Abstract
RPTPσ is a cell adhesion molecule-like receptor protein tyrosine phosphatase involved in nervous system development. Its avian orthologue, known as cPTPσ or CRYPα, promotes intraretinal axon growth and controls the morphology of growth cones. The molecular mechanisms underlying the functions of cPTPσ are still to be determined, since neither its physiological ligand(s) nor its substrates have been described. Nevertheless, a major class of ligand(s) is present in the retinal basal lamina and glial endfeet, the potent native growth substrate for retinal axons. We demonstrate here that cPTPσ is a heparin-binding protein and that its basal lamina ligands include the heparan sulfate proteoglycans (HSPGs) agrin and collagen XVIII. These molecules interact with high affinity with cPTPσ in vitro, and this binding is totally dependent upon their heparan sulfate chains. Using molecular modelling and site-directed mutagenesis, a binding site for heparin and heparan sulfate was identified in the first immunoglobulin-like domain of cPTPσ. HSPGs are therefore a novel class of heterotypic ligand for cPTPσ, suggesting that cPTPσ signaling in axons and growth cones is directly responsive to matrix-associated cues.
The complex pattern of neural connectivity established during nervous system development relies on the ability of the axon's motile tip, the growth cone, to receive, transduce, and integrate multiple environmental signals. Protein phosphorylation on tyrosine residues plays a key role in these processes (21, 29). Two major families of enzymes, the protein tyrosine kinases and the protein tyrosine phosphatases (PTPs), control cellular phosphotyrosine levels. These enzymes are found in both cytoplasmic and transmembrane (receptor-like) forms, and the biochemical interactions between them lead to a diversity of cellular behaviors (25, 76).
Receptor protein tyrosine phosphatases (RPTPs) have recently joined the list of molecules involved in neural development and in particular in axon growth and guidance (reviewed in references 6, 68, 72, and 79). Type 2 RPTPs, containing cell adhesion molecule (CAM)-like extracellular regions, may be particularly well equipped to trigger signals involving cell-cell or cell-extracellular matrix contacts (68). Recent experiments with Drosophila have demonstrated the involvement of the RPTPs DLAR and DPTP69D in motor (19, 20, 50), retinal (27, 57), and midline (73) axon guidance. In leech, a LAR gene-related RPTP (HmLAR2) is implicated in Comb cell behavior, specifically in process outgrowth and mutual avoidance by sibling growth cones (2, 28). Several vertebrate RPTPs have been shown to promote neurite outgrowth in cell culture, including cPTPσ (51), RPTPκ (23), RPTPμ (10), and RPTPδ (82). Moreover, it has recently been shown that RPTPδ also has a potential guidance function, at least in vitro (74).
In mice, gene deficiencies in type 2 RPTPs lead to various abnormalities. LAR deficiency leads to a reduction in size of basal forebrain cholinergic neurons, diminished hippocampal innervation, and defects in other tissues, such as the mammary gland (63, 78, 86). RPTPδ deficiency leads to impaired learning and enhanced hippocampal long-term potentiation (77). The most extreme defects are seen in RPTPσ-deficient mice, which show poor fecundity, hypomyelination of peripheral nerves, ataxias, and abnormalities in development of the hypothalamus and pituitary (24, 80). Although the developmental mechanism of these defects is not known, it is of interest that the avian orthologue of RPTPσ, cPTPσ (69, 85), regulates axon outgrowth of embryonic neurons (51).
Despite this accumulation of functional data, much less is known about the extracellular cues that trigger signal transduction though RPTPs. Several RPTPs interact homophilically in trans, including RPTPμ (8), RPTPκ (62), and RPTPδ (82), but the effects of such homophilic interactions on enzyme function are as yet unclear. The laminin-nidogen complex has been shown to be a heterotypic ligand for a nonneural isoform of LAR (59), while type 5 RPTPζ can bind to several molecules, including contactin and tenascin and the cytokines midkine and pleiotrophin (60). Significantly, pleiotrophin has also recently been shown to suppress the catalytic activity of RPTPζ (53).
cPTPσ, originally described as CRYPα (69), is a type 2 RPTP expressed as two major isoforms: cPTPσ1 (CRYPα1) has three immunoglobulin-like (Ig) domains and four fibronectin type III (FNIII) domains in its extracellular region, while cPTPσ2 (CRYPα2) has four extra FNIII domains. Both isoforms are strongly expressed in the chicken embryo nervous system, in particular within retinal and tectal axons and on their growth cones (70, 71). Moreover cPTPσ1 promotes intraretinal axon growth in vitro and controls growth cone morphology via the maintenance of lamellipodia (51). One or more ligands for cPTPσ are localized in the retinal and tectal basal lamina (BL) and on the glial endfeet of these tissues (33, 51), but the identity of these ligands has remained elusive. To understand how cPTPσ may function at the molecular and cellular levels, we sought to identify the molecular nature of these ligands. Here we show that cPTPσ is a heparin binding protein and that extracellular matrix heparan sulfate proteoglycans (HSPGs), in particular agrin and collagen XVIII, are binding partners for cPTPσ in vitro. Receptor affinity probe assays on tissue sections reveal that the binding of cPTPσ ectodomains to HSPGs is absolutely dependent on the presence of heparan sulfate (HS) side chains. Site-directed mutagenesis in a putative heparin and HS (heparin/HS) binding site in cPTPσ completely abolished this interaction. These data, together with the overlapping expression patterns of cPTPσ, agrin, and collagen XVIII in the developing chick retina, suggest that the reported interactions are physiologically relevant and that HSPGs could be a major ligand class for cPTPσ.
MATERIALS AND METHODS
Expression constructs, fusion protein production, and site-directed mutagenesis.
The pBG plasmid was constructed by subcloning the HindIII-XbaI fragment coding for human placental alkaline phosphatase (AP) from APTag2 (13) into pcDNA3.1 (Invitrogen). A HindIII fragment coding for the extracellular region of cPTPσ1 (amino acids 1 to 721; GenBank accession number L32780) was subcloned into pBG, in frame with AP, resulting in pBGσ1-AP. pBGσ1-AP was transfected into 293T cells (grown in Dulbecco's modified Eagle medium, 10% fetal calf serum, 1% penicillin-streptomycin mixture; sigma) using Superfect (Qiagen). Conditioned medium containing the secreted cPTPσ1-AP fusion protein was collected after 6 to 7 days, sterile filtered, buffered to pH 7.4 with 20 mM HEPES, and stored at 4°C. The fusion protein was quantified by measuring the AP activity as described previously (17).
The pσ1VST expression vector was constructed by subcloning the HindIII fragment coding for the cPTPσ1 extracellular region into the pCS3N-VST shuttle vector (A. R. Aricescu and A. W. Stoker, unpublished data), which contains a HindIII site, a linker coding for the vesicular stomatitis virus (VSV) peptide tag, a spacer sequence, and an AclI site, all flanked by two NotI sites. pσ1VST was used to express the secreted cPTPσ1-VSV fusion protein in 293T cells as described above, or for higher expression levels, the cPTPσ1-VSV coding fragment was transferred into the RCAS(A) avian retrovirus (46) as follows: pσ1VST was linearized with AclI and ligated into the ClaI opened RCAS(A); the shuttle vector backbone was then removed via NotI digestion and self-ligation, resulting in the RCAS-σ1VST retrovirus. This construct was transfected into line 0 chicken embryo fibroblasts (grown in Dulbecco's modified Eagle medium plus 10% fetal calf serum plus 2% chick serum) using Superfect. After six passages the conditioned medium containing cPTPσ1-VSV was collected, sterile filtered, and buffered to pH 7.4 with 20 mM HEPES.
The pFN3Δ-AP expression plasmid, containing a cPTPσ1 extracellular construct lacking the membrane-proximal FNIII-like domain, fused to the amino terminus of AP, was a gift from John Chilton. This plasmid was used as a template for site-directed mutagenesis according to Stratagene's QuickChange mutagenesis kit protocol. Primer sequences can be obtained from A. R. Aricescu. All mutant constructs were verified by DNA sequencing and transfected into 293T cells, and conditioned media were collected as described above.
Solid-phase binding assays.
Heparin-albumin (Sigma), agrin (purified as described in reference 36), and collagen XVIII (purified as described in reference 38) were immobilized on 96-well microtiter plates at a concentration of 2 μg/ml (heparin-albumin) or 5 μg/ml (agrin and collagen XVIII) for 2 h at room temperature. Remaining binding sites were saturated by overnight incubation at 4°C in phosphate-buffered saline (PBS) buffer (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.4]) containing 3% bovine serum albumin (BSA). Wells were incubated for 3 h at room temperature with conditioned media from 293T cultures, containing AP fusion proteins. After four washes in PBS and one wash in SEAP buffer (0.5 mM MgCl2, 1 M diethanolamine [pH 9.8]), the bound AP activity was determined by adding 200 μl SEAP buffer containing 10 mM p-nitrophenyl phosphate. Progress curves were recorded for 1 h at room temperature, at 405 nm, using a Dynex MRX microplate reader, and the initial rates were determined using the SigmaPlot 4.01 software (SPSS, Inc.). Each data point represents the average of triplicate wells ± the standard deviation. Nonspecific binding was determined from cPTPσ1-AP bound to BSA (immobilized at a concentration of 3 mg/ml) and was subtracted from each data point.
