cdt1/Dup in geminin knockout cells. We found that Chk1/Grapes cosilencing with geminin had a partial rescue effect on the level of Cdt1/Dup compared with the effect of geminin single knockout (Fig. 7D). However, since the rescue was not complete, we could not rule out the possibility that an additional mechanism for the expression of Cdt1/Dup was missing in geminin-deficient cells.
Loss of cyclin A is dominant over geminin-induced partial overreplication.
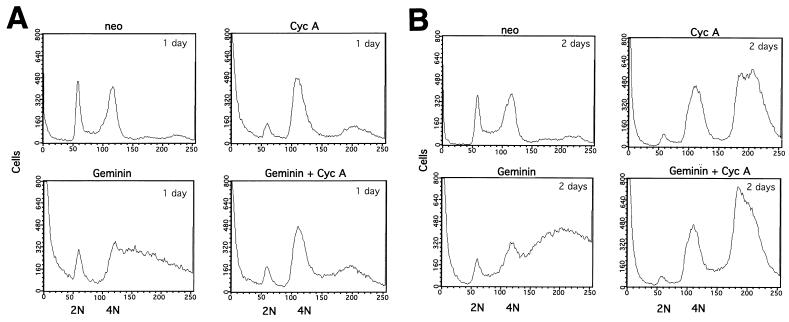
Our study suggests that the silencing of both cyclin A and geminin induces overreplication with different DNA ploidy profiles (Fig. 3 and 5). To determine the relationship between the losses of these proteins in inducing DNA overreplication, we have examined DNA overreplication profiles by flow cytometry analysis for cyclin A and geminin double-knockout cells compared with those for single-knockout cells (Fig. 8A and B). Although geminin single knockout produces extensive and yet partial overreplication of the genome, such an effect was suppressed by cyclin A cosilencing on the first day (Fig. 8A). The geminin and cyclin A double-knockout cells showed no overreplication but did show G2 arrest that was similar to the G2 arrest profile induced by cyclin A single knockout (Fig. 8A). On the second day, the double knockouts overreplicated their genome to 8N DNA content in a pattern that was again similar to that of the cyclin A single knockout (Fig. 8B). These data suggest that the loss of cyclin A is dominant over geminin deficiency for cell cycle arrest and overreplication.
FIG. 8.
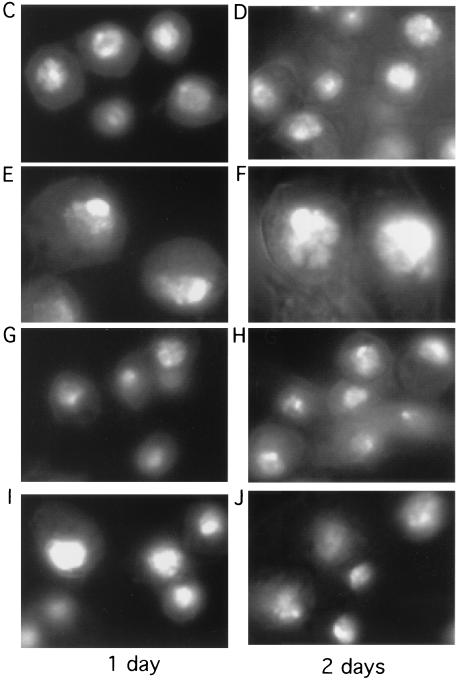
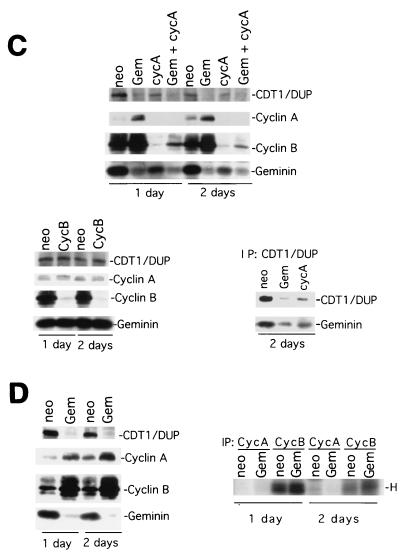
Cyclin A deficiency is dominant over geminin deficiency for cell cycle arrest and overreplication. (A and B) Flow cytometry analysis of control, cyclin A, geminin, and cyclin A plus geminin knockouts for 1 (A) or 2 (B) days. (C) Protein analysis of equal amounts of cell lysates from geminin, cyclin A, and cyclin B knockout cells. The top panel shows an examination of Cdt1/Dup, cyclin A, cyclin B, and geminin levels in the control, geminin, cyclin A, and cyclin A plus geminin knockout cells by direct Western blotting of equal amounts of cell lysates with respective antibodies. The lower left panel shows cyclin A, Cdt1/Dup, and geminin levels in cyclin B knockout cells. The lower right panel shows an analysis of the Cdt1/geminin complex in geminin and cyclin A knockout cells. The Cdt1/Dup complex was immunoprecipitated from control, geminin, and cyclin A knockout cells and then analyzed by Western blotting with Cdt1/Dup and geminin antibodies. (D) Examination of cyclin B-associated histone H1 kinase activity in geminin knockout cells. The left panel shows an analysis of Cdt1/Dup, cyclin A, cyclin B, and geminin protein levels in control and geminin knockout cells by direct Western blotting of equal amounts of cell lysates with respective antibodies. The right panel shows the results of cyclin B being immunoprecipitated from the same set of cell lysates as shown in the left panel and assayed for kinase activity towards histone H1 (H). The cyclin A antibody could not immunoprecipitate the cyclin A protein and therefore has no associated protein kinase activity. Neo, control; CycA, cyclin A; CycB, cyclin B; Gem, geminin; Gem + cycA, geminin plus cyclin A; IP, immunoprecipitate.
