Abstract
Mammalian Lin-2 (mLin-2)/CASK is a membrane-associated guanylate kinase (MAGUK) and contains multidomain modules that mediate protein-protein interactions important for the establishment and maintenance of neuronal and epithelial cell polarization. The importance of mLin-2/CASK in mammalian development is demonstrated by the fact that mutations in mLin-2/CASK or SAP97, another MAGUK protein, lead to cleft palate in mice. We recently identified a new protein-protein interaction domain, called the L27 domain, which is present twice in mLin-2/CASK. In this report, we further define the binding of the L27C domain of mLin-2/CASK to the L27 domain of mLin-7 and identify the binding partner for L27N of mLin-2/CASK. Biochemical analysis reveals that this L27N domain binds to the N terminus of SAP97, a region that was previously reported to be essential for the lateral membrane recruitment of SAP97 in epithelia. Our colocalization studies, using dominant-negative mLin-2/CASK, show that the association with mLin-2/CASK is crucial for lateral localization of SAP97 in MDCK cells. We also report the identification of a novel isoform of Discs Large, a Drosophila melanogaster orthologue of SAP97, which contains a region highly related to the SAP97 N terminus and which binds Camguk, a Drosophila orthologue of mLin-2/CASK. Our data identify evolutionarily conserved protein-protein interaction domains that link mLin-2/CASK to SAP97 and account for their common phenotype when mutated in mice.
Establishing and maintaining the proper spatial distribution of integral membrane proteins is essential for all cells. The polarized expression of receptors and ion channels is especially crucial for the proper function of neurons and epithelial cells. These cells are composed of unique plasma membrane domains with distinct structural and biochemical properties: axonal and dendritic for neurons and apical and basolateral for epithelial cells. This polarized expression of membrane proteins for appropriate plasma membrane regions and the subsequent maintenance of their asymmetry are mediated by regulated protein-protein interactions.
Recent studies from several groups have shown that PSD95/Dlg/ZO1 (PDZ) domain-containing protein complexes play a pivotal role in localizing their target membrane proteins to specialized membrane domains of neuronal and epithelial cells (11). Many cell surface proteins contain conserved sequences at their extreme C-terminal ends, which are target motifs recognized by PDZ domains found in membrane-associated proteins (31). Genetic studies of Drosophila melanogaster and Caenorhabditis elegans provide important clues to understanding the principles that govern protein targeting to the polarized membrane in both neurons and epithelial cells. For example, the Drosophila PDZ domain-containing protein, Discs Large (Dlg), is essential for epithelial polarity (42) and protein targeting in neuroblasts (30, 32). A heterotrimeric complex containing C. elegans PDZ proteins Lin-2, Lin-7, and Lin-10 localizes tyrosine kinase receptor Let-23 to the basolateral surfaces of vulval precursor cells (18). The C-terminal PDZ recognition motif of Let-23 mediates binding to the PDZ domain of Lin-7 (18). Mutations in Lin-2, Lin-7, or Lin-10 lead to a vulvaless phenotype, presumably due to improper localization of Let-23 and disruption of the signal transduction cascade (18, 37)
The mammalian orthologs of Lin-2, Lin-7, and Lin-10 have been identified and are known as mammalian Lin-2 (mLin-2)/CASK, mLin-7/VELIS/MALS, and mLin-10/X11α/MINT1, respectively (3, 5, 13, 16, 17). Studies have shown that this tripartite complex exists in brain (3, 5), while mLin-2/CASK and mLin-7 form a complex and localize to the basolateral surfaces of renal epithelia and MDCK (Madin-Darby canine kidney) cells (7, 39). mLin-2/CASK is a member of the membrane-associated guanylate kinase (MAGUK) family of proteins and contains multiple protein-protein interaction domains: N-terminal CaM kinase-like (CKII) domain (2), L27 domain, PDZ domain, SH3 domain, Hook domain (also known as the 4.1 binding domain), and C-terminal guanylate kinase-like domain. Recent studies have revealed several binding partners of mLin-2/CASK. Its CKII binds to X11α in brain, and the region between the CKII domain and the PDZ domain mediates binding to mLin-7 (3, 5, 18). The PDZ domain of mLin-2/CASK binds to ubiquitously expressed cell surface syndecans (7), brain-specific proteins such as neurexin (13), and an integral membrane protein junctional adhesion molecule (26). Furthermore, the Hook domain of mLin-2/CASK has also been shown to interact with protein 4.1 (7) and mediate stable association with the cytoskeleton. Despite the identification of these mLin-2/CASK-interacting proteins, it is not entirely clear how mLin-2/CASK is localized to the basolateral membrane. Previous studies from our laboratory revealed that neither the amino-terminal nor the carboxyl-terminal half of mLin-2/CASK properly localized to the basolateral membrane of MDCK cells, indicating that localization of mLin-2/CASK was a complex process, possibly involving multiple protein-binding modules (39).
mLin-7 is a smaller protein with an N-terminal mLin-2/CASK binding region and a C-terminal PDZ domain. The role of the mLin-7 PDZ domain in localization of membrane proteins to the basolateral surface has been demonstrated by our laboratory (38) and others (34). The N terminus interacts with mLin-2/CASK and mediates mLin-7 membrane localization (39); however, the precise molecular mechanism used by the mLin-7- and mLin-2/CASK-containing complex to localize its target proteins to the basolateral membrane remains to be elucidated. In efforts to define the mechanism of protein targeting by the mLin-7-containing complexes, several proteins that interact with the amino-terminal region of mLin-7 were identified in our laboratory (19) and others (5, 41). These include novel proteins we identified called proteins associated with Lin seven (PALS), as well as DLG2 and DLG3.
PALS are also members of the MAGUK family proteins and are highly related to mLin-2/CASK. Sequence alignment reveals an approximately 55-amino-acid (aa) domain conserved in PALS1, PALS2, DLG2, DLG3, and mLin-2/CASK that mediates interaction with mLin-7 (8). Interestingly, this domain is duplicated at the amino termini of all mLin-7 binding MAGUK proteins, resulting in adjacent L27 domains, referred to as L27N and L27C. Furthermore, the amino-terminal region of mLin-7 also comprises an L27 domain that mediates interaction with the L27C domain of mLin-2/CASK and each PALS. Hence, the domain was named L27, having been found in both mLin-2/CASK and mLin-7 . These observations suggest that the L27N domains of these MAGUK proteins represent novel protein-protein interaction domains. In this report, we elucidate the binding partner for the L27N domain of mLin-2/CASK and demonstrate its important role in protein targeting.
MATERIALS AND METHODS
DNA constructs.
Full-length Myc-SAP97 (Myc-SAP97 FL) was a generous gift from Morgan Sheng. Yellow fluorescent protein (YFP)-tagged SAP97 FL was generated by subcloning the coding sequence for Myc-SAP97 FL into the pEYFP-N1 vector (Clontech) via EcoRI sites. Deletion mutants SAP97ΔN-PDZ2(1-368) and SAP97ΔGK(745-911) were constructed by subcloning EcoRI-HindIII and EcoRI-BamHI restriction enzyme-digested inserts, respectively, in frame with the coding sequence for an N-terminal Myc tag in the pCDNA3.1 vector. The SAP97ΔN construct, which is devoid of the first 64 amino acids, was made by PCR using a sense primer containing an XhoI site and an antisense primer containing an EcoRI site. PCR products were cloned into the pEYFP-C1 (Clontech) vector. SAP97ΔN-PDZ2(1-368) was generated by PCR using a sense primer containing an XhoI site and an antisense primer containing EcoRI sites, and subsequent PCR products were cloned into the MycRK5 vector. Internal deletion-containing constructs, such as SAP97ΔPDZ3-SH3(466-701) and SAP97N(1-160)+GK(702-911), were generated by a single-primer PCR deletion method as described previously (24). Glutathione S-transferase (GST)-SAP97 constructs, such as GST-SAP97GK, GST-SAP97(1-158), GST-SAP97(1-78), GST-SAP97(65-104), and GST-SAP97(89-158), were generated by PCR using a sense primer containing an XhoI site and an antisense primer containing HindIII sites, and subsequent PCR products were cloned into the pGSTag vector. The SAP97(1-158)-YFP construct was generated by subcloning restriction enzyme-digested inserts from pGStag-SAP97(1-158) into pEYFP-N1 via XhoI and HindIII. Cloning of full-length human mLin-2/CASK DNA and Myc-mLin-2/CASK, has been previously described (39). pCDNA3.1 Myc-mLin-2/CASK L27N(335-390) deletion, pCDNA3.1 Myc-mLin-2/CASK L27C(405-454) deletion, MycRK5 Myc-mLin-2/CASK Hook deletion, MycRK5 Myc-mLin-2/CASK 1-612 L27N(335-390), L27C(440-453), and Myc-mLin-2/CASK Hook band (670-687) deletion constructs were generated by methods published previously (24). GST-mLin-7 L27(13-68), GST-PALS2 L27N(1-55), and GST-mLin-2/CASK L27N(329-403) were generated by PCR using a sense primer containing an XhoI site and an antisense primer containing a HindIII site, and subsequent products were cloned into the pGSTag vector. The YFP-mLin-2/CASK L27N construct was generated by subcloning restriction enzyme-digested inserts into pEYFP-C1 (Clontech) via EcoRI and BamHI sites. CPD constructs were generated by PCR using a sense primer containing a SalI site, an antisense primer containing a HindIII site, and an expressed sequence tag (EST; LP07807) as a template. The subsequent PCR product was cloned into the pGSTag vector. Myc-Camguk (1-500) was also generated by PCR using EST as a template and subcloned into the Myc-RK5 vector. To identify the 5′ end of the coding sequence of the alternate splice variant of Dlg, we isolated total RNA by homogenizing two subsequent mixed stages (0 to 8 h and 8 to 16 h) of fly embryos in Trizol (Gibco BRL) and subsequently performing extractions. Reverse transcription-PCR was conducted simultaneously by reverse transcribing 4 μg of total RNA with a reverse transcriptase-Taq mixture (Invitrogen) and subsequently amplifying DNA fragments from the cDNA pool with gene-specific primers. Resulting fragments were cloned into the pGEMT-easy vector (Promega) and sequenced.
