Abstract
The Ral proteins are members of the Ras superfamily of GTPases. Because they reside in synaptic vesicles, we used transgenic mice expressing a dominant inhibitory form of Ral to investigate the role of Ral in neurosecretion. Using a synaptosomal secretion assay, we found that while K+-evoked secretion of glutamate was normal, protein kinase C-mediated enhancement of glutamate secretion was suppressed in the mutant mice. Since protein kinase C effects on secretion have been shown to be due to enhancement of the size of the readily releasable pool of synaptic vesicles docked at the plasma membrane, we directly measured the refilling of this readily releasable pool of synaptic vesicles after Ca2+-triggered exocytosis. Refilling of the readily releasable pool was suppressed in synaptosomes from mice expressing dominant inhibitory Ral. Moreover, we found that protein kinase C and calcium-induced phosphorylation of proteins thought to influence synaptic vesicle function, such as MARCKS, synapsin, and SNAP-25, were all reduced in synaptosomes from these transgenic mice. Concomitant with these studies, we searched for new functions of Ral by detecting proteins that specifically bind to it in cells. Consistent with the phenotype of the transgenic mice described above, we found that active but not inactive RalA binds to the Sec6/8 (exocyst) complex, whose yeast counterpart is essential for targeting exocytic vesicles to specific docking sites on the plasma membrane. These findings demonstrate a role for Ral-GTPase signaling in the modulation of the readily releasable pool of synaptic vesicles and suggest the possible involvement of Ral-Sec6/8 (exocyst) binding in modulation of synaptic strength.
The presynaptic nerve terminal contains hundreds of synaptic vesicles, but only a small number of these vesicles are docked and fusion competent. This population of vesicles, referred to as the readily releasable pool (RRP), is thought to be in equilibrium with the majority of vesicles that comprise the reserve pool such that the RRP is replenished following a release event (for a review see reference [46]). The probability of release upon depolarization is proportional to the size of the RRP (8, 40). Thus, regulating the equilibrium between the RRP and the reserve pool is an effective means of modulating synaptic strength (46).
Regulation of the size of the RRP is incompletely understood. At least two signaling pathways can influence the RRP, apparently by altering the recruitment of vesicles from the reserve pool. The first involves protein kinase C (PKC) (32, 43), which increases the size of the RRP. Since PKC is activated by presynaptic metabatropic receptors, it may participate in a positive feedback mechanism involved in synaptic modulation (32). In parallel, presynaptic Ca2+ entry associated with membrane depolarization may play a similar role by enhancing the rate of refilling of the RRP (42, 45), at least in part through its activation of calmodulin (CaM) kinase II (3, 31). These two signaling pathways function through distinct mechanisms, yet they appear to share a common ill-defined final pathway (43).
Efforts to elucidate molecular mechanisms of neurosecretion and its regulation have identified and characterized synaptic components that may participate in regulating the RRP. Among them is the Ras superfamily of GTPases. Within this superfamily, members of the Rab subfamily of GTPases have emerged as key mediators of a variety of vesicle sorting processes, including those involved in neurosecretion (14). However, recent biochemical data have hinted that the Ral family GTPases, RalA and RalB, may also play an important role in vesicle regulation. First, like Rab3A, Ral proteins are present at high levels in synaptic vesicles and other secretory vesicles (4, 34, 36). Interestingly, calmodulin can bind to Ral in vitro and elute it from membranes, raising the possibility that Ral may cycle between the cytoplasm and membrane fractions of cells like the related Rab proteins (37). Moreover, Ral proteins participate in the regulation of Arf-dependent phospholipase D (PLD) (26, 33), an enzyme implicated in vesicle function (10). Finally, a downstream target of Ral proteins is RalBP1 (6, 27, 38), which forms a complex with proteins involved in clathrin-mediated endocytosis, such as the eps-15 homology (EH) domain proteins Reps (51), its close relative POB (29), and the clathrin-binding protein adaptin (28, 35).
Ral activity is tightly coupled to extracellular signals. Like all GTPases, Ral is activated by guanine nucleotide exchange factors (GEF), which promote the exchange of GTP for GDP bound to GTPases. Many Ral-GEFs become activated by binding active GTP-bound Ras, making Ral activation one of the three known downstream signaling pathways emanating from Ras proteins (12). Ral proteins can also be activated by elevated calcium levels in cells through an ill-defined Ras-independent mechanism (22).
Here we show that expression of a dominant inhibitory Ral mutant in transgenic mice suppressed the enhancement of the RRP of synaptic vesicles induced by PKC and calcium, thus implicating Ral function in the regulation of neurosecretion. Consistent with this result, we find that the Sec6/8 (exocyst) complex, which is known to target secretory vesicles to specific sites on the plasma membrane in cells, binds specifically to active Ral in cells.
MATERIALS AND METHODS
Generation of RalA28N transgenic mice.
Simian RalA28N was subcloned into pNSE(13) containing the neuronal-specific enolase promoter sequence. Pronuclear injection was carried out, and two independent heterozygous transgenic lines were established and used for this study. Subsequent genotyping of mice was conducted by PCR of genomic DNA with primers that detected both vector sequence and Ral sequence. Male mice 2 months to 1 year old from two independent strains expressing RalA28N were used in all experiments.
RT-PCR.
Whole-brain total RNAs were isolated from RalA28N and wild-type control mice. The same RalA-specific set of primers described above to genotype the mice was used for both reverse transcription (RT) to generate cDNA and for PCR amplification of the cDNA.
Synaptosomal secretion assay.
Synaptosomes from the cortex, prepared by using discontinuous Percoll (Pharmacia) gradients (19), were resuspended in basal buffer (145 mM NaCl, 2.7 mM KCl, 2.4 mM MgCl2, 10 mM glucose, 10 mM HEPES-Tris, pH 7.4) and kept on ice for up to 2 h. For experiments employing phorbol esters, synaptosomes were combined with a small volume of 12,13-phorbol dibutyrate (PDBu) (LC Laboratories, Woburn, Mass.) or vehicle control (dimethyl sulfoxide [DMSO]) to give the desired final PDBu concentration. A portion of the suspension was combined with [3H]glutamate (56.0 Ci/mMol; Amersham International, Piscataway, N.J.) and was incubated for 12 min, at which time the reaction was stopped by adding 600 μl of basal buffer and applying the suspension to a filtration sandwich composed of cellulose ester and glass fiber filters, as described previously (48). Release was measured with a superfusion device in conjunction with a fraction collector modified from a phonograph turntable (48). Synaptosomes were superfused using various stimulus buffers, and fractions of 35 or 100 ms were collected. For experiments where depolarization-induced glutamate release was measured, we routinely included a data set where elevated K+ and no added Ca2+ (nominal [Ca2+] was ∼3 μM) was used as a stimulus buffer. Release evoked by elevated K+ without Ca2+ was eliminated by 3-hydroxy aspartate, an inhibitor of the Na+-dependent glutamate transporter present in the plasma membrane, indicating that this component of release was mediated by the reversal of the transporter and was not due to exocytosis. Ca2+-dependent release was defined as the difference between total release and Ca2+-independent release measured at each [Ca2+].