To further test the effect of geminin deficiency on cyclin A and cyclin B, we examined the levels of cyclin A and cyclin B as well as their intrinsic histone H1 kinase activities in the geminin knockout cells. Our results suggest that geminin deficiency did not induce downregulation of cyclin A or cyclin B proteins (Fig. 8C). Instead, the silencing of geminin caused a significant increase in both the cyclin A and cyclin B protein levels, possibly as a consequence of prolonged S phase in geminin-deficient cells. There was also a slight increase in the histone H1 kinase activity of cyclin B in the geminin knockout samples (we could not assay for cyclin A, because the antibody did not immunoprecipitate the protein) (Fig. 8D). In the cyclin A knockout cells, we found that geminin levels decreased by about two- to fivefold but never diminished completely as in the geminin knockout cells (Fig. 8C). This downregulation of geminin, however, was accompanied by similar decreases in the Cdt1/Dup protein levels on both day 1 and day 2 after the silencing of cyclin A (Fig. 8C and data not shown). The ratio of Cdt1 to geminin, therefore, does not appear to be significantly affected by cyclin A knockout. Immunoprecipitation of the Cdt1/Dup complex suggests that it associates with geminin in the cyclin A-deficient cells (Fig. 8C). Cyclin B knockout had no effect on the level of either Cdt1/Dup or geminin (Fig. 8C). These studies suggest that overreplication caused by geminin deficiency does not undergo a process that requires the downregulation of cyclin A or B.
DISCUSSION
We have used the RNAi-silencing technique in Drosophila SD2 cells to investigate the control of overreplication and chromosome ploidy. Although geminin has recently been implicated in replication licensing in Xenopus egg extract (43, 49), previous studies have suggested that the depletion of geminin did not cause overreplication in the Xenopus egg extract (27). Our data for SD2 cells clearly indicate that geminin participates in overreplication control in high eukaryotic cells (Fig. 3 and 6). A similar conclusion has also been reached by a recent study (34). One possible explanation for these discrepancies could be that the maternal levels of free Cdt1 in Xenopus egg extract are not significantly affected by geminin depletion. Tada et al. reported that only a fraction of Cdt1 in the interphase of Xenopus egg extracts is complexed with geminin (43). There are substantial amounts of free Cdt1 in the egg extract which are capable of forming complexes with exogenously added recombinant geminin. If this is the case, other mechanisms, such as Cdk activity, should also operate to prevent overreplication in the egg extract even in the absence of geminin. For example, it has been shown that even though the cyclin E/Cdk2 activity is required for S phase, the sustained high levels of nuclear cyclin E/Cdk2 prevent the binding of Mcm3 to chromatin in the Xenopus egg extracts (16). Studies using 6-dimethylaminopurine and roscovitine, which can inhibit Cdk activity, also suggested that Cdks play a role in inhibiting relicensing of replication in Xenopus egg extracts (43). However, it is possible that the control of overreplication in egg extract, which undergoes alternating S phase and mitosis, might be somewhat different from that in cultured SD2 or other somatic cells which show well-defined G1 and G2 phases.
