Abstract
Pdx1 (IPF-1 in humans, which is altered in MODY-4) is essential for pancreas development and mature β-cell function. Pdx1 is expressed dynamically within the developing foregut, but how its expression characteristics are linked to the various steps of organ specification, differentiation, and function is unknown. Deletion of a conserved enhancer region (Area I-II-III) from Pdx1 produced a hypomorphic allele (Pdx1ΔI-II-III) with altered timing and level of expression, which was studied in combination with wild-type and protein-null alleles. Lineage labeling in homozygous Area I-II-III deletion mutants (Pdx1ΔI-II-III/ΔI-II-III) revealed lack of ventral pancreatic bud specification and early-onset hypoplasia in the dorsal bud. Acinar tissue formed in the hypoplastic dorsal bud, but endocrine maturation was greatly impaired. While Pdx1-/- (protein-null) mice have nonpancreatic abnormalities (e.g., distorted pylorus, absent Brunner's glands), these structures formed normally in Pdx1ΔI-II-III/ΔI-II-III and Pdx1ΔI-II-III/- mice. Surprisingly, heterozygous (Pdx1+/ΔI-II-III) mice had abnormal islets and a more severe prediabetic condition than Pdx1+/- mice. These findings provide in vivo evidence of the differential requirements for the level of Pdx1 gene activity in the specification and differentiation of the various organs of the posterior foregut, as well as in pancreas and gut endocrine cell differentiation.
Keywords: Organogenesis, pancreatic β cells, Pdx1, MODY, Enhancer, cis-element, foregut differentiation
Studies in several vertebrate species have started to define conserved developmental pathways controlled by extrinsic and intrinsic factors that are essential for the development of the endocrine and exocrine pancreas (Kim and Hebrok 2001; Wilson et al. 2003; Jensen 2004). It is generally accepted that signals from mesodermal tissues control the early stage of pancreas development. Dorsal bud outgrowth depends on inductive signals from the nearby notochord (Kim et al. 1997; Hebrok et al. 1998), and its subsequent expansion and differentiation require signals from embryonic blood vessel cells (Lammert et al. 2001; Yoshitomi and Zaret 2004) and other signaling pathways, including Activin, FGFs, and Notch (Kim and Hebrok 2001; Wilson et al. 2003; Jensen 2004). These signals affect the expression of transcription factors that execute the pancreatic developmental program.
Among the transcription factors identified as essential for proper pancreas differentiation, the homeodomain protein Pdx1 (also known as IPF-1, STF-1, or IDX-1) (Wright et al. 1989; Leonard et al. 1993; Ohlsson et al. 1993; Miller et al. 1994) is both expressed in precursors of the endocrine and exocrine compartments of the pancreas (Gu et al. 2002), and is essential for their development and maintenance, especially the insulin-producing β cells (Jonsson et al. 1994; Offield et al. 1996; Ahlgren et al. 1998; Holland et al. 2002). In addition to pancreatic agenesis, Pdx1-/- mouse mutants have defects throughout the posterior foregut region, including distorted gastro-duodenal junction, loss of Brunner's glands, and deficiency of enteroendocrine differentiation in the stomach and duodenum (Larsson et al. 1996; Offield et al. 1996; Jepeal et al. 2005). Loss of IPF-1 function also results in pancreatic agenesis in humans (Stoffers et al. 1997).
In mature β cells, Pdx1 transactivates the Insulin gene and other genes involved in glucose sensing and metabolism, such as Glut2 and Glucokinase (Waeber et al. 1996; Watada et al. 1996). Pdx1+/- mice are glucose intolerant, indicating that gene dosage for Pdx1/IPF-1 is crucial for normal glucose homeostasis (Ahlgren et al. 1998; Dutta et al. 1998; Brissova et al. 2002; Johnson et al. 2003). These findings are concordant with the prior discovery that humans heterozygous for an inactivating mutation of IPF-1 are at elevated risk for adult-onset type II diabetes and are linked to a dominant heritable condition, maturity onset diabetes of the young type 4 (MODY4) (Stoffers et al. 1997, 1998).
The regulatory mechanisms that orchestrate the complex developmental transitions involved in cellular differentiation and function during pancreatic organogenesis are far from elucidated. Normal endocrine pancreas development and function depends on a highly integrated transcription-factor network, and even subtle abnormalities in islet tissue caused by heterozygosity or reduced gene dosage of MODY susceptibility genes can substantially predispose toward severe diabetes in humans (Bell and Polonsky 2001). Recent promoter analyses of genes involved in islet differentiation and function suggest complex genetic interactions among these factors (Shih et al. 2002; Servitja and Ferrer 2004). Most of this evidence, however, is inferred from in vitro studies using immortalized β-cell lines or other cells such as ES cells, which are perhaps of limited relevance to normal ontogeny and organogenesis.