Cell culture and transfections.
Human embryonic kidney (HEK) 293 cells, Madin-Darby canine kidney (MDCK) cells, and A-172, a human neuroblastoma cell line, were grown in Dulbecco's modified Eagle's medium containing 100 U of penicillin/ml and 100 μg of streptomycin sulfate/ml, supplemented with 10% fetal bovine serum. HEK 293 cells and MDCK cells were transfected with Fugene6 reagent (Roche) for both transient and stable expression of proteins. Stable cell lines were picked from clones remaining after selection with appropriate antibiotics, G-418 (Gibco BRL) or Zeocin (Invitrogen).
Protein procedures.
Cells were washed twice with cold phosphate-buffered saline and lysed in lysis buffer (50 mM HEPES [pH 7.5], 10% glycerol, 150 mM NaCl, 1% Triton X-100, 1.5 mM MgCl2) supplemented with 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, and 10 μg of leupeptin/ml. After centrifugation at 16,000 × g for 30 min to remove insoluble debris, the cleared lysate was isolated. For immunoprecipitation, lysates were incubated with antibodies overnight at 4°C. Protein A-agarose beads were added, and immune complexes bound to beads were recovered after 1 h, washed three times with HNTG (50 mM HEPES [pH 7.5], 10% glycerol, 150 mM NaCl, 0.1% Triton X-100), boiled in 1× sample buffer, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Transfer and immunoblotting on nitrocellulose using the horseradish peroxidase (HRP)-protein A or HRP-antimouse antibody chemiluminescence method were performed as described previously (3). GST binding assays were performed as described previously (3).
Far-Western overlay assay.
GST-mLin-2/CASK L27N, GST-SAP97N(1-78), and GST-mLin-7 were used as 32P-labeled probes. Inserts were cloned into the pGSTag vector, which encodes GST and a protein kinase A phosphorylation site. Details of labeling probes and the far-Western overlay assay were previously described (19).
Antibodies.
The anti-Myc monoclonal antibody (clone 9E10), anti-mLin-7 polyclonal antibody, anti-mLin-2/CASK polyclonal antibody, and anti-X11α polyclonal antibody were used for immunoprecipitation, immunoblotting, and immunostaining. The production, characterization, and purification of all these antibodies have been described previously (3, 39). The anti-CASK antibody used for immunostaining was purchased from Zymed (San Francisco, Calif.). The anti-SAP97 monoclonal antibody (Stressgene; generated against the N-terminal 163 aa residues) was used for immunoprecipitation, immunoblotting, and immunostaining. The polyclonal anti-SAP97C antibody, which recognizes the C terminus of the protein, was generated by immunizing rabbits with the GST-SAP97GK protein. Affinity-purified rabbit polyclonal anti-ZO-1 antibodies and rat anti-uvomorulin/E-cadherin monoclonal antibodies used for control immunostaining were purchased from Zymed and Sigma Chemical Co. (St. Louis, Mo.), respectively. Fluorochrome-conjugated secondary antibodies used in immunostaining procedures were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.) and Molecular Probes, Inc. (Eugene, Oreg.).
Immunostaining of MDCK cells and confocal microscopy.
MDCK cells were grown for 5 to 7 days at confluence on Transwell membrane filters (0.4 μm pore size; Corning Costar Corp., Cambridge, Mass.) in order to form a polarized monolayer. Monolayer formation by the MDCK cell lines used in these studies was assayed by immunostaining with anti-ZO-1 and anti-E-cadherin antibodies. Cells were fixed and permeabilized and then blocked with goat serum. Primary antibodies were diluted in 2% goat serum-phosphate-buffered saline (anti-SAP97 at 1:1,000, anti-CASK at 1:500, anti-Myc at 1:1,000, anti-ZO-1 at 1:500, and anti-E-cadherin at 1:2,000). The cells were incubated with secondary antibodies coupled to fluorescein isothiocyanate, Cy5, or Texas red (diluted 1:500). The specificities of all antibodies were determined from appropriate positive- and negative-control immunostaining. Immunostained filters were mounted with ProLong antifade reagent (Molecular Probes, Inc.). Confocal laser scanning microscopy was performed at the Morphology and Image Analysis Core of the University of Michigan Diabetes Research Center. Typically, 20 to 30 serial images were taken in 0.5-μm steps, beginning 1 to 2 μm below the focal plane of the bottom of the cell monolayer and proceeding upward to 1 to 2 μm above the top of the monolayer. Adobe Photoshop (Adobe Systems Incorporated; San Jose, Calif.) was used to overlay channels and other paper images for publication. Detailed protocols for the immunostaining confocal microscopy of MDCK cell lines have been previously described (38).
RESULTS
The L27N domain of mLin-2/CASK is a novel protein-protein interaction domain.
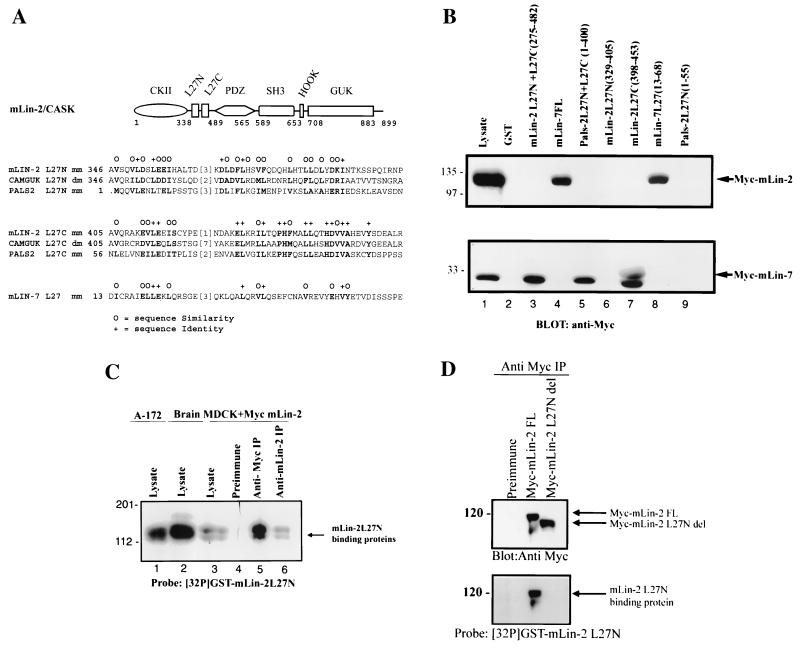
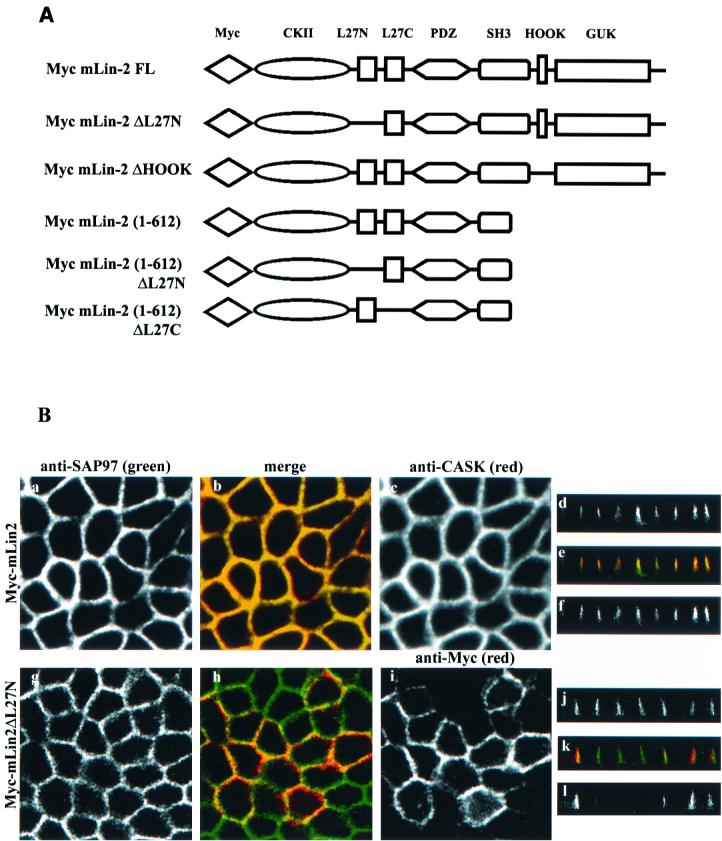
Figure 1A, top, is a schematic of the recognized protein interaction domains in mLin-2/CASK, showing the L27 domains between the CKII domain and PDZ domain; the bottom portion displays an alignment of L27 domains (8). Included are the deduced amino acid sequences of MAGUKs, including mLin-2/CASK, Drosophila Camguk, PALS2/VAM-1, and mLin-7. This alignment revealed two adjacent regions of high homology, which we named the L27N and L27C domains and previously reported as L27A and L27B (8), respectively. After examining its sequence similarity to the adjacent L27C domain, which mediates interaction with mLin-7, we postulated that the L27N domain was also a novel protein-protein interaction domain that mediates homo- or heterodimerization. To test this hypothesis, several proteins consisting of fusions of GST and the L27N and L27C domains of mLin-2/CASK, mLin-7, and PALS2 were constructed and an in vitro affinity binding assay was performed to examine each domain's ability to bind either Myc-tagged mLin-2/CASK or mLin-7. GST fusion proteins containing both L27N and L27C were used as positive controls. In Fig. 1B, we show that the GST fusion protein containing the minimal L27C domain of mLin-2/CASK interacted with mLin-7 while the L27N domain, previously considered to be part of the mLin-7 interaction domain, did not. In addition, the minimal L27 domain of mLin-7 interacted with mLin-2/CASK. The assay also revealed that the L27N domain of mLin-2/CASK does not function as a homodimerization domain (Fig. 1B, lanes 3 and 6). Next, we tested whether the L27N domain of mLin-2/CASK interacts with another protein using a far-Western overlay assay. A 32P-labeled GST-mLin-2/CASK L27N domain probe was overlaid on a nitrocellulose membrane containing Triton X-100-extracted cell lysates from brain, A-172, and MDCK cells stably expressing Myc-mLin-2/CASK as well as protein complexes immunoprecipitated with either the anti-Myc antibody or the anti-mLin-2/CASK antibody. As shown in Fig. 1C, the GST-mLin-2/CASK L27N domain bound to a protein doublet with a molecular mass of 120 to 130 kDa. Further biochemical analysis of multiple tissue lysates, including brain, heart, lung, kidney, and spleen, using a radiolabeled probe showed that this 120- to 130-kDa doublet was widely expressed (data not shown). In addition, the binding specificity of this protein interaction was confirmed by using an L27N-domain-deleted mLin-2/CASK protein, which was unable to bind this 120- to 130-kDa doublet (Fig. 1D).
