Abstract
The double-stranded RNA-dependent protein kinase (PKR) is one of the four mammalian kinases that phosphorylates the translation initiation factor 2α in response to virus infection. This kinase is induced by interferon and activated by double-stranded RNA (dsRNA). Phosphorylation of eukaryotic initiation factor 2α (eIF2α) blocks translation initiation of both cellular and viral mRNA, inhibiting virus replication. To counteract this effect, most viruses express inhibitors that prevent PKR activation in infected cells. Here we report that PKR is highly activated following infection with alphaviruses Sindbis (SV) and Semliki Forest virus (SFV), leading to the almost complete phosphorylation of eIF2α. Notably, subgenomic SV 26S mRNA is translated efficiently in the presence of phosphorylated eIF2α. This modification of eIF2 does not restrict viral replication; SV 26S mRNA initiates translation with canonical methionine in the presence of high levels of phosphorylated eIF2α. Genetic and biochemical data showed a highly stable RNA hairpin loop located downstream of the AUG initiator codon that is necessary to provide translational resistance to eIF2α phosphorylation. This structure can stall the ribosomes on the correct site to initiate translation of SV 26S mRNA, thus bypassing the requirement for a functional eIF2. Our findings show the existence of an alternative way to locate the ribosomes on the initiation codon of mRNA that is exploited by a family of viruses to counteract the antiviral effect of PKR.
Keywords: Translation, eIF2, eIF2A, PKR, alphaviruses, antiviral response
The activity of eukaryotic initiation factor 2 (eIF2) is a key target in the overall control of protein synthesis in mammalian cells (Dever 2002). eIF2 is an oligomer composed of three subunits (α, β, γ) that interact with GTP and initiator methionyl-tRNA (Met-tRNAi). This ternary complex associates with the small 40S ribosomal subunits to form the 43S initiation complex (Hershey 1991; Pestova et al. 2001). According to the scanning model, the 43S complex binds to the 5′ end of mRNA through the interaction of the cap-binding complex (eIF4F). The resulting 48S complex moves downstream to reach the first AUG in an appropriate context (Kozak 1980; Gingras et al. 1999). Once positioned on the initiation codon (AUGi), the 60S subunit joins to the small ribosomal subunit to form the 80S ribosome; concomitantly, eIF2 is released following GTP hydrolysis. The eIF2-GDP complex is continuously recycled by GDP–GTP exchange in a process catalyzed by eIF-2B (Yang and Hinnebusch 1996; Kimball et al. 1998; Kimball 1999). The function of eIF2 in protein synthesis is thus the delivery of Met-tRNAi to the P ribosomal site to initiate protein synthesis starting at AUGi. Recent data also indicate that initiation factors 1 and 1A are required for correct ribosome location on the initiation codons (Pestova et al. 1998).
Several stress signals induce transient inactivation of eIF2α by phosphorylation, leading to a general down-regulation of protein synthesis, accompanied by the activation of genes implicated in stress response (Harding et al. 2000; Dever 2002). Four different kinases regulate eIF2 activity in response to specific environmental stresses in mammalian cells: HRI, RNA-dependent protein kinase (PKR), GCN2, and PERK (de Haro et al. 1996; Dever 2002). These kinases catalyze the phosphorylation of eIF2α at Ser 51; phosphorylated eIF2-GDP binds eIF2B in an irreversible manner, thus preventing the regeneration of active eIF2-GDP, which results in a general inhibition of protein synthesis (Sudhakar et al. 2000). Translation directed by certain cellular and viral mRNAs is nonetheless induced by eIF2α phosphorylation. The best-illustrated examples are the expression of three genes involved in the response to nutrient deprivation: yeast GCN4, and ATF4 and Cat-1 in mammalian cells (Mueller and Hinnebusch 1986; Harding et al. 2000; Yaman et al. 2003; Vattem and Wek 2004). In these three cases, eIF2α phosphorylation may promote leaky scanning of ribosomes through the small open reading frames (uORF) at the 5′ leader sequence of these mRNAs to initiate translation at the downstream bona fide AUG codon (Dever 2002). One of the most striking cases of eIF2 independence for initiation of protein synthesis is the IRES-driven translation of the second cistron of the cricket paralysis virus (CrPV) genomic RNA. This cistron directs incorporation of the first amino acid (Ala), rather than the canonical methionine, into the A ribosomal site (Wilson et al. 2000).
The double-stranded RNA (dsRNA)-activated PKR has been implicated in antiviral defense due to its ability to respond to viral infection. PKR binds to and is activated by double-stranded RNA, a molecule usually generated during replication and transcription of viral genomes. eIF2α phosphorylation by PKR leads to inhibition of translation, blocking viral replication (Meurs et al. 1990; Manche et al. 1992; Gunnery and Mathews 1998; Williams 1999). A large body of evidence supports the idea that PKR activity is intimately linked to the antiviral effect of interferons (IFN) (Stark et al. 1998). PKR expression is induced by type I IFN, and PKR-deficient mice are not protected against several animal viruses, lacking the antiviral response after IFNγ priming (Yang et al. 1995; Balachandran et al. 2000; Stojdl et al. 2000). The importance of PKR in antiviral defense is further supported by the majority of animal viruses, which have evolved diverse strategies to prevent PKR activation in infected cells (Kaufman 1999). PKR is thus rapidly degraded in picornavirus-infected cells (Black et al. 1993), whereas other animal viruses encode proteins that directly or indirectly block PKR activation. Some of these viral proteins, such as influenza (FLU) NS1, vaccinia E3L, or reovirus σ3, are able to sequester the dsRNA generated in the infected cells (Carroll et al. 1993; Davies et al. 1993; Lu et al. 1995; Yue and Shatkin 1997; Bergmann et al. 2000). Other viral products, such as adenovirus VAI RNA or HCV NS5A and E2 proteins, appear to prevent PKR activation by direct binding to the kinase (Kitajewski et al. 1986; Gale et al. 1998; Taylor et al. 1999). On the contrary, HSV-1 expresses a gene that promotes dephosphorylation of eIF2α by activating cell phosphatase PP1α (He et al. 1997). In contrast to these strategies followed by most animal viruses, we describe that alphavirus (Sindbis and Semliki forest virus) infection induces strong PKR activation, which results in almost complete phosphorylation of eIF2α. Notably, translation of alphavirus 26S mRNA takes place efficiently in the presence of phosphorylated eIF2α. Our findings support a novel model for the initiation of translation, in which eIF2 activity appears dispensable. This represents a new strategy, used by this group of viruses to overcome the antiviral effect of PKR.
Results
PKR activation and eIF2α phosphorylation in sindbis (SV)-infected cells
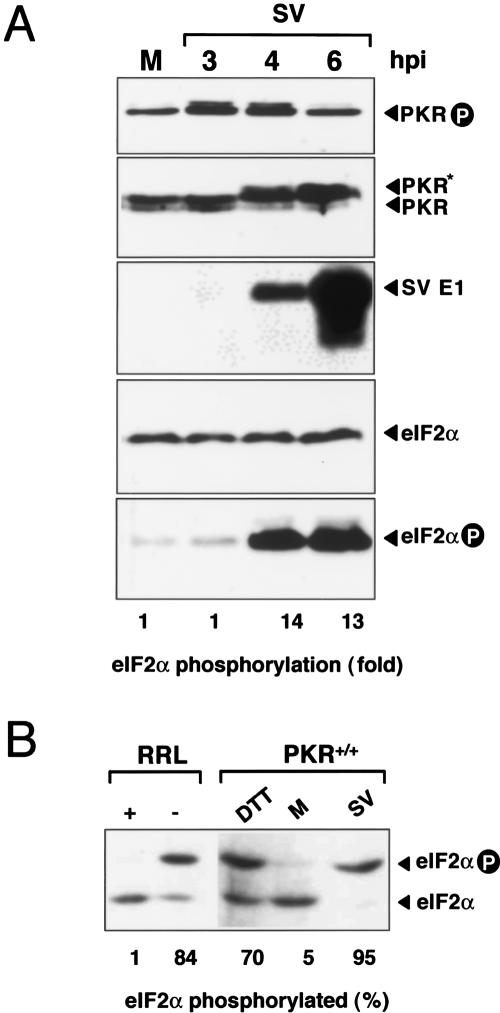
Alphaviruses are a group of positive single-stranded RNA (ssRNA) viruses that infect several invertebrate and mammalian hosts. After uncoating, genomic 49S RNA is translated to produce the nonstructural proteins, involved in the synthesis of viral genomes and subgenomic 26S mRNA. Beginning at ∼3–5 h post-infection (hpi), subgenomic mRNAs are efficiently synthesized and translated, generating the precursors of structural proteins (p130) that are proteolytically processed to the mature virion structural proteins (Strauss and Strauss 1994). During the course of our experiments, we observed that translation of subgenomic 26S mRNA proceeded at very high rates in the presence of phosphorylated eIF2α in SV-infected cells. To examine this in detail, we analyzed the time course of eIF2α phosphorylation and protein synthesis in SV-infected 3T3 cells. Extensive phosphorylation of eIF2α was already apparent at 4 hpi, and did not increase further with time (Fig. 1A). Using a phosphospecific antibody, we estimated that the level of phosphorylated eIF2α increased 10- to 15-fold in SV-infected cells at 4–6 hpi compared with mock-infected cells. Despite this, viral structural proteins were able to accumulate in infected cells at a very high rate (Fig. 1A). Western blotting of total or Thr 451 phosphospecific PKR forms showed strong activation of the kinase following infection. The level of phosphorylated PKR at residue Thr 451 increased early in time (3 h), diminishing to basal levels at 5–6 hpi. This transient increase precedes the change in electrophoretic mobility observed for PKR at 3–4 hpi, indicative of extensive autophosphorylation and activation (Gorchakov et al. 2004).