The phenotype of geminin deficiency is intriguing. The asynchronous and partial overreplication of the genome suggests that the elimination of geminin may result in only a limited capacity for replication of the entire genome and that this replication capacity might be consumed by the replication process itself. Alternatively, geminin may have other functions that limit genome duplication in its absence. For example, geminin may affect Cdt1/Dup localization within the cell or the stability of the Cdt1/Dup protein. We found that geminin deficiency caused rapid downregulation of its binding partner, Cdt1/Dup (Fig. 4 and 7). This effect appears to occur at the level of Cdt1/Dup RNA, suggesting that geminin deficiency may cause the downregulation of a factor required for Cdt1/Dup expression. It is possible that Cdt1 transcription is regulated by a checkpoint in response to overreplication. Such a possibility is supported by our observation that the cosilencing of Chk1 had a partial rescue effect on the levels of Cdt1 in geminin-deficient cells (Fig. 7). However, these observations do not rule out the possibility that the loss of geminin also affects Cdt1/Dup protein stability or localization in the cell. A recent study suggests that Cdt1 protein, but not RNA, is regulated in a cell cycle-dependent fashion. Cdt1 protein is stable in G1 but is degraded by the ubiquitin-dependent proteolysis upon the entry of S phase (31). This observation is consistent with our data showing that limited Cdt1 protein is available for each S phase. Geminin knockout may release a limited amount of Cdt1 which is in complex with geminin, promoting the partial overreplication. In addition to the downregulation of Cdt1 RNA, we found that the loss of Cdt1 is partially sensitive to MG132, an inhibitor of 26S proteasome that degrades polyubiquitinated proteins (data not shown). Thus, in our study, the S phase induced by geminin deficiency may also work to destabilize the Cdt1 protein.
In contrast to the effect of geminin deficiency, we found that the silencing of cyclin A caused an initial G2 block followed by duplication of the entire genome, as judged by flow cytometry and morphology studies (Fig. 5). This effect is similar to those seen in previous observations of the fission yeast cdc13 mutant (11), which encodes a mitotic cyclin. However, it is surprising that the silencing of cyclin B did not cause overreplication in SD2 cells. Our analysis further suggests that cyclin A deficiency leads to the downregulation of cyclin B, but not vice versa. It has been shown that mutation of the cyclin A gene in Drosophila causes thoracic epidermis cells to skip the mitosis between S phases 16 and 17 and to undergo endoreduplication (38). Our results are consistent with these observations. In addition, our data unequivocally show that deficiency of cyclin A, unlike that of geminin, causes duplication of the entire genome (Fig. 5). Furthermore, our studies indicate that in cyclin A-deficient cells, cyclin B is downregulated (Fig. 5). The downregulation of both cyclin A and cyclin B in the cyclin A-deficient cells might explain why overreplication is not observed in the cyclin B-deficient cells, as they still contain relatively normal levels of cyclin A (Fig. 5).
Our data indicate that the silencing of cyclin A induces an overreplication that is quite different from the one caused by geminin deficiency under our assay conditions. The loss of geminin causes only partial overreplication, while the silencing of cyclin A induces the full duplication of the genome. In addition, it appears that geminin deficiency induces substantial cell death (Fig. 3) while cyclin A silencing does not (Fig. 5). The geminin deficiency-induced cell death can be rescued by Cdt1 cosilencing (Fig. 3). These observations suggest that mechanisms for suppressing overreplication might be different for geminin and cyclin A. This notion is supported by our finding that the overreplication induced by geminin deficiency may not require the downregulation of cyclin A or cyclin B. Geminin deficiency does not appear to induce a decrease in cyclin A or cyclin B protein levels. Instead, the loss of geminin causes a marked increase in cyclin A and cyclin B protein levels (Fig. 8). The overall kinase activity of cyclin B is slightly enhanced with the in vitro histone H1 kinase assay (Fig. 8). Conversely, our studies show that cyclin A deficiency does cause a substantial decrease in geminin levels on days 1 and 2 after silencing (Fig. 8C and data not shown). However, the downregulation of geminin is still far from complete compared with that caused by geminin knockout (Fig. 4 and 7). Furthermore, we also found that cyclin A deficiency induces the downregulation of Cdt1 at the same time points (days 1 and 2) (Fig. 8C and data not shown). It thus appears that the ratio of geminin to Cdt1/Dup is not significantly altered by cyclin A deficiency. Moreover, because the silencing of cyclin A causes only G2 arrest on day 1, the downregulation of geminin in cyclin A-deficient cells does not appear to be sufficient to induce overreplication (Fig. 8C, cyc A knockout, 1 day; also see the discussion of double knockout below). These studies suggest that other events, independent of or in addition to the downregulation of geminin, may be required for the overreplication induced by cyclin A deficiency.