FIG. 1.
L27N domain of mLin-2/CASK is a novel protein-protein interaction domain. (A) (Top) Schematic of the recognized protein interaction domains in mLin-2/CASK, showing the L27 domains between the CKII and PDZ domains. mLin-2/CASK consists of a CKII domain, a PDZ (PSD-95/Dlg/ZO-1) domain found in other MAGUKs, an Src homology domain (SH3), the Hook binding domain, which mediates cytoskeletal attachment, and a GUK domain. (Bottom) Alignment of the deduced amino acid sequences of MAGUKs including mLin-2/CASK, D. melanogaster (dm) Camguk, PALS2/Vam-1, and mLin-7, which revealed two adjacent regions of high homology, the L27N and L27C domains (8). mm, mammalian. (B) GST fusion protein affinity precipitation assay using L27 domains of mLin-2/CASK, mLin-7, and PALS2. HEK 293T cells were transiently transfected with plasmids, pRK5Myc-mLin-2/CASK, and pRK5Myc-mLin-7. Proteins collected from HEK 293 cell lysates were precipitated with various GST fusion proteins including GST, GST-mLin-2/CASK-L27N+L27C, GST-mLin-7FL, GST-PALS2-L27N+L27C, GST-mLin-2/CASK-L27N, GST-mLin-2/CASK-L27C, GST-mLin-7-L27, and GST-PALS2-L27N. Precipitated proteins were detected by an anti-Myc antibody. One-tenth of the lysates used for precipitation was run as input control (Lysate). (C) Far-Western overlay assay of different cell lysates and immunoprecipitated protein complexes with a 32P-labeled GST-mLin-2/CASK L27N protein probe. Triton X-100-extracted cell lysates collected from human glioblastoma cell line A-172 (lane 1), mouse brain (lane 2), an MDCK cell line stably expressing Myc-mLin-2/CASK (lane 3), proteins precipitated with preimmune serum (lane 4), proteins precipitated with an anti-Myc antibody (lane 5), and proteins precipitated with an anti-mLin-2/CASK antibody (lane 6) were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was then probed with a 32P-labeled GST-mLin-2/CASK L27N protein probe. (D) Far-Western overlay assay of lysates immunoprecipitated with a preimmune or anti-Myc antibody from MDCK cells stably expressing full-length (FL) Myc-mLin-2/CASK and Myc-mLin-2/CASK ΔL27N. Top, presence of each of the Myc-tagged mLin-2/CASK proteins; bottom, coprecipitated unknown protein that binds to the L27N domain of mLin-2/CASK. Relative molecular masses in kilodaltons are shown to the left. IP, immunoprecipitation.
SAP97 binds to the L27N domain of mLin-2/CASK.
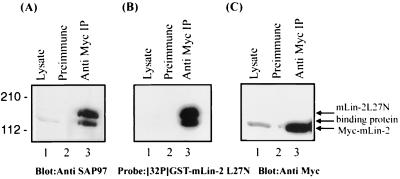
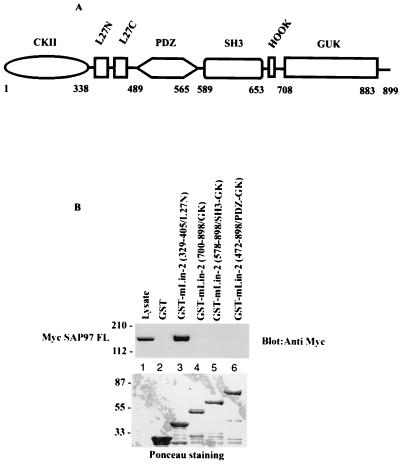
To identify the protein(s) that interacts with the L27N domain of mLin-2/CASK, we initially screened a human brain λ-screen library using the radiolabeled GST-mLin-2/CASK L27N domain probe. However, this method yielded several proteins that could not represent the 120- to 130-kDa protein seen on far-Western blotting. Therefore, as an alternative approach, candidate proteins were screened by immunoblotting. Potential binding partners were coimmunoprecipitated with mLin-2/CASK from Triton X-100-extracted MDCK cell lysates stably overexpressing Myc-mLin-2/CASK. Protein complexes were electrophoretically separated on SDS-PAGE gels and transferred to a nitrocellulose membrane, and Western blotting was performed using antibodies against candidate proteins with molecular masses of about 130 kDa. A few of the many proteins tested, all of which are involved in protein targeting or cell adhesion in neurons and/or epithelial cells, included kinesin heavy chain, E-cadherin, and the PDZ domain-containing MAGUK protein, SAP97. Subsequently, a radiolabeled GST-mLin-2/CASK L27N fusion protein was overlaid on the same membrane to confirm the interaction. As a result, SAP97, a mammalian homologue of Drosophila tumor suppressor protein Dlg, was identified as an mLin-2/CASK L27N-interacting protein as shown in Fig. 2A to C. This result was reconfirmed by testing different domains of mLin-2/CASK proteins for their ability to bind to Myc-tagged SAP97 by a GST affinity binding assay (Fig. 3B). Only the L27N domain of mLin-2/CASK interacted with SAP97, while the guanylate kinase-like (GUK) domain-containing GST fusion protein did not interact with SAP97 (Fig. 3B). Previously, the GUK domain of mLin-2/CASK was reported to interact with the SH3 domain of SAP97 (29). However, we found that the SH3-deleted SAP97 protein precipitated with mLin-2/CASK, demonstrating that the SH3 domain was not necessary for the interaction (Fig. 4C). Nonetheless, this result does not exclude a potential role for the SH3 domain in stabilizing the interaction.
FIG. 2.
Identification of SAP97 as an mLin-2/CASK L27N domain binding partner. (A) Lysate (lane 1) and proteins immunoprecipitated (IP) with preimmune serum (lane 2) or with an anti-Myc antibody from MDCK cell lysates stably expressing mLin-2/CASK (lane 3) were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was then probed with an anti-SAP97N terminus antibody (A), stripped and probed with a 32P-labeled GST-mLin-2/CASK L27N protein probe (B), or stripped and probed with an anti-Myc antibody (C).
FIG. 3.
Direct interaction between L27N domain of mLin-2/CASK and SAP97. (A) Domain structure of mLin-2/CASK. Numbers, amino acid positions of various domain limits. (B) HEK 293T cells were transiently transfected with Myc-SAP97. Myc-SAP97 from Triton X-100-extracted cell lysates was precipitated with GST (lane 2), GST-mLin-2/CASK (L27N) (lane 3), GST-mLin-2/CASK (GK) (lane 4), GST-mLin-2/CASK (SH3-GK) (lane 5), or GST-mLin-2/CASK (PDZ-SH3-GK) (lane 6). The precipitated mLin-2/CASK proteins were detected with an anti-Myc antibody (top). Equivalent amounts of GST fusion proteins were revealed with Ponceau red stain (bottom). Molecular masses in kilodaltons are shown to the left. FL, full length.