The data were plotted for Scatchard analysis in order to get initial estimates of the binding parameters. cPTPσ1-AP was found to bind HSPGs with very high affinities, and at low concentrations, more than 10% of the initial AP activity was bound. Therefore, in order to obtain more accurate Kd values, we fitted the saturation curve by nonlinear regression to the following equation, which takes into account significant ligand depletion, with negligible nonspecific binding (75): b*tot2 − b*tot[β(Kd + L*tot) + rtot] + β L*tot rtot = 0, where b*tot is the total amount of bound ligand (cPTPσ1-AP) expressed in optical density units (OD) per minute, β is the specific AP activity expressed in OD per minute per molar concentration units, Kd is the equilibrium dissociation constant, L*tot is the total ligand (cPTPσ1-AP) concentration, and rtot is the maximal specific binding expressed in OD per minute. The specific activity used for cPTPσ1-AP fusion protein was 1.12 OD/min/nM.
In vivo viral injection.
White Leghorn eggs were obtained from Needle Farm (Herts) and incubated at 38°C to the desired stage. The RCAS-σ1VST vector was used to generate stocks of viral inoculi as described previously (54). Virus (0.1 μl) was injected into the optic vesicle of stage 11 embryos (∼33 h) (40) using a PM1000 microinjector (MicroData Instrument Inc.). E6 embryos were processed for immunohistochemistry or in situ hybridization.
Immunohistochemistry and in situ hybridization.
E6 or E10 chicken embryo heads were fixed in 4% paraformaldehyde in PBS, cryoprotected in 30% sucrose in PBS, and frozen in OCT compound (TissueTek). Cryosections (10 to 12 μm) were mounted on 3-aminopropyltriethoxysilane-coated glass slides. For immunohistochemistry, sections were blocked with 1% BSA-0.25% Triton X-100 in PBS for 15 min at room temperature. Primary antibodies used were IG2 (anti-cPTPσ rabbit polyclonal) (70) diluted at 1:500; P5D4 (anti-VSV glycoprotein monoclonal; Sigma) at 1:500; 6D2 (anti-agrin monoclonal) (35) at 1:20, and 6C4 (anti-collagen XVIII monoclonal) (38) at 1:20, all in 3% BSA-0.25% Triton X-100 in PBS. After 1 h of incubation at room temperature, the sections were washed three times in 0.1% BSA-0.05% Triton X-100 in PBS and secondary antibodies were added for 1 h at room temperature: goat anti-rabbit peroxidase-conjugated (Promega), diluted at 1:100; rabbit anti-mouse peroxidase-conjugated (Promega), at 1:100; and goat anti-mouse fluorescein isothiocyanate-conjugated (Jackson Labs), at 1:200. After three final washes the peroxidase reactions were performed using the DAB substrate kit (Vector Laboratories); the fluorescein isothiocyanate-labeled sections were mounted in Fluorosave (Calbiochem), and all sections were analyzed using an Axiophot fluorescence microscope (Zeiss).
For in situ hybridization, the PRD4A viral probe (58) was used, as described previously (52).
Receptor affinity probe (RAP) in situ.
The in situ localization of cPTPσ ligands on tissue sections and flat-mounted retinal basal laminae was performed using the cPTPσ1-AP conditioned medium, as previously described (33, 51). In modified RAP assays, the cPTPσ1-AP probe was preincubated with 100 μg of heparin (Sigma), bovine kidney HS (Sigma), or chondroitin sulfate (Calbiochem)/ml for 1 h at room temperature before addition to the tissue samples. Alternatively, the tissue samples were pretreated for 2 h at 37°C with 0.5 U of heparinase III (Sigma) in 50 μl of PBS, pH 7.4, containing 0.1% BSA or with 0.1 U of chondroitinase ABC (Sigma) in 50 μl of Tris-acetate buffer, pH 8. The protease inhibitor 4-(2-aminoethyl)-benzenesulfonyl fluoride was added to both reactions at a final concentration of 2 mM.
Immunoblotting and blot-overlay assays.
An HSPG-enriched fraction (HfV) from E9 chicken embryo vitreous bodies homogenized in Ca2+-free and Mg2+-free Hanks' balanced salt solution, pH 7.3 (CMF), was partially purified by ion-exchange chromatography as follows. The vitreous homogenate was supplemented with 1:100 protease inhibitors cocktail with no metal chelators (Sigma) and centrifuged at 12,000 × g for 30 min at 4°C to remove debris. The supernatant was applied on a Q-Sepharose Fast Flow column (Amersham Pharmacia Biotech) and washed with 0.5 M NaCl in CMF, and the HfV fraction was eluted with 1.5 M NaCl in CMF. Eluted fractions were concentrated on a Centricon YM-30 (Amicon), and the buffer was changed to CMF. Aliquots of the HfV, 100 ng of immunopurified agrin, and collagen XVIII (as described in references 36 and 38) were preincubated for 2 h at 37°C with either 0.5 U of heparinase III, 0.1 U of chondroitinase ABC, or 0.1 U of collagenase (Sigma type VII) in PBS, pH 7.4, containing 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride.
For immunoblotting, the reactions were mixed with equal volumes of Laemmli sample loading buffer (62 mM Tris [pH 6.8], 2% sodium dodecyl sulfate (SDS), 10% glycerol, 5% β-mercaptoethanol, 0.001% bromophenol blue), boiled, and separated by SDS-6% polyacrylamide gel electrophoresis. Proteins were transferred onto Hybond-C Extra nitrocellulose membranes (Amersham Pharmacia Biotech), blocked overnight at 4°C in 5% nonfat milk in PBS, and probed for 1 h at room temperature with antiagrin (6D2; 1:20) or anticollagen XVIII (6C4; 1:20) monoclonal antibodies. The secondary antibody used was rabbit anti-mouse horseradish peroxidase (Dako) diluted at 1:2,000. Chemiluminescence detection was performed using the ECL Western blotting kit (Amersham Pharmacia Biotech).
For blot overlay assays, the samples were processed as described above except that following overnight blocking, the membranes were incubated for 3 h at room temperature with cPTPσ1-VSV conditioned medium supplemented with 0.5% Igepal CA-630 (Sigma). The membranes were then washed three times with PBS containing 0.5% Igepal CA-630, and bound cPTPσ1-VSV was detected using the anti-VSV monoclonal antibody (P5D4; 1:1,000) as described above.
Homology modelling of cPTPσ domains.
Individual domains of the extracellular region of cPTPσ were defined using the Pfam server (http://www.sanger.ac.uk/Pfam [5]). The corresponding sequences were submitted to the SWISS-MODEL v3.5 protein modelling server (http://www.expasy.ch/swissmod/SWISS-MODEL.html [31]) for analysis. Suitable modelling templates were identified in the ExNRL-3D database using SWISS-MODEL Blast. The sequence alignments obtained were analyzed, and the most appropriate ExPDB entries were selected as templates. All the models generated were quality checked by the WHAT IF verification routines (WHAT-CHECK [45]) and the Biotech protein validation suite (http://biotech.embl-heidelberg.de:8400/). Energy minimization was done with the GROMOS implementation of Swiss-PdbViewer v3.6b2 (30) (http://www.expasy.ch/spdbv/).
RESULTS
cPTPσ binds to the retinal BL in vivo and in vitro.
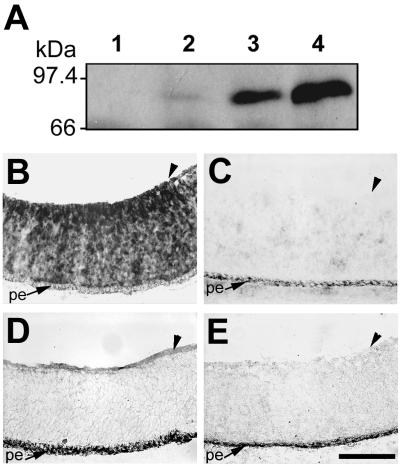
Our previous results established that the BL and glial endfeet of the chicken embryo retina, optic stalk, and chiasm contain a prominent ligand for the cPTPσ1 ectodomain (33). This was detected in vitro using the cPTPσ1-AP fusion protein in a RAP in situ technique (26).To test whether a related binding pattern occurs in vivo, we constructed the RCAS-α1VST retrovirus that encodes a secretable cPTPσ1 ectodomain fused with the VSV peptide tag (cPTPσ1-VSV). cPTPσ1-VSV was readily detected as it accumulated in conditioned medium from infected cells in culture (Fig. 1A). RCAS-α1VST virus was injected into the optic vesicle of chick embryos at stage 11 (∼33 h), and by embryonic day 6 (E6) the retrovirus had infected the whole retina (Fig. 1B). The cPTPσ1-VSV protein, secreted by infected retinal cells in situ, accumulated specifically in the ligand-rich BL region (Fig. 1D), providing good evidence in vivo that at least one physiological ligand for cPTPσ is located in this region.
FIG. 1.