Our analyses of cyclin A and geminin double-knockout cells suggest that the loss of cyclin A is dominant over geminin deficiency. In these experiments, even though geminin is completely silenced, coelimination of cyclin A caused only G2 cell cycle arrest on day 1 (Fig. 8A). The cosilencing of cyclin A and geminin on day 2 induced overreplication of the genome which, unlike that induced by geminin deficiency, produced a discrete 8N peak similar to that caused by cyclin A single knockout (Fig. 8). These data suggest either that cyclin A is required for subsequent geminin-mediated replication control or that the loss of cyclin A may cause the replication to proceed in a geminin-independent mechanism.
We originally isolated human geminin during the analysis of proteins that are associated with human Chk2 protein. While this interaction appeared to be relatively weak during later verification, we sought to address its potential significance for SD2 cells. We found that in SD2 cells, Chk2 knockout did not have a significant effect on geminin deficiency-induced overreplication or the formation of giant nuclei (data not shown). However, we found that Chk1/Grapes deficiency significantly suppressed the geminin knockout phenotype (Fig. 6), suggesting that Chk1/Grapes possesses a checkpoint function for the overreplication induced by geminin deficiency. Our result is consistent with those of previous studies indicating that Chk1/Grapes regulates the DNA replication checkpoint for Drosophila (12, 40, 41, 50). These studies have shown that interference of Drosophila nuclear division cycles 12 and 13 by X-irradiation or the DNA replication inhibitor aphidicolin activates the Chk1/Grapes signaling pathway. It has been shown that the activated Chk1 kinase phosphorylates Cdc25, promoting its complex formation with 14-3-3 and its subsequent retention in cytoplasm (47). Consequently, the activated Chk1/Grapes promotes the inhibitory phosphorylation of Cdc2 at threonine14 and tyrosine15 in a Cdc25/String-dependent process. In our studies, it is likely that overreplication caused by geminin deficiency induces the Chk1/Grapes-mediated checkpoint, leading to the inhibition of the Cdc2 kinase activity and mitosis. This effect may be reflected in part by our observation that cyclin B-associated kinase activity is not dramatically induced by geminin deficiency compared to the marked increase of cyclin B protein levels in these cells (Fig. 8D). Since the loss of Chk1/Grapes or Cdt1 can either partially or completely rescue the geminin deficiency-induced phenotypes (Fig. 3, 4, and 6), our studies indicate that the loss of Chk1/Grapes does not suppress geminin deficiency through downregulation of Cdt1. Instead, the loss of Chk1/Grapes partially restores the Cdt1 levels in geminin-deficient cells (Fig. 7). In mouse cells, Chk1 deficiency causes an aberrant G2/M cell cycle checkpoint during development or in response to DNA damage (24, 44), causing the formation of nuclei containing highly condensed and aggregated chromatin and, consequently, massive apoptotic cell death. Although we did not observe extensive cell death in Chk1/Grapes knockout SD2 cells (Fig. 6 and data not shown), it is possible that Chk1/Grapes silencing allows cells to undergo aberrant mitosis, even though they are overreplicating their genome. This would produce a pseudorescue effect on the geminin deficiency-induced phenotypes. It is unclear how Chk1/Grapes rescues Cdt1/Dup expression. It is possible that Chk1/Grapes may be involved in the suppression of Cdt1/Dup transcription during overreplication. Alternatively, since Cdt1/Dup protein levels are regulated in a cell cycle-dependent fashion (31), being high in G1 and low in S and G2/M phases, the aberrant mitosis and possibly subsequent G1 phase induced by Chk1/Grapes deficiency may allow Cdt1/Dup to be expressed in G1. Further work is required to clarify these issues. Although we do not see a significant effect of Chk2, Chk2 may play a regulatory role for geminin under certain conditions.
Genome instability is often associated with cancer (17, 39). It is still not clear how these processes are linked to the alteration of DNA replication, mitosis, or G1 cell cycle regulation. Our present work suggests that dysregulation of geminin/Cdt1 and cyclin A contributes to genome instability in Drosophila cells. Further studies are necessary to link alterations in the activities of geminin/Cdt1 and mitotic cyclins to human cancer.