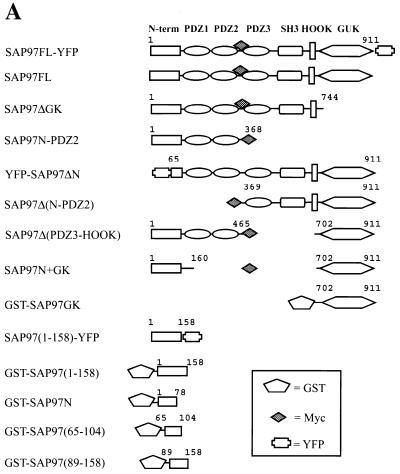
FIG. 4.
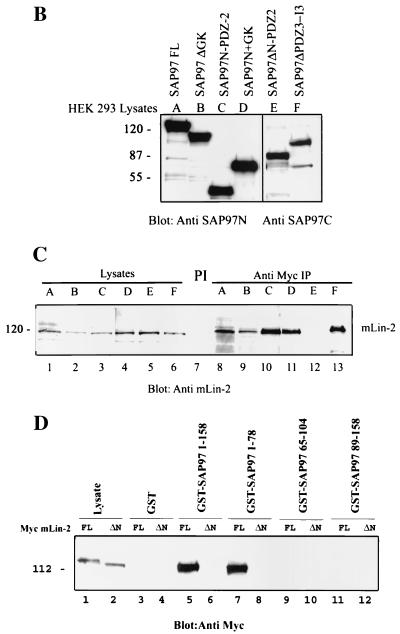
Determination of minimum mLin-2/CASK L27N binding domain of SAP97. (A) Schematic representation of SAP97 deletion constructs. The generation of these constructs is described in Materials and Methods. The amino acid limits relative to full-length SAP97 are shown above each construct. FL, full length. (B) HEK 293T cells were transiently transfected with Myc-SAP97 deletion constructs. The expression levels of transfected proteins were analyzed by immunoblotting lysates of each protein with a polyclonal anti-SAP97N antibody (lanes A to D) and a polyclonal anti-SAP97C antibody (lanes E and F). (C) Proteins from the above lysates were immunoprecipitated (IP) with preimmune serum (PI) and an anti-Myc antibody and then blotted with a polyclonal anti-mLin-2/CASK antibody to detect endogenous mLin-2/CASK. (D) HEK 293T cells were transiently transfected with full-length Myc-mLin-2/CASK and Myc-mLin-2/CASK L27N deletion (ΔN) constructs. Proteins from the collected lysates (lanes 1 and 2) were precipitated with bacterially expressed GST (lanes 3 and 4), GST-SAP97(1-158) (lanes 5 and 6), GST-SAP97(1-78) (lanes 7 and 8), GST-SAP97(65-104) (lanes 9 and 10), and GST-SAP97(89-158) (lanes 11 and 12). Precipitated Myc-SAP97 proteins were detected by immunoblotting with an anti-Myc antibody. Molecular masses in kilodaltons are shown at the left.
The N-terminal domain of SAP97 interacts with the L27N domain of mLin-2/CASK.
Next, we mapped the region of SAP97 that interacts with the mLin-2/CASK L27N domain. Figure 4A shows representations of SAP97 deletion constructs used to determine the interaction domain. These constructs were transiently expressed in HEK 293 cells and precipitated with an anti-Myc antibody to examine whether or not they bind endogenous mLin-2/CASK. Figure 4B shows that all the transfected proteins were equally expressed. As shown in Fig. 4C, a SAP97 protein missing the first 368 amino acids failed to coprecipitate mLin-2/CASK while SAP97ΔSH3 (protein missing the SH3 domain) precipitated mLin-2/CASK as well as full-length SAP97. Further biochemical analysis using a GST fusion protein affinity pull-down assay revealed that the first 65 amino acids of SAP97 were responsible for binding to the mLin-2/CASK L27N domain (Fig. 4D). To confirm the specificity of the interaction, an mLin-2/CASK protein lacking the L27N domain was used as a negative control.
SAP97, mLin-2/CASK, mLin-7 form a stable complex in a brain cell line, HEK 293 cells, and MDCK cells.
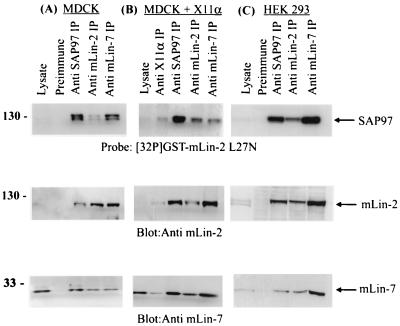
Next we wanted to identify other protein components in the SAP97- and mLin-2-containing protein complex from both neuronal and epithelial cells (Fig. 5). We postulated that mLin-7 was in the complex with SAP97 through interaction with mLin-2/CASK, because the interaction between mLin-7 and mLin-2/CASK has been previously demonstrated (3, 5). In MDCK and HEK 293 cells we found that immunoprecipitation of mLin-7 or mLin-2 coimmunoprecipitated SAP97. Interestingly, mLin-7 appeared to precipitate with SAP97 more strongly than mLin-2/CASK. However, we could not detect a direct interaction between mLin-7 and SAP97 using radiolabeled mLin-7 fused to GST in far-Western blots, but it is possible that multiple protein complexes contain both mLin-7 and SAP97 (see below). We also demonstrated complex formation between mLin-7 and the mLin-2/CASK protein with L27N deleted to show that the mLin-2-mLin-7 interaction was independent of SAP97 (data not shown). We then tested whether or not X11α/Mint1, which is known to interact with the CKII domain of mLin-2/CASK and form a tripartite complex containing X11α/Mint1, mLin-2/CASK, and mLin-7 in brain (3), was part of the SAP97-mLin-2/CASK complex. We took lysates from MDCK cells stably expressing X11α and subjected them to immunoprecipitation with appropriate antibodies. As shown in Fig. 5 (middle), the anti-X11α antibody coprecipitated SAP97, mLin-2/CASK, and mLin-7 although with weaker affinity than the rest of the antibodies. As a negative control, MDCK cells stably expressing X11γ were used, because X11γ does not interact with mLin-2/CASK; as expected, X11γ was shown not to be in a complex with SAP97 (data not shown). All together, we demonstrated that the complex comprising SAP97 and mLin-2/CASK can also contain mLin-7 and X11α.
FIG. 5.
SAP97, mLin-2/CASK, and mLin-7 form a stable complex in a HEK 293 and MDCK cells. (A) Collected lysates from MDCK cells were precipitated with preimmune serum, an anti-SAP97 antibody, an anti-mLin-2/CASK antibody, and an anti-mLin-7 antibody. Precipitated proteins were separated on an SDS-10% PAGE gel and transferred to a nitrocellulose membrane. The top portion of the membrane was immunoblotted with an anti-mLin-2/CASK antibody, and the bottom portion of the membrane was immunoblotted with an anti-mLin-7 antibody. The top portion of the membrane was then stripped and reprobed with radiolabeled 32P-GST-L27N to detect SAP97. IP, immunoprecipitation. (B and C) The same procedures were performed with lysate from MDCK cells stably expressing X11α (B) and HEK 293 cells (C).
The N-terminal domain of SAP97 binds to another mLin-7 binding protein as well as mLin-2/CASK.
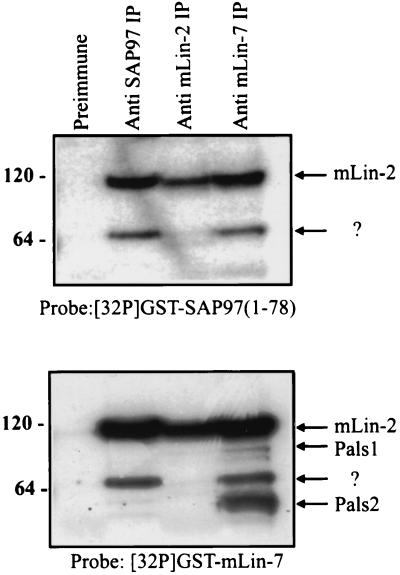
To examine whether mLin-2/CASK was the only binding partner of the N-terminal domain of SAP97, the first 78 aa residues of SAP97 were fused to GST and used as a radiolabeled probe in a far-Western blot. Proteins were coimmunoprecipitated from Triton X-100-extracted MDCK cell lysates with the anti-SAP97 antibody, the anti-mLin-2/CASK antibody, and the anti-mLin-7 antibody. Precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose. Subsequently, the membrane was overlaid with 32P-labeled GST-SAP97 N1(1-78). As expected, the N terminus of SAP97 recognized mLin-2/CASK (Fig. 6, top). The identity of mLin-2/CASK was confirmed by far-Western blotting using a radiolabeled mLin-7 protein probe (Fig. 6, bottom). Interestingly, the N-terminal domain of SAP97 also interacted with another protein of about 65 kDa that also coprecipitated with mLin-7. Furthermore, the level of the 65-kDa protein that precipitated with SAP97 was reduced when the exogenous mLin-2 construct was overexpressed, suggesting that the 65-kDa protein competes with mLin-2/CASK for the N terminus of SAP97 (data not shown). Figure 6, top, shows two protein bands that are recognized by the SAP97 N-terminal probe, while the bottom portion shows that the second band is also recognized by a probe containing the N terminus of mLin-7. Current work is focused on identifying this additional SAP97 binding protein.