Radioactivity in each fraction and the amount remaining on the filter at the end of the experiment were determined by adding 1.5 ml of liquid scintillation cocktail (BioSafe II; Research Products Inc., Mt. Prospect, Ill.) and counting in a Packard Instruments Tri-Carb 2100 liquid scintillation analyzer. Results were expressed as the ratio of counts per minute in each fraction to the total radioactivity remaining on the filter (×100). Peak release rates were calculated by summing the Ca-dependent release over a 140-ms period immediately after the end of the switching surge and are expressed as percentages of total [3H]glutamate content per second (% s−1). Data reported are the averages of at least three separate experiments performed on different days with freshly prepared synaptosomes. Standard deviations (error bars are omitted from kinetic plots for clarity) were generally less than 10% and never exceeded 20%. In order to account for any time-dependent changes in release rates, the order of the experimental conditions was randomized for each experiment.
Protein immunoblots.
Synaptosomes were lysed in 1% Triton X-100 or radioimmunopreciptation assay buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM MgCl2, and the protease inhibitors aprotinin and leupeptin. Equal amounts of protein were loaded on a 12% sodium dodecyl sulfate (SDS) gel, and proteins were detected with one of the following antibodies: mouse monoclonal anti-RalA (Transduction Labs), α-Sec8 antibodies (Stressgen), anti-SNAP-25 (Upstate Biotechnology), rabbit polyclonal anti-synapsins (Novac), anti-phospho-Erk antibodies (New England Bio-Labs), and rabbit polyclonal anti-MARCKS (a gift of James K. T. Wang). The enhanced chemiluminescence detection system was used to visualize immunoreactive bands. To quantify differences in protein expression signals, dilutions of each sample were compared.
Immunoprecipitation.
Synaptosomes were metabolically labeled with [32P]H3PO4 at 1.5 mCi/ml for 45 to 50 min at 35°C. Labeled synaptosomes were stimulated with 2.5 μM PDBu or an equal volume of DMSO alone for 12 min at 37°C. Synaptosomal pellets were lysed in a buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM MgCl2) containing either 1% Triton X-100 (for MARCKS) or 0.1% SDS, 1% Na-cholate (for SNAP-25 and Synapsins). The extracts were immunoprecipitated, and following SDS-polyacrylamide gel electrophoresis (PAGE) the radioactive bands were quantified by PhosphorImager analysis.
Detection of Ral-Sec6/8 complexes.
Three 100-mm-diameter dishes of semiconfluent HEK-293 cells were transfected with each mutant Ral gene in pCDNA3 by using Lipofectamine (Invitrogen). Cells were lysed with buffer (50 mM Tris-HCl, pH 7.4, plus 150 mM NaCl, 1 mM MgCl2, and aprotinin) containing 1% Triton X-100 and were immunoprecipitated with α-Myc antibodies (Transduction Labs). Proteins were eluted from Protein A beads with 1.0 M NaCl, concentrated, and loaded on SDS gels. After colloidal Coomassie staining, bands containing Ral-binding proteins were cut out of gels and analyzed by mass spectroscopy.
RESULTS
Expression of a dominant-inhibitory Ral GTPase mutant in mice.
To investigate the possible involvement of Ral GTPases in neuronal function in vivo, we generated transgenic mouse lines in which a dominant-inhibitory mutant of Ral, RalA28N, was expressed in the adult central nervous system. RalA28N is analogous to widely used inhibitory RasH17N. This class of GTPase mutant is in a constitutively inactive conformation and has approximately fivefold higher affinity for guanine nucleotide exchange factors than its normal counterpart (11). As such, RalA28N forms an inactive complex with Ral-GEFs and prevents activation of endogenous RalA and RalB in cells (50). Expression of the transgene was driven by the neuronal-specific enolase promoter, which becomes active around the time of synaptogenesis (13). Two independent mouse lines, TG5 and TG7, were established and used in this study. The transgenic animals were fertile, and all major organs, including the brain, were normal at the light microscopic level (data not shown).
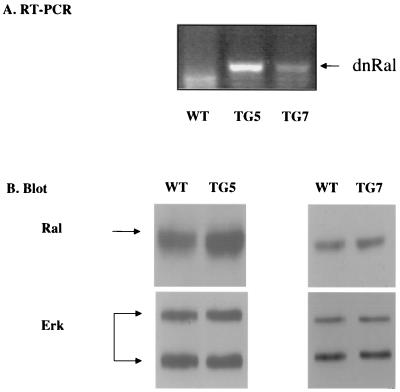
Transgenic mRNA in the brain was detected by amplifying RalA28N-specific sequences by RT-PCR. Both mouse lines showed the expected PCR product of 540 bp, while a wild-type animal did not (Fig. 1A). To assess mutant RalA protein expression, synaptosomes used in experiments described below were analyzed by immunoblotting. Because we found previously that epitope-tagging RalA28N suppressed its activity, we used untagged RalA28N in these experiments. This required us to estimate the transgene product expression by comparing total Ral immunoreactivity in synaptosomes from mutant mice and wild-type littermates. RalA levels were elevated in both transgenic cell lines while Erk levels, assayed on the same immunoblots, were not (Fig. 1B). By comparing signals generated from dilutions of wild-type and mutant samples (data not shown), we estimated that RalA28N was expressed at a level that was ∼50% that of endogenous Ral in the TG5 line and ∼30% that of endogenous Ral in the TG7 line.
FIG. 1.
Expression of RalA28N RNA in the brain of RalA28N transgenic animals. (A) Total tissue RNAs were prepared from the whole brain of adult RalA28N and wild-type (WT) animals. Following an RT reaction to generate cDNAs from the cellular RNAs, primers that specifically recognize the transgene sequence were used to amplify the simian RalA28N sequence. Lane 1, wild-type mice; lane 2, transgene line TG5; lane 3, transgenic line TG7. (B) Synaptosomes lysates (50 μg) from either transgenic lines TG5 and TG7 or wild-type mice were run in SDS gels and immunoblotted with α-RalA antibodies or Erk antibodies. The data are representative of tissue from four independent sets of wild-type and transgenic mice.
Expression of RalA28N suppresses phorbol-enhanced secretion of glutamate from synaptosomes.