Figure 1.
PKR activation and eIF2α phosphorylation in SV-infected cells. (A) 3T3 cells were infected with SV at an MOI of 25 PFU/cell. At the indicated times, cell extracts were made and analyzed by Western blot using the indicated antibodies. Bands corresponding to viral structural proteins E1, PKR, phospho-PKR, eIF2α, and phopho-eIF2α are shown. (Lower panel) The ratio of phosphorylated versus total eIF2α was estimated by densitometry of corresponding bands. (B) IEF analysis of eIF2α phosphorylation. 3T3 cells were SV-infected (4 h) or treated with 1 mM DTT (1 h), then analyzed by IEF (see Materials and Methods). Mock-infected cells (M) were included, as well as RRL-treated (+) or untreated (–), with hemine and EDTA as negative and positive controls of eIF2α phosphorylation, respectively. Phosphorylated and unphosphorylated eIF2α forms were quantified by densitometry.
To better quantify the percentage of eIF2α that became phosphorylated upon SV infection, IEF analysis of protein extracts was performed. We found that virtually all eIF2α (>95%) was phosphorylated in SV-infected cells at 4 hpi (Fig. 1B). Similar results were obtained using MEF (data not shown).
Translation of subgenomic 26S mRNA is resistant to eIF2α phosphorylation as compared with genomic RNA and reporter (EGFP) mRNA
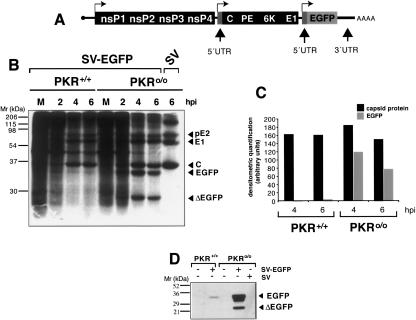
Two possibilities were considered to explain how 26S mRNA translation proceeds in the presence of phosphorylated eIF2α. SV could express a protein that replaces eIF2 function in trans, or SV 26S mRNA does not require eIF2 to initiate translation. To distinguish between these options, we engineered a recombinant SV expressing the EGFP gene under the control of a second subgenomic promoter (Fig. 2A; Levis et al. 1990). The recombinant SV simultaneously expresses RNA from the genuine subgenomic promoter, 26S mRNA, and from a duplicate subgenomic promoter, EGFP mRNA (Fig. 2A). Moreover, EGFP mRNA contains the 5′ and 3′ UTRs present in SV 26S mRNA. Notably, EGFP-encoding mRNA was efficiently translated in PKR0/0, but not in PKR+/+ cells. In addition to the 33-kDa band corresponding to whole EGFP, we also detected a smaller 26-kDa band that reacted with anti-EGFP antibodies (Fig. 2B,D); this band may correspond to a proteolyzed form of EGFP or to internal initiation of translation. Densitometric analysis of the 33-kDa band showed that translation of EGFP mRNA was inhibited >25-fold in PKR+/+ compared with PKR0/0 cells, whereas translation of 26S subgenomic mRNA was similar in both cell types (Fig. 2C). The results indicate that SV 26S mRNA is endowed with specific features to initiate translation in the presence of phosphorylated eIF2α.
Figure 2.
SV 26S mRNA is specifically translated in PKR+/+ cells. (A) Scheme of recombinant SV expressing the EGFP gene under a second subgenomic promoter. Note that EGFP mRNA also contains the natural 5′ and 3′ UTRs present in 26S mRNA. Arrows indicate transcription initiation sites. (B) PKR+/+ and PKR0/0 cells were infected with SV-EGFP virus and metabolically labeled at indicated times. Bands corresponding to EGFP and a truncated form of the protein (ΔEGFP) are marked (see text for explanation). (C) Comparative analysis of EGFP versus SV C protein levels synthesized in PKR+/+ and PKR0/0 cells. Protein bands from the film shown in B were quantified by densitometry and plotted in arbitrary units. (D) Western blot analysis of EGFP accumulated in PKR+/+ and PKR0/0 cells. The blot was probed with a monoclonal anti-EGFP antibody (Clontech).
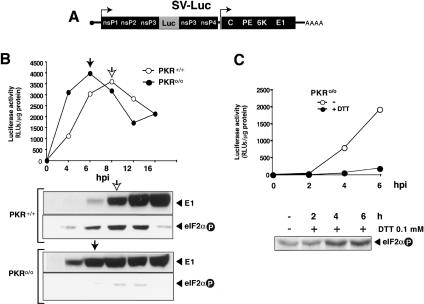
Since translation of alphavirus genomic RNA takes place after virus uncoating and precedes the synthesis of structural proteins, we tested the resistance of genomic RNA translation to eIF2α phosphorylation. To analyze the time course of translation directed from genomic and subgenomic RNA in SV-infected cells, we used a recombinant SV expressing the luciferase gene as part of a nonstructural polyprotein precursor (SV-luc) (Fig. 3A). Luciferase activity thus directly reports translation of genomic RNA. It should be noted, however, that this recombinant virus has a delayed replication cycle compared with the wild type. In SV-Luc-infected PKR+/+ cells, luciferase activity increased until 7–8 hpi, although it slowly decreased at later times (Fig. 3B). Considering the estimated half-life for luciferase activity in infected cells (3 h), it appears that genomic RNA stops translation at 6–7 hpi. Notably, the late glycoprotein E1 began to accumulate when translation of genomic RNA had ceased. These data support the concept that translation of genomic and subgenomic RNA is subject to a strict temporal regulation. Luciferase activity increased more rapidly in SV-luc-infected PKR0/0 cells than in PKR+/+ cells, reaching maximal activity at 4–5 hpi. SV structural proteins consequently appeared earlier in the infection in PKR0/0 cells. These findings agree well with the results described above (Fig. 2) and suggest that translation of genomic SV RNA is improved in PKR0/0 cells. The bulk of eIF2α phosphorylation took placed immediately on termination of genomic RNA translation, and correlated with the appearance of structural proteins (Fig. 3B). Since translation of genomic RNA precedes eIF2α phosphorylation, we analyzed the effect of this modification on translation of genomic RNA. SV-infected PKR0/0 cells were incubated with low concentrations of DTT (0.1 mM) from 0 hpi. This treatment induced a slight increase in eIF2α phosphorylation from 1 hpi, as well as a drastic inhibition of luciferase activity (Fig. 3C). These data suggest that, unlike 26S mRNA, translation of genomic RNA is very sensitive to eIF2α phosphorylation.
Figure 3.
Premature phosphorylation of eIF2α blocks translation of genomic SV RNA. (A) Scheme of recombinant SV expressing luciferase from the genomic RNA. (B) Timing of eIF2α phosphorylation and translation of genomic and subgenomic 26S mRNA in PKR+/+ and PKR0/0 cells. Cells were infected with Toto-Luc1101 virus (MOI: 10 PFU/cell), and extracts were prepared at the times indicated. Luciferase activity was measured and used to quantitate translation from genomic SV RNA. Arrows indicate the time at which genomic RNA stopped translating. Translation from subgenomic mRNA was measured by Western blot of viral glycoprotein E1. (C) Effect of premature eIF2α phosphorylation on SV genomic RNA translation. PKR0/0 cells were infected with TotoLuc1101 and treated with 0.1 mM DTT from 0 hpi. At the times indicated, luciferase activity and the phosphorylation status of eIF2α were measured in extracts.