FIG. 6.
Identification of proteins that bind to the N-terminal domain of SAP97. Triton X-100-extracted lysates from MDCK cells were immunoprecipitated (IP) with preimmune serum (lane 1), with an anti-SAP97N antibody (lane 2), with an anti-mLin-2/CASK antibody (lane 3), and with an anti-mLin-7 antibody (lane 4). Precipitated proteins were resolved on a 4 to 12% Novex gel and transferred to nitrocellulose. The membrane was overlaid with 32P-labeled GST-SAP97(1-78) (top), stripped, and then probed with 32P-labeled GST-mLin-7N protein (bottom). Molecular masses in kilodaltons are shown at the left. ?, identity of the protein is not known.
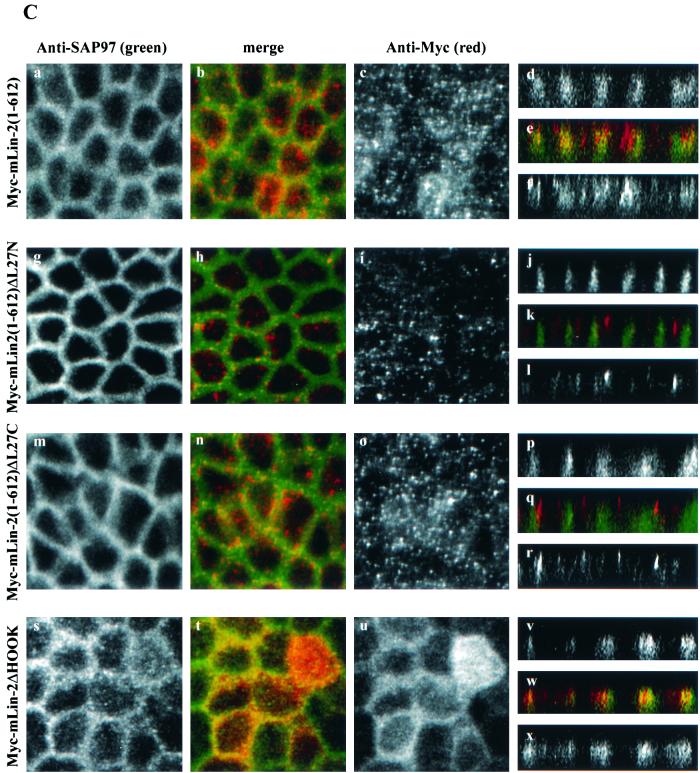
Basolateral membrane localization of SAP97 depends on its interaction with mLin-2/CASK.
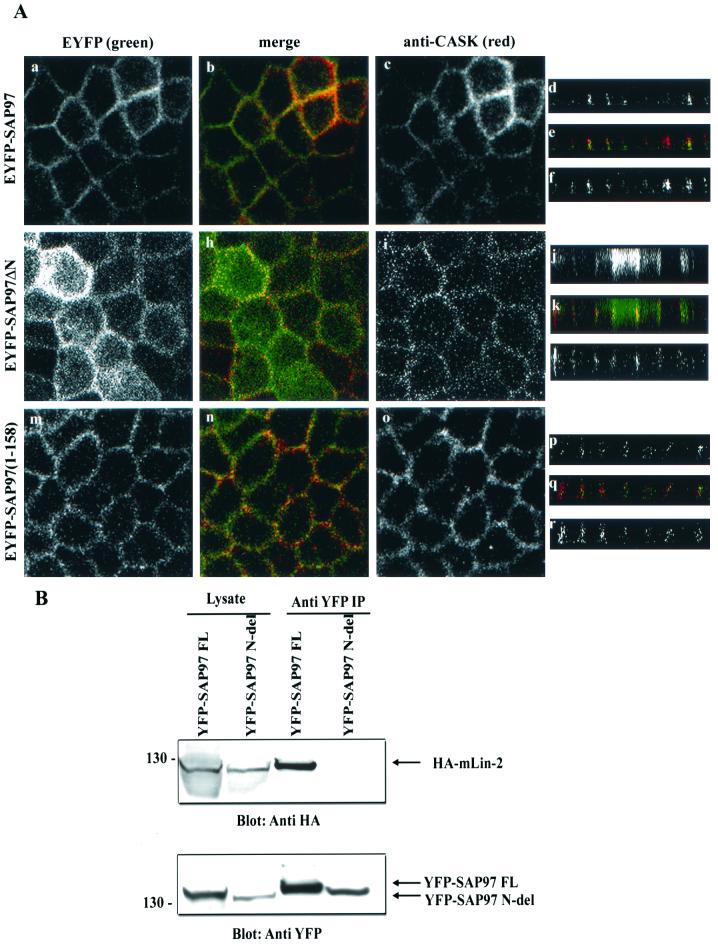
To this point our data have demonstrated a strong interaction between the L27N domain of mLin-2/CASK and the first 65 aa of SAP97. Next we wanted to examine the role of these domains in the targeting of these two proteins to the lateral surfaces of epithelial cells. The role of the N terminus in the targeting of SAP97 to the basolateral surface in Caco-2 cells has already been described by Wu and coworkers (43). To confirm these findings, we analyzed the role of the N terminus of SAP97 in the targeting of SAP97 in MDCK cells. We first determined the localization pattern of both SAP97 and mLin-2/CASK in MDCK cells. Both proteins localized to the lateral membrane very efficiently (Fig. 7A, a to f). However, we found that the YFP-SAP97 protein with the N-terminal domain deleted failed to localize to the basolateral membrane (g to l) while SAP97(1-158)-YFP (m to r) localized properly. The basolateral targeting of endogenous mLin-2/CASK was not affected by the stable expression of YFP-SAP97 with the N-terminal domain deleted, suggesting that basolateral membrane targeting of mLin-2/CASK does not depend on SAP97 (i and l). We also demonstrated that the YFP-SAP97 protein with the N-terminal domain deleted failed to coprecipitate hemagglutinin (HA)-tagged mLin-2/CASK (Fig. 7B).
FIG. 7.
The N terminus of SAP97 is important for its basolateral membrane localization. MDCK cells stably expressing YFP-tagged full-length SAP97 (a to f), YFP-SAP97ΔN (g to l), and YFP-SAP97(1-158) (m to r) were stained with anti-CASK antibodies (c, i, o, f, l, and r). Cells were then stained with secondary antibodies coupled to fluorochromes and examined by confocal laser scanning microscopy. YFP-tagged protein localization was observed at an excitation wavelength of 513 nm and an emission wavelength 527 nm (a, g, m, d, j, and p). Squares, digital photomicrographs (the X-Y dimension of the Z series); rectangles, X-Z dimension (Z section). The apical side of the membrane is on the top of the Z sections and the basolateral sides are at the bottom. (B) The N-terminus-deleted SAP97 does not interact with mLin-2/CASK. HEK 293 cells were cotransfected with HA-tagged full-length mLin-2/CASK and either full-length YFP-tagged SAP97 or YFP-tagged N-terminus-deleted SAP97. Lysates and proteins immunoprecipitated (IP) with a YFP antibody were resolved on a 4 to 12% Novex gel and transferred to a nitrocellulose membrane. Subsequently, the membrane was probed with an anti-HA antibody to detect coprecipitated HA-mLin-2/CASK (top), stripped, and then probed with an anti-YFP antibody to detect both full-length YFP-SAP97 and N-terminus-deleted YFP-SAP97 (bottom).