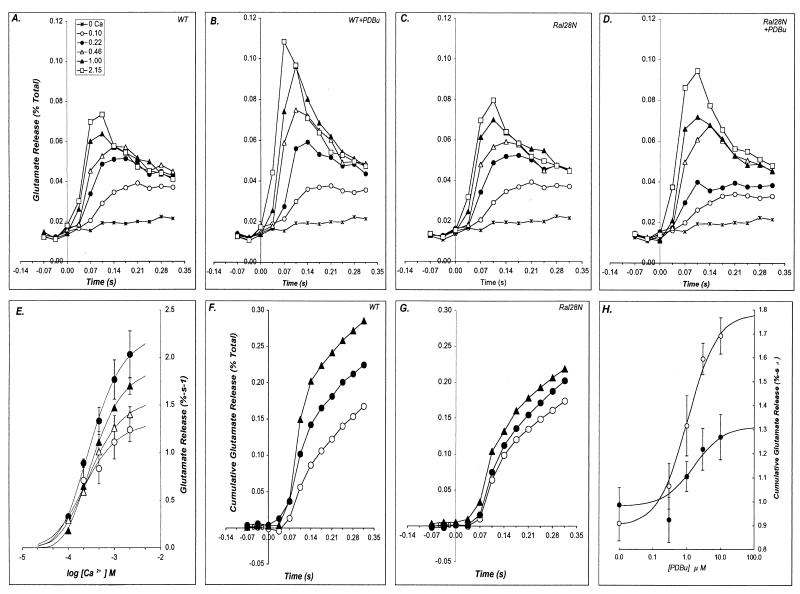
In all of the experiments described below both RalA28N transgenic lines displayed similar phenotypes. Thus, the data from both were pooled throughout this study. Because Ral proteins are known to be responsive to a variety of extracellular signals and because they are present in nerve terminals, we hypothesized that Ral might participate in the modulation of neurosecretion by PKC. Thus, we assessed the ability of PDBu, an exogenous activator of PKC, to modulate [3H]glutamate release from synaptosomes prepared from the cortex of RalA28N and wild-type littermates by using a rapid superfusion method (48). Synaptosomes labeled with [3H]glutamate were immobilized on filter discs and placed in a superfusion device capable of 25-ms temporal resolution. The synaptosomes were superfused with HEPES-buffered saline to establish a baseline for release. Exocytosis was triggered by switching the superfusion buffer to a stimulating saline that contained 60 mM KCl (substituted for NaCl) and the desired concentration of Ca2+ ranging between 0.1 and 2.15 mM. Depolarization-induced release of glutamate in wild-type and RalA28N synaptosomes were not significantly different at all Ca2+ concentrations tested (Fig. 2A and C). In contrast, RalA28N expression suppressed the ability of PDBu to increase the initial rate of glutamate release (Fig. 2B and D). In particular, pretreatment of wild-type synaptosomes with 2.5 μM PDBu enhanced Ca2+-dependent glutamate release, with a prominent increase in the initial rate of release observed in the first 70 ms of stimulation, especially at the higher Ca2+ concentrations (Fig. 2B). The effect of 2.5 μM PDBu on synaptosomes prepared from RalA28N animals was significantly diminished (P < 0.05 for [Ca2+] 0.46 mM and higher) (Fig. 2D).
FIG. 2.
Ral28N suppresses PDBu enhancement of synaptosomal [3H]glutamate release. The influence of Ral28N expression on neurosecretion was evaluated by measuring glutamate release from synaptosomes prepared from Ral28N mice and wild-type (WT) littermates. (A to D) Synaptosomes from wild-type (A and B) or Ral28N (C and D) animals were metabolically labeled with [3H]glutamate for 12 min and then were immobilized on a glass fiber filter sandwich placed in a superfusion device as described previously (47). At t = 0 the synaptosomes were depolarized by being superfused with a stimulus buffer containing 60 mM KCl (substituted isosmotically for NaCl) and 0 (∗), 0.1 (○), 0.22 (•), 0.46 (▵), 1.0 (▴), or 2.2 (□) mM Ca2+. In parallel, aliquot portions of synaptosomes were incubated with 2.5 μM PDBu (B and D) for 12 min (using 0.1% [vol/vol] DMSO as a vehicle) to evaluate the ability of PDBu to enhance Ca2+-dependent glutamate release. Results are plotted as the mean values of three experiments performed in triplicate. (E) The apparent affinity of the release process for Ca2+ was analyzed by plotting the net Ca2+-dependent glutamate release (i.e., the release in the presence of a given [Ca2+] and the Ca2+-independent release observed with no added Ca2+) integrated over the first 140 ms of depolarization versus the log[Ca2+] for wild-type (○, •) and Ral8N (▵, ▴) synaptosomes. The data were fit to the Dodge-Rahamimoff equation by nonlinear least-squares regression analysis. The best-fit parameters were used to generate the smooth curves for each data set. The effect of 2.5 μM PDBu on glutamate release from wild-type synaptosomes (•) was judged to be significantly different from control values (P < 0.05; Student's t test) at the Ca2+ concentrations of 0.46, 1.0, and 2.2 mM, while the differences between PDBu-treated and control release for Ral28N synaptosomes (▴) were not significant. The action of PDBu was further evaluated by concentration-response analysis (F to H). Wild-type (F) and Ral28N (G) synaptosomes were pretreated with 0 (○), 1.0 (•), or 10 μM (▴) PDBu, and glutamate release was evoked with 60 mM KCl, 1.0 mM Ca2+. The net Ca2+-dependent glutamate release was averaged from six experiments performed in triplicate; these values were integrated (to emphasize the small amount of enhancement observed in Ral28N synaptosomes) and plotted as a function of stimulus duration for wild-type (F) and Ral28N synaptosomes (G). Net Ca2+-dependent release for each PDBu concentration was integrated over the 0.28-s stimulus interval, and the values were fit using a simple hyperbolic (Michaelis-Menten) equation (H), yielding the following kinetic parameters for PDBu: wild type, Km = 1.07 μM, Vmax = 1.79% (99% enhancement); Ral28N, Km = 1.64 μM, Vmax = 1.31% (34% enhancement). The data at 10 μM PDBu are statistically significant (P < 0.02; level two-tailed paired t test, n = 6).