As one key entry point into understanding the operation of these gene regulatory networks in vivo, we have examined the cis- and trans-regulation of Pdx1. Alignment of the mouse and human Pdx1 gene sequences revealed three phylogenetically conserved regions referred to collectively as Area I-II-III (Gerrish et al. 2000), which are also characterized as PH-1, PH-2, and PH-3 in human (Melloul et al. 2002). The Area I-II-III region harbors binding sites for MODY transcription factors such as HNF-1α and Pdx1 itself (Marshak et al. 2000; Gerrish et al. 2001), as well as other endodermal and islet-enriched transcriptional regulators, including Foxa2/HNF3β (Gerrish et al. 2000; Ben-Shushan et al. 2001), Pax6 (Samaras et al. 2002), Maf/RIPE3b1 (Samaras et al. 2003), and HNF-6/OC-1 (Jacquemin et al. 2003). We have reported on sufficiency tests in transgenic mice and cultured cell lines that identified islet-specific (PstI-BstEII fragment: containing Area I-II) and β-cell-specific (XhoI-BglII fragment: containing Area III and an adjacent 3′ region) cis-regulatory regions that overlap with Area I-II-III, suggesting that Area I-II-III functions specifically in differentiation and maintenance of pancreatic islets (Wu et al. 1997; Gerrish et al. 2000, 2001; Gannon et al. 2001; Samaras et al. 2002). A slightly more distal Area IV, which can act in vitro to drive β-cell-selective and glucocorticoid-regulated transcription of rat Pdx1 (STF-1) (Sharma et al. 1997), may also potentiate Area I/II-mediated activity in β cells (Gerrish et al. 2004).
Here we demonstrate that removal of conserved sequences from the endogenous Pdx1 locus results in a decreased level and abnormal spatiotemporal expression of Pdx1 protein. Our studies overcome a main caveat of transgenic studies, which do not elucidate the function of transcriptional elements in a native chromosomal context. This study lays the foundation for further in vivo dissection of the networks that control pancreas organogenesis via the direct and precise manipulation of Pdx1 and its trans-regulatory genes.
Results
Targeting of the Pdx1 locus with an Area I-II-III floxed sequence
Gene targeting in ES cells was used to delete Area I-II-III from the endogenous Pdx1 locus (Fig. 1). The targeting vector contained the 1.2-kb Area I-II-III flanked by loxP sites and FRT-flanked PGKneopA (neo-) cassette in the 3′ adjacent region. Vector electroporation into ES cells, positive-negative selection, and Southern blot analysis (data not shown) gave rise to frt-neor heterozygous ES cell clones. These cells were injected into C57BL/6 blastocysts, and chimeric mutant mice derived. The neor cassette was excised in vivo by crossing chimeras to mice expressing the Flpe recombinase (ACTB:FLPe), leading to Pdx1floxI-II-III/+ offspring.
Figure 1.
Strategy for deleting Area I-II-III within Pdx1. (Top) Pdx1 genomic structure. Area I-II-III lies ∼2 kb 5′ of the transcription start site. Homologous recombination with the targeting vector generated the frt-neo allele; Flp deletion created the flox I-II-III allele; subsequent Cre deletion led to the ΔI-II-III allele. (Black boxes) Exons; (gray box) B-X external probe for Southern blot analysis (diagnostic band lengths pre/post-recombination indicated). (neoR) Neomycin resistance cassette; (TK) thymidine kinase cassette. Black and white arrowheads indicate LoxP and FRT sites, respectively. (B) BamHI; (Bs) BstEII; (P) PstI; (RV) EcoRV; (X) XbaI.
After neor cassette removal, the remaining FRT site and adjacent loxP site accounted for ∼200 base pairs (bp) of exogenous bystander sequence in the Pdx1floxI-II-III allele. We ascertained that this extra sequence does not disrupt the function of the floxed allele by examining litters derived from mating Pdx1floxI-II-III/+ mice to Pdx1+/- mice (Offield et al. 1996). All genotypes arose at the expected Mendelian frequency. No significant differences in appearance or body weight were observed at birth or 4 wk of age between Pdx1floxI-II-III/- mice and their controls: Pdx1floxI-II-III/+, Pdx1+/-, and Pdx1+/+ (data not shown). Immunohistochemical analyses revealed that Pdx1floxI-II-III/- mice have normal islets with all four major endocrine cell types (α, β, δ, and PP; producing glucagon, insulin, somastatin, or pancreatic polypeptide, respectively) arranged indistinguishably from those in Pdx1+/- mice (data not shown). Pdx1floxI-II-III/floxI-II-III mice also showed normal islet morphology and architecture. Intraperitoneal glucose tolerance test (IP-GTT) demonstrated that glucose clearance in Pdx1floxI-II-III/+ mice was comparable to wild-type mice (data not shown). We therefore conclude that the floxI-II-III allele is functionally equivalent to the wild-type allele.