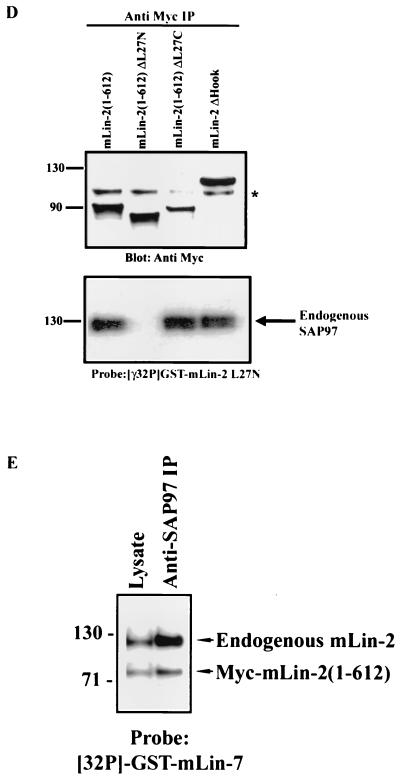
We next analyzed the role of the L27N domain on the basolateral targeting of mLin-2/CASK. The basolateral membrane localization of the mLin-2/CASK protein was not compromised by the deletion of the L27N domain (Fig. 8B). Furthermore, this mLin-2/CASK mutant had no significant impact on the basolateral localization of endogenous SAP97 that was free to bind to endogenous mLin-2/CASK in these cells (Fig. 8B, g to l). We then expressed Myc-mLin-2/CASK(1-612), a protein that lacks its C-terminal half (Fig. 8A) and that was improperly localized in epithelia (39), as a dominant-negative mutant to examine its effect on SAP97. This dominant-negative protein can bind to SAP97 (Fig. 8D). As shown in Fig. 8C, a to f, endogenous SAP97 did not localize properly to the basolateral membrane in cells expressing this truncated mLin-2/CASK (seen best on the Z section [d]). We then examined the effects of Myc-mLin-2/CASK(1-612) missing either the L27N or the L27C domain on the targeting of SAP97. Myc-mLin-2/CASK(1-612) missing the L27N domain does not bind SAP97 (Fig. 8D) and should not affect SAP97 targeting. Indeed as shown in Fig. 8C, g to l (seen best on the Z section [j]), endogenous SAP97 was efficiently localized to the basolateral membrane when the L27N domain was deleted from dominant-negative mLin-2/CASK(1-612). This lack of effect was not due to the expression level, as the dominant-negative Myc-mLin-2/CASK(1-612) missing the L27N domain and the intact Myc-mLin-2/CASK(1-612) were expressed at similar levels (Fig. 8D). In contrast, deletion of the L27C region did not affect the ability of mLin-2/CASK(1-612) to bind (Fig. 8D) or mislocalize SAP97 (Fig. 8C, m to r). We also analyzed another mLin-2/CASK mutant that is mislocalized in MDCK cells. We have found that a 14-aa deletion that disrupts the Hook domain of mLin-2/CASK prevents its proper localization to the basolateral membranes of MDCK cells (S. Fan and B. Margolis, unpublished observations). SAP97 was also mislocalized in MDCK cells expressing this mLin-2/CASK mutant, in agreement with our results with the Myc-mLin-2/CASK(1-612) mutant (Fig. 8C, s to x). We noted that all our dominant-negative constructs had an incomplete effect on SAP97 localization, leading us to believe that the expression level of dominant-negative mLin-2/CASK might be less than that of the endogenous mLin-2/CASK present in these cells. We found that the level of endogenous mLin-2/CASK bound to SAP97 was still higher than that of the dominant-negative Myc-mLin-2/CASK(1-612) mutant (Fig. 8E). This suggests that our results may, if anything, underestimate the importance of mLin-2/CASK in SAP97 targeting.
FIG. 8.
Basolateral membrane localization of SAP97 is disrupted by expression of dominant-negative mLin-2/CASK construct in MDCK cells. (A) Schematic representation of various mLin-2/CASK dominant-negative constructs. FL, full length. (B)The role of the L27N domain of mLin-2/CASK in the membrane localization of both mLin-2/CASK and SAP97 was analyzed. MDCK cells stably expressing either wild-type Myc-mLin-2/CASK (a to f) or L27N-domain-deleted mLin-2/CASK (g to l) were costained with both an anti-SAP97 antibody (a, d, g, and j) and an anti-CASK antibody (c and f) or anti-Myc (i and l). (C) Effects of expressing Myc-mLin-2/CASK(1-612) (a to f), L27N-domain-deleted Myc-mLin-2/CASK (g to l), L27C-domain-deleted Myc-mLin-2/CASK (m to r), and Hook-domain-deleted Myc-mLin-2/CASK (s to x) on basolateral localization of SAP97 were analyzed. MDCK cells were costained with both anti-SAP97 antibodies (a, d, g, j, m, p, s, and v) and anti-Myc antibodies (c, f, l, i, l, o, r, u, and x). (D) Expression level of Myc-mLin-2/CASK(1-612) and mutants with the L27N, L27C, and Hook domains deleted from stable MDCK cells were analyzed. Proteins were precipitated with anti-Myc antibodies and resolved on a 4 to 12% Novex gel. A nitrocellulose membrane was subjected to anti-Myc Western blotting to detect different Myc-mLin-2/CASK deletion proteins (top), stripped, and then overlaid with a 32P-GST-mLin-2 L27N probe to detect coprecipitated SAP97 (bottom). ∗, nonspecific protein band. IP, immunoprecipitation. (E) MDCK cells stably expressing Myc-mLin-2/CASK(1-612) were precipitated with anti-SAP97 antibodies to examine the level of endogenous full-length mLin-2/CASK bound to SAP97 versus that of the exogenous Myc-mLin-2/CASK(1-612). The precipitated protein complex was resolved on a 4 to 12% Novex gel, transferred to a nitrocellulose membrane, and then probed with a 32P-GST-mLin-7 probe.
Evolutionary conservation of the L27N binding region of SAP97 in Drosophila.
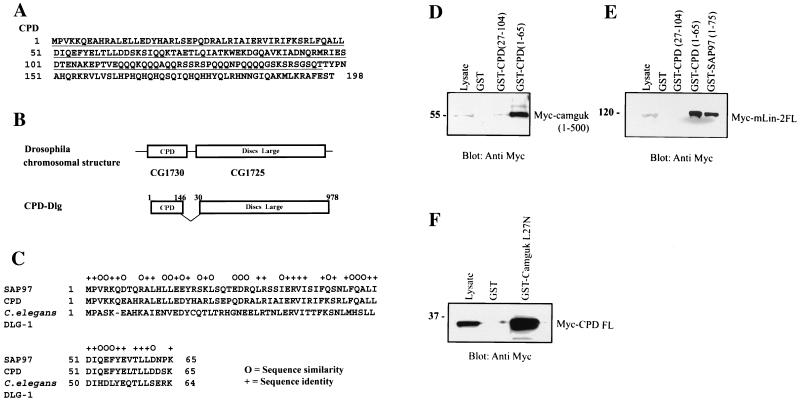
Next we wanted to determine whether or not the interaction between the N terminus of SAP97 and the L27N domain of mLin-2/CASK was conserved in other organisms. We postulated that, if the L27N domain-mediated interaction between MAGUK proteins is one of the mechanisms that govern the targeting of membrane proteins, it should be evolutionarily conserved. To test the hypothesis, we searched for other proteins containing sequences that are similar to the first 75 aa residues of SAP97 using BLAST analysis. As a result, we identified a small Drosophila gene product, called CG1730 (accession no. AAF48037), which contained N-terminal sequences that are highly homologous to the N-terminal domain of SAP97. After analyzing the genomic sequence encoding CG1730 and its chromosomal location, we realized that it was located near the beginning of the gene encoding Drosophila Dlg (CG1725) (Fig. 9B). Subsequently the full-length CG1730 protein, containing 198 aa, was cloned by PCR using an EST (LP07807) as a template (Fig. 9A). To confirm that we cloned the full-length CPD, we compared the CPD genomic sequence to three ESTs and identified the common stop codon from all sequences. We have named the protein CPD, component protein of Dlg, for its potential role as a missing N-terminal domain of the Drosophila Dlg protein, which is also present in other orthologues such as C. elegans DLG-1. Figure 9C shows a sequence alignment of different SAP97 homologues that revealed the high degree of sequence similarity within the first 65 aa.
FIG. 9.
Evolutionary conservation of the interaction between the SAP97 N terminus and the mLin-2 L27N domain in Drosophila. (A) Amino acid sequence of full-length CPD protein (B) Schematic of Drosophila chromosomal region containing the coding sequences for CPD and Dlg and the primary structure of the CPD-Dlg splice variant. (C) Sequence alignment of the first 65 aa residues of SAP97, CPD, and C. elegans DLG-1 proteins. (D) Interaction between Myc-Camguk(1-500) and GST-CPD. Lysates from HEK 293 cells transiently expressing Myc-Camguk(1-500) were incubated with GST, GST-CPD(27-104), and GST-CPD(1-65) proteins. (E) Interaction between Myc-mLin-2/CASK and GST-CPD. Lysates from MDCK cells stably expressing Myc-mLin-2/CASK proteins were precipitated with GST, GST-CPD(25-104), GST-CPD(1-65), and GST-SAP97(1-78). (F) Interaction between GST-Camguk L27N domain and the full-length Myc-CPD protein. Lysates from HEK 293 cells transiently expressing full-length Myc-CPD were incubated with GST and the GST-Camguk L27N domain.
We also wanted to examine the possibility that a splice variant of the Drosophila Dlg gene might contain regions of the CPD gene due to their close genomic location. To identify the transcript, we isolated and reverse transcribed RNA from two different mixed stages of Drosophila embryos (0 to 8 h and 8 to 16 h). The CPD gene-Dlg gene hybrid 5′ end was amplified by reverse transcription-PCR from cDNA using primers specific for CPD gene sequences, and the 3′ end was amplified by using primers specific for the Dlg (aa 1 to 493) coding sequence. When primers specific for sequences generating full-length CPD (aa 1 to 198) and full-length DLG (aa 1 to 978) were used as internal controls, all three fragments with the predicted sizes were amplified. Subsequent sequencing of those DNA fragments revealed the existence of a unique CPD-Dlg hybrid transcript consisting of the first 146 aa of CPD fused to aa 30 of Dlg, resulting in a protein of 1,094 amino acids (Fig. 9B).