Initiation of SV 26S mRNA translation with Met in the presence of phosphorylated eIF2α
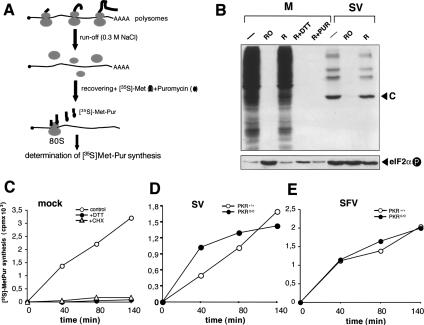
It was of interest to analyze whether SV 26S mRNA was able to initiate translation at the initiator AUG codon in the virtual absence of a functional ternary complex. We developed a protocol to measure the synthesis of methionyl-puromycin (Met-Pur) catalyzed by the 80S initiation complex directed by SV and Semliki Forest virus (SFV) 26S mRNA. Infected cells were first treated with hypertonic medium to induce polysome run-off, followed by a recovery period in normal medium to allow reassembly of the 80S initiation complex in the presence of puromycin and [35S]-Met (Fig. 4A). Since infected cells exclusively translate 26S mRNA from 3 to 4 hpi, [35S]-Met-Pur synthesis after polysome run-off reflects translation initiation of viral mRNAs. During hypertonic shock, translation was completely inhibited in both mock and infected cells, recovering to control levels after 1 h of incubation in normal medium (Fig. 4B). Strikingly, hypertonic medium induced strong but reversible eIF2α phosphorylation, as described for yeast (Goossens et al. 2001). [35S]-Met-Pur synthesis increased progressively during the recovery period in control cells (Fig. 4C). As a control of [35S]-Met-Pur synthesis, we used cycloheximide (CHX), another inhibitor of the elongation step of protein synthesis, which does not form a peptidyl bond with Met. No radioactivity was recovered in the organic phase of CHX-treated cells, validating the experimental protocol. Notably, [35S]-Met-Pur synthesis was blocked in cells treated with DTT during the recovery period, indicating that functional eIF2 is required to reinitiate translation of cellular mRNA. In SV- or SFV-infected cells, progressive [35S]-Met-Pur accumulation was found during the recovery period at levels comparable to those in uninfected cells, despite massive eIF2α phosphorylation. Moreover, only small differences in [35S]-Met-Pur synthesis were observed between SV- or SFV-infected PKR+/+ and PKR0/0 cells (Fig. 4D,E). These data suggest that SV and SFV 26S mRNAs initiate translation with methionine in the presence of high levels of phosphorylated eIF2α. To confirm this, we carried out sequence determination of the N-terminal tryptic peptide of capsid proteins from SV and SFV virions synthesized in PKR+/+ and PKR0/0 cells. The N-terminal sequence, acetyl-MNYIPTQTFYGR, was identical for SFV capsid protein synthesized in PKR+/+ and PKR0/0 cells (see Supplementary Fig. S1). We were unable to determine the N-terminal peptide sequence for the SV capsid protein due to the presence of Arg at position 3, which yields a tryptic peptide too small for MALDI-TOF detection. N-terminal blockade by acetylation in SV and SFV capsids did not permit direct sequencing of the N terminus of capsid protein by Edman degradation. Finally, TLC chromatographic analysis was carried out of [35S]Met-labeled tryptic peptides of capsid protein synthesized in PKR+/+ and PKR0/0 cells. The pattern of [35S]Met-labeled fragments for SV and SFV capsid proteins was identical in PKR+/+ and PKR0/0 cells (see Supplemental Material). Altogether, the data show that in the presence of phosphorylated eIF2α, SV and SFV are able to initiate translation of subgenomic mRNA with Met.
Figure 4.
SV and SFV 26S mRNA initiate translation with Met in the presence of phosphorylated eIF2α.(A) Schematic overview of in vivo Met-Pur ([35S]Met-Pur) synthesis assay (see Materials and Methods for details). (B) Effect of different treatments on protein synthesis and eIF2α phosphorylation in mock- and SV-infected cells (MOI: 25 PFU/cell). (–) Untreated cells; (RO) cells treated with hypertonic medium (polysome run-off) for 40 min; (R) translation recovered in normal medium for 1 h after polysome run-off; (R + DTT) translation recovered in presence of 0.5 mM DTT; (R + Pur) translation recovered in presence of 50 μg/mL puromycin. In SV-infected cells, polysome run-off was initiated at 3.5 hpi followed by 1 h of recovery in normal medium. (Upper panel) SDS-PAGE followed by autoradiography of [35S]-labeled proteins. (Lower panel) Western blot for phosphorylated eIF2α. (C) [35S]Met-Pur synthesis in uninfected cells recovered in normal medium for 40, 80, and 140 min after polysome run-off (control). (+DTT) Recovery in 0.5 mM DTT; (CHX) recovery in 50 μg/mL CHX instead of puromycin. [35S]Met/Pur synthesis in PKR+/+ and PKR0/0 cells infected with SV (D) or SFV (E). All experiments were performed in parallel. (M) Mock-infected cells.
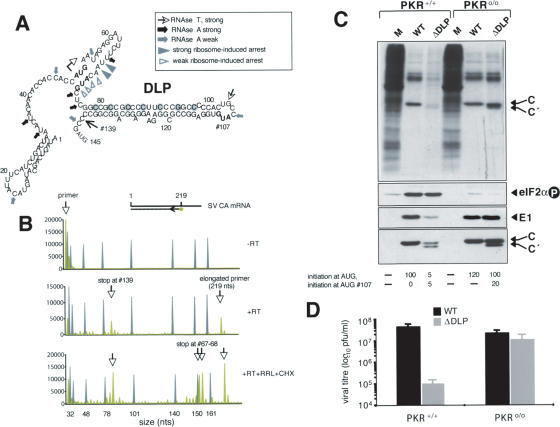
A hairpin loop RNA structure (DLP) downstream of AUGi promotes translational resistance of SV 26S mRNA to eIF2α phosphorylation
We attempted to determine how 26S mRNA initiates protein synthesis in the presence of phosphorylated eIF2α. Since our data (Fig. 2) ruled out the involvement of 5′ or 3′ UTR regions in translational resistance to eIF2α phosphorylation, we considered that sequences within the SV 26S mRNA-coding region might promote eIF2-independent translation. Previous works by Frolov and Schlesinger (1994b, 1996) showed that the first 180 nucleotides (nt) of 26S mRNA act as a translational enhancer of subgenomic mRNA in SV-infected cells. This region includes the 50-nt 5′ UTR, followed by 130 nt corresponding to the capsid protein-coding sequence. Site-directed mutagenesis revealed a DLP involved in the translation, located downstream of the initiation codon (AUGi + 50) (Frolov and Schlesinger 1996). This loop encompasses nucleotides 77–139, containing an extensive G-C pairing stretch that could form a very stable structure (ΔG° = –45 kcal/mol). The existence of this loop was confirmed by enzymatic probing using RNAses followed by primer extension analysis of the fragments generated (Fig. 5A). The RNA sequences encompassing nucleotides 77–102 and 109–139, predicted to form the dsRNA stretch of DLP, were resistant to single-strand-specific RNAse A and T1, whereas the loop itself was sensitive to these enzymes. In addition, primer extension detected a premature elongation halt of RT at 26S mRNA nucleotide 139, corresponding to the 3′ base of the hairpin loop (Fig. 5A). We also analyzed ribosomal initiation complex formation by primer extension using RRL programmed with SV-CA mRNA in the presence of CHX. This showed two major toeprints at positions U67 and U68 and four minor toeprints at A69, C70, U72, and G73 (Fig. 7B, below). The data indicate that 80S initiation complexes immobilized on SV CA mRNA in the presence of CHX protected 18–19 nt 3′ from the AUGi (where A is +1), concurring with results reported for other mRNAs (Pestova and Hellen 2003). Furthermore, given the significant protection observed at G73, our data suggest that the leading edge of the 80S complex could be extended a few nucleotides downstream of the AUGi.
Figure 5.
Analysis of DLP in SV 26S mRNA. Effect of disruption on viral translation and virus replication in PKR+/+ and PKR0/0 cells. (A) Structural analysis of a DLP of 26S mRNA. The MFold program secondary structure prediction for the first 145 nt of SV 26S mRNA is shown. The initiation codon (bold) is marked with a starting arrow; data from enzymatic probing are indicated (arrows). In-frame AUG at nucleotides 71 and 107 are shown in bold. The RT elongation arrest position at nucleotide 139 is marked. Toeprints generated by ribosome attachment to mRNA are indicated (arrowheads). The nucleotides replaced in ΔDLP virus are circled in gray. (B) Fluorochrome-based toeprinting analysis of 80S bound to SV CA mRNA. Negative control of reaction without RT addition (upper panel), primer extensions generated by RT addition (middle panel), and results after programming translation in RRL with SV CA mRNA in the presence of CHX, followed by RT addition (lower panel). Numbers indicate the position from the 5′ end at which primer extension was arrested (green lines). DNA weight markers are in gray. (C) Analysis of protein synthesis in PKR+/+ and PKR0/0 cells infected with wild-type or ΔDLP viruses (MOI: 25 PFU/cell). (Upper panels) Autoradiography of cell extracts metabolically labeled with [35S]Met/Cys at 5 hpi. (Middle panels) Western blot analysis of extracts using SV E1, SV capsid, and phospho eIF2α antibody, respectively. The initiation from AUGi and AUG#107 was quantified by densitometry of capsid bands and expressed in arbitrary units. (D) Replication of wild-type and ΔDLP mutant viruses in PKR+/+ and PKR0/0 cells. Cells were infected with the indicated viruses (MOI: 0.1 PFU/cell) and viral yields at 24 hpi were titrated on PKR0/0 cells.