Pancreatic agenesis in Pdx1ΔI-II-III/- mutants
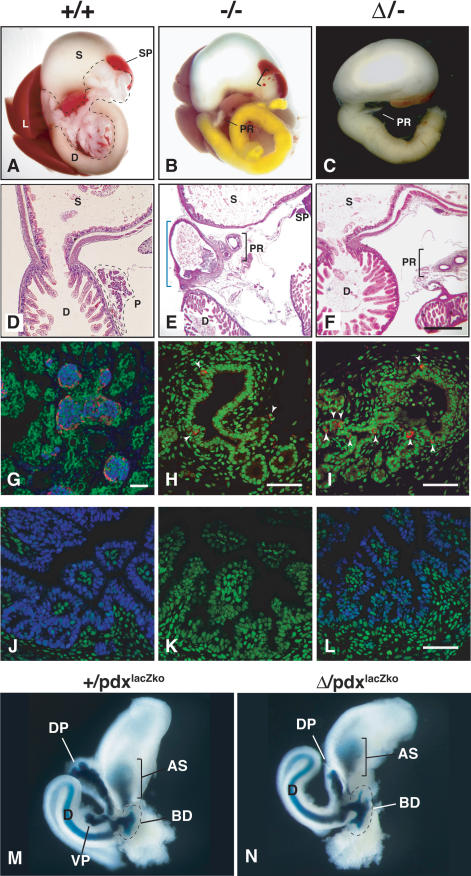
Germline deletion of Area I-II-III was accomplished by crossing Pdx1floxI-II-III/+ mice to EIIaCre transgenic mice. Because EIIaCre produces a mosaic pattern of recombination by paternal transmission, candidate F1 mutants were screened by Southern blot and PCR (data not shown) to confirm deletion of Area I-II-III, yielding the Pdx1ΔI-II-III allele (Fig. 1). Pdx1ΔI-II-III/+ and Pdx1+/- mice were mated to generate Pdx1ΔI-II-III/- progeny. Neonatal Pdx1ΔI-II-III/- mice were outwardly indistinguishable from Pdx1+/+ or Pdx1+/- littermates. By postnatal day 2 (P2), however, Pdx1ΔI-II-III/- animals showed growth retardation and dehydration. Most Pdx1ΔI-II-III/- pups died by P9, and none survived past 17 d. Internal examination of P1 Pdx1ΔI-II-III/- pups revealed that the liver, gall bladder, spleen, and stomach were present and grossly normal. Also, the common bile duct was of similar diameter and length to that of wild-type littermates (data not shown). In all Pdx1ΔI-II-III/- pups, the pancreas was replaced by a small rudiment protruding from the duodenal wall representing a stunted dorsal pancreatic outgrowth (see below). The dorsal outgrowth contained numerous small clusters of glucagon-producing cells at the tips of a ductular tree that displayed limited branching (Fig. 2I). No other endocrine hormones (insulin, somatostatin, PP), nor exocrine markers (e.g., amylase), were detected in the dorsal rudiment at birth, and Pdx1 protein was not detected in its epithelium at this stage (data not shown). Comparison of several animals of each genotype showed that the block to pancreas development in Pdx1ΔI-II-III/- mice was indistinguishable from that in homozygous protein-null mice (Fig. 2H; Jonsson et al. 1994; Offield et al. 1996).
Figure 2.
Characterization of Pdx1ΔI-II-III/- animals. (A-C) Gross analysis of pancreas differentiation in newborn Pdx+/+, Pdx+/-, and PdxΔI-II-III/- animals (labeled here as +/+, +/-, and Δ/-, respectively). (A) Pancreas tissue outlined. (D) Duodenum; (S) stomach; (SP) spleen; (L) liver. (B,C) (PR) Pancreatic rudiment derived from stunted outgrowth of dorsal pancreatic bud. The bracket in B indicates ectopic spleen tissue frequently observed in Pdx1-/- (-/-) or Pdx1ΔI-II-III/- (Δ/-) mutants. Yellow bile color in duodenum is often seen in -/- mutant animals. (D-F) Histological analysis of gastro-duodenal region (H&E stain). (D) Normal gastro-duodenal junction with a well-defined pylorus in P1 pup, with continuous lumen joining stomach and rostral duodenum. (E) This region is malformed in -/- littermates (blue bracket). In many -/- animals, the region forms a tortuous, sometimes blind-ended tube that lacks villi and is lined by cuboidal epithelium continuous with the pancreatic rudiment. (F) All Δ/- animals examined (n = 9) have a normal gastro-duodenal junction. (G) At P1, endocrine cell aggregations in +/+ animals represent immature islets (confocal immunofluroescence: peripheral glucagon-producing cells [red] surround insulin-producing cells [blue]; nuclei counterstained green by Yo-Pro-1). (H,I) Similar confocal immunofluorescence analysis—at higher magnification—of P1 dorsal bud-derived pancreatic rudiment in -/- and Δ/- mutants showed simple cuboidal epithelium similar to bile duct. Glucagon-producing cells (red, indicated by white arrowheads; nuclei green) were observed adjoining slight evaginations of the endodermal epithelium. (J-L) Immunofluorescence analysis, E16.5 tissues. A lower level of nuclear Pdx1 (blue; nuclei green) was found in the rostral duodenum of Δ/- animals compared with +/+ tissue (-/- tissue lacks signal). (M,N) E12.0 foregut organ development visualized with a lacZ knock-in allele of Pdx1 (Pdx1lacZko). X-gal staining labels (AS) antral stomach, (D) duodenum, (BD) common bile duct, and (DP) dorsal pancreas in both Pdx1lacZko and Δ-lacZko animals. The ventral pancreatic growth (VP) present in +/lacZko animals is undetected in Δ/lacZko animals, in which dorsal pancreatic growth is also stunted. Bars: D-F, 400 μm; G-L, 50 μm.