The cumulative Ca2+-dependent release upon depolarization was integrated over the initial 0.14 s of the stimulation period and was plotted as a function of Ca2+ in the stimulation buffer, and the data were fit to the Dodge-Rahamimoff equation (9) (Fig. 2E). The maximal amplitude of glutamate release derived from this analysis was 1.33% s−1 in wild-type synaptosomes and was slightly, but not significantly, higher (1.57% s−1) in RalA28N synaptosomes (compare open circles and triangles in Fig. 2E). The maximal amplitude of glutamate release from wild-type synaptosomes was increased by 69.9% by 2.5 μM PDBu to 2.26% s−1 (see Fig. 2E, filled circles), but the enhancement of release was significantly smaller from synaptosomes prepared from RalA28N expressing mice (1.91% s−1, only a 21.7% increase over untreated RalA28N) (see filled triangles in Fig. 2E). In order to evaluate whether the suppression of PDBu effects was due to a decreased apparent affinity for phorbol, a separate set of experiments was conducted to measure the concentration-response relationship for PDBu enhancement of glutamate release data from normal and RalA28N mice (Fig. 2F and G). Synaptosomes were preincubated with either 0, 1, or 10 μM PDBu, and release was evoked with a stimulus buffer that contained 60 mM K+, 1.0 mM Ca2+. The cumulative Ca2+-dependent glutamate release plotted as a function of time indicated that the PDBu-dependent enhancement of glutamate release was suppressed in RalA28N synaptosomes at both PDBu concentrations. The values for total glutamate release (integrated over the initial 0.315 s of stimulation) were plotted as a function of PDBu concentration and fit to the Michaelis-Menten equation by least-squares regression analysis (Fig. 2H). This analysis indicated that the efficacy (99% versus 34% increase; P < 0.05) of PDBu was diminished in the RalA28N synaptosomes, with a small effect on the apparent potency (1.07 μM versus 1.64 μM). Taken together, these results show that expression of RalA28N suppresses phorbol-induced enhancement of glutamate release.
Expression of RalA28N suppresses the calcium-dependent refilling of the readily releasable pool of vesicles.
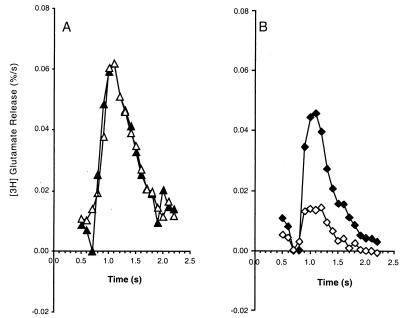
Ca2+ has multiple effects on the synaptic vesicle cycle (45). In addition to regulating the exocytosis/fusion step, Ca2+ also regulates the steps that lead to refilling of the RRP. Therefore, we investigated whether RalA28N also suppressed Ca2+-dependent regulation of the RRP in synaptosomes. We used hypertonic sucrose solutions as a Ca2+-independent means of measuring the size of the RRP in synaptosomes (32, 40). Application of 0.5 M sucrose produced a marked increase in the rate of glutamate release that peaked within 0.5 s and decayed to a plateau rate within ∼2 s (Fig. 3). The cumulative sucrose-evoked [3H]glutamate release collected over a 2-s period was defined as the RRP. We found that the RRP in synaptosomes from RalA28N mice was not significantly different from that from wild-type mice (Fig. 3A), consistent with our observations that depolarization-evoked release was also normal. However, when we measured the recovery of the RRP after depletion by triggering Ca2+-dependent exocytosis and then applying a sucrose pulse 200 ms later (Fig. 3B), clear differences were seen between wild-type and RalA28N synaptosomes. The sucrose-stimulated [3H]glutamate release in wild-type synaptosomes had recovered to 57% (0.28% versus 0.48% of total [3H]glutamate content) of the response recorded without calcium-induced depletion, while RalA28N synaptosomes had only recovered to 18% (0.09% versus 0.47% total [3H]glutamate content).
FIG. 3.
RalA28N suppresses refilling of the RRP after Ca2+-triggered depletion. The ability to refill the RRP of [3H]glutamate after a depleting stimulus was measured with synaptosomes from wild-type (▴ and ♦) and RalA28N mice (▵ and ⋄). (A) A 500-ms depolarizing pulse containing 60 mM K+ without added Ca2+ was delivered, followed 200 ms later by a test pulse containing saline only or by saline made hypertonic by the addition of 0.5 M sucrose (▴ and ⋄). The net sucrose-evoked release was calculated as the difference between the baseline release and the total release measured in the presence of 0.5 M sucrose. (B) Refilling of the RRP was assessed by delivering a 500-ms depolarizing pulse that contained 60 mM K+ plus 1 mM Ca2+. This was followed 200 ms later by a test pulse that contained 0.5 M sucrose (♦ and ⋄). In control animals, the test pulse released 0.28% of total [3H]glutamate content (♦), or 43% less than the size of the RRP assessed after the Ca2+-free conditioning pulse shown in panel A (▴). By contrast, the size of the sucrose response in the RalA28N animals was only 0.06% of total [3H]glutamate (⋄), 82% less than the respective control value shown in panel A (▵). Data were calculated as the mean values from four experiments, each conducted in triplicate. Standard deviations were generally less than 10% and never exceeded 20%.
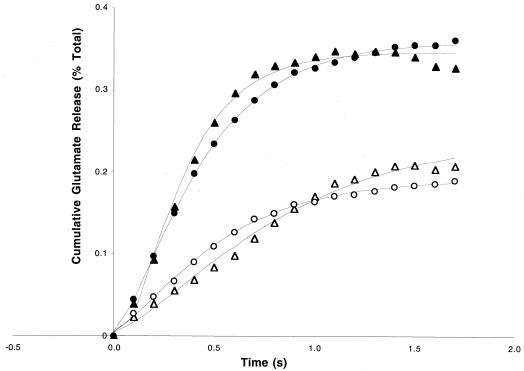
Thus, expression of RalA28N suppressed both PKC and calcium effects on the RRP. However, not all perturbations that increased the size of the RRP were suppressed in RalA28N synaptosomes. N-ethylmaleimide (NEM) pretreatment has been shown to increase the RRP of synaptosomes, possibly by enhancing the assembly of the SNARE core complex after vesicles have docked at the plasma membrane. NEM pretreatment affected mutant and wild-type synaptosomes equally (Fig. 4).
FIG. 4.
Enhancement of the size of the RRP by NEM treatment is unaltered by dnRal expression. Synaptosomes prepared from normal (circles) or RalA28N expressing (triangles) mice were loaded with [3H]glutamate for 12 min. The synaptosomes were then treated with either 30 μM NEM buffer (filled symbols) or buffer only (open symbols) for 10 min. The samples were then exposed to sucrose solution to release the RRP of vesicles. Results shown are the average of data from two independent experiments, each performed in triplicate.
Expression of RalA28N suppresses PKC- and calcium-induced phosphorylation of target proteins.