Figure 7.
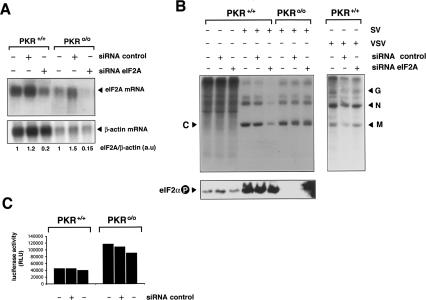
Silencing of eIF2A expression inhibits translation of SV 26S mRNA in PKR+/+, but not in PKR0/0 cells. The indicated cell type was transfected with eIF2A-specific or control siRNAs as described in Materials and Methods. (A) Fifty hours later, poly(A)+ mRNAs were isolated from cells and subjected to Northern blot analysis using a murine eIF2A probe. The ∼2-kb eIF2A transcript is indicated. The blot was also probed with a β-actin probe as a loading control. The level of eIF2A mRNA was quantified by densitometry and corrected by the amount of β-actin mRNA detected in each sample. The amount of eIF2A mRNA with respect to β-actin mRNA found in PKR+/+ and PKR0/0 cells was arbitrarily assigned as 1 a.u., arbitrary units. (B) Effect of eIF2A silencing on SV 26S mRNA translation. Cells transfected with the indicated siRNA were infected with SV or VSV at an MOI of 25 PFU/cell. (Upper panel) Six hours later, cells were pulsed with [35S]Met-Cys for 30 min and analyzed by SDS-PAGE followed by autoradiography. (Lower panel) Analysis of eIF2α phosphorylation in these samples is also shown. (C) Silencing of eIF2A does not affect translation of genomic SV mRNA. Transfected cells were infected with recombinant SV-Luc, and the luciferase activity of cell extracts was assayed 6 hpi.
To test the role of DLP in translation initiation of 26S mRNA, we engineered an SV mutant lacking the DLP. This was achieved by changing C (or G) residues to A to destroy the G-C pairings of the loop, with no effect on the coding sequence except a conservative Leu-to-Phe change at position 14 of the capsid protein. RNA folding programs predicted no stable structures for viral RNA lacking the DLP. RNA from wild-type or ΔDLP cDNA was electroporated in BHK-21 cells, and the resulting viruses were amplified in these cells to obtain high-titer stocks. SV ΔDLP virus was viable, although the viral yield was 10-fold less than wild-type SV in BHK-21 cells. We compared protein synthesis of these viruses in PKR+/+ and PKR0/0 cells; notably, translation of ΔDLP 26S mRNA was impaired in PKR+/+, but not in PKR0/0 cells (Fig. 5C). Consequently, replication of ΔDLP virus was greatly diminished in PKR+/+ cells, giving ∼2 log less progeny than wild type. On the contrary, ΔDLP virus replicated at similar levels to SV wild type in PKR0/0 cells (Fig. 5D). We found that host translation shut-off occurred in SV ΔDLP-infected cells, suggesting that the initial steps of viral replication took place in these cells. We detected a short form of capsid protein (C′) in cells infected with ΔDLP virus, probably generated by initiation at a downstream in-frame AUG. This shorter capsid protein form has the same electrophoretic mobility as that synthesized by an SV mutant lacking the first two AUG codons (data not shown). C′ thus appears to initiate at the third AUG, 107. Together, these results suggest that DLP integrity contributes to efficient, accurate translation initiation in the absence of functional eIF2α.
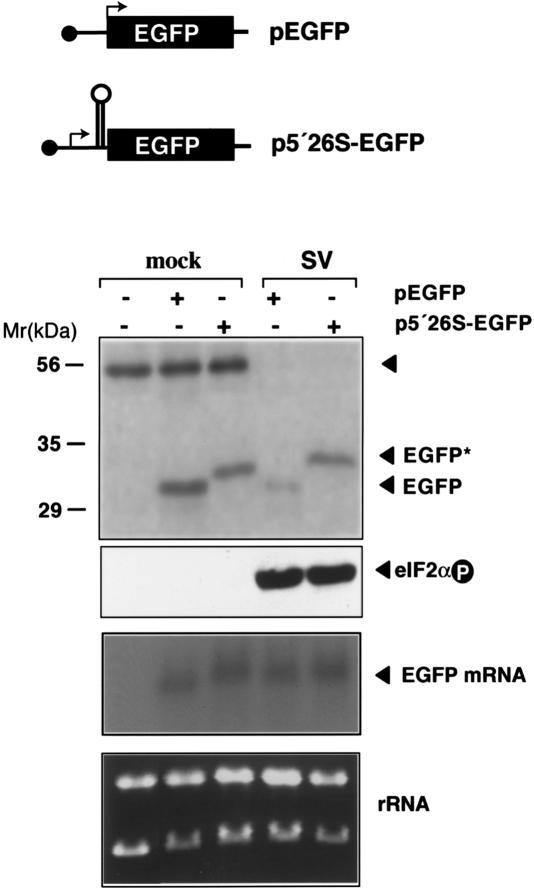
To analyze whether the 5′ end of SV 26S mRNA confers translational resistance to eIF2α phosphorylation, we examined the translation of a hybrid mRNA containing the first 140 nt of SV 26S mRNA followed by the EGFP sequence. The resulting construct (p5′26S-EGFP) expresses a protein with the first 31 amino acids of the SV capsid protein fused to EGFP. As predicted, the hybrid protein shows delayed electrophoretic mobility compared with EGFP alone (Fig. 6). To test the effect of eIF2α phosphorylation on translation of mRNA derived from pEGFP and p5′26S-EGFP constructs, BHK-21 cells were transfected with the plasmids, then infected with SV. EGFP synthesis was estimated by immunoprecipitation (Fig. 6). The presence of 140 nt of SV 26S mRNA had little effect on EGFP translation in mock-infected cells. Nonetheless, in SV-infected cells with phosphorylated eIF2α, translation of p5′26S-EGFP mRNA resisted inhibition, whereas translation of EGFP alone was greatly reduced. These data show that the first 140 nt of SV 26S mRNA were sufficient to confer translational resistance to eIF2α phosphorylation.
Figure 6.
Translation resistance to eIF2α phosphorylation promoted by the 5′ extreme of SV 26S mRNA. Diagram of EGFP constructs. p5′26S-EGFP contains the first 140 nt of SV before the EGFP-coding sequence. Arrows indicate translation initiation sites. BHK-21 cells were transfected with 2 μg of the indicated plasmids using JetPEI (Poly-Plus Transfection) and infected (SV) or not (mock) 48 h later with SV (MOI: 25 PFU/cell). (Upper panel) At 5 hpi, cells were labeled with [35S]Met/Cys (1 h) and immunoprecipitated with anti-EFGP antibodies. The autoradiogram of labeled products is shown. The protein band that cross-precipitated with anti-EGFP antibodies probably corresponds to actin and serves as an internal control. Western blot analysis of eIF2α phosphorylation and Northern blot analysis of EGFP mRNA levels are also shown (middle panel), as well as ethidium bromide staining of total RNA loaded in each sample (bottom panel). For Northern blot analysis, the membrane was probed with a 32P-labeled DNA fragment corresponding to the first 600 nt of the EGFP gene.