The early failure of ventral pancreatic bud formation was confirmed by using the Pdx1lacZko allele (Offield et al. 1996), in which Pdx1 exon 2 is replaced by nuclear-targeted β-galactosidase, to analyze the ontogeny of pancreas malformation in Pdx1ΔI-II-III/- embryos. As previously reported (Offield et al. 1996), in Pdx1 heterozygous embryonic day 12.0 (E12.0) embryos (Pdx1+/lacZko), β-galactosidase labels the endogenous Pdx1 expression domain, including the dorsal and ventral pancreatic buds, caudal stomach, rostral duodenum, and common bile duct (Fig. 2M). In Pdx1ΔI-II-III/lacZko mutants, the dorsal pancreatic bud was markedly smaller, with no sign of branching morphogenesis at this stage; a separate ventral pancreatic bud was not present (Fig. 2N).
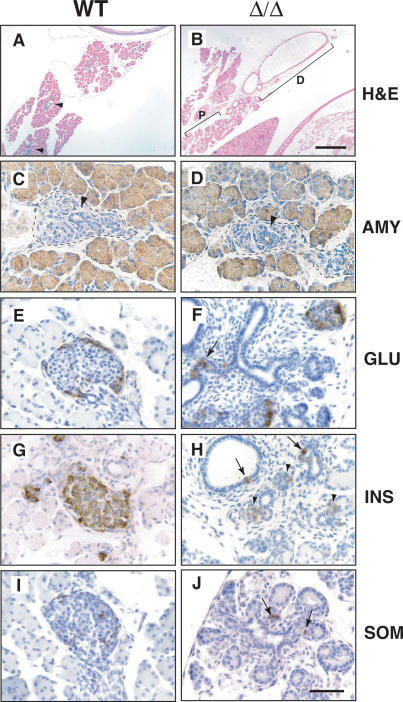
Pdx1ΔI-II-III/ΔI-II-III animals show dorsal pancreas hypoplasia and loss of ventral pancreas
Intercrossing of Pdx1ΔI-II-III/+ mice generated Pdx1ΔI-II-III/ΔI-II-III mice. The Pdx1ΔI-II-III/ΔI-II-III pups lived at least 7 d, but none survived beyond weaning (n = 17). While the dorsal pancreas of Pdx1ΔI-II-III/ΔI-II-III mice was hypoplastic (Fig. 3A,B), it developed substantially more than that of Pdx1ΔI-II-III/- mutants (Fig. 2C). Proximal to the duodenum, the pancreatic remnant contained a relatively expansive region of zymogen granule-laden acinar cells connected by a well-formed ductal network (Fig. 4C,D). Pancreatic endocrine cell differentiation in this zone was highly defective, with no well-differentiated islets observed (Fig. 4E-J). While glucagon-producing cells were abundant, they were less clustered than in wild-type tissue and did not surround a core of insulin-producing cells. All four major endocrine cell types (α, β, δ, and PP) were detected, but only as small cell clusters, or single cells tightly apposed to ductal structures (arrows in Fig. 4F,H,J). The number of insulin-producing cells was dramatically decreased in Pdx1ΔI-II-III/ΔI-II-III mutants (Fig. 4G,H), and these cells had a uniformly much lower insulin level. The portion of the dorsal pancreatic remnant distal to the duodenum (bracket in Fig. 3B) developed as a cyst-like structure lined by a simple cuboidal epithelium (Fig. 4B); no pancreatic endocrine or acinar markers were detected in or near it. The cystic epithelium produced cytokeratins typical of pancreatic ductal epithelium and lacked expression of Nkx6.1, a marker of the early, progenitor-stage pancreatic epithelium (data not shown). These findings provide evidence for regional (distal vs. proximal) differences in the differentiation program of the dorsal pancreatic bud as related to the gene dosage or spatial and temporal expression characteristics of the Pdx1ΔI-II-III allele.
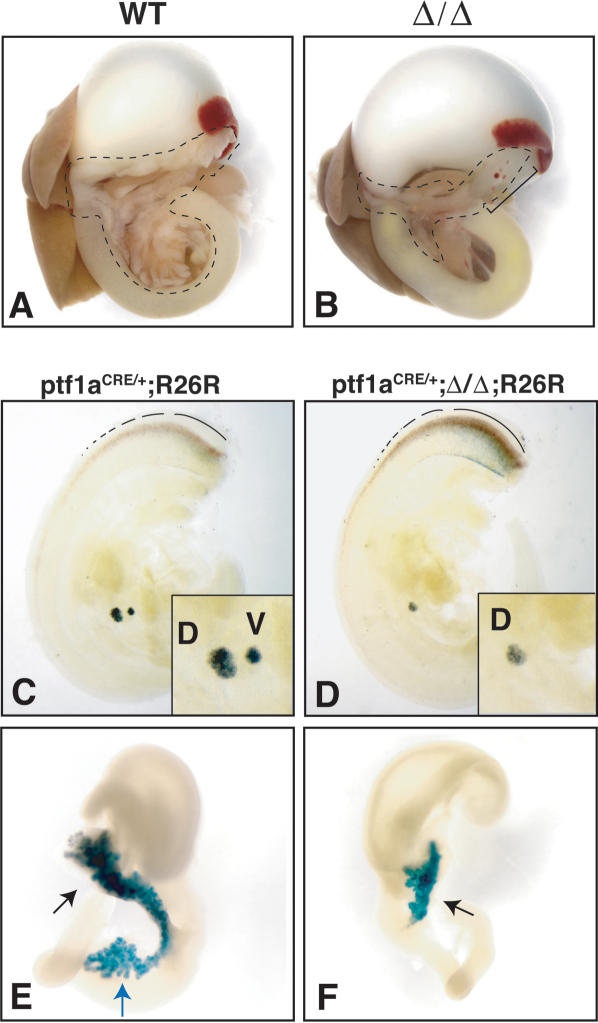
Figure 3.