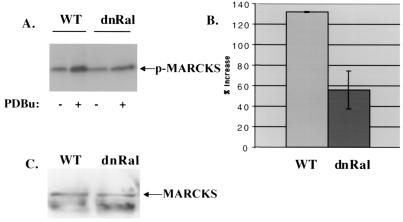
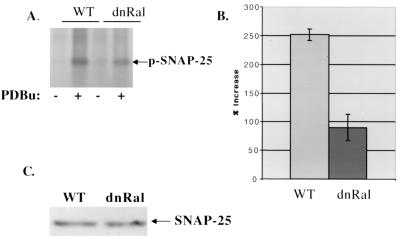
To begin to understand why enhancement of the RRP by PKC was suppressed in RalA28N synaptosomes, the function of PKC in synaptosomes was investigated. First we found that representative phorbol responsive PKC isozymes highly expressed in synaptosomes, such as PKCα, PKCβ, and PKCɛ (15), were present at similar levels in wild-type and RalA28N synaptosomes, and they translocated to the particulate fraction of cells similarly in response to phorbol treatment (data not shown). PKCγ was not studied because it has been shown previously to be postsynaptic and plays no role in the regulation of the RRP (1). We then investigated the effect of RalA28N expression on PKC kinase function in synaptosomes by measuring PDBu-induced phosphorylation of synaptic proteins known to be phosphorylated upon PKC activation, such as MARCKS and SNAP-25. In control synaptosomes, PDBu increased the phosphorylation of MARCKS by approximately 130% (Fig. 5A and B). However, in RalA28N synaptosomes PDBu increased MARCKS phosphorylation by only ∼58%, a ∼55% reduction. Total MARCKS protein in synaptosomes from the two mouse lines was indistinguishable by immunoblot analysis (Fig. 5C). A similar result was obtained with SNAP-25 (Fig. 6), where PDBu-enhanced phosphorylation of SNAP-25 was reduced by ∼65% in RalA28N synaptosomes. These effects of PDBu were mediated by PKC, since the PKC inhibitor GF109203X abolished the PDBu-enhanced phosphorylation of these proteins (data not shown).
FIG. 5.
RalA28N expression reduces phorbol-induced phosphorylation of MARCKS. (A) Wild-type (WT) and mutant synaptosomes were metabolically labeled with [32P]H3PO4 and then were treated with phorbol ester (+) or 50 mM K+ (+) or buffer only (−). Phosphorylation of MARCKS was measured by immunoprecipitation followed by quantitation with a PhosphorImager. (B) The average percentage of increase in phosphate incorporation upon PDBu treatment for 30 min ± standard deviation from three independent experiments is shown. (C) Total MARCKS proteins in wild-type and RalA28N synaptosomes were detected by immunoblotting of synaptosomal lysates.
FIG. 6.
Ral28N expression reduces phorbol-induced SNAP-25 phosphorylation. (A) SNAP-25 was immunoprecipitated from [32P]H3PO4-labeled wild-type (WT) and mutant synaptosomes stimulated with PDBU and analyzed as described for MARCKS in the legend to Fig. 6. (B) The average percentage of increase in SNAP-25 phosphorylation upon PDBu treatment from three independent experiments is shown. (C) Total SNAP-25 in wild-type and mutant synaptosomes was detected by immunoblotting of synaptosomal lysates.
Significantly, not all phorbol ester-responsive pathways were inhibited by RalA28N expression. Phospho-specific antibodies showed that PDBu treatment enhanced the phosphorylation of ERK and AKT by similar degrees in RalA28N and control synaptosomes (Fig. 7). In addition, phorbol ester treatment of synaptosomes is known to enhance PLD activity (41). This effect was also normal in RalA28N synaptosomes (data not shown).
FIG. 7.
Ral28N expression does not reduce phorbol-induced ERK or Akt phosphorylation. Cell lysates from control (−) and PDBu-stimulated synaptosomes from wild-type (WT) or RalA28N synaptosomes were immunoblotted with phosphorylation-specific antibodies to either ERK or Akt.
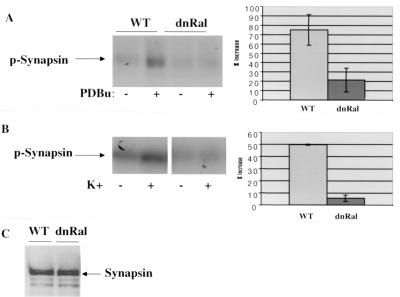
Synapsin was also investigated, since it not only is phosphorylated in response to PKC activation but is also a substrate of calcium-activated CaM kinase II, which becomes activated upon depolarization of neurons (3). Both PDBu and K+-induced phosphate incorporation into synapsins was suppressed in RalA28N synaptosomes compared to that in wild-type synaptosomes (Fig. 8) (∼70 and ∼90%, respectively). Overall, these results show that RalA28N expression suppressed both calcium-induced and PKC-induced phosphorylation of a subset of potential substrates.
FIG. 8.
RalA28N expression reduces both PDBu and depolarization-induced phosphorylation of synapsin. (A) Synapsin was immunoprecipitated from [32P]H3PO4-labeled wild-type (WT) and mutant synaptosomes stimulated with PDBu, as described in the legend to Fig. 6 for MARCKs. The percentage of increase in phorbol-induced phosphate incorporation averaged from three independent experiments is also shown. (B) Labeled synaptosomes were also depolarized by exposure to 50 mM K+ for 5 min, and synapsin was immunoprecipitated and analyzed as described for panel A. (C) Total synapsin in wild-type and mutant synaptosomes was detected by immunoblotting synaptosomal lysates.
Sec6/8 binds specifically to active Ral in cells.
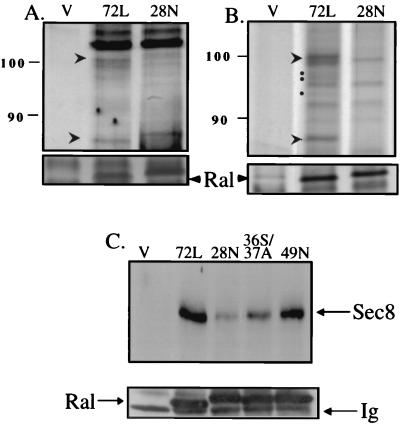
Concomitant with our characterization of the dominant inhibitory Ral transgenic mice, we were searching for novel downstream targets of Ral by identifying proteins that form a stable complex specifically with the activated GTP-bound form of Ral in cells. HEK-293 cells were transfected with mutant Ral proteins tagged with a Myc epitope. Results obtained with empty-vector-transfected cells were compared with those obtained with both RalA-72L, a constitutively activated GTP-bound form, and RalA-28N, a constitutively inactive form. Ral-binding partners were immunoprecipitated with anti-Myc antibodies, and the immunoprecipitates were separated by SDS electrophoresis. Two major bands appeared in silver-stained gels (Mr of ∼110 and ∼86 kDa) of immunoprecipitates of RalA-72 but not of RalA28N or empty-vector-transfected cells (Fig. 9A). We detected additional minor bands with Mrs between ∼100 and ∼90 kDa that also formed complexes specifically with activated Ral72L (Fig. 9B) when [35S]methionine/cysteine-labeled cells transfected with Ral proteins were used.
FIG. 9.