Evidence that initiation factor 2A is involved in translation initiation of SV 26S mRNA in the absence of functional eIF2
The data presented above raised the question as to how ribosomes incorporate the first methionine on initiation complex of SV subgenomic mRNA. We tested the possibility that eukaryotic initiation factor 2A (eIF2A) could act by delivering the Met-tRNAi on initiation complex containing SV 26S mRNA in the absence of functional eIF2. Initiation factor 2A has been shown to direct binding of the Met-tRNAi to 40S ribosomal subunits in a AUG codon-dependent manner (Merrick and Anderson 1975). In contrast to eIF2, mammalian eIF2A consists of a single polypeptide of 68 kDa that does not require GTP to bind the Met-tRNAi (Adams et al. 1975). To test the involvement of eIF2A in translation of SV 26S mRNA, we silenced the expression of murine eIF2A by means of small interfering RNA (siRNA) interference. A murine cDNA clone (GenBank: NM_001005509) is predicted to encode a 65-kDa polypeptide that shows 90% identity in amino acid sequence to human eIF2A (Zoll et al. 2002; see Supplementary Figure S3). Giving this high degree of sequence homology, we considered this gene as the murine ortholog of human eIF2A. Cells were transfected with a siRNA targeted to eIF2A mRNA as described in Materials and Methods, and the effect on SV translation was assayed 50 h post-transfection. As a control, we transfected in parallel an unrelated siRNA labeled with FITC fluorochrome. Silencing of eIF2A expression was confirmed by Northern blot (Fig. 7A). Hybridization of blots with a specific probe revealed a single mRNA transcript with the expected size (∼2 kb). Transfection with specific siRNA gave a consistent 70%–80% reduction in the amount of eIF2A mRNA presented at 50 h post-transfection in both PKR+/+ and PKR0/0 cells. This agrees well with the percentage of transfection estimated by using FITC-labeled control siRNA (data not shown). Silencing of eIF2A neither induced any apparent phenotype in uninfected cells, nor affected steady-state general protein synthesis. This agrees with previous data showing that deletion of yeast eIF2A did not affect translation (Komar et al. 2005). Interestingly, interference of eIF2A expression led to a considerable reduction in the synthesis of SV structural proteins in PKR+/+ cells, but not in PKR0/0 cells. Densitometric quantification revealed a 80% reduction in the synthesis of SV capsid protein, which agrees well with the percentage of transfection achieved. As expected, eIF2α phosphorylation was only observed in PKR+/+ cells infected with SV irrespective of siRNA treatment. The effect of eIF2A silencing on SV was restricted to translation of 26S mRNA and did not affect translation of genomic mRNAs as demonstrated by using the recombinant SV expressing the luciferase gene (SV-luc) (Fig. 7C). Finally, the specific effect of eIF2A silencing on SV translation was further confirmed by the lack of effect on translation of vesicular stomatitis virus (VSV) proteins (Fig. 7B).
Discussion
Considering the role of PKR kinase in antiviral defense, it is not surprising that viruses have evolved mechanisms to prevent activation of this kinase in infected cells. Inhibition of PKR activity could favor viral replication at two levels. The deleterious effect of early eIF2α phosphorylation by dsRNA on viral protein synthesis would be avoided, and production of IFN and proinflammatory cytokines through activation of IRF and NFκB, respectively, would be also limited (Yang et al. 1995; Stark et al. 1998). Here we report that infection with alphaviruses (SV and SFV) induces PKR activation, resulting in phosphorylation of virtually all eIF2α. Accumulation of dsRNA replicative forms in SV-infected cells probably triggers PKR activation, as described for other viruses (Bischoff and Samuel 1989). The synthesis of large amounts of 26S mRNA from ∼3–4 hpi may also contribute to PKR activation and subsequent eIF2α phosphorylation. Furthermore, our data indicate that unlike other animal viruses, alphaviruses express no specific PKR inhibitor. Expression of these PKR-blocking agents in other viruses frequently enhances viral replication by preventing the deleterious effect of dsRNA accumulation. In some cases, these inhibitors can even confer resistance to IFN (Gale et al. 1998; Xiang et al. 2002).
Phosphorylation of eIF2α impairs translation initiation in mammalian cells (Kimball et al. 1998; Kimball 1999). Since the amount of eIF2B in the cell is limited with respect to eIF2, small increases in phosphorylated eIF2α levels could cause severe inhibition of protein synthesis due to eIF2B sequestration (Yang and Hinnebusch 1996; Sudhakar et al. 2000; Krishnamoorthy et al. 2001; Balachandran and Barber 2004). Here we show that virtually all eIF2α is phosphorylated following alphavirus infection of 3T3 cells. Phosphorylation of this factor severely impairs translation of cellular or reporter (EGFP) mRNA, but not translation directed by viral SV subgenomic mRNA. That the remaining ∼5% of intact eIF2α might support 26S mRNA translation seems unlikely for three reasons. First, translation of SV structural proteins represents 30%–40% of protein synthesis in uninfected cells; it is difficult to envisage how a very small percentage of functional eIF2 could support the massive synthesis of viral structural proteins in SV-infected cells. Second, the recycling of eIF2 necessary to support viral translation would be limited due to the inhibitory effect of phosphorylated eIF2α on eIF2B activity. Third, translation of EGFP and ΔDLP 26S mRNAs was abrogated in PKR+/+ cells infected with recombinant SV-EGFP and SV ΔDLP, respectively, indicating that the small fraction of unphosphorylated eIF2 that remaining in these cells cannot support canonical translation.
In contrast to 26S mRNA, we found that translation of SV genomic mRNA was very sensitive to eIF2α phosphorylation. Consistent with this, RNA folding programs did not predict a similar structure as DLP at the 5′ end of SV genomic mRNA. However, given the temporal regulation of translation found for the different types of SV mRNAs in infected cells, translation of genomic mRNA can efficiently proceed before the extensive phosphorylation of eIF2α. Although SV genomic and subgenomic mRNAs appear to have different requirements for eIF2 to initiate translation, phosphorylation of eIF2α does not seem to be the event that switches translation from genomic to subgenomic mRNAs, since temporal regulation of SV mRNAs translation was also observed in the absence of eIF2α phosphorylation (PKR0/0 cells). It is interesting to note that the translational switch from genomic to subgenomic mRNA of SV is temporally correlated with the onset of host translation shut-off. Moreover, SV replicons lacking the structural region of the genome can induce an inhibition of host translation similar to wild-type virus (Frolov and Schlesinger 1994a). These observations suggest that the activity of one or more nonstructural proteins of SV is involved in this process, which is largely independent of eIF2α phosphorylation.
Mechanism of translation resistance to eIF2α phosphorylation
To counteract the effect of PKR activation, our data support a translational mechanism that promotes the synthesis of SV structural proteins irrespective of the functional status of eIF2. mRNA that is translated after eIF2α phosphorylation was reported for yeast GCN4 and mammalian Cat-1 and ATF-4 genes, and for the second cistron of CrPV genomic RNA (Mueller and Hinnebusch 1986; Dever 2002; Yaman et al. 2003; Vattem and Wek 2004). Translation of mRNA for the first three genes is very low under normal (unstressed) conditions, but is induced by eIF2α phosphorylation. The mechanism involved is still poorly understood, but appears to involve reinitiation of ribosomes from upstream short ORFs under limited concentrations of active eIF2 (Dever 2002). For CrPV, however, translation is initiated by Ala on the A ribosomal site, obviating eIF2 participation in delivery of the initiator Met-tRNA (Wilson et al. 2000). Several of our observations strongly suggest that 26S mRNA translation is resistant to eIF2α phosphorylation through a mechanism that differs from those described above. 26S mRNA is translated efficiently, irrespective of the functional status of eIF2α, and translation does not involve short upstream ORFs, since 26S mRNA initiates at the first AUG from the 5′ end. Furthermore, in contrast to CrPV and CAT-1 mRNAs, 5′ UTR + DLP of SV 26S mRNA did not promote internal initiation in bicistronic constructs, suggesting that it does not act as an IRES element (see Supplementary Figure S2).
Our data reveal that the translational resistance of 26S mRNA to eIF2 phosphorylation requires an RNA structure (DLP) located downstream of the initiator AUG triplet. The existence of this DLP was predicted by computer folding, and confirmed by enzymatic tests. Downstream secondary RNA structures were reported to facilitate recognition of the initiator codon on artificial mRNA (Kozak 1990), although the biological role of these structures in natural mRNA has not been addressed. This structure exerts a dual effect on SV 26S mRNA translation. It can act as a translational enhancer, since disruption of DLP structure decreases translational efficiency of 26S mRNA (Frolov and Schlesinger 1996). In addition, DLP allows initiation of translation in the absence of functional eIF2. This conclusion is based on the finding that ΔDLP 26S mRNA translation is very inefficient in PKR+/+, but not in PKR0/0 cells. Consequently, ΔDLP virus replicates poorly in PKR+/+ cells compared with wild-type SV. Strikingly, a replacement of only seven nucleotides in the SV genome renders this virus sensitive to the antiviral effect of PKR. The impact of DLP disruption on tissue tropism of SV in mice is currently under study.