Acknowledgments
We thank Terry Orr-Weaver for generously providing the anti-Dup antibody and Helena Richardson for communicating unpublished results.
This work was supported by grants from the National Cancer Institute, National Institutes of Health (CA72878), and the American Cancer Society (RPG-98-340-01-CCG) to H.Z.
REFERENCES
- 1.Blasina, A., I. V. de Weyer, M. C. Laus, W. H. Luyten, A. E. Parker, and C. H. McGowan. 1999. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Calzada, A., M. Sanchez, E. Sanchez, and A. Bueno. 2000. The stability of the Cdc6 protein is regulated by cyclin-dependent kinase/cyclin B complexes in Saccharomyces cerevisiae. J. Biol. Chem. 275:9734-9741. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18:4047-4054. [DOI] [PubMed] [Google Scholar]
- 4.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diffley, J. F. 1996. Once and only once upon a time: specifying and regulating origins of DNA replication in eukaryotic cells. Genes Dev. 10:2819-2830. [DOI] [PubMed] [Google Scholar]
- 6.Dong, X., K. H. Zavitz, B. J. Thomas, M. Lin, S. Campbell, and S. L. Zipursky. 1997. Control of G1 in the developing Drosophila eye: rca1 regulates cyclin A. Genes Dev. 11:94-105. [DOI] [PubMed] [Google Scholar]
- 7.Drury, L. S., G. Perkins, and J. F. Diffley. 2000. The cyclin-dependent kinase Cdc28p regulates distinct modes of Cdc6p proteolysis during the budding yeast cell cycle. Curr. Biol. 10:231-240. [DOI] [PubMed] [Google Scholar]
- 8.Duronio, R. J., and P. H. O'Farrell. 1995. Developmental control of the G1 to S transition in Drosophila: cyclin E is a limiting downstream target of E2F. Genes Dev. 9:1456-1468. [DOI] [PubMed] [Google Scholar]
- 9.Elledge, S. J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 274:1664-1672. [DOI] [PubMed] [Google Scholar]
- 10.Elsasser, S., Y. Chi, P. Yang, and J. L. Campbell. 1999. Phosphorylation controls timing of Cdc6p destruction: a biochemical analysis. Mol. Biol. Cell 10:3263-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher, D. L., and P. Nurse. 1996. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 15:850-860. [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty, P., S. D. Campbell, R. Abu-Shumays, B. S. Phalle, K. R. Yu, G. L. Uy, M. L. Goldberg, and W. Sullivan. 1997. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7:418-426. [DOI] [PubMed] [Google Scholar]
- 13.Furnari, B., A. Blasina, M. N. Boddy, C. H. McGowan, and P. Russell. 1999. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell 10:833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirao, A., Y. Y. Kong, S. Matsuoka, A. Wakeham, J. Ruland, H. Yoshida, D. Liu, S. J. Elledge, and T. W. Mak. 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287:1824-1827. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann, J. F., and D. Beach. 1994. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J. 13:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua, X. H., H. Yan, and J. Newport. 1997. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J. Cell Biol. 137:183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter, T., and J. Pines. 1994. Cyclins and cancer. II. Cyclin D and CDK inhibitors come of age. Cell 79:573-582. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, W., N. J. Wells, and T. Hunter. 1999. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc. Natl. Acad. Sci. USA 96:6193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoblich, J. A., K. Sauer, L. Jones, H. Richardson, R. Saint, and C. F. Lehner. 1994. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77:107-120. [DOI] [PubMed] [Google Scholar]
- 20.Labib, K., J. F. Diffley, and S. E. Kearsey. 1999. G1-phase and B-type cyclins exclude the DNA-replication factor Mcm4 from the nucleus. Nat. Cell Biol. 1:415-422. [DOI] [PubMed] [Google Scholar]
- 21.Lehner, C. F., and P. H. O'Farrell. 1989. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell 56:957-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehner, C. F., and P. H. O'Farrell. 1990. The roles of Drosophila cyclins A and B in mitotic control. Cell 61:535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehner, C. F., N. Yakubovich, and P. H. O'Farrell. 1991. Exploring the role of Drosophila cyclin A in the regulation of S phase. Cold Spring Harbor Symp. Quant. Biol. 56:465-475. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 25.Maiorano, D., J. Moreau, and M. Mechali. 2000. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature 404:622-625. [DOI] [PubMed] [Google Scholar]