Coimmunoprecipitation of Sec6/8 (exocyst) complex with active but not inactive RalA. (A) Active Myc-tagged RalA72L (72L), inactive RalA28N (28N), or empty expression vector (V) were transfected into 293 cells. The Ral proteins were immunoprecipitated and then run on SDS-PAGE. The gel was then stained with silver. Bands reproducibly immunoprecipitated only with active RalA72L are marked with arrows. (B) Myc-tagged active RalA72L SAAX, inactive RalA28N SAAX, or empty vector were transfected into 293 cells. (C to S mutants in the CAAX boxes at the C termini of these proteins were used in these experiments, because we found they expressed at higher levels than normal proteins, which allowed us to detect minor coprecipitated bands). The cells were then metabolically labeled with [35S]methionine and cysteine. Ral proteins were then immunoprecipitated, run on gels, and exposed to a PhosphorImager. Bands reproducibly detected only in immunoprecipitates of activated Ral are marked with arrows and dots. Results are representative of experiments performed at least three times. Molecular size markers are indicated on the left. (C) Myc-tagged active RalA72L, inactive RalA28N, RalA72L-36S/37A, RalA72L-49N, or empty vector were transfected and immunoprecipitated as above except that the immunoprecipitates were immunoblotted with α-Sec8 antibodies. Ral protein content in the immunoprecipitates was determined by immunoblotting and is shown below each panel. Ig, immunoglobulin.
Mass spectroscopy was used to identify the 110-kDa band as Sec8 and the 86-kDa band as two proteins, Sec6 and Exo84 (30). These proteins constitute three of eight protein components of the 734-kDa Sec6/8 complex (30), also known as the exocyst in yeast (47). The molecular weights of the fainter staining coprecipitated proteins depicted in Fig. 1B suggest that they represent some of the remaining Sec6/8 components, such as p102, Sec5, and Sec15 (30). We confirmed the specific association of the Sec6/8 complex with active Ral by blotting Ral immunoprecipitates with anti-Sec8 antibodies (Fig. 9C). A mutation in the effector domain of Ral72L(Y36S/D37A) that preserved Ral activation of the Src/Stat signaling cascade in cells (16) (data not shown) suppressed Ral association with Sec8. In contrast, Ral72L(49N), which inhibits Ral binding to another target protein, RalBP1 (6), did not block complex formation between Ral and Sec8. These findings identify the Sec6/8 (exocyst) complex as a potential downstream target of Ral GTPases in cells. Since the Sec6/8 (exocyst) complex in yeast has been shown to be involved in targeting secretory vesicles to specific sites in the plasma membrane, the finding that Sec6/8 is a potential effector of Ral is consistent with the suppression of neurosecretion in the transgenic animals described above.
DISCUSSION
Ral GTPases, RalA and RalB, display many properties expected of a protein that could regulate synaptic vesicle trafficking. They are enriched in the synaptic vesicle fraction of neurons (4), they are known to function as molecular switches much like their relative, Rab3a, and they can be activated by several signaling pathways, including those initiated by Ca2+ transients (22). However, little is known about Ral-dependent regulatory pathways in the nerve terminal.
The phenotype induced by expression of RalA28N in mice was a clear and specific defect in the regulation of neurosecretion. In one set of experiments, while depolarization-induced secretion of glutamate was normal, the ability of phorbol ester treatment to enhance this secretion was suppressed in mutant synaptosomes. Previous studies in both cultured neurons and isolated synaptosomes have shown that this phorbol ester effect is due to PKC-induced increase in the size of the RRP of synaptic vesicles (32, 43). Depolarization-induced calcium influx has also been shown to affect the RRP by enhancing the rate at which it is refilled from the reserve pool (42, 43, 45). In a second set of experiments, direct measurement of the size of the RRP showed that RalA28N expression suppressed its refilling after depletion by K+-induced depolarization. Thus, these findings argue that Ral GTPases participate in the regulation of the readily releasable pool of secretory vesicles by multiple signaling cascades.
Precisely how Ral activity may participate in the regulation of the RRP of vesicles by both PKC and calcium remains to be determined. However, we obtained one clue from the observation that RalA28N expression suppressed both phorbol ester- and calcium-induced enhancement in the phosphorylation of proteins that may participate in synaptic vesicle function, such as SNAP-25, MARCKS, and synapsin. Importantly, there was selectivity in the effects of RalA28N, since phorbol ester-induced ERK and AKT phosphorylation was normal in RalA28N synaptosomes. MARCKS is a well-characterized PKC substrate (2), and synapsin is known to be phosphorylated directly by CaM kinase II (18). Since RalA28N affected the signaling of more than one kinase, it may have influenced their substrates rather than the kinases themselves. Accordingly, Ral may function to target specific substrates to phorbol- and calcium-stimulated kinases. This function for Ral is similar to the one recently proposed following studies showing that Ral promotes c-Src kinase activity toward only a subset of its potential substrates (16).
The inhibition of synapsin phosphorylation by PKC or calcium in RalA28N synaptosomes may be particularly important for the phenotype we observed. Synapsins have been suggested to participate in regulating the distribution of vesicles between the reserve and RRP due to their ability to tether vesicles to the actin cytoskeleton (7). Interestingly, this tethering is removed upon synapsin phosphorylation (23). Moreover, as for RalA28N mice, synapsin I knockout mice display resistance to PKC-mediated increase in the RRP of vesicles (49). Thus, in RalA28N mice the reduced phosphorylation of synapsin observed after exposure to phorbol ester or depolarization may account at least in part for the defect observed in the regulation of the RRP of synaptic vesicles.
Another clue has come from our finding that the active but not inactive form of Ral binds to the Sec6/8 (exocyst) complex in cells. In addition, we found that a mutation in the effector domain of Ral, which is known to participate in signaling to downstream targets, blocks the interaction in vivo. These findings, together with those of a recently published study demonstrating that Ral can bind to the exocyst complex in vitro (5), strongly argue that the exocyst is a newly identified downstream target of Ral proteins. This conclusion is striking because studies with yeast have shown that the function of the exocyst complex is to mark the plasma membrane as a delivery site for exocytic vesicles (24, 47). The eight proteins that make up this complex are Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p, and Exo84p, and mammalian counterparts for each of the proteins have been identified (30). Thus, it is plausible that an analogous function for these complexes in mammalian cells is to regulate the RRP of synaptic vesicles in neurons. In fact, Sec6/8 complexes have been detected in the plasma membrane of synaptosomes (isolated synaptic endings) from adult animals, where it has been suggested that this protein complex has a role in synaptic vesicle docking (5). In a study of developing neurons, Sec6/8 complexes were found at the highest levels in regions of the brain undergoing synaptogenesis and in regions of cultured neurons where synapses will subsequently develop. In contrast, the level of Sec6/8 was downregulated in mature synapses (21). This led to the hypothesis that the main function of the Sec6/8 complex is in formation of synapses rather than in their function once formed (21). However, since there are thought to be only one or two dozen docked vesicles at each central nervous system synapse (44), the level of Sec6/8 required to participate in the modulation of the equilibrium between the reserve pool and the docked pool of synaptic vesicles in mature synapses may be quite low.