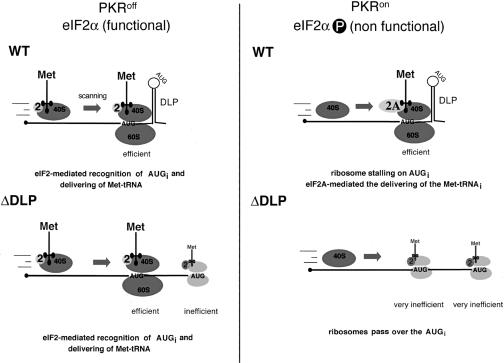
Based on the findings presented here, we propose that the DLP structure could transiently stall ribosomes at the correct site to initiate translation from the AUGi at nucleotide 50. The presence of DLP could thus slow down ribosomes in the scanning process to stop at AUGi (Fig. 8). Signaling of the correct AUGi by intact DLP is supported by the fact that, in the absence of DLP, a fraction of ribosomes passed by the AUGi to initiate from a downstream AUG not normally used as an initiation codon. This is particularly evident in PKR+/+ cells, in which half of the initiation events on ΔDLP 26S mRNA take place at an internal AUG at position 107. This is reminiscent of reinitiation at downstream initiation codons on GCN4 or ATF-4 mRNAs under limiting concentrations of active eIF2 (Mueller and Hinnebusch 1986; Vattem and Wek 2004). Toeprint analysis of immobilized 80S/SV-CA mRNA complexes showed that ribosomes located on AUGi protected mainly +18–19 nt downstream of the initiation codon. Nonetheless, we also detected weak arrests of primer extension at positions +20, +23, and +24 with respect to AUGi. Assuming that RT can penetrate a few nucleotides into the 80S complex (Kozak 1998), the data indicate that the leading edge of 80S ribosomes could be extended several nucleotides forward, to locate just behind DLP, or contacting the base of this structure (Fig. 7). In this model, AUGi and DLP in 26S mRNA would be sufficiently separated to allow precise accommodation of ribosomes on the AUGi. In fact, the AUGi–DLP distance (25–28 nt) is conserved among members of the alphavirus group, despite their lack of sequence homology.
Figure 8.
A model for translational resistance to eIF2α phosphorylation of SV 26S mRNA. Proposed mechanism for translation initiation of SV 26S mRNA. When eIF2 is available (PKR0/0 cells), 26S mRNA can initiate by canonical eIF2-mediated recognition of AUGi. In this situation, ΔDLP can efficiently initiate translation and replicate. Under conditions in which eIF2 activity is absent or greatly limited (PKR+/+ cells), ribosomes could still position on AUGi by the stalling effect of DLP. Then, eIF2A could transfer the Met-tRNAi to the initiation complex. For virus lacking DLP, ribosomes cannot position on AUGi in the absence of eIF2 and continue scanning. Low-frequency initiation at AUG 107 in ΔDLP SV is shown in light gray. The efficient and inefficient translation shown is based on data in Figure 5. Met-tRNAi and eIF2 are in dark blue and yellow, respectively. eIF2A is marked in light blue. For simplicity, the rest of the eIFs were omitted.
Here we present evidence that, in the absence of functional eIF2, eukaryotic initiation factor 2A (eIF2A) can support initiation of SV 26S mRNA. This is based on the fact that silencing of eIF2A expression inhibited translation of SV 26S mRNA in PKR+/+ cells, but not in PKR0/0 cells. Initial characterization of eIF2A from rabbit reticulocytes showed that this factor can bind and transfer the Met-tRNAi to 40S subunits only in the presence of the AUG codon (Merrick and Anderson 1975). However, the biological function of this factor still remains to be determined, although data from yeast showed that it is not essential for cell growth and translation (Komar et al. 2005). We propose that eIF2A could deliver the Met-tRNAi to the 40S ribosome stalled on the 26S mRNA by the effect of DLP structure. According to this model, both DLP and eIF2A factor would be necessary to confer eIF2-independent initiation of SV 26S mRNA, as supported by the data presented here.
Taken together, these results reveal the existence of an alternative mechanism to locate ribosomes on the initiation codon. We identified a novel mechanism by which the alphaviruses escape the antiviral action of PKR. This mechanism has implications for a better understanding of the function of eIF2 and eIF2A in mammalian cells, as well as for unraveling the intricate strategy developed by viruses to counteract the cellular antiviral response. Our findings open the way to analyze if a similar translation initiation mechanism operates in cellular mRNAs, in particular those involved in the stress response.
Materials and methods
Cells and virus infection
Murine embryonic fibroblasts (MEFs) derived from normal and PKR knockout mice were described previously (Yang et al. 1995); these mice have a mixed C57BL/6 and 129 SV genetic background. SV, SFV, encephalomyocarditis virus (EMC), VSV, and FLU were grown in 3T3 cells and purified through sucrose cushions. Virus was titrated by the standard plaque assay method. For infections, ∼5 × 105 cells were infected with viruses (multiplicity of infection [MOI]: 25–50 plaque-forming units [PFU]/cell) in serum-free DMEM. After 30 min of adsorption, viral inoculum was removed and fresh medium containing 10% fetal serum was added to the plates. At the times indicated, cells were washed briefly with cold PBS and lysed in 100 μL of sample buffer (0.15 M Tris-HCl at pH 6.8, 2% SDS, 10% glycerol, 0.1 M DTT, 0.02% bromophenol blue).
Plasmids and recombinant DNA procedures
The SV-EGFP virus expressing the green fluorescence protein from a second 26S subgenomic promoter was constructed as follows: A plasmid encoding the EGFP gene (pEGFP-N1; Clontech) was digested with BglII and BamHI and religated to eliminate the XhoI site of the polylinker. The EGFP gene was then cloned into the SV-2p26S infectious clone of SV using the XbaI site (Levis et al. 1990). The Sindbis virus expressing the luciferase gene as part of NSP3 protein (Toto1101/Luc) was generously provided by Charles Rice (Rockefeller University, New York).
The p5′C-EGFP plasmid was constructed by placing the first 140 nt of the SV 26S mRNA before the EGFP-coding region. Primers 5′L26S forward (CGCGGCTAGCATAGTCAGCAT AGTAC) and 5′L26S reverse (CGGTGGATCCCGCGGGGCC GCCTGCCTC) were used to PCR-amplify the first 140 nt of SV 26S cDNA. This fragment was NheI- and BamHI-digested and cloned into the pEGFP-N1 plasmid (Clontech) using the same enzymes.
Site-directed mutagenesis at the 5′ end of SV 26S cDNA was carried out by PCR (Ventoso and Carrasco 1995). To disrupt the SV DLP, we replaced nucleotides C79, C82, C85, C88, C91, G94, and C97 with A (+1 is the start site of 26S mRNA). The primers used were CATGCTCGGACGACGACCAUUACCAG CACCCCTGCCATG (forward) and CATGGCAGTGGGTGC TGGTAATGGTCGTCGTCCGAGCATG (reverse) (substitutions in bold).
pT7-CA plasmid expresses the natural 5′ leader of SV 26S mRNA, followed by the capsid protein-coding region, and was obtained by cloning a SacI–AatII PCR fragment corresponding to the first 398 nt of 26S mRNA into the SV replicon that express the capsid protein using the same enzymes (Sanz et al. 2003).
To construct plasmids that express bicistronic mRNAs, the coding sequence of the DsRed2 gene from pDsRed2-N1 (Clontech) was cloned into pEGFP-N1, p5′C-EGFP-N1, or pIRES-2 EGFP plasmids using the NheI enzyme, so that DsRed-2- and EGFP-coding regions were placed as the first and second cistron, respectively. The distance between the stop codon of the DsRed-coding sequence and the ATG of EGFP is 100 and 70 nt for pRed-EGFP and pRed-5′26S-EGFP, respectively. For pRed-5′26S-EGFP, the second cistron initiates at the natural ATG of 26S mRNA and includes the first 31 amino acids of the capsid protein fused to EGFP.
Electroporation of cells with viral RNA
To generate viruses from infectious clones, SV-EGFP and Toto1101/luc plasmids were linearized with XhoI and transcribed in vitro with T7 or SP6 RNA polymerases, respectively, in the presence of cap analog (New England Biolabs). Transcription mixtures (2 μg of RNA) were used directly to electroporate ∼106 cells in a Bio-Rad electroporator (1500 V, 25 μF). Virus was collected from medium after 48 h and further amplified to obtain high-titer stocks. Toto1101/luc has a slow-growth phenotype compared with SV or SV-EGFP viruses.
Metabolic labeling
Cells were labeled with [35S]-Met/Cys (25 μCi/mL; ProMix, Amersham) in medium lacking Met (30 min), washed with complete medium, and lysed in sample buffer. Proteins were analyzed by SDS-PAGE followed by fluorography and autoradiography.