- 26.Mamillapalli, R., N. Gavrilova, V. T. Mihaylova, L. M. Tsvetkov, H. Wu, H. Zhang, and H. Sun. 2001. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2). Curr. Biol. 11:263-267. [DOI] [PubMed] [Google Scholar]
- 27.McGarry, T. J., and M. W. Kirschner. 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93:1043-1053. [DOI] [PubMed] [Google Scholar]
- 28.Murray, A. 1995. Cyclin ubiquitination: the destructive end of mitosis. Cell 81:149-152. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, V. Q., C. Co, K. Irie, and J. J. Li. 2000. Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Curr. Biol. 10:195-205. [DOI] [PubMed] [Google Scholar]
- 30.Nishitani, H., Z. Lygerou, T. Nishimoto, and P. Nurse. 2000. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404:625-628. [DOI] [PubMed] [Google Scholar]
- 31.Nishitani, H., S. Taraviras, Z. Lygerou, and T. Nishimoto. 2001. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J. Biol. Chem. 276:44905-44911. [DOI] [PubMed] [Google Scholar]
- 32.Peng, C. Y., P. R. Graves, R. S. Thoma, Z. Wu, A. S. Shaw, and H. Piwnica-Worms. 1997. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277:1501-1505. [DOI] [PubMed] [Google Scholar]
- 33.Petersen, B. O., J. Lukas, C. S. Sorensen, J. Bartek, and K. Helin. 1999. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 18:396-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn, L. M., A. Herr, T. J. McGarry, and H. Richardson. 2001. The Drosophila geminin homolog: roles for geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev. 15:2741-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson, H., L. V. O'Keefe, T. Marty, and R. Saint. 1995. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development 121:3371-3379. [DOI] [PubMed] [Google Scholar]
- 36.Saha, P., J. Chen, K. C. Thome, S. J. Lawlis, Z.-H. Hou, M. Hendricks, J. D. Parvin, and A. Dutta. 1998. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol. 18:2758-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez, Y., C. Wong, R. S. Thoma, R. Richman, Z. Wu, H. Piwnica-Worms, and S. J. Elledge. 1997. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497-1501. [DOI] [PubMed] [Google Scholar]
- 38.Sauer, K., J. A. Knoblich, H. Richardson, and C. F. Lehner. 1995. Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev. 9:1327-1339. [DOI] [PubMed] [Google Scholar]
- 39.Sherr, C. J. 1996. Cancer cell cycle. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 40.Sibon, O. C., A. Laurencon, R. Hawley, and W. E. Theurkauf. 1999. The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol. 9:302-312. [DOI] [PubMed] [Google Scholar]
- 41.Sibon, O. C., V. A. Stevenson, and W. E. Theurkauf. 1997. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature 388:93-97. [DOI] [PubMed] [Google Scholar]
- 42.Sprenger, F., N. Yakubovich, and P. H. O'Farrell. 1997. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr. Biol. 7:488-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tada, S., A. Li, D. Maiorano, M. Mechali, and J. J. Blow. 2001. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 3:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, M. Nakanishi, and K. Nakayama. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 45.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9:661-664. [DOI] [PubMed] [Google Scholar]
- 46.Walworth, N. C. 2000. Cell-cycle checkpoint kinases: checking in on the cell cycle. Curr. Opin. Cell Biol. 12:697-704. [DOI] [PubMed] [Google Scholar]
- 47.Walworth, N. C. 2001. DNA damage: Chk1 and Cdc25, more than meets the eye. Curr. Opin. Genet. Dev. 11:78-82. [DOI] [PubMed] [Google Scholar]
- 48.Whittaker, A. J., I. Royzman, and T. L. Orr-Weaver. 2000. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 14:1765-1776. [PMC free article] [PubMed] [Google Scholar]
- 49.Wohlschlegel, J. A., B. T. Dwyer, S. K. Dhar, C. Cvetic, J. C. Walter, and A. Dutta. 2000. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290:2309-2312. [DOI] [PubMed] [Google Scholar]
- 50.Yu, K. R., R. B. Saint, and W. Sullivan. 2000. The Grapes checkpoint coordinates nuclear envelope breakdown and chromosome condensation. Nat. Cell Biol. 2:609-615. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, H., R. Kobayashi, K. Galaktionov, and D. Beach. 1995. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 82:915-925. [DOI] [PubMed] [Google Scholar]
- 52.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]