Both Ral proteins and their binding partner, the Sec6/8 (exocyst) complex, are present at easily measurable levels in synaptosomes from adult mice (25; unpublished observations). Thus, it is reasonable to speculate that the interaction between Ral, which resides on the cytoplasmic side of vesicles (4), and the Sec6/8 exocyst complex, which resides on the inner surface of the plasma membrane (25), could promote the recruitment of synaptic vesicles from the reserve pool in the cytoplasm to the plasma membrane in response to stimuli like phorbol esters and calcium. The Ral-Sec6/8 complex may also participate in targeting the components of the vesicle, like synapsin to phorbol and calcium-responsive kinases in the plasma membrane, which could explain the defect in synapsin phosphorylation in RalA28N mice described above. Unfortunately, we have been unable to add additional support to this model by detecting a complex between Ral and Sec6/8 proteins in synaptosomes, possibly because the majority of the Sec6/8 complex (25) and a large fraction of Ral is in the detergent-insoluble fraction of synaptosomes (data not shown). This observation is consistent with previous results suggesting that the Sec6/8 complex is associated with cytoskeletal elements associated with the plasma membrane (25). In addition, as described above only a very small fraction (<5%) of synaptic vesicles are docked at any one time at the plasma membrane (46); thus, only a very small fraction of Ral in the synaptic ending would be expected to be bound to the Sec6/8 complex.
While Ral proteins are present in higher eukaryotes, such as mammals, drosophila, and Caenorhabditis elegans, they do not exist in yeast. In yeast, other GTPase family members have been shown to interact with the exocyst complex, such as Rho3 (39), Rho1 (17), and Rab3 (20). Thus, although Ral may play a role in targeting vesicles to the plasma membrane, it is clear that Ral proteins are not needed for the basic processes of polarized exocytosis that yeast perform. Perhaps the appearance of Ral proteins in evolution coincided with the requirement for regulated secretion, allowing extracellular signals (e.g., those that activate PKC or raise Ca2+) to modulate the maximal rate of exocytosis, since refilling of the RRP after depletion is likely to be an important physiological parameter that dictates synaptic strength during bursts of activity.
Regardless of the mechanism underlying Ral effects, the present study implicates the Ral-GTPase signaling cascade in a key aspect of neurosecretion. The size of the RRP of vesicles is an important factor contributing to synaptic strength. For example, the rate of refilling of the RRP, which is normally enhanced by calcium influx, likely determines how extensively synapses fatigue during bursts of action potentials (45). Other signals, such as metabotropic glutamate autoreceptors coupled to phospholipase C enhance the RRP and synaptic strength (32). The transgenic mouse model we have developed has revealed an important role for Ral signaling in both of these signaling systems that modulate neuronal activity. This model may also be useful to study the consequence of suppressing the enhancement of the RRP by calcium and PKC pathways on higher-order neuronal functions that are thought to rely on changes in presynaptic plasticity.
Acknowledgments
This study was supported by a Public Health Service grant from the NIGMS to L.A.F. and DARPA to T.J.T.
Atsuko Polzin and Michail Shipitsin contributed equally to this work.
REFERENCES
- 1.Abeliovich, A., C. Chen, Y. Goda, A. J. Silva, C. F. Stevens, and S. Tonegawa. 1993. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell 75:1253-1262. [DOI] [PubMed] [Google Scholar]
- 2.Aderem, A. 1995. The MARCKS family of protein kinase-C substrates. Biochem. Soc. Trans. 23:587-591. [DOI] [PubMed] [Google Scholar]
- 3.Benfenati, F., F. Valtorta, J. L. Rubenstein, F. S. Gorelick, P. Greengard, and A. J. Czernik. 1992. Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature 359:417-420. [DOI] [PubMed] [Google Scholar]
- 4.Bielinski, D. F., N. Y. Pyun, K. Linko-Stentz, I. Macara, and R. E. Fine. 1993. Ral and Rab3a are major GTP binding proteins of axonal rapid transport vesicles of synaptic vesicles and do not redistribute following depolarization stimulated synaptosomal exocytosis. Biochem. Biophys. Acta 1151:246-256. [DOI] [PubMed] [Google Scholar]
- 5.Brymora, A., V. A. Valova, M. R. Larsen, B. D. Roufogalis, and P. J. Robinson. 2001. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 276:29792-29797. [DOI] [PubMed] [Google Scholar]
- 6.Cantor, S., T. Urano, and L. A. Feig. 1995. Identification and characterization of RalBP1, a potential downstream target of Ral GTPases. Mol. Cell. Biol. 15:4578-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccaldi, P. E., F. Grohovaz, F. Benfenati, E. Chieregatti, P. Greengard, and F. Valtorta. 1995. Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J. Cell Biol. 128:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrunz, L. E., and C. F. Stevens. 1997. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18:995-1008. [DOI] [PubMed] [Google Scholar]
- 9.Dodge, F. A., Jr., and R. Rahamimoff. 1967. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 193:419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exton, J. H. 1997. New developments in phospholipase D. J. Biol. Chem. 272:15579-15582. [DOI] [PubMed] [Google Scholar]
- 11.Feig, L. A. 1999. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1:E25-E27. [DOI] [PubMed]
- 12.Feig, L. A., T. Urano, and S. Cantor. 1996. Evidence for a Ras/Ral signaling cascade. Trends Biochem. Sci. 21:438-441. [DOI] [PubMed] [Google Scholar]
- 13.Forss-Petter, S., P. E. Danielson, S. Catsicas, E. Battenberg, J. Price, M. Nerenberg, and J. G. Sutcliffe. 1990. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron 5:187-197. [DOI] [PubMed] [Google Scholar]
- 14.Geppert, M., and T. C. Sudhof. 1998. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu. Rev. Neurosci. 21:75-95. [DOI] [PubMed] [Google Scholar]
- 15.Gil, C., P. Pelliccioni, E. Itarte, and J. Aguilera. 1999. Differential action of nerve growth factor and phorbol ester TPA on rat synaptosomal PKC isoenzymes. Neurochem. Int. 35:281-291. [DOI] [PubMed] [Google Scholar]
- 16.Goi, T., M. Shipistin, Z. Lu, D. A. Foster, S. Klinz, and L. A. Feig. 2000. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. EMBO J. 19:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo, W., F. Tamanoi, and P. Novick. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353-360. [DOI] [PubMed] [Google Scholar]