Reduced Pdx1 function leads to dysmorphic growth from the dorsal bud and loss of ventral bud specification. (A,B) Pancreas is outlined in newborn pups. Ventral pancreatic lobe (intraduodenal loop region) appears absent in Pdx1ΔI-II-III/ΔI-II-III (Δ/Δ) mutants. Bracket in B indicates cystic pancreatic epithelium with associated ectopic splenic tissue. (C-F) Heterozygous mice carrying a Ptf1aCRE knock-in allele (Ptf1aCRE/+) together with the Cre-activatable R26R reporter allele were used to assess dorsal (d) and ventral (v) bud pancreas specification at E10.5 and E14.5. (C,D) In contrast to normal pancreas specification (C), E10.5 Δ/Δ mutants (D) lack the ventral bud region-specific activation of Ptf1a expression and have a reduced dorsal bud. (E,F) At E14.5, the dorsal outgrowth (black arrows) is highly stunted compared with normal, and there is no evidence of ventral lobe-derived cells (blue arrow in normal situation in E). Dotted lines in C and D denote Ptf1a expression in developing spinal cord.
Figure 4.
Distal dysmorphogenesis and proximal quasipancreatic differentiation in dorsal pancreas bud of Pdx1ΔI-II-III homozygous mice. All tissues are from P1 animals. (A,B) Distal region of dorsal bud tissue (D) differentiates as a simple dilated cystic epithelium (H&E staining), while gut-proximal tissue (P) undergoes quasipancreatic differentiation (cf. A), with a transitional zone between these regions. Blue hematoxylin-stained regions in A indicate endocrine compartment forming in newborn +/+ pancreas. (C-J) Immunohistochemical comparison of normal and gut-proximal pancreatic tissue of Pdx1ΔI-II-III/ΔI-II-III (Δ/Δ) animals. Well-polarized acinar cells express almost normal levels of amylase (AMY) (C,D), and there are substantial numbers of disorganized glucagon-producing cells (GLU) (E,F), but only a few, weakly positive insulin-expressing cells (G,H; INS, arrowheads in H). (H) Some insulin-producing cells are located in or adjacent to ductal epithelium (arrows). (I,J) Somatostatin-producing cells (SOM) are found as small cell clusters or isolated cells in close proximity to ducts. Forming endocrine compartments are outlined in C and D. Ducts are denoted by black arrowheads. Bars: A-B, 400 μm; C-J, 50 μm.
To gain insight into the etiology of pancreatic hypoplasia in Pdx1ΔI-II-III/ΔI-II-III mice, we investigated embryonic stages. Analysis at E12.0 revealed progressive pancreas-specific reduction in Pdx1 protein immunoreactivity in the Pdx1ΔI-II-III/ΔI-II-III and Pdx1ΔI-II-III/- mutants (cf. signal in caudal stomach vs. dorsal pancreas; Fig. 5A-C). The relative signal intensities were consistent in careful side-by-side processing of several serial sections of the pancreatic region across three embryos of each genotype. Examination of gut-proximal dorsal pancreatic tissue at E16.5 (Fig. 5D-F) revealed less intense Pdx1 signal in the developing acini, the epithelial cords, and ductal regions. In addition, there was a notable absence of focal high-level expressing cells, which could represent the precursors of endocrine islet cells based on their number and location tightly abutting the epithelial cords. The reduced level of Pdx1 throughout gestation may account for the reduced mass of acinar tissue and failed β-cell differentiation in Pdx1ΔI-II-III/ΔI-II-III mutants. While it has been reported recently that mature islets in Pdx1+/- animals are susceptible to apoptosis (Johnson et al. 2003), the phenomenon was observed in old mice (∼5 mo to 1 yr of age). Together with the finding of an early stage hypoplasia of the Pdx1ΔI-II-III/ΔI-II-III pancreatic bud (Figs. 3, 5), we therefore consider it unlikely that apoptosis is a major contributor to the reduced pancreas mass in Pdx1ΔI-II-III/ΔI-II-III animals. Furthermore, immunodetection of the proapoptotic cleaved form of caspase-3 revealed no increase in apoptosis in E16.5 or E18.5 Pdx1ΔI-II-III/ΔI-II-III tissue (data not shown).