Since the SAP97 N terminus mediates interaction with mLin-2 via the L27N domain, we wanted to test whether regions of CPD interacted with the L27N domain of mLin2/CASK as well as with Drosophila Camguk, the orthologue of mLin-2/CASK, by using a GST fusion protein affinity binding assay. Bacterially expressed GST fusion proteins were incubated with lysate from MDCK cells stably expressing either Myc-mLin-2/CASK or HEK 293 cell lysate transiently expressing Myc-Camguk proteins (aa 1 to 500). As shown in Fig. 9D and E, full-length CPD interacted with both mammalian and Drosophila proteins. The binding region was narrowed to the first 65 aa of CPD in agreement with the alignment in Fig. 9C. We also determined that the first 26 aa residues were necessary for the interaction. Upon deletion of these residues, the interaction between CPD and either Camguk or mLin-2/CASK was significantly reduced or abolished, respectively. We also found that the L27N domain of mLin-2/CASK is conserved in Camguk by demonstrating that the L27N domain of Camguk can bind CPD (Fig. 9F).
DISCUSSION
Identification of the L27N domain of mLin-2/CASK as a functional protein-protein interaction domain and its binding partner.
In this study, we investigated the role of the newly identified L27N domain of mLin-2/CASK (8). This region of mLin-2/CASK is also conserved in many other MAGUK proteins that interact with mLin-7. Here, we demonstrated that the L27N domain of mLin-2/CASK is a novel protein-protein interaction domain that mediates interaction with SAP97. The L27N domain-mediated interaction with SAP97 yields some unique insights into the function of MAGUK proteins. First, it suggests that the L27N domains of other MAGUK proteins, such as PALS1, DLG2, and DLG3, could also function as potential protein interaction domains and play a crucial role in targeting other membrane proteins. Second, the amino acid sequence similarity of these L27N domains, and thus their implied structural conservation, among different organisms suggests the evolutionary conservation of L27N domain function.
Functional significance of interaction between the mLin-2/CASK L27N domain and SAP97: localization of SAP97 to the basolateral membrane in epithelia.
In our study, we identify a novel interaction between the L27N domain of mLin-2/CASK and SAP97, a mammalian homologue of Drosophila Dlg tumor suppressor protein and another MAGUK family member. Here we show that the association of SAP97 with the L27N domain of mLin-2/CASK requires the N-terminal domain by using in vitro GST fusion protein affinity pull-down assays and coimmunoprecipitation studies of living cells. Recently, the SH3 domain of SAP97 was reported to associate with the GUK domain of mLin-2/CASK (29). However, our experiments showed that association between the L27N domain of mLin-2/CASK and the N terminus of SAP97 was stronger than the interaction between the GUK and SH3 domains of these proteins. It is possible that there is more than one point of attachment between these proteins. Furthermore, their association site may vary upon binding to other interaction partners since, like other MAGUK proteins, SAP97 is also a multidomain scaffolding protein that recruits transmembrane proteins and signaling molecules to the plasma membrane sites.
SAP97 contains three PDZ domains as well as one SH3 domain, one Hook domain, and one GUK domain (23). In neuronal cells, SAP97 is expressed in presynaptic membranes and mediates the clustering of receptor molecules, such as the voltage-gated Shaker K+ channels and the NR2 subunits of the N-methyl d-aspartate receptor, by interacting with PDZ domains (36). It is also expressed at the basolateral membrane of epithelial cells and at the lymphocyte immune synapse, but its precise role in epithelia remains to be characterized (23, 28). The PDZ domains of SAP97 interact with adenomatous polyposis coli (27), PTEN tumor suppressor protein (1), and several viral oncoproteins (22), while the GUK domain interacts with the GKAP/SAPAP1/DAP1 family (20, 40). There have been reports suggesting the possibility of oligomerization of SAP97 through the N-terminal protein sequences (aa 106 to 120), which are also conserved in other SAP proteins such as PSD-95 and SAP102 (25). In addition, the functional significance of the N termini of these MAGUK proteins in targeting and receptor clustering has been previously demonstrated (9, 14, 15). The first 65 aa residues of SAP97 have been reported to direct selective subcellular localization and the attachment to the cytoskeleton assembly at sites of cell-cell contact (43). However, the protein that interacts with the N terminus of SAP97 and that is responsible for its targeting to the lateral membrane was unknown. In this report, our experiments using dominant-negative mLin-2/CASK revealed that the membrane localization of SAP97 was dependent on its association with mLin-2/CASK. When dominant-negative protein mLin-2/CASK(1-612) was overexpressed, SAP97 failed to properly localize to the lateral membrane, while the dominant-negative effect was removed upon deletion of the interacting L27N domain. We further supported the hypothesis that SAP97 basolateral membrane localization was mediated through its interaction with the mLin-2/CASK protein by demonstrating that expression of a dominant-negative mLin-2/CASK missing the Hook domain also results in mislocalization of SAP97 in the epithelia. However, it was difficult to show complete mislocalization of all SAP97 proteins due to high expression of the endogenous full-length mLin-2/CASK protein present in cells.
Combinatorial multiprotein complex formation of MAGUKs in epithelial cell membrane.
We also examined the protein complex containing SAP97 and mLin-2/CASK to determine other proteins associated with the complex. Coimmunoprecipitation studies from both neuronal and epithelial cells revealed that both mLin-7 and X11α can exist in the complex. One can hypothesize possible roles for each protein in this complex. For example, mLin-7 was reported to directly interact with β-catenin through the PDZ domains in both neurons and epithelia in a calcium-dependent manner (33). In brain, X11α is known to form a tripartite complex with mLin-2/CASK and mLin-7 (5) as well as with kinesin-like motor protein KIF17, which may play a role in vesicle transport (35). Together, both proteins may be crucial for targeting the complex to the membrane either by stabilizing cytoskeletal attachment or mediating the interaction between the complex and the vesicle transport machinery.
Genetic association between mLin-2/CASK and SAP97.
In spite of many binding partners that have been identified for both mLin-2/CASK and SAP97, the specific biological function of these two proteins still remains unclear. However, recent genetic and phenotypic reports on both mLin-2/CASK and SAP97 from transgenic-mouse models provided us with clues to understanding their biological function as well as their functional association. Laverty and his colleagues reported that disruption of the murine CASK (mCASK) gene resulted in a sex-linked cleft palate mouse mutant (21). In their studies, the transgene was inserted within the calmodulin-binding domain of mCASK, which is located adjacent to the L27N domain. Meanwhile, Caruana and Bernstein reported that replacing the SAP97 gene with a construct lacking the C-terminal half, the SH3 and GUK domains, causes craniofacial dysmorphogenesis including cleft palate in mice (6). Also, both mLin-2/CASK and SAP97 mutant mice displayed kinked tails. This similarity in the phenotypes of mice carrying mutations in either mLin-2/CASK or SAP97, and thus their functional association, may be explained by the physical interaction of these two proteins, which we have demonstrated in this report.
Evolutionary conservation of the interaction between the L27N domain of Camguk and the SAP97 N terminus homologue of D. melanogaster.
Drosophila Dlg is a tumor suppressor protein that is an orthologue of mammalian SAP97. Dlg is reported to be essential for prohibiting cell overgrowth during oogenesis (12), maintaining cell adhesion and cell polarity in both embryonic and adult tissues (42), development of proper synapse structure and function (4), and formation of neuroblasts (30, 32). Dlg is very similar to SAP97 in structure, containing three PDZ domains, an SH3 domain, and a GUK domain. Protein sequence analysis of reported homologous proteins revealed that Dlg lacks N-terminal sequences corresponding to those of SAP97. However, the C. elegans orthologue of Dlg, DLG-1, contains these N-terminal sequences, and the N terminus of DLG-1 has recently been reported to be crucial for its localization to the adherens junctions (10). Here we report the discovery of a small Drosophila gene product (CG1730), which we named CPD, containing a protein sequence highly homologous to that of the SAP97 N terminus. We also report finding a unique Drosophila Dlg splice variant containing a region of CPD at its N terminus. When CPD was expressed as a separate protein, it interacted with the L27N domains of both Camguk and mLin-2/CASK, and the first 65 aa of CPD were sufficient for this binding in our in vitro binding assays. Therefore, the CPD-Dlg hybrid protein should interact with Camguk and likely plays a role separate from those previously identified for Dlg. It is also possible that CPD is an evolutionary relic that was initially separated from but later fused to Dlg over the course of evolution. The fact that the C. elegans counterpart of Dlg contains this N-terminal region suggests this possibility, since Drosophila and C. elegans branched from the phylogenic tree at similar times. CPD alone could also behave as a negative regulator of Dlg acting to prevent proteins such as Camguk from interacting with the N terminus of the CPD-Dlg hybrid. Therefore, further study of CPD and CPD-Dlg will be necessary to understand their biological significance in Drosophila development. Nonetheless the finding that the domains that bind SAP97 to mLin-2/CASK are highly evolutionarily conserved suggests that these domains play an important role in protein targeting and cell polarity.
Acknowledgments
We thank Morgan Sheng for the Myc-tagged SAP97 construct and Ken Cadigan for helpful discussion. We also thank Robert Kruger and the laboratory of Jack Dixon for his help in maintaining a large-scale fly cage and collecting embryos.