- 18.Hanson, P. I., and H. Schulman. 1992. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem. 61:559-601. [DOI] [PubMed] [Google Scholar]
- 19.Harrison, S. M., P. E. Jarvie, and P. R. Dunkley. 1988. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: viability of subcellular fractions. Brain Res. 441:72-80. [DOI] [PubMed] [Google Scholar]
- 20.Harsay, E., and A. Bretscher. 1995. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131:297-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazuka, C. D., D. L. Foletti, S. C. Hsu, Y. Kee, F. W. Hopf, and R. H. Scheller. 1999. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 19:1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer, F., R. Berdeaux, and G. S. Martin. 1998. Ras-independent activation of Ral by a Ca(2+)-dependent pathway. Curr. Biol. 14:839-842. [DOI] [PubMed] [Google Scholar]
- 23.Hosaka, M., R. E. Hammer, and T. C. Sudhof. 1999. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 24:377-387. [DOI] [PubMed] [Google Scholar]
- 24.Hsu, S. C., C. D. Hazuka, D. L. Foletti, and R. H. Scheller. 1999. Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol. 9:150-153. [DOI] [PubMed] [Google Scholar]
- 25.Hsu, S. C., A. E. Ting, C. D. Hazuka, S. Davanger, J. W. Kenny, Y. Kee, and R. H. Scheller. 1996. The mammalian brain rsec6/8 complex. Neuron 17:1209-1219. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, H., J. Q. Luo, T. Urano, P. Frankel, Z. Lu, D. A. Foster, and L. A. Feig. 1995. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature 378:409-412. [DOI] [PubMed] [Google Scholar]
- 27.Jullien-Flores, V., O. Dorseuil, F. Romero, F. Letourneur, S. Saragosti, R. Berger, A. Tavitian, G. Gacon, and J. H. Camonis. 1995. Bridging Ral GTPase to Rho pathways. J. Biol. Chem. 270:22473-22477. [DOI] [PubMed] [Google Scholar]
- 28.Jullien-Flores, V., Y. Mahe, G. Mirey, C. Leprince, B. Meunier-Bisceuil, A. Sorkin, and J. H. Camonis. 2000. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J. Cell Sci. 113:2837-2844. [DOI] [PubMed] [Google Scholar]
- 29.Kariya, K., S. Koyama, S. Nakashima, T. Oshiro, K. Morinaka, and A. Kikuchi. 2000. Regulation of complex formation of POB1/Epsin/AP-2 by mitotic phosphorylation. J. Biol. Chem. 275:18399-18406. [DOI] [PubMed] [Google Scholar]
- 30.Kee, Y., J. S. Yoo, C. D. Hazuka, K. E. Peterson, S. C. Hsu, and R. H. Scheller. 1997. Subunit structure of the mammalian exocyst complex. Proc. Natl. Acad. Sci. USA 94:14438-14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llinas, R., J. A. Gruner, M. Sugimori, T. L. McGuinness, and P. Greengard. 1991. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J. Physiol. 436:257-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonart, G., and T. C. Sudhof. 2000. Assembly of SNARE core complexes prior to neurotransmitter release sets the readily releasable pool of synaptic vesicles. J. Biol. Chem. 275:27703-27707. [PubMed] [Google Scholar]
- 33.Luo, J. Q., X. Liu, S. M. Hammond, W. C. Colley, L. A. Feig, M. A. Frohman, A. J. Morris, and D. A. Foster. 1997. RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1. Biochem. Biophys. Res. Commun. 235:854-859. [DOI] [PubMed] [Google Scholar]
- 34.Mark, B. L., O. Jilkina, and R. P. Bhullar. 1996. Association of Ral GTP-binding protein with human platelet dense granules. Biochem. Biophys. Res. Commun. 225:40-46. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima, S., K. Morinaka, S. Koyama, M. Ikeda, M. Kishida, K. Okawa, A. Iwamatsu, S. Kishida, and A. Kikuchi. 1999. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 18:3629-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngsee, J. K., L. A. Elferink, and R. H. Scheller. 1991. A family of Ras-like GTP-binding proteins expressed in electromotor neurons. J. Biol. Chem. 266:2675-2680. [PubMed] [Google Scholar]
- 37.Park, J. B. 2001. Regulation of GTP-binding state in RalA through Ca2+ and calmodulin. Exp. Mol. Med. 33:54-58. [DOI] [PubMed] [Google Scholar]
- 38.Park, S. H., and R. A. Weinberg. 1995. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene 11:2349-2355. [PubMed] [Google Scholar]
- 39.Robinson, N. G., L. Guo, J. Imai, E. A. Toh, Y. Matsui, and F. Tamanoi. 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19:3580-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenmund, C., and C. F. Stevens. 1996. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16:1197-1207. [DOI] [PubMed] [Google Scholar]
- 41.Sarri, E., I. Bockmann, U. Kempter, A. Valeva, C. von Eichel-Streiber, O. Weichel, and J. Klein. 1998. Regulation of phospholipase D activity in synaptosomes permeabilized with Staphylococcus aureus alpha-toxin. FEBS Lett. 440:287-290. [DOI] [PubMed] [Google Scholar]
- 42.Smith, C., T. Moser, T. Xu, and E. Neher. 1998. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron 20:1243-1253. [DOI] [PubMed] [Google Scholar]
- 43.Stevens, C. F., and J. M. Sullivan. 1998. Regulation of the readily releasable vesicle pool by protein kinase C. Neuron 21:885-893. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, C. F., and T. Tsujimoto. 1995. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc. Natl. Acad. Sci. USA 92:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens, C. F., and J. F. Wesseling. 1998. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron 21:415-424. [DOI] [PubMed] [Google Scholar]
- 46.Sudhof, T. C. 2000. The synaptic vesicle cycle revisited. Neuron 28:317-320. [DOI] [PubMed] [Google Scholar]
- 47.TerBush, D. R., T. Maurice, D. Roth, and P. Novick. 1996. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483-6494. [PMC free article] [PubMed] [Google Scholar]
- 48.Turner, T. J., and K. Dunlap. 1995. Pharmacological characterization of presynaptic calcium channels using subsecond biochemical measurements of synaptosomal neurosecretion. Neuropharmacology 34:1469-1478. [DOI] [PubMed] [Google Scholar]
- 49.Walaas, S. I., S. Hilfiker, L. Li, L. S. Chin, and P. Greengard. 2000. Decrease in phorbol ester-induced potentiation of noradrenaline release in synapsin I-deficient mice. Synapse 36:114-119. [DOI] [PubMed] [Google Scholar]
- 50.Wolthuis, R. M., N. D. de Ruiter, R. H. Cool, and J. L. Bos. 1997. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 16:6748-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi, A., T. Urano, T. Goi, and L. A. Feig. 1997. An Eps homology (EH) domain protein that binds to the Ral-GTPase target, RalBP1. J. Biol. Chem 272:31230-31234. [DOI] [PubMed] [Google Scholar]