Binding of cPTPσ to the E6 chick retinal basal lamina in vivo. (A) Time course of cPTPσ1-VSV production by chicken embryo fibroblasts infected with RCAS-α1VST retrovirus. Samples of culture medium conditioned for 1 (lane 2), 2 (lane 3) or 3 (lane 4) days after transfection were analyzed by immunoblotting using antibody specific for the VSV epitope. Lane 1 contains conditioned medium from untransfected cells. (B and D) RCAS-α1VSV retrovirus-infected retinas at E6, probed with a digoxigenin-labeled viral probe by in situ hybridization (B) or with VSV antibody (D). Noninfected tissues were probed by in situ hybridization (C) or with VSV antibody (E). Arrowheads indicate the retinal BL. pe, pigmented epithelium. Scale bar, 0.1 mm.
cPTPσ is a heparin-binding protein.
We screened for reagents that could modulate RAP in situ binding of the cPTPσ ectodomain to the retinal BL. Increasing concentrations of sodium chloride abolished this interaction, whereas the nonionic detergent Igepal CA-630 had no effect even at high concentrations. We hypothesized therefore that the cPTPσ-ligand interaction was predominantly ionic rather than hydrophobic and that it may involve charged glycosaminoglycan chains.
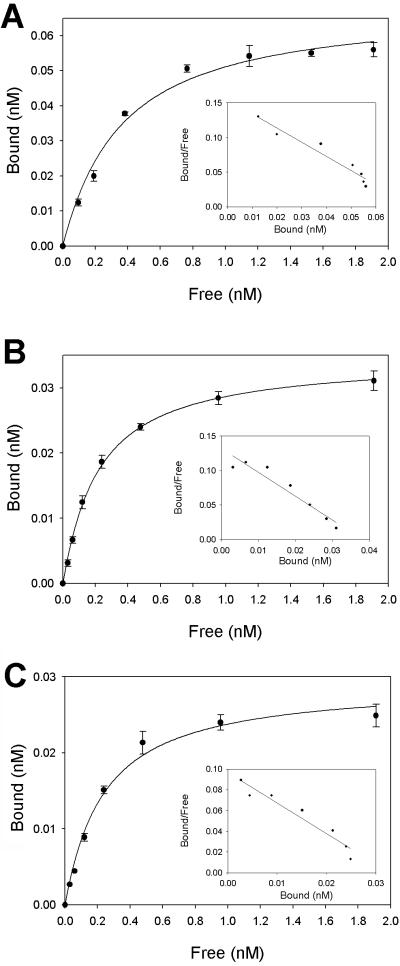
Using a secreted cPTPσ1 ectodomain fused with AP at its C terminus (cPTPσ1-AP) (33), we found that in solid-phase binding assays cPTPσ1-AP bound to heparin-albumin at subnanomolar concentrations (Fig. 2A). Using a nonlinear regression analysis, a Kd value of 0.32 ± 0.05 nM was calculated, demonstrating that cPTPσ binds heparin with very high affinity. Control constructs containing AP alone or AP fused with the ectodomain of RPTPμ did not bind to heparin-albumin in similar assays (data not shown). In addition, the nonspecific binding of cPTPσ1-AP on wells coated just with albumin was negligible (data not shown). Therefore, we propose that the cPTPσ1-AP construct binds heparin via the extracellular region of cPTPσ.
FIG. 2.
Saturation curves and Scatchard plots for the binding of cPTPσ to heparin, agrin, and collagen XVIII. Microtiter plates were coated with heparin-albumin (A), agrin (B), or collagen XVIII (C) and incubated with a range of cPTPσ1-AP concentrations. Saturation curves were fitted by nonlinear regression analysis as described in Materials and Methods. Each value represents the mean ± standard deviation of three measurements.
cPTPσ binds to retinal HSPGs.
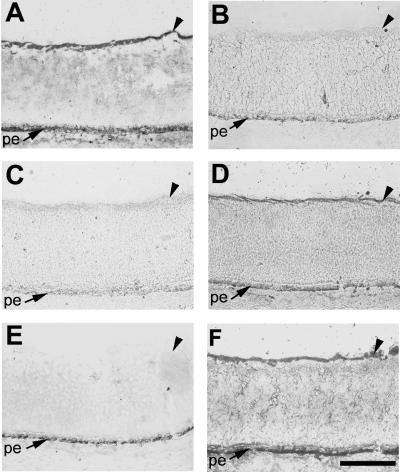
We next investigated whether ligand interactions detected in RAP assays (33) are mediated by HS chains and whether heparin and soluble HS can compete in binding. Preincubation of cPTPσ1-AP conditioned medium with heparin completely blocked the BL signal (Fig. 3B). HS also interfered with binding but did not completely block it (Fig. 3C); this may be due to the type of HS we used (bovine kidney), since it is known that significant variations in the HS structure (the degree of sulfation, for example) exist between different tissues. No effect was observed when cPTPσ1-AP was preincubated with the same amount of chondroitin sulfate (Fig. 3D), itself a highly charged polyanionic molecule.
FIG. 3.
Binding of cPTPσ to the E6 chick retinal BL is mediated by HS chains. Results of receptor affinity probe assays using the extracellular region of cPTPσ1 fused to alkaline phosphatase are shown. Retina sections were untreated (A to D) or pretreated with heparinase III (E) or chondroitinase ABC (F). cPTPσ1-AP conditioned medium was used alone (A) or preincubated with heparin (B), HS (C), or chondroitin sulfate (D). The BL staining indicates cPTPσ1-AP binding. Arrowheads indicate the retinal BL. pe, pigmented epithelium. Scale bar, 0.1 mm.
To establish whether heparin and HS modulate the binding because they bound cPTPσ1-AP directly, or because they simply masked the binding site(s) on the BL ligand, the sections were pretreated with heparinase III or chondroitinase ABC. Heparinase III completely removed the BL signal (Fig. 3E), while chondroitinase ABC had no effect (Fig. 3F). Therefore, we conclude that the cPTPσ1-AP binding observed on the retinal BL is mediated by HS chains and (since free heparin is not present in the retina) that the ligand(s) is an HSPG(s).
cPTPσ binds to the extracellular matrix HSPGs agrin and collagen XVIII.
The major HSPGs present in the retinal BL are agrin and collagen XVIII (39). Perlecan can also be detected at very low levels. Agrin and collagen XVIII immunopurified from the vitreous body (36, 38) were tested as candidate ligands for cPTPσ in solid-phase binding assays. cPTPσ1-AP bound at picomolar concentrations to both agrin and collagen XVIII (Fig. 2B and C). The equilibrium dissociation constants measured were 0.18 ± 0.01 nM for agrin and 0.21 ± 0.02 nM for collagen XVIII, demonstrating that cPTPσ binds these HSPGs with very high affinity. This experiment shows that native agrin and collagen XVIII behave as potential ligands for cPTPσ, at least in vitro.
These interactions were investigated in a further assay to determine whether cPTPσ-proteoglycan interactions do occur mainly via the HS chain, as predicted above. An HSPG-enriched (HfV) fraction from E9 chicken embryo vitreous bodies was prepared by ion-exchange chromatography and, together with pure agrin and collagen XVIII, was tested on a blot overlay assay. The HfV fraction contained both agrin and collagen XVIII (Fig. 4A). Agrin, a large proteoglycan with an apparent molecular mass of 500 kDa, appeared in the HfV fraction and was not digested by treatment with collagenase (lane c) or chondroitinase ABC (lane d). However, treatment with heparinase III (lane b) reduced agrin's size to 250 kDa, corresponding to its core protein. Similarly, the molecular mass of collagen XVIII was reduced from 300 kDa to the 180-kDa core protein (lane f). Chondroitinase ABC treatment had no effect (lane h), whereas collagenase removed any trace of collagen XVIII (lane g). When the same HfV samples were probed with the cPTPσ1-VSV conditioned medium in the blot overlay assay, two bands, corresponding in size to agrin and collagen XVIII, were observed in the untreated (lane i) and chondroitinase ABC-treated (lane l) samples. Heparinase III digestion (lane j) completely removed any signal on the overlay, showing that cPTPσ binding requires the presence of HS chains. This agrees with the RAP in situ results described above. Finally, collagenase preincubation (lane k) specifically removed the lower-molecular-weight band, corresponding to collagen XVIII.
FIG. 4.
cPTPσ binds agrin and collagen XVIII via their HS chains. An HSPG-enriched fraction from chicken embryo vitreous bodies (A), purified agrin (B), and purified collagen XVIII (C) samples, separated by SDS-6% polyacrylamide gel electrophoresis, were transferred to nitrocellulose and probed with antibodies specific for agrin or collagen XVIII or were incubated with cPTPσ1-VSV conditioned medium. cPTPσ1-VSV was detected using antibody to VSV. Samples were either not treated with enzymes (lanes a, e, and i) or predigested with heparinase III (lanes b, f, and j), collagenase (lanes c, g, and k), or chondroitinase ABC (lanes d, h, and l). Arrows in panel A indicate the two bands corresponding in molecular mass to agrin (upper arrow) and collagen XVIII (lower arrow) observed in the blot overlay assay.