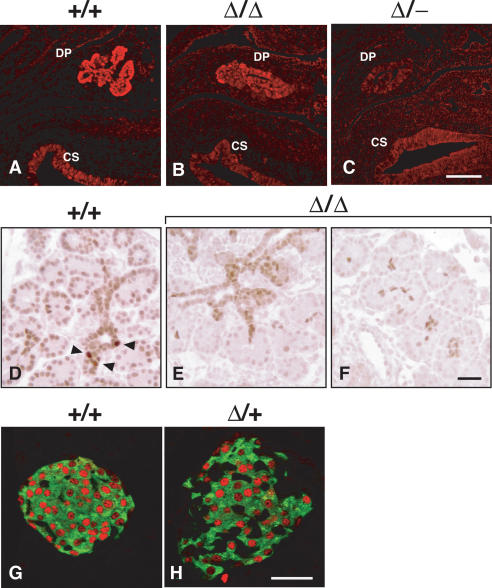
Figure 5.
Immunohistochemical assessment of Pdx1 protein expression levels with various combinations of Pdx1 deletion alleles. Tissue processing and immunostaining in parallel allowed comparison of cellular Pdx1 level; representative images are from serial section analysis of at least three embryos for each genotype. Confocal imaging and post-processing was identical for all samples; background is shown deliberately, so that the specific Pdx1 signal in the dorsal pancreas (DP) can be judged against it. (A-C) E12.0 tissues. Different combinations of mutant alleles affect Pdx1 levels in the early pancreas anlagen; signal intensity is similar in all cases in caudal stomach epithelium (CS). (D-F) E16.5 tissues. Embryos homozygous for the Pdx1ΔI-II-III allele (Δ/Δ) lack the focal high-level Pdx1-expressing cells (arrowheads) seen in normal tissue, with a significantly lower signal through the developing epithelial cords and acinar clusters. (G,H) Confocal immunofluorescence analysis on parallel-processed tissues from 4-wk-old mice from +/+ and Δ/+ adult islets (insulin, green; Pdx1, red). We noted an increased number of non-β cells (insulin-) in Δ/+ islets (Fig. 7, Supplementary Fig. 3). Bars: A-C, 50 μm; D-F, 50 μm; G,H, 50 μm.
Differential requirement for Area I-II-III for dorsal versus ventral pancreas specification
Since newborn Pdx1ΔI-II-III/ΔI-II-III animals appear to lack the ventral pancreas lobe (Fig. 3B), we used the genetic lineage-tracing approach of Kawaguchi et al. (2002) to test whether any ventral pancreatic tissue was specified in these mutants. This approach is powerful because it allows the progeny of cells that activate Ptf1a—an early marker that, within endoderm, is exquisitely specific for only the region that acquires the pancreatic fate—to be followed even if their abnormal genetic constitution causes them to move toward nonpancreatic fates, for example, taking up residence in and differentiating as duodenal cell types (Kawaguchi et al. 2002). We crossed Ptf1aCRE/+;Pdx1ΔI-II-III/+ with R26R;Pdx1ΔI-II-III/+ mice to obtain Ptf1aCRE/+;R26R and littermate Ptf1aCRE/+; R26R;Pdx1ΔI-II-III/ΔI-II-III mice. In these animals, Ptf1a-driven Cre expression excises a floxed stop cassette and activates β-galactosidase expression in a heritable cell-type-independent manner. In normal animals, Ptf1aCRE/+; R26R labeling traces pancreatic progenitors and their descendants in the duct, acinar, and endocrine lineages (Kawaguchi et al. 2002). Therefore, both dorsal and ventral pancreatic buds at E10.5 and both pancreatic lobes at E14.5 were labeled robustly in Ptf1aCRE/+;R26R mice (Fig. 3C,E). In contrast, at both the early and midgestation time points, there was no formation of putative ventral pancreas or its progenitors in Pdx1ΔI-II-III/ΔI-II-III mutants. Importantly, no ventral pancreas-specified progenitors led to progeny that differentiated as either pancreatic or intestinal cell types in the gut tube. This labeling strategy also showed that the dorsal bud was already stunted at the earliest time point, and was much smaller than normal at midgestation (Fig. 3D,F). The failure to activate Ptf1a expression shows that a specific level and/or timing of Pdx1 expression is required for the specification of the ventral pancreas; i.e., the production or survival of Ptf1a-expressing progenitor cells.