IEF and Western blot
To analyze eIF2α by isoelectric focusing (IEF), infected cells were lysed in buffer (20 mM HEPES-KOH at pH 7.5, 150 mM NaCl, 10% [v/v] glycerol, 1% [v/v] Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 100 mM sodium fluoride, 17.5 mM β-glycerophosphate, 10 mM tetrasodium diphosphate, and a protease inhibitor cocktail [Complete; Boehringer Mannheim]). Cell debris was removed by centrifugation (15,000 × g for 5 min at 4°C); supernatant was snap-frozen on dry ice and stored at –70°C. Equivalent amounts of protein extract (50 μg) were precipitated with trichloracetic acid (10%). Protein pellets were resuspended in 60-μL vertical slab isoelectric focusing (VSIEF) gel sample buffer and resolved as described (Savinova and Jagus 1997). To control eIF2α phosphorylation status, we used rabbit reticulocyte lysate (RRL) incubated with 500 μM hemin and 2 mM EDTA (5 min at 30°C; nonphosphorylated) or minus hemin (10 min), followed by 5 min in the presence of 17.5 mM β-glycerophosphate (phosphorylated). RRL samples (2 μL) were added to 100 μL of VSIEF sample buffer, 20 μL of which was resolved. After VSIEF, proteins were transferred to Immobilon-P membranes and probed with eIF2α antibody (Santa Cruz). The rest of the antibodies used were anti-PKR (polyclonal no. sc-1702; Santa Cruz), anti-phospho eIF2α and anti-phospho PKR (Biosource); and anti-EGFP (Clontech). The rabbit antibody to the SV E1 glycoprotein has been described (Sanz et al. 2003). Polyclonal serum to the SV capsid protein was generously provided by Dr. M.J. Schlesinger (Karolinska Institute, Stockholm, Sweden). Blots were developed using the ECL system (Amersham Biosciences).
Proteomic analysis
[35S]Met/Cys-labeled peptide maps of alphavirus capsid proteins were analyzed by TLC as described (Maroto et al. 2000). Briefly, PKR+/+ or PKR0/0 cells were infected with SV or FSV (MOI: 50 PFU/cell). At 4.5 hpi, cells were labeled with 50 μCi/mL of [35S]Met/Cys (1 h), extracted, and analyzed by SDS-PAGE followed by transfer to nitrocellulose membrane. The membrane was exposed to X-ray films (2 h) and the band corresponding to capsid proteins was excised, blocked with polyvinylpyrrolidone, and trypsin-digested (18 h; Promega). Peptides were then oxidized with performic acid, rinsed in distilled water, and analyzed by one-dimensional TLC electrophoresis, and the TLC plates were exposed to X-ray films.
For N-terminal sequence determination of SFV capsid proteins, PKR+/+ and PKR0/0 cells were infected with SFV (MOI: 10 PFU/cell); virus released to the culture medium at 16 hpi was purified by sedimentation through a 20/50% sucrose cushion (160,000 × g for 3 h). Bands corresponding to capsid protein were excised from SDS-PAGE gels, then subjected to tryptic digestion and fingerprint analysis (MALDI-TOF), followed by sequence verification using fragmentation coupled to ESI (electrospray ionization) analysis.
Met-Pur synthesis assay
Synthesis of Met-Pur dipeptide in vivo was carried out as follows: Cells were incubated in hypertonic medium containing 0.31 M NaCl (40 min) to dissociate ribosomes from mRNA. 80S initiation complexes were then allowed to reassemble in normal medium (0.12 M NaCl) containing 25 μCi/mL [35S]Met and 50 μg/mL puromycin for 40–140 min. Cells were washed extensively in cold PBS and lysed in TNE buffer containing 1% Triton X-100. Post-nuclear supernatant was treated with 1 μg/mL RNAse A (15 min, 37°C) and diluted fivefold in 0.1 M phosphate buffer (pH 8.0). Samples were extracted twice with ethyl acetate, and the radioactivity in the organic phase was counted in a liquid scintillation counter as described previously (Suzuki and Goldberg 1974).
Toeprinting analysis
We used a nonradiactive modification of primer extension analysis (Anthony and Merrick 1992; Kozak 1998). In brief, 0.1 μg of in vitro synthesized SV CA mRNA was preannealed to 5′-fluorochrome-labeled (VIC) primer: GCCTGTCCAATGAC TAGGGCACTGACGG by heating (1 min, 65°C), then cooling slowly to 37°C. For enzymatic probing, the RNA-primer mixture was treated with 0.01 U of RNase A or T1 (10 min, room temperature), extracted twice with phenol/chloroform, precipitated with ethanol, and resuspended in RT buffer (50 mM Tris-HCl at pH 7.5, 40 mM KCl, 6 mM MgCl2, 5 mM DTT, 0.5 mM dNTPs). Following addition of 2 U of SuperScript II reverse transcriptase (GIBCO-BRL), primer extension reactions were incubated (20 min at 25°C), phenol-extracted, precipitated with ethanol, resuspended in 40% formamide/8 mM EDTA, and heated (5 min at 95°C). Samples were analyzed in an ABI Prism 3700 DNA Analyzer using GeneScan software (Applied Biosystems). To analyze formation of ribosomal initiation complex, nuclease-treated RRL (Promega) was programmed with RNA/primer mixture in the presence of 100 μg/mL CHX and incubated (25°C, 10 min) in a final volume of 25 μL. Samples were then diluted 20-fold in RT buffer supplemented with 100 μg/mL CHX, and primer extension analysis was carried out as described above.
Gene silencing by siRNA
To knock down the expression of murine eIF2A gene, an siRNA was designed (GUAAGGAUGGGACAUUGUUtt) corresponding to nucleotides 177–195 from the 5′ extreme of mRNA sequence of murine eIF2A cDNA (GenBank: NM_001005509). PKR+/+ and PKR0/0 cells were transfected with 40 pmol of siRNA eIF2A or with an unrelated siRNA labeled with FITC using oligofectamine (GIBCO-BRL) according to the manufacturer's recommendation. At 50 h post-transfection, cells were infected with SV or VSV at an MOI of 25 PFU/cell. Six hours later, cells were metabolically labeled and analyzed as describe above. Silencing of eIF2A expression was confirmed by Northern blot analysis using an [α-32P]dCTP-labeled probe encompassing nucleotides 287–886 of murine eIF2A cDNA derived from clone ID 30606936 provided by the I.M.A.G.E consortium (MRC Geneservice). Poly(A)+ mRNAs were isolated by the QuickPrep micro mRNA purification kit (Pharmacia Biotech). Hybridization was performed in ExpressHyb solution (BD Biosciences) according to the manufacturer's recommendation.
Acknowledgments
We thank A. Paradela and A. Varas for technical support in proteomic and DNA sequence analysis, respectively; S. Guerra and J.M. Almendral for help with TLC analysis; C. Rice, M.J. Schlesinger, and M. Nieto for providing plasmid Toto1101/Luc, anti-SV C, and pIRES-2EGFP; and A. Fraile for his help with siRNA transfection. We also thank C. Mark for editorial assistance and J. Benavente for critical reading of the manuscript. This research was supported by grants BME2002-03246 and EU(ALK2-CT-2002-00954) to the CNB group and Grant BMC2003/00494 to the CBM group. The institutional grant to the Centro de Biología Molecular by the Fundación Ramón Areces is acknowledged.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.357006.