The same patterns were obtained when purified agrin (Fig. 4B) or purified collagen XVIII (Fig. 4C) samples were enzyme treated and probed in immunoblots or overlay assays. The collagen XVIII blots (Fig. 4C) are more smeared than the fresh HfV samples, presumably due to partial degradation during the purification steps. Altogether, these data suggest that agrin and collagen XVIII can act as binding partners for cPTPσ, irrespective of the cPTPσ fusion tag used (AP or VSV), and that these interactions occur necessarily via their HS chains.
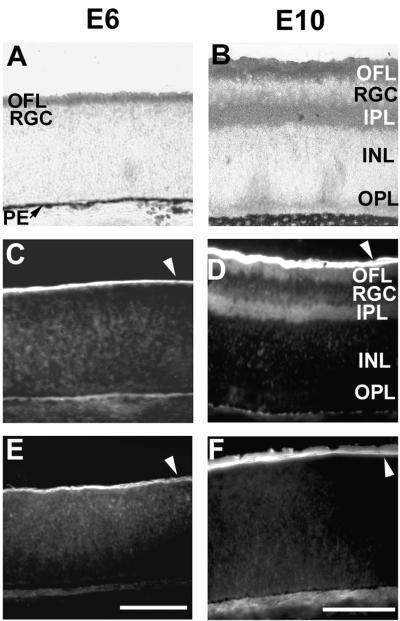
Overlapping expression of cPTPσ, agrin, and collagen XVIII in the developing chick retina.
cPTPσ, agrin, and collagen XVIII are expressed in the embryonic nervous system in a developmentally regulated manner (36, 38, 52, 71). We examined whether the expression patterns of cPTPσ and the proposed ligands are juxtaposed, as would be expected if they were to interact in vivo. At E6 cPTPσ is localized in retinal axons in the optic fiber layer (OFL) in close contact with the BL (Fig. 5A). Agrin (Fig. 5C) and collagen XVIII (Fig. 5E) are localized at E6 mainly in the BL, which forms part of the complex substrate on which the retinal ganglion cells (RGCs) extend their axons. At E10, when the retinal plexiform layers have formed, cPTPσ is localized mainly in the OFL and in the inner plexiform layer (IPL) (Fig. 5B). Agrin, produced like cPTPσ by the RGCs, is present in the OFL and IPL but also accumulates in large amounts in the BL (Fig. 5D). Collagen XVIII, however, produced by the ciliary body (39), is present only on the retinal inner surface, representing the BL (Fig. 5F).
FIG. 5.
Expression patterns of cPTPσ, agrin, and collagen XVIII in the developing chick retina. E6 (A, C, and E) and E10 (B, D, and F) chick retina sections were probed with antibodies to cPTPσ (A and B), agrin (C and D), and collagen XVIII (E and F). At E6, cPTPσ is strongly expressed in the retinal axons (outer fiber layer [OFL]), while both agrin and collagen XVIII show strong expression in the juxtaposed BL. At E10, cPTPσ shows strong expression, mainly in neurite layers: OFL and IPL (inner plexiform layer). Agrin expression overlaps in fiber layers and is also a major constituent of the BL along with collagen XVIII. RGC, retinal ganglion cell layer; INL, inner nuclear layer; OPL, outer plexiform layer; PE, pigmented epithelium. White arrowheads indicate the BL staining. Scale bar, 0.1 mm.
The expression patterns described above suggest that cPTPσ would indeed be able to interact with both agrin and collagen XVIII during eye development.
The first Ig domain of cPTPσ contains a putative heparin-binding site.
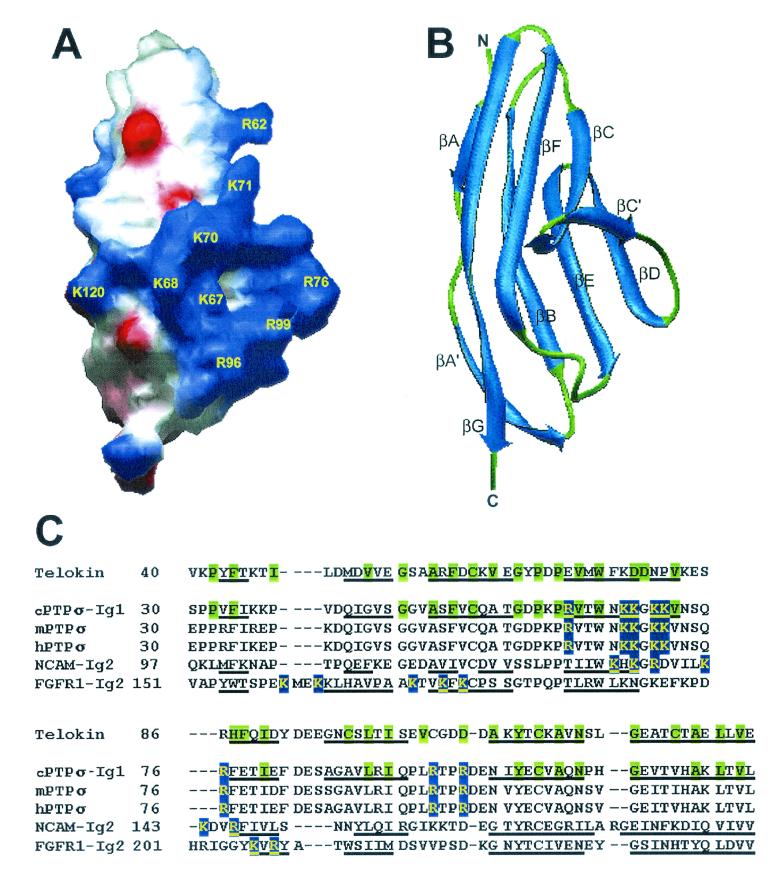
Basic amino acid clusters located on the surface of extracellular domains of proteins are likely to bind polyanionic glycosaminoglycan chains (for a review, see reference 42). Using the SWISS-MODEL (v3.5) automated protein modelling server (31), we obtained three-dimensional (3D) models for all the Ig domains and three out of four FNIII domains of cPTPσ1 (except the FNIII-4 domain, which had less than 30% identity to any 3D structure in the Protein Data Bank [PDB]). Several positively charged clusters were observed on the molecular surfaces (computed using the Swiss-PdbViewer v3.6b2 program [30]), the most prominent being in the Ig-1 domain (Fig. 6A). This cluster consists mainly of a β-hairpin formed by strands βC and βC′ (Fig. 6B) and contains the sequence R62VTWNKKGKKVNSQR76, plus the side chains of Arg96 and Arg99, from a loop between the βE and βF strands. The Ig-1 domain homology model was generated using telokin as a template (39.3% identity; PDB codes 1FHG and 1TLK), a typical Ig superfamily I-set structure. Twenty-four out of the 35 key structural residues as defined in reference 4 are identical, representing all the major secondary structures and most of the peripheral regions (Fig. 6C).
FIG. 6.
Structural model of the Ig-1 domain of cPTPσ and sequence alignment with the Ig domains of telokin, NCAM, and FGFR1. (A) Electrostatic surface representation of the Ig-1 domain of cPTPσ; blue and red represent positive and negative electrostatic potential, respectively. The large positive-potential patch represents the putative heparin binding site. Basic residues are labeled in yellow and numbered according to Stoker (69). (B) Ribbon view of the predicted folding with the same orientation as in panel A. N and C denote the amino and carboxyl termini. The β strands are labeled, from A to G, according to the telokin fold. (C) Structure-based sequence alignment between telokin (the template used for modelling) and the heparin-binding Ig domains of cPTPσ, NCAM, and FGFR1. The corresponding sequences of the mouse and human orthologues of cPTPσ (mPTPσ and hPTPσ) are included. The heparin-binding sites are highlighted in blue (proposed site for cPTPσ). The telokin key structural residues and their conserved equivalents in cPTPσ are highlighted in green. The β strands are underlined. The secondary structure definitions were reported in the following sources: for telokin, reference 44; for NCAM-Ig2, reference 47; and for FGFR1-Ig2, reference 61. This figure was made using the Swiss-PdbViewer v3.6b2 (30).
The cluster described above in the cPTPσ Ig-1 domain contains a BBxBB motif suggested to interact optimally with HS chains (where B represents Arg or Lys) (42). Other proteins containing heparin/HS binding sites located in I-set Ig domains have been described, including the classical examples: neural cell adhesion molecule (NCAM) and fibroblast growth factor (FGF) receptors (FGFRs). High-resolution 3D structures of both NCAM (1EPF, X-ray structure) (47) and FGFR1 (1CVS, X-ray structure) (61) have recently been published, and these were aligned with the cPTPσ Ig-1 model using Swiss-PdbViewer (Fig. 6C). The proposed heparin-binding site of cPTPσ is perfectly conserved in its mouse and human orthologues and aligns well with the heparin-binding site found in NCAM (14). However, the location of the FGFR1 heparin-binding site does not seem to overlap, suggesting a different molecular architecture of the FGFR1-heparin complex.
The heparin-binding site of cPTPσ is essential for binding to the retinal BL.