Gastro-duodenal defects limited to enteroendocrine cells in Pdx1ΔI-II-III/- mutants
It was previously shown that Pdx1-/- (global protein-null) animals display various defects in addition to pancreas agenesis, including a distorted pylorus, absence of Brunner's glands, and localized reductions in enteroendocrine cell numbers (Larsson et al. 1996; Offield et al. 1996; Jepeal et al. 2005). Many Pdx1-/- animals had pyloric atresia and the rostral-most duodenal cavity had an undulating appearance, lacking villi, that was lined by a simple cuboidal epithelium continuous with the common bile duct and dorsal pancreatic ductule (Fig. 2E; Offield et al. 1996). At P1, a substantial proportion of Pdx1-/- pups showed stomach distension caused by poor gastric emptying likely associated with the malformed gastro-duodenal junction (Offield et al. 1996). In contrast, a distended stomach was not observed in Pdx1ΔI-II-III/- mutants (n = 12). The gastro-duodenal junction developed normally in Pdx1ΔI-II-III/- mice, and the smooth muscle bands of the pyloric ring, columnar epithelium of the rostral duodenal villi (Fig. 2F), and Brunner's glands (Supplementary Fig. 1) were all well formed. Pdx1 protein was detected at E16.5 in the rostral duodenum (Fig. 2J-L) and antral stomach epithelium (data not shown) of Pdx1ΔI-II-III/- animals, although the level was lower than in Pdx1+/+ animals (Fig. 2J,L); similar findings were made at E18.5 (data not shown). Therefore, while Pdx1 expression in the Pdx1ΔI-II-III/- animals is sufficient for normal morphological formation of the gastro-duodenal junction and Brunner's gland differentiation, the reduced level, or abnormalities in the spatiotemporal expression profile, cannot support pancreatic development over and above the level seen in Pdx1-/- mutants. The very low level of Pdx1 in the Pdx1ΔI-II-III/- dorsal pancreas detected at E12.0 (Fig. 5) was transient, not being detected at late gestation or in neonates (data not shown).
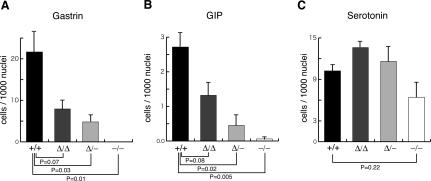
We and others have reported that Pdx1-/- (protein-null) animals have reduced numbers of enteroendocrine cells in the rostral duodenum and adjacent antral stomach (Larsson et al. 1996; Offield et al. 1996; Jepeal et al. 2005). Because Area I-II-III contains enhancer-like elements that drive reporter expression in a pancreatic endocrine-specific manner in transgenic animals and cell culture (Wu et al. 1997; Gerrish et al. 2000; Gannon et al. 2001), it may also allow Pdx1 expression to reach a specific threshold associated with specification, differentiation, and/or maintenance of enteroendocrine cell types. As shown in Figure 6, there was a dramatic reduction in Pdx1-/- animals of gastrin-producing cells in antral stomach (100% loss at P7) and GIP-producing cells in rostral duodenum (96.5% reduction at E18.5), confirming a central requirement for Pdx1 in establishing these cell populations. Moving from Pdx1ΔI-II-III/ΔI-II-III to Pdx1ΔI-II-III/- led to a step-wise reduction in gastrin-producing cells (Fig. 6A; 63.4% and 77.8% reduction, respectively) and GIP-producing cells (Fig. 6B; 51.3% and 83.0% reduction, respectively), without significant alteration in the number of serotonin-producing cells (Fig. 6C). These results suggest that gastrin- and GIP-producing cells require wild-type levels and/or timing of Pdx1 expression that is dependent upon the cis-activity of Area I-II-III.
Figure 6.
Frequency of gastrin- and GIP-producing cells as a function of Pdx1 genotype. Sections analyzed were E18.5 for GIP- and serotonin-producing cells, and P7 for gastrin-producing cells. Results are from analyzing three mice of each genotype (see Materials and Methods for tissue sampling protocol). The Y-axis represents the number of gastrin-, GIP-, or serotonin-producing cells per 1000 epithelial nuclei; data expressed as mean ± SE (n = 3).
Pdx1ΔI-II-III/+ animals have impaired islet architecture and maturity-onset diabetes
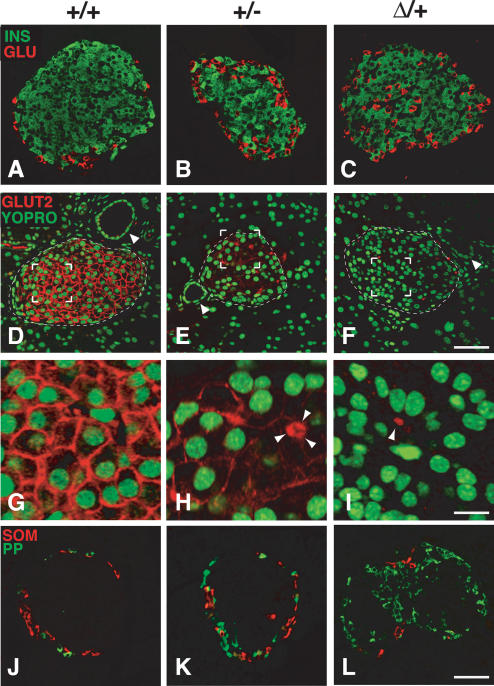
Mice with a heterozygous deletion of Area I-II-III (Pdx1ΔI-II-III/+) were fully viable, fertile, and indistinguishable in body weight from control littermates. They had a normal-sized pancreas (data not shown), with an islet size distribution similar to wild type (Supplementary Fig. 2). However, the islets of Pdx1ΔI-II-III/+ animals had a severely altered structure, containing significantly increased numbers of PP- and glucagon-producing cells compared with age-matched wild-type littermates (Supplementary Fig. 3). Glucagon-producing cells in Pdx1ΔI-II-III/+ islets were most often intermixed with β cells, and not peripherally located as in wild type (Fig. 7A-C). In wild-type islets, Glut2, a key component of the glucose-stimulated insulin-secretion machinery (Thorens 2001), was localized to the β-cell membrane (Fig. 7D,G), but undetectable in the majority of islets in Pdx1ΔI-II-III/+ animals, while Pdx1+/- littermates showed moderately reduced Glut2 (Fig. 7, cf. E,H and F,I), as reported previously (Ahlgren et al. 1998; Brissova et al. 2002; Johnson et al. 2003). These results indicate that Area I-II-III plays a key role in establishing and/or maintaining the normal ratio of pancreatic endocrine cell types, with heterozygous deletion also affecting the differentiated state of the β cells.