Seonok Lee was supported by NIH Genetics training grant (5-T32 GM07544). Ben Margolis is an investigator of the Howard Hughes Medical Institute. This work was supported in part by NIDDK grant (2P50DK39255). We are also grateful to Thomas Komorowski, manager of the University of Michigan Diabetes Research Center Morphology and Image Analysis Core (NIH grant 5-P60-DK20572), for his assistance with confocal microscopy.
S. Lee and S. Fan made approximately equal contributions to this work.
REFERENCES
- 1.Adey, N. B., L. Huang, P. A. Ormonde, M. L. Baumgard, R. Pero, D. V. Byreddy, S. V. Tavtigian, and P. L. Bartel. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60:35-37. [PubMed] [Google Scholar]
- 2.Borg, J. P., M. O. Lopez-Figueroa, M. de Taddeo-Borg, D. E. Kroon, R. S. Turner, S. J. Watson, and B. Margolis. 1999. Molecular analysis of the X11-mLin-2/CASK complex in brain. J. Neurosci. 19:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg, J. P., S. W. Straight, S. M. Kaech, M. de Taddeo-Borg, D. E. Kroon, D. Karnak, R. S. Turner, S. K. Kim, and B. Margolis. 1998. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem. 273:31633-31636. [DOI] [PubMed] [Google Scholar]
- 4.Budnik, V. 1996. Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr. Opin. Neurobiol. 6:858-867. [DOI] [PubMed] [Google Scholar]
- 5.Butz, S., M. Okamoto, and T. C. Sudhof. 1998. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell 94:773-782. [DOI] [PubMed] [Google Scholar]
- 6.Caruana, G., and A. Bernstein. 2001. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol. Cell. Biol. 21:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, A. R., D. F. Woods, S. M. Marfatia, Z. Walther, A. H. Chishti, J. M. Anderson, and D. F. Wood. 1998. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J. Cell Biol. 142:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerks, T., P. Bork, E. Kamberov, O. Makarova, S. Muecke, and B. Margolis. 2000. L27, a novel heterodimerization domain in receptor targeting proteins Lin-2 and Lin-7. Trends Biochem. Sci. 25:317-318. [DOI] [PubMed] [Google Scholar]
- 9.El-Husseini, A. E., J. R. Topinka, J. E. Lehrer-Graiwer, B. L. Firestein, S. E. Craven, C. Aoki, and D. S. Bredt. 2000. Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs. J. Biol. Chem. 275:23904-23910. [DOI] [PubMed] [Google Scholar]
- 10.Firestein, B. L., and C. Rongo. 2001. DLG-1 is a MAGUK similar to SAP97 and is required for adherens junction formation. Mol. Biol. Cell 12:3465-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Mariscal, L., A. Betanzos, and A. Avila-Flores. 2000. MAGUK proteins: structure and role in the tight junction. Semin. Cell Dev. Biol. 11:315-324. [DOI] [PubMed] [Google Scholar]
- 12.Goode, S., and N. Perrimon. 1997. Inhibition of patterned cell shape change and cell invasion by Discs large during Drosophila oogenesis. Genes Dev. 11:2532-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hata, Y., S. Butz, and T. C. Sudhof. 1996. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J. Neurosci. 16:2488-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsueh, Y. P., E. Kim, and M. Sheng. 1997. Disulfide-linked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron 18:803-814. [DOI] [PubMed] [Google Scholar]
- 15.Hsueh, Y. P., and M. Sheng. 1999. Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel Kv1.4. J. Biol. Chem. 274:532-536. [DOI] [PubMed] [Google Scholar]
- 16.Irie, M., Y. Hata, M. Deguchi, N. Ide, K. Hirao, I. Yao, H. Nishioka, and Y. Takai. 1999. Isolation and characterization of mammalian homologues of Caenorhabditis elegans lin-7: localization at cell-cell junctions. Oncogene 18:2811-2817. [DOI] [PubMed] [Google Scholar]
- 17.Jo, K., R. Derin, M. Li, and D. S. Bredt. 1999. Characterization of MALS/Velis-1, -2, and -3: a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J. Neurosci. 19:4189-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaech, S. M., C. W. Whitfield, and S. K. Kim. 1998. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamberov, E., O. Makarova, M. Roh, A. Liu, D. Karnak, S. Straight, and B. Margolis. 2000. Molecular cloning and characterization of Pals, proteins associated with mLin-7. J. Biol. Chem. 275:11425-11431. [DOI] [PubMed] [Google Scholar]
- 20.Kim, E., S. Naisbitt, Y. P. Hsueh, A. Rao, A. Rothschild, A. M. Craig, and M. Sheng. 1997. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J. Cell Biol. 136:669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laverty, H. G., and J. B. Wilson. 1998. Murine CASK is disrupted in a sex-linked cleft palate mouse mutant. Genomics 53:29-41. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. S., R. S. Weiss, and R. T. Javier. 1997. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 94:6670-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lue, R. A., S. M. Marfatia, D. Branton, and A. H. Chishti. 1994. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc. Natl. Acad. Sci. USA 91:9818-9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarova, O., E. Kamberov, and B. Margolis. 2000. Generation of deletion and point mutations with one primer in a single cloning step. BioTechniques 29:970-972. [DOI] [PubMed] [Google Scholar]
- 25.Marfatia, S. M., O. Byron, G. Campbell, S. C. Liu, and A. H. Chishti. 2000. Human homologue of the Drosophila discs large tumor suppressor protein forms an oligomer in solution. Identification of the self-association site. J. Biol. Chem. 275:13759-13770. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Estrada, O. M., A. Villa, F. Breviario, F. Orsenigo, E. Dejana, and G. Bazzoni. 2001. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J. Biol. Chem. 276:9291-9296. [DOI] [PubMed] [Google Scholar]
- 27.Matsumine, A., A. Ogai, T. Senda, N. Okumura, K. Satoh, G. H. Baeg, T. Kawahara, S. Kobayashi, M. Okada, K. Toyoshima, and T. Akiyama. 1996. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272:1020-1023. [DOI] [PubMed] [Google Scholar]
- 28.Muller, B. M., U. Kistner, R. W. Veh, C. Cases-Langhoff, B. Becker, E. D. Gundelfinger, and C. C. Garner. 1995. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J. Neurosci. 15:2354-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nix, S. L., A. H. Chishti, J. M. Anderson, and Z. Walther. 2000. hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions. J. Biol. Chem. 275:41192-41200. [DOI] [PubMed] [Google Scholar]
- 30.Ohshiro, T., T. Yagami, C. Zhang, and F. Matsuzaki. 2000. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408:593-596. [DOI] [PubMed] [Google Scholar]
- 31.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 32.Peng, C. Y., L. Manning, R. Albertson, and C. Q. Doe. 2000. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408:596-600. [DOI] [PubMed] [Google Scholar]
- 33.Perego, C., C. Vanoni, S. Massari, R. Longhi, and G. Pietrini. 2000. Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell junctions of epithelia and neurons. EMBO J. 19:3978-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perego, C., C. Vanoni, A. Villa, R. Longhi, S. M. Kaech, E. Frohli, A. Hajnal, S. K. Kim, and G. Pietrini. 1999. PDZ-mediated interactions retain the epithelial GABA transporter on the basolateral surface of polarized epithelial cells. EMBO J. 18:2384-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setou, M., T. Nakagawa, D. H. Seog, and N. Hirokawa. 2000. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288:1796-1802. [DOI] [PubMed] [Google Scholar]
- 36.Sheng, M., and D. T. Pak. 2000. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu. Rev. Physiol. 62:755-778. [DOI] [PubMed] [Google Scholar]
- 37.Simske, J. S., S. M. Kaech, S. A. Harp, and S. K. Kim. 1996. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell 85:195-204. [DOI] [PubMed] [Google Scholar]
- 38.Straight, S. W., L. Chen, D. Karnak, and B. Margolis. 2001. Interaction with mLin-7 alters the targeting of endocytosed transmembrane proteins in mammalian epithelial cells. Mol. Biol. Cell 12:1329-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straight, S. W., D. Karnak, J. P. Borg, E. Kamberov, H. Dare, B. Margolis, and J. B. Wade. 2000. mLin-7 is localized to the basolateral surface of renal epithelia via its NH(2) terminus. Am. J. Physiol. 278:F464-F475. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, M., Y. Hata, K. Hirao, A. Toyoda, M. Irie, and Y. Takai. 1997. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J. Biol. Chem. 272:11943-11951. [DOI] [PubMed] [Google Scholar]
- 41.Tseng, T. C., S. M. Marfatia, P. J. Bryant, S. Pack, Z. Zhuang, J. E. O'Brien, L. Lin, T. Hanada, and A. H. Chishti. 2001. VAM-1: a new member of the MAGUK family binds to human Veli-1 through a conserved domain. Biochim. Biophys. Acta 1518:249-259. [DOI] [PubMed] [Google Scholar]
- 42.Woods, D. F., C. Hough, D. Peel, G. Callaini, and P. J. Bryant. 1996. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 134:1469-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, H., S. M. Reuver, S. Kuhlendahl, W. J. Chung, and C. C. Garner. 1998. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J. Cell Sci. 111:2365-2376. [DOI] [PubMed] [Google Scholar]