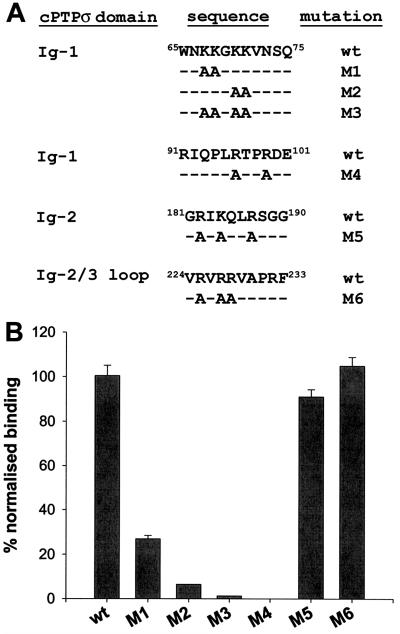
To test the accuracy of the molecular modelling prediction, we mutated the charged amino acids in the putative heparin-binding site of the Ig-1 domain and, as controls, other basic clusters present in cPTPσ Ig domains 2 and 3. Several basic clusters are also present in the membrane-proximal FNIII-4 domain. However, a cPTPσ1 extracellular construct lacking this FNIII-4 domain, termed FN3Δ-AP, bound in a similar way to full-length cPTPσ1 in RAP in situ assays (see Fig. 8A and B). Therefore, FNIII-4 does not contain the BL HSPG binding site. The FN3Δ-AP construct was used as a backbone for incorporation of further mutations, as shown in Fig. 7A. Mutations M1, M2, M3, and M4 are all directed to the charged amino acids from the putative heparin/HS binding site in Ig-1. Mutation M5 targets a basic cluster in Ig-2, and M6 targets a similar cluster in the loop connecting Ig-2 and Ig-3. All constructs were transfected into 293T cells, and the cells produced essentially equal amounts of fusion protein in the conditioned media (data not shown). All the mutations directed at the large basic cluster in Ig-1 (Fig. 6A) showed impaired heparin binding in solid-phase assays (Fig. 7B). Binding of M5 and M6 constructs was not significantly affected. The severely reduced binding properties of the M1 to M4 mutants precluded the measurement of Kd values.
FIG. 8.
The heparin/HS binding site in domain Ig-1 is essential for retinal BL binding. Receptor affinity probe assays were performed using cPTPσ1-AP (A), FN3Δ-AP (B), M1 (C), M2 (D), M3 (E), M4 (F), M5 (G), and M6 (H) conditioned media, respectively. The BL staining indicates fusion protein binding. Arrowheads indicate the retinal BL. pe, pigmented epithelium. Scale bar, 0.1 mm.
FIG. 7.
Identification of the cPTPσ heparin-binding site by site-directed mutagenesis. (A) Basic residues in the heparin-binding site in domain Ig-1 (mutations M1, M2, M3, and M4), another cluster in Ig-2 (mutation M5), or in the loop connecting Ig-2 and Ig-3 (mutation M6) were replaced with alanine. wt corresponds to the original cPTPσ sequence. (B) The mutated proteins were tested in solid-phase binding assays for binding to heparin-BSA. Bars represent means ± standard errors of three determinations.
The mutated constructs were tested on E6 chick retina sections by RAP in situ. The BL binding pattern observed correlated with the solid-phase heparin binding profile (Fig. 8). Therefore, we conclude that the heparin/HS binding site was correctly predicted by molecular modelling. This site, which is highly conserved in the orthologues of cPTPσ (Fig. 6C), is essential for the binding of cPTPσ1 ectodomains to the BL HSPGs.
Müller glia endfeet contain HSPG and non-HSPG binding sites for cPTPσ.
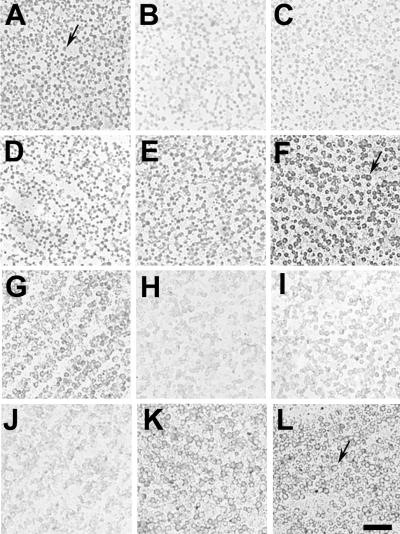
Radial Müller glial cells are the only nonneuronal cell type in the chicken retina. RAP in situ experiments demonstrate that in addition to the BL ligand(s), cPTPσ binds strongly to radial glia endfeet in contact with the BL. This interaction contributes to the role of cPTPσ in neurite growth and in maintenance of growth cone lamellipodia (33, 51). Here we have assessed whether or not the endfeet binding sites are also HSPGs.
Unfixed preparations of retinal basal laminae, with glial endfeet attached, were used for RAP in situ experiments. Both heparin addition and heparinase III treatment diminished cPTPσ1-AP binding on glial endfeet significantly (Fig. 9B and C). Unlike results in fixed cryosections, however (Fig. 3B), this interaction was not blocked completely. In addition, several cPTPσ1 mutant constructs exhibited reduced binding to the glial endfeet (Fig. 9H, I, and J), but again there was residual cPTPσ binding. Neither chondroitin sulfate nor chondroitinase ABC pretreatment could significantly impair the interaction between cPTPσ and its ligands expressed on glial endfeet (Fig. 9D and E). These observations suggest that the glial endfeet, a highly differentiated area of the Müller glia, express both an HSPG and a non-HSPG class of cPTPσ ligands.
FIG. 9.
Müller glia endfeet express two classes of cPTPσ ligands. Retinal basal laminae with glial endfeet attached were flat mounted and probed by RAP in situ with conditioned media containing cPTPσ1-AP (A to E), FN3Δ-AP (F), M1 (G), M2 (H), M3 (I), M4 (J), M5 (K), and M6 (L). Heparin addition (B) or heparinase III pretreatment (C) reduces binding compared to control treatments (A and F) but does not abolish it. cPTPσ mutants with impaired heparin-binding properties also show reduced binding on glial endfeet (H to J). Chondroitin sulfate addition (D) or chondroitinase ABC pretreatment (E) does not affect cPTPσ binding significantly. Arrows indicate ringlike endfeet. Scale bar, 10 μm.
DISCUSSION
Cell adhesion molecule-like RPTPs are emerging as a major class of molecules involved in regulating neural development, including axon growth and guidance, synaptic function, and nerve repair (41, 78, 84; also reviewed in references 6, 55, 72, and 79). Currently, little is understood regarding the molecular mechanisms of RPTP signaling, one reason being that in only a few cases are their interaction partners known. Where homophilic binding has been demonstrated between RPTPs, for example with RPTPμ (8), RPTPκ (62), or RPTPδ (82), it is not understood how, or even if, such binding directly influences RPTP signaling. Where heterotypic ligands have been proposed, it is clearer that ligand binding can influence phosphatase activity, either positively (66) or negatively (53). To advance our understanding it will be necessary to define many more ligand classes for the RPTPs.
cPTPσ has recently been implicated in promoting intraretinal axon growth and controlling the growth cone morphology of RGCs (51). Putative, heterotypic ligands for cPTPσ have been localized on Müller glia endfeet and RGC neurites and in the retinal BL (33, 51). The retinal BL (inner limiting membrane) is a 50- to 70-nm-thick extracellular matrix sheet separating the retina from the vitreous body. Its major constituents include laminins, nidogen, collagen IV, HSPGs (mainly agrin and collagen XVIII), and a chondroitin sulfate proteoglycan (39). The retinal BL is an excellent substrate for axon growth in vitro. In vivo, during the intraretinal axon outgrowth (E3 to E7 in chick), axons grow initially on the BL and a layer formed by radial glia endfeet (34). Accordingly, enzymatic disruption of the BL severely perturbs retinal architecture and axonal outgrowth (37). Our solid-phase binding assays have not detected interactions between cPTPσ and laminin 1, laminin 2, fibronectin, or collagen IV (McKinnell and Stoker, unpublished data). However, cPTPσ binds heparin with high affinity as well as the HS chains of HSPGs agrin and collagen XVIII. In addition, using molecular modelling, we have identified a heparin/HS binding site in the first Ig domain of cPTPσ, which is necessary for these interactions. In our previous work we showed that both the Ig and FNIII domains of cPTPσ are required for ligand binding, at least under RAP in situ conditions (33). Therefore, it appears that the HS binding site in the first Ig domain described here is essential but not sufficient for the interaction with HSPGs. The FNIII domains may be required either to achieve an appropriate functional conformation or to interact with other ligands in a putative cPTPσ signaling complex.
HSPGs are well established as modulators of neurite outgrowth. Previous studies have reported that HSPGs enhance the growth-promoting abilities of laminin and NCAM (reviewed in reference 7) and of basic FGF (12). Heparinase III treatment of cell-free basal laminae greatly inhibited the growth rate and distribution density of RGC axons (12) and induced aberrant growth of axons in cultured insect wing appendages (83). In Xenopus, heparitinase removal of HS chains during optic tract formation retards retinal axon elongation; later HS removal, after axons have extended to the tract, elicits a tectal bypass phenotype (81). Moreover, addition of exogenous HS chains to the developing Xenopus optic pathway severely disrupted target recognition (81). These effects have been attributed, at least in part, to the disruption of FGF signaling. Our present data suggest that further signaling receptors that bind to HSPGs, including cPTPσ, may also be directly affected in these assays.