References
- Adams S.L., Safer, B., Anderson, W.F., and Merrick, W.C. 1975. Eukaryotic initiation complex formation. Evidence for two distinct pathways. J. Biol. Chem. 250: 9083–9089. [PubMed] [Google Scholar]
- Anthony D.D. and Merrick, W.C. 1992. Analysis of 40 S and 80 S complexes with mRNA as measured by sucrose density gradients and primer extension inhibition. J. Biol. Chem. 267: 1554–1562. [PubMed] [Google Scholar]
- Balachandran S. and Barber, G.N. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5: 51–65. [DOI] [PubMed] [Google Scholar]
- Balachandran S., Roberts, P.C., Brown, L.E., Truong, H., Pattnaik, A.K., Archer, D.R., and Barber, G.N. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13: 129–141. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Garcia-Sastre, A., Carnero, E., Pehamberger, H., Wolff, K., Palese, P., and Muster, T. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74: 6203–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff J.R. and Samuel, C.E. 1989. Mechanism of interferon action. Activation of the human P1/eIF-2 α protein kinase by individual reovirus s-class mRNAs: s1 mRNA is a potent activator relative to s4 mRNA. Virology 172: 106–115. [DOI] [PubMed] [Google Scholar]
- Black T.L., Barber, G.N., and Katze, M.G. 1993. Degradation of the interferon-induced 68,000-M(r) protein kinase by poliovirus requires RNA. J. Virol. 67: 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K., Elroy-Stein, O., Moss, B., and Jagus, R. 1993. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 α-specific protein kinase. J. Biol. Chem. 268: 12837–12842. [PubMed] [Google Scholar]
- Davies M.V., Chang, H.W., Jacobs, B.L., and Kaufman, R.J. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67: 1688–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Mendez, R., and Santoyo, J. 1996. The eIF-2α kinases and the control of protein synthesis. FASEB J. 10: 1378–1387. [DOI] [PubMed] [Google Scholar]
- Dever T.E. 2002. Gene-specific regulation by general translation factors. Cell 108: 545–556. [DOI] [PubMed] [Google Scholar]
- Frolov I. and Schlesinger, S. 1994a. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J. Virol. 68: 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1994b. Translation of Sindbis virus mRNA: Effects of sequences downstream of the initiating codon. J. Virol. 68: 8111–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1996. Translation of Sindbis virus mRNA: Analysis of sequences downstream of the initiating AUG codon that enhance translation. J. Virol. 70: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M., Blakely, C.M., Kwieciszewski, B., Tan, S.L., Dossett, M., Tang, N.M., Korth, M.J., Polyak, S.J., Gretch, D.R., and Katze, M.G. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: Molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18: 5208–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Raught, B., and Sonenberg, N. 1999. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68: 913–963. [DOI] [PubMed] [Google Scholar]
- Goossens A., Dever, T.E., Pascual-Ahuir, A., and Serrano, R. 2001. The protein kinase Gcn2p mediates sodium toxicity in yeast. J. Biol. Chem. 276: 30753–30760. [DOI] [PubMed] [Google Scholar]
- Gorchakov R., Frolova, E., Williams, B.R., Rice, C.M., and Frolov, I. 2004. PKR-dependent and -independent mechanisms are involved in translational shutoff during Sindbis virus infection. J. Virol. 78: 8455–8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnery S. and Mathews, M.B. 1998. RNA binding and modulation of PKR activity. Methods 15: 189–198. [DOI] [PubMed] [Google Scholar]
- Harding H.P., Novoa, I., Zhang, Y., Zeng, H., Wek, R., Schapira, M., and Ron, D. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6: 1099–1108. [DOI] [PubMed] [Google Scholar]
- He B., Gross, M., and Roizman, B. 1997. The γ(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. 94: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J.W. 1991. Translational control in mammalian cells. Annu. Rev. Biochem. 60: 717–755. [DOI] [PubMed] [Google Scholar]
- Kaufman R.J. 1999. Double-stranded RNA-activated protein kinase mediates virus-induced apoptosis: A new role for an old actor. Proc. Natl. Acad. Sci. 96: 11693–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S.R. 1999. Eukaryotic initiation factor eIF2. Int. J. Biochem. Cell Biol. 31: 25–29. [DOI] [PubMed] [Google Scholar]
- Kimball S.R., Fabian, J.R., Pavitt, G.D., Hinnebusch, A.G., and Jefferson, L.S. 1998. Regulation of guanine nucleotide exchange through phosphorylation of eukaryotic initiation factor eIF2α. Role of the α- and δ-subunits of eiF2b. J. Biol. Chem. 273: 12841–12845. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider, R.J., Safer, B., Munemitsu, S.M., Samuel, C.E., Thimmappaya, B., and Shenk, T. 1986. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 α kinase. Cell 45: 195–200. [DOI] [PubMed] [Google Scholar]
- Komar A.A., Gross, S.R., Barth-Baus, D., Strachan, R., Hensold, J.O., Goss Kinzy, T., and Merrick, W.C. 2005. Novel characteristics of the biological properties of the yeast Saccharomyces cerevisiae eukaryotic initiation factor 2A. J. Biol. Chem. 280: 15601–15611. [DOI] [PubMed] [Google Scholar]
- Kozak M. 1980. Evaluation of the `scanning model' for initiation of protein synthesis in eucaryotes. Cell 22 (1 Pt 1): 7–8. [DOI] [PubMed] [Google Scholar]
- ____. 1990. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl. Acad. Sci. 87: 8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1998. Primer extension analysis of eukaryotic ribosome–mRNA complexes. Nucleic Acids Res. 26: 4853–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy T., Pavitt, G.D., Zhang, F., Dever, T.E., and Hinnebusch, A.G. 2001. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 21: 5018–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Schlesinger, S., and Huang, H.V. 1990. Promoter for Sindbis virus RNA-dependent subgenomic RNA transcription. J. Virol. 64: 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wambach, M., Katze, M.G., and Krug, R.M. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214: 222–228. [DOI] [PubMed] [Google Scholar]
- Manche L., Green, S.R., Schmedt, C., and Mathews, M.B. 1992. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol. 12: 5238–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto B., Ramirez, J.C., and Almendral, J.M. 2000. Phosphorylation status of the parvovirus minute virus of mice particle: Mapping and biological relevance of the major phosphorylation sites. J. Virol. 74: 10892–10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W.C. and Anderson, W.F. 1975. Purification and characterization of homogeneous protein synthesis initiation factor M1 from rabbit reticulocytes. J. Biol. Chem. 250: 1197–1206. [PubMed] [Google Scholar]
- Meurs E., Chong, K., Galabru, J., Thomas, N.S., Kerr, I.M., Williams, B.R., and Hovanessian, A.G. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62: 379–390. [DOI] [PubMed] [Google Scholar]
- Mueller P.P. and Hinnebusch, A.G. 1986. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45: 201–207. [DOI] [PubMed] [Google Scholar]
- Pestova T.V. and Hellen, C.U. 2003. Translation elongation after assembly of ribosomes on the Cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes & Dev. 17: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Borukhov, S.I., and Hellen, C.U. 1998. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 394: 854–859. [DOI] [PubMed] [Google Scholar]
- Pestova T.V., Kolupaeva, V.G., Lomakin, I.B., Pilipenko, E.V., Shatsky, I.N., Agol, V.I., and Hellen, C.U. 2001. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. 98: 7029–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz M.A., Madan, V., Carrasco, L., and Nieva, J.L. 2003. Interfacial domains in Sindbis virus 6K protein. Detection and functional characterization. J. Biol. Chem. 278: 2051–2057. [DOI] [PubMed] [Google Scholar]
- Savinova O. and Jagus, R. 1997. Use of vertical slab isoelectric focusing and immunoblotting to evaluate steady-state phosphorylation of eIF2 α in cultured cells. Methods 11: 419–425. [DOI] [PubMed] [Google Scholar]
- Stark G.R., Kerr, I.M., Williams, B.R., Silverman, R.H., and Schreiber, R.D. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67: 227–264. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F., Abraham, N., Knowles, S., Marius, R., Brasey, A., Lichty, B.D., Brown, E.G., Sonenberg, N., and Bell, J.C. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74: 9580–9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.H. and Strauss, E.G. 1994. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 58: 491–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A., Ramachandran, A., Ghosh, S., Hasnain, S.E., Kaufman, R.J., and Ramaiah, K.V. 2000. Phosphorylation of serine 51 in initiation factor 2 α (eIF2 α) promotes complex formation between eIF2 α(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry 39: 12929–12938. [DOI] [PubMed] [Google Scholar]
- Suzuki H. and Goldberg, I.H. 1974. Reversal of pactamycin inhibition of methionyl-puromycin synthesis and 80S initiation complex formation by a ribosomal joining factor. Proc. Natl. Acad. Sci. 71: 4259–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.R., Shi, S.T., Romano, P.R., Barber, G.N., and Lai, M.M. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285: 107–110. [DOI] [PubMed] [Google Scholar]
- Vattem K.M. and Wek, R.C. 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. 101: 11269–11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventoso I. and Carrasco, L. 1995. A poliovirus 2A(pro) mutant unable to cleave 3CD shows inefficient viral protein synthesis and transactivation defects. J. Virol. 69: 6280–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.R. 1999. PKR: A sentinel kinase for cellular stress. Oncogene 18: 6112–6120. [DOI] [PubMed] [Google Scholar]
- Wilson J.E., Pestova, T.V., Hellen, C.U., and Sarnow, P. 2000. Initiation of protein synthesis from the A site of the ribosome. Cell 102: 511–520. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Condit, R.C., Vijaysri, S., Jacobs, B., Williams, B.R., and Silverman, R.H. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76: 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman I., Fernandez, J., Liu, H., Caprara, M., Komar, A.A., Koromilas, A.E., Zhou, L., Snider, M.D., Scheuner, D., Kaufman, R.J., et al. 2003. The zipper model of translational control: A small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 113: 519–531. [DOI] [PubMed] [Google Scholar]
- Yang W. and Hinnebusch, A.G. 1996. Identification of a regulatory subcomplex in the guanine nucleotide exchange factor eIF2B that mediates inhibition by phosphorylated eIF2. Mol. Cell. Biol. 16: 6603–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Reis, L.F., Pavlovic, J., Aguzzi, A., Schafer, R., Kumar, A., Williams, B.R., Aguet, M., and Weissmann, C. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14: 6095–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z. and Shatkin, A.J. 1997. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology 234: 364–371. [DOI] [PubMed] [Google Scholar]
- Zoll W.L., Horton, L.E., Komar, A.A., Hensold, J.O., and Merrick, W.C. 2002. Characterization of mammalian eIF2A and identification of the yeast homolog. J. Biol. Chem. 277: 37079–37087. [DOI] [PubMed] [Google Scholar]