The HSPGs agrin and collagen XVIII are the first heterotypic binding partners described for cPTPσ. These interactions can not only bring further insight to our understanding of the molecular mechanisms behind currently known cPTPσ functions but also suggest novel ones. Agrin, for example, is abundant in axonal pathways of the chick central nervous system, such as the optic nerve and the tecto-bulbar pathway (36). The temporal coincidence of its expression with axonal outgrowth suggests a growth-promoting role for agrin, as has also been proposed for cPTPσ (51). Surprisingly, though, purified agrin displays an inhibitory activity for neurite outgrowth in vitro (36). However, a large variety of molecules bind in vivo to agrin via its HS chains, protein core, or both, including laminin-1, thrombospondin, fibronectin, FGF2, merosin (laminin-2), and pleiotrophin (15). All of these are in turn potent substrates for neurite extension, and their binding to HSPGs is important for modulating this activity. A functional interaction between agrin and cPTPσ may or may not involve such molecules as well. Furthermore, agrin is expressed in several isoforms. Alternative promoter and first (N-terminal) exon usage defines a long (LN) and short (SN) isoform (11, 56). LN is secreted and incorporated into the BL, while SN remains attached on the neuronal surface. The purified agrin used as a substrate for axon growth experiments in vitro was LN, whereas the antibody we have used in this study does not discriminate between the two isoforms (36). It is possible, therefore, that the agrin involved in neurite outgrowth modulation is of the SN type. Both isoforms mentioned above have the potential to interact with cPTPσ either as a ligand (LN agrin) or as a coreceptor on the neuronal surface (SN agrin). Which one of these interactions has functional consequences is still to be determined.
The best-characterized function for agrin remains its ability to induce acetylcholine receptor clustering at neuromuscular junctions (43). Transgenic animals have provided convincing evidence for the essential role of LN agrin in triggering synaptic differentiation (11; reviewed in reference 32). cPTPσ1 is expressed on motor neurons and also binds to sites on muscles in RAP in situ experiments (J. Chilton, F. Haj, and A. Stoker, unpublished data). This interaction appears to be HS independent, however, and its relationship, if any, to agrin has yet to be determined.
Despite the large amount of data supporting the role of agrin at neuromuscular junctions, less is known about its roles in the brain, where it is a major HSPG. Recent reports (16, 22) have revealed a potential role in Alzheimer's disease. The β-amyloid peptide (Aβ) accumulates as aggregates within senile plaques and cerebrovascular deposits. Aβ binds to the HS chains of agrin in vitro, leading to the acceleration of Aβ fibril formation (16). Agrin also localizes to Aβ deposits in brains affected by Alzheimer's disease. Interestingly, senile plaques promote neurite outgrowth and usually contain numerous dystrophic neurites, events that may be dependent on FGF2, which can associate with agrin's HS chains (18). It would be interesting to see whether PTPσ plays any role in this process. In the avian retina, agrin is found mainly in the BL and retinal plexiform layers (from E8 onwards) (35, 48), consistent with a possible role in interneuronal synapse formation (49). Since cPTPσ is present at high levels in the IPL (71) and, as reported here, binds to agrin, this raises the possibility that cPTPσ might be involved in retinal synaptogenesis. There is evidence already that the highly related RPTPδ may be involved in modulating synaptic plasticity (77).
Another BL ligand for cPTPσ is collagen XVIII. Collagen XVIII has recently emerged as a modulator of cell migration and axon guidance (1), and a mutation in the human COL18A1 gene causes Knobloch syndrome, a disease involving vitreoretinal degeneration (65). A 38-kDa C-terminal fragment termed NC1 appears to be responsible for stimulating cell and axon migration (1). Although this fragment has heparin-binding ability, its neuronal receptor has not been identified. We found that binding of cPTPσ to collagen XVIII is dependent on the integrity of the latter's HS chains, at least in blot-overlay assays, although we cannot rule out direct protein-protein interactions. NC1 contains a putative O-glycosylation site, although it is not clear yet whether it is glycosylated in vivo. It will be of interest to see if RPTPσ also acts as a receptor for NC1/endostatin, considering the involvement of both proteins in neurite growth and guidance.
The HS chains of agrin and collagen XVIII may directly regulate the phosphatase activity of cPTPσ as is seen with ligands interacting with other RPTPs (53, 66). Whether or not such regulation occurs through receptor dimerization, which occurs with certain RPTPs (reviewed in reference 3), remains to be seen. Given that HS chains are highly variable and complex, it may be unlikely that there is a singular HS structure that acts as a specific ligand for cPTPσ. Instead, one likely way in which HSPGs could interact with cPTPσ is by acting as a facilitator of either receptor oligomerization or the binding of further, cognate ligands. An example of this is seen with the cobinding of FGF and HS to the FGF receptor, producing a supramolecular complex with full signaling potential (64). If HSPG-cPTPσ interactions represent part of a multiprotein signaling complex necessary for regulating the RPTP, then the characterization of additional components of this complex will be essential. It is also feasible that binding of HS to cPTPσ represents an adhesive function rather than a signaling function, and this will require further investigation.
We provide evidence that glial endfeet contain at least two binding partners for cPTPσ. Previous work of members of our group demonstrated that Müller glia endfeet contain ligands for cPTPσ and that interactions with these ligands sustain neurite outgrowth (51). Here we have shown that endfeet contain HS-dependent binding sites for cPTPσ. It is possible that interactions between these sites and cPTPσ lead at least in part to the axonal phenotype observed earlier (51). However, we also report that endfeet contain non-HS binding sites for cPTPσ, which remain to be characterized. These binding sites were not evident on cultured Müller glia, where cPTPσ binding was entirely sensitive to heparinase treatment and heparin competition (Aricescu, unpublished data). The non-HS binding sites on endfeet are also apparently removed or masked by methanol fixation, both in flat-mounted basal laminae and in tissue sections. Glial endfeet, in both the retina and tectum, are highly differentiated structures (9, 67). Tectal endfeet, for example, specifically accumulate ephrin B1 (9), and axon outgrowth experiments in vitro demonstrate that Müller glia are highly polarized (67). As previously suggested (51), the endfeet contain a rich mixture of positive and negative cues for neurite outgrowth, and it is therefore possible that cPTPσ is interacting, perhaps simultaneously, with at least two ligand types on endfeet, with the net effect of promoting neurite elongation.
In conclusion, we demonstrate that cPTPσ has a novel, heparin binding activity and that its ability to bind to the retinal BL is mediated by HS chains of extracellular matrix HSPGs. These matrix HSPGs are identified as agrin and collagen XVIII and are the first heterotypic ligands identified for cPTPσ. These HSPGs may be ligands in their own right or may act as partners in presenting other ligand proteins to cPTPσ. Further investigations will clarify the direct effect of HSPGs on cPTPσ function, helping us to understand how extracellular ligands regulate RPTP function and how such interactions in turn influence axon development.
Acknowledgments
We thank John Flanagan for providing us with the plasmid AP-Tag2, Bruce Morgan for the PRD4A viral probe and the pFN3Δ-AP plasmid, Stéphane Swillens for advice regarding the nonlinear regression analysis, and Caroline Paternotte for help with DNA sequencing. We also thank Michael Hurley for critical comments on the manuscript.
This work was supported by a University College London Graduate Research Scholarship (A.A.), the Medical Research Council (I.M.), and grants from the University of London Central Research Fund (A.S.) and the National Institutes of Health (NS33981-02) (W.H.).
REFERENCES
- 1.Ackley, B. D., J. R. Crew, H. Elamaa, T. Pihlajaniemi, C. J. Kuo, and J. M. Kramer. 2001. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 152:1219-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, M. W., and E. R. Macagno. 2000. The role of a LAR-like receptor tyrosine phosphatase in growth cone collapse and mutual-avoidance by sibling processes. J. Neurobiol. 44:194-203. [DOI] [PubMed] [Google Scholar]
- 3.Barford, D., A. K. Das, and M. P. Egloff. 1998. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct. 27:133-164. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, A., M. Jouet, J. MacFarlane, J. S. Du, S. Kenwrick, and C. Chothia. 1996. Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological disorders. EMBO J. 15:6050-6059. [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, K. L. Howe, and E. L. Sonnhammer. 2000. The Pfam protein families database. Nucleic Acids Res. 28:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bixby, J. L. 2000. Receptor tyrosine phosphatases in axon growth and guidance. Neuroreport 11:R5-R10. [DOI] [PubMed] [Google Scholar]
- 7.Bovolenta, P., and I. Fernaud-Espinosa. 2000. Nervous system proteoglycans as modulators of neurite outgrowth. Prog. Neurobiol. 61:113-132. [DOI] [PubMed] [Google Scholar]
- 8.Brady-Kalnay, S. M., A. J. Flint, and N. K. Tonks. 1993. Homophilic binding of PTPmu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J. Cell Biol. 122:961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braisted, J. E., T. McLaughlin, H. U. Wang, G. C. Friedman, D. J. Anderson, and D. O'Leary. 1997. Graded and lamina-specific distributions of ligands of EphB receptor tyrosine kinases in the developing retinotectal system. Dev. Biol. 191:14-28. [DOI] [PubMed] [Google Scholar]
- 10.Burden-Gulley, S. M., and S. M. Brady-Kalnay. 1999. PTPmu regulates N-cadherin-dependent neurite outgrowth. J. Cell Biol. 144:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess, R. W., W. C. Skarnes, and J. R. Sanes. 2000. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J. Cell Biol. 151:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai, L., and J. E. Morris. 1999. Heparan sulfate in the inner limiting membrane of embryonic chicken retina binds basic fibroblast growth factor to promote axonal outgrowth. Exp. Neurol. 160:175-185. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, H. J., and J. G. Flanagan. 1994. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell 79:157-168. [DOI] [PubMed] [Google Scholar]
- 14.Cole, G. J., and R. Akeson. 1989. Identification of a heparin binding domain of the neural cell adhesion molecule N-CAM using synthetic peptides. Neuron 2:1157-1165. [DOI] [PubMed] [Google Scholar]
- 15.Cotman, S. L., W. Halfter, and G. J. Cole. 1999. Identification of extracellular matrix ligands for the heparan sulfate proteoglycan agrin. Exp. Cell Res. 249:54-64. [DOI] [PubMed] [Google Scholar]
- 16.Cotman, S. L., W. Halfter, and G. J. Cole. 2000. Agrin binds to beta-amyloid (Abeta), accelerates abeta fibril formation, and is localized to Abeta deposits in Alzheimer's disease brain. Mol. Cell. Neurosci. 15:183-198. [DOI] [PubMed] [Google Scholar]
- 17.Cullen, B. R., and M. H. Malim. 1992. Secreted placental alkaline phosphatase as a eukaryotic reporter gene. Methods Enzymol. 216:362-368. [DOI] [PubMed] [Google Scholar]
- 18.Cummings, B. J., J. H. Su, and C. W. Cotman. 1993. Neuritic involvement within bFGF immunopositive plaques of Alzheimer's disease. Exp. Neurol. 124:315-325. [DOI] [PubMed] [Google Scholar]
- 19.Desai, C. J., J. G. Gindhart, Jr., L. S. Goldstein, and K. Zinn. 1996. Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell 84:599-609. [DOI] [PubMed] [Google Scholar]
- 20.Desai, C. J., N. X. Krueger, H. Saito, and K. Zinn. 1997. Competition and cooperation among receptor tyrosine phosphatases control motoneuron growth cone guidance in Drosophila. Development 124:1941-1952. [DOI] [PubMed] [Google Scholar]
- 21.Desai, C. J., Q. Sun, and K. Zinn. 1997. Tyrosine phosphorylation and axon guidance: of mice and flies. Curr. Opin. Neurobiol. 7:70-74. [DOI] [PubMed] [Google Scholar]
- 22.Donahue, J. E., T. M. Berzin, M. S. Rafii, D. J. Glass, G. D. Yancopoulos, J. R. Fallon, and E. G. Stopa. 1999. Agrin in Alzheimer's disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc. Natl. Acad. Sci. USA 96:6468-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drosopoulos, N. E., F. S. Walsh, and P. Doherty. 1999. A soluble version of the receptor-like protein tyrosine phosphatase kappa stimulates neurite outgrowth via a Grb2/MEK1-dependent signaling cascade. Mol. Cell. Neurosci. 13:441-449. [DOI] [PubMed] [Google Scholar]
- 24.Elchebly, M., J. Wagner, T. E. Kennedy, C. Lanctot, E. Michaliszyn, A. Itie, J. Drouin, and M. L. Tremblay. 1999. Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat. Genet. 21:330-333. [DOI] [PubMed] [Google Scholar]
- 25.Fischer, E. H. 1999. Cell signaling by protein tyrosine phosphorylation. Adv. Enzyme Regul. 39:359-369. [DOI] [PubMed] [Google Scholar]
- 26.Flanagan, J. G., and P. Leder. 1990. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell 63:185-194. [DOI] [PubMed] [Google Scholar]
- 27.Garrity, P. A., C. H. Lee, I. Salecker, H. C. Robertson, C. J. Desai, K. Zinn, and S. L. Zipursky. 1999. Retinal axon target selection in Drosophila is regulated by a receptor protein tyrosine phosphatase. Neuron 22:707-717. [DOI] [PubMed] [Google Scholar]
- 28.Gershon, T. R., M. W. Baker, M. Nitabach, and E. R. Macagno. 1998. The leech receptor protein tyrosine phosphatase HmLAR2 is concentrated in growth cones and is involved in process outgrowth. Development 125:1183-1190. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg, D. J., and D. Y. Wu. 1996. Tyrosine phosphorylation and protrusive structures of the growth cone. Perspect. Dev. Neurobiol. 4:183-192. [PubMed] [Google Scholar]
- 30.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 31.Guex, N., A. Diemand, and M. C. Peitsch. 1999. Protein modelling for all. Trends Biochem. Sci. 24:364-367. [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara, H., and J. R. Fallon. 2001. Shaping membrane architecture: agrins in and out of the synapse. J. Cell Biol. 153:F39-F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haj, F., I. McKinnell, and A. Stoker. 1999. Retinotectal ligands for the receptor tyrosine phosphatase CRYPalpha. Mol. Cell. Neurosci. 14:225-240. [DOI] [PubMed] [Google Scholar]
- 34.Halfter, W., and Y. VonBoxberg. 1992. Axonal growth on solubilized and reconstituted matrix from the embryonic chicken retina inner limiting membrane. Eur. J. Neurosci. 4:840-852. [DOI] [PubMed] [Google Scholar]
- 35.Halfter, W. 1993. A heparan sulfate proteoglycan in developing avian axonal tracts. J. Neurosci. 13:2863-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halfter, W., B. Schurer, J. Yip, L. Yip, G. Tsen, J. A. Lee, and G. J. Cole. 1997. Distribution and substrate properties of agrin, a heparan sulfate proteoglycan of developing axonal pathways. J. Comp. Neurol. 383:1-17. [PubMed] [Google Scholar]
- 37.Halfter, W. 1998. Disruption of the retinal basal lamina during early embryonic development leads to a retraction of vitreal end feet, an increased number of ganglion cells, and aberrant axonal outgrowth. J. Comp. Neurol. 397:89-104. [DOI] [PubMed] [Google Scholar]
- 38.Halfter, W., S. Dong, B. Schurer, and G. J. Cole. 1998. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 273:25404-25412. [DOI] [PubMed] [Google Scholar]
- 39.Halfter, W., S. Dong, B. Schurer, A. Osanger, W. Schneider, M. Ruegg, and G. J. Cole. 2000. Composition, synthesis, and assembly of the embryonic chick retinal basal lamina. Dev. Biol. 220:111-128. [DOI] [PubMed] [Google Scholar]
- 40.Hamburger, V., and H. L. Hamilton. 1992. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195:231-272. [DOI] [PubMed] [Google Scholar]
- 41.Haworth, K., K. K. Shu, A. Stokes, R. Morris, and A. Stoker. 1998. The expression of receptor tyrosine phosphatases is responsive to sciatic nerve crush. Mol. Cell. Neurosci. 12:93-104. [DOI] [PubMed] [Google Scholar]
- 42.Hileman, R. E., J. R. Fromm, J. M. Weiler, and R. J. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156-167. [DOI] [PubMed] [Google Scholar]
- 43.Hoch, W. 1999. Formation of the neuromuscular junction. Agrin and its unusual receptors. Eur. J. Biochem. 265:1-10. [DOI] [PubMed] [Google Scholar]
- 44.Holden, H. M., M. Ito, D. J. Hartshorne, and I. Rayment. 1992. X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 A resolution. J. Mol. Biol. 227:840-851. [DOI] [PubMed] [Google Scholar]
- 45.Hooft, R. W., G. Vriend, C. Sander, and E. E. Abola. 1996. Errors in protein structures. Nature 381:272.. [DOI] [PubMed] [Google Scholar]
- 46.Hughes, S. H., J. J. Greenhouse, C. J. Petropoulos, and P. Sutrave. 1987. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 61:3004-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasper, C., H. Rasmussen, J. S. Kastrup, S. Ikemizu, E. Y. Jones, V. Berezin, E. Bock, and I. K. Larsen. 2000. Structural basis of cell-cell adhesion by NCAM. Nat. Struct. Biol. 7:389-393. [DOI] [PubMed] [Google Scholar]
- 48.Kroger, S., S. E. Horton, and L. S. Honig. 1996. The developing avian retina expresses agrin isoforms during synaptogenesis. J. Neurobiol. 29:165-182. [DOI] [PubMed] [Google Scholar]
- 49.Kroger, S. 1997. Differential distribution of agrin isoforms in the developing and adult avian retina. Mol. Cell. Neurosci. 10:149-161. [DOI] [PubMed] [Google Scholar]
- 50.Krueger, N. X., D. VanVactor, H. I. Wan, W. M. Gelbart, C. S. Goodman, and H. Saito. 1996. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell 84:611-622. [DOI] [PubMed] [Google Scholar]
- 51.Ledig, M. M., F. Haj, J. L. Bixby, A. W. Stoker, and B. K. Mueller. 1999. The receptor tyrosine phosphatase CRYPalpha promotes intraretinal axon growth. J. Cell Biol. 147:375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledig, M. M., I. W. McKinnell, T. Mrsic-Flogel, J. Wang, C. Alvares, I. Mason, J. L. Bixby, B. K. Mueller, and A. W. Stoker. 1999. Expression of receptor tyrosine phosphatases during development of the retinotectal projection of the chick. J. Neurobiol. 39:81-96. [DOI] [PubMed] [Google Scholar]
- 53.Meng, K., A. Rodriguez-Pena, T. Dimitrov, W. Chen, M. Yamin, M. Noda, and T. F. Deuel. 2000. Pleiotrophin signals increased tyrosine phosphorylation of beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc. Natl. Acad. Sci. USA 97:2603-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan, B. A., and D. M. Fekete. 1996. Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol. 51:185-218. [DOI] [PubMed] [Google Scholar]
- 55.Mueller, B. K., M. M. Ledig, and S. Wahl. 2000. The receptor tyrosine phosphatase CRYPalpha affects growth cone morphology. J. Neurobiol. 44:204-218. [PubMed] [Google Scholar]
- 56.Neumann, F. R., G. Bittcher, M. Annies, B. Schumacher, S. Kroger, and M. A. Ruegg. 2001. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol. Cell. Neurosci. 17:208-225. [DOI] [PubMed] [Google Scholar]
- 57.Newsome, T. P., B. Asling, and B. J. Dickson. 2000. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127:851-860. [DOI] [PubMed] [Google Scholar]
- 58.Noramly, S., and B. A. Morgan. 1998. BMPs mediate lateral inhibition at successive stages in feather tract development. Development 125:3775-3787. [DOI] [PubMed] [Google Scholar]
- 59.O'Grady, P., T. C. Thai, and H. Saito. 1998. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J. Cell Biol. 141:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peles, E., J. Schlessinger, and M. Grumet. 1998. Multi-ligand interactions with receptor-like protein tyrosine phosphatase beta: implications for intercellular signaling. Trends Biochem. Sci. 23:121-124. [DOI] [PubMed] [Google Scholar]
- 61.Plotnikov, A. N., J. Schlessinger, S. R. Hubbard, and M. Mohammadi. 1999. Structural basis for FGF receptor dimerization and activation. Cell 98:641-650. [DOI] [PubMed] [Google Scholar]
- 62.Sap, J., Y. P. Jiang, D. Friedlander, M. Grumet, and J. Schlessinger. 1994. Receptor tyrosine phosphatase R-PTP-kappa mediates homophilic binding. Mol. Cell. Biol. 14:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaapveld, R. Q., J. T. Schepens, G. W. Robinson, J. Attema, F. T. Oerlemans, J. A. Fransen, M. Streuli, B. Wieringa, L. Hennighausen, and W. J. Hendriks. 1997. Impaired mammary gland development and function in mice lacking LAR receptor-like tyrosine phosphatase activity. Dev. Biol. 188:134-146. [DOI] [PubMed] [Google Scholar]
- 64.Schlessinger, J., A. N. Plotnikov, O. A. Ibrahimi, A. V. Eliseenkova, B. K. Yeh, A. Yayon, R. J. Linhardt, and M. Mohammadi. 2000. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 6:743-750. [DOI] [PubMed] [Google Scholar]
- 65.Sertie, A. L., V. Sossi, A. A. Camargo, M. Zatz, C. Brahe, and M. R. Passos-Bueno. 2000. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum. Mol. Genet. 9:2051-2058. [DOI] [PubMed] [Google Scholar]
- 66.Sörby, M., J. Sandström, and A. Östman. 2001. An extracellular ligand increases the specific activity of the receptor-like protein tyrosine phosphatase DEP-1. Oncogene 20:5219-5224. [DOI] [PubMed] [Google Scholar]
- 67.Stier, H., and B. Schlosshauer. 1998. Different cell surface areas of polarized radial glia having opposite effects on axonal outgrowth. Eur. J. Neurosci. 10:1000-1010. [DOI] [PubMed] [Google Scholar]
- 68.Stoker, A., and R. Dutta. 1998. Protein tyrosine phosphatases and neural development. Bioessays 20:463-472. [DOI] [PubMed] [Google Scholar]
- 69.Stoker, A. W. 1994. Isoforms of a novel cell adhesion molecule-like protein tyrosine phosphatase are implicated in neural development. Mech. Dev. 46:201-217. [DOI] [PubMed] [Google Scholar]
- 70.Stoker, A. W., B. Gehrig, F. Haj, and B. H. Bay. 1995. Axonal localisation of the CAM-like tyrosine phosphatase CRYP alpha: a signalling molecule of embryonic growth cones. Development 121:1833-1844. [DOI] [PubMed] [Google Scholar]
- 71.Stoker, A. W., B. Gehrig, M. R. Newton, and B. H. Bay. 1995. Comparative localisation of CRYP alpha, a CAM-like tyrosine phosphatase, and NgCAM in the developing chick visual system. Brain Res. Dev. Brain Res. 90:129-140. [DOI] [PubMed] [Google Scholar]
- 72.Stoker, A. W. 2001. Receptor tyrosine phosphatases in axon growth and guidance. Curr. Opin. Neurobiol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 73.Sun, Q., S. Bahri, A. Schmid, W. Chia, and K. Zinn. 2000. Receptor tyrosine phosphatases regulate axon guidance across the midline of the Drosophila embryo. Development 127:801-812. [DOI] [PubMed] [Google Scholar]
- 74.Sun, Q. L., J. Wang, R. J. Bookman, and J. L. Bixby. 2000. Growth cone steering by receptor tyrosine phosphatase delta defines a distinct class of guidance cue. Mol. Cell. Neurosci. 16:686-695. [DOI] [PubMed] [Google Scholar]
- 75.Swillens, S. 1995. Interpretation of binding curves obtained with high receptor concentrations: practical aid for computer analysis. Mol. Pharmacol. 47:1197-1203. [PubMed] [Google Scholar]
- 76.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 77.Uetani, N., K. Kato, H. Ogura, K. Mizuno, K. Kawano, K. Mikoshiba, H. Yakura, M. Asano, and Y. Iwakura. 2000. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 19:2775-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Lieshout, E. M., I. Van der Heijden, W. J. Hendriks, and C. E. Van der Zee. 2001. A decrease in size and number of basal forebrain cholinergic neurons is paralleled by diminished hippocampal cholinergic innervation in mice lacking leukocyte common antigen-related protein tyrosine phosphatase activity. Neuroscience 102:833-841. [DOI] [PubMed] [Google Scholar]
- 79.Van Vactor, D. 1998. Protein tyrosine phosphatases in the developing nervous system. Curr. Opin. Cell Biol. 10:174-181. [DOI] [PubMed] [Google Scholar]
- 80.Wallace, M. J., J. Batt, C. A. Fladd, J. T. Henderson, W. Skarnes, and D. Rotin. 1999. Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma. Nat. Genet. 21:334-338. [DOI] [PubMed] [Google Scholar]
- 81.Walz, A., S. McFarlane, Y. G. Brickman, V. Nurcombe, P. F. Bartlett, and C. E. Holt. 1997. Essential role of heparan sulfates in axon navigation and targeting in the developing visual system. Development 124:2421-2430. [DOI] [PubMed] [Google Scholar]
- 82.Wang, J., and J. L. Bixby. 1999. Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecule for CNS neurons. Mol. Cell. Neurosci. 14:370-384. [DOI] [PubMed] [Google Scholar]
- 83.Wang, L., and J. L. Denburg. 1992. A role for proteoglycans in the guidance of a subset of pioneer axons in cultured embryos of the cockroach. Neuron 8:701-714. [DOI] [PubMed] [Google Scholar]
- 84.Xie, Y., T. T. Yeo, C. Zhang, T. Yang, M. A. Tisi, S. M. Massa, F. M. Longo. 2001. The leukocyte common antigen-related protein tyrosine phosphatase receptor regulates regenerative neurite outgrowth in vivo. J. Neurosci. 21:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan, H., A. Grossman, H. Wang, P. D'Eustachio, K. Mossie, J. M. Musacchio, O. Silvennoinen, and J. Schlessinger. 1993. A novel receptor tyrosine phosphatase-sigma that is highly expressed in the nervous system. J. Biol. Chem. 268:24880-24886. [PubMed] [Google Scholar]
- 86.Yeo, T. T., T. Yang, S. M. Massa, J. S. Zhang, J. Honkaniemi, L. L. Butcher, and F. M. Longo. 1997. Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J. Neurosci. Res. 47:348-360. [DOI] [PubMed] [Google Scholar]