Figure 7.
The Pdx1ΔI-II-III/+ genotype leads to severely compromised mature islet architecture and endocrine differentiation. All tissues analyzed were from 4-wk-old mice. (A-C) Pdx1ΔI-II-III/+ (Δ/+) mice show a reproducible increase and intra-islet scattering of glucagon cells (see also Supplementary Fig. 3) (D-F) β-Cell-selective expression of Glut2 is modestly decreased in Pdx1+/- (+/-) animals, but is absent in Δ/+ animals (arrowheads indicate ducts). (G-I) High magnification of bracketed region in D-F (arrowheads in H,I indicate autofluorescent erythrocytes in capillaries to show gain setting equivalence across the three panels; three erythrocytes clustered in H). (J-L) Frequency of PP cells is dramatically increased in Δ/+ animals; somatostatin cells are relatively unchanged in number and location (see also Supplementary Fig. 3). Bars: A-F, 50 μm; G-I, 12.5 μm; J-L, 50 μm.
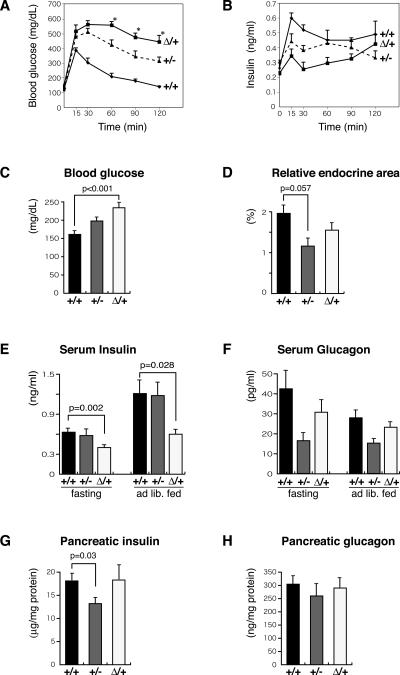
The islet architecture alterations in Pdx1ΔI-II-III/+ mice were associated with highly defective glucose homeostasis. Blood glucose levels were similar in newly weaned 3-wk-old ad libitum-fed Pdx1ΔI-II-III/+, Pdx1+/+, and Pdx1+/- littermates (data not shown). At 10 wk, however, Pdx1ΔI-II-III/+ mice showed statistically higher glucose levels (Fig. 8C) and significantly reduced serum insulin (Fig. 8E), suggesting the onset of defective islet function. A gross evaluation of overall islet function was made by IP-GTT at 8-11 wk of age (Fig. 8A). Consistent with previous reports (Dutta et al. 1998; Brissova et al. 2002; Johnson et al. 2003), Pdx1+/- mutant animals had impaired glucose clearance compared with wild type. However, Pdx1ΔI-II-III/+ animals that were, importantly, derived from the same litters (see Discussion), displayed even worse glucose clearance. A substantial number of the Pdx1ΔI-II-III/+ animals showed off-scale glucose measurements (i.e., >600 mg/dL) at the early IP-GTT time points (see the legend for Fig. 8). Measuring plasma insulin from the same samples shown in Figure 8A showed that it was inappropriately low in Pdx1ΔI-II-III/+ mice at 15 min after glucose challenge, with an overall delayed and smaller increase (Fig. 8B). There was no appreciable alteration in total cellular insulin content in Pdx1ΔI-II-III/+ pancreata (Fig. 8G); furthermore, there was no hyperglucagonemia or change in pancreatic glucagon (Fig. 8F,H). Thus, disruption of insulin secretion in Pdx1ΔI-II-III/+ mice appears to be the major contributor to the observed hyperglycemia, and an underlying mechanistic defect could be the drastically reduced Glut2 in the β cells of these mice.
Figure 8.
Impaired glucose homeostasis in Pdx1ΔI-II-III/+ animals. Glucose clearance (A) and corresponding serum insulin levels (B) during IP-GTT (data from 8- to 11-wk-old female offspring from three litters resulting from crosses of Pdx1+/- [+/-] with Pdx1ΔI-II-III/+ [Δ/+] mice; N analyzed = 4 +/+, 5 +/-, and 5 Δ/+). (*) P < 0.05 compared with +/- mice. Note: In A, two mice showed 30 and 60 min off-scale measurments, which were assigned values of 600 mg/dL for the mean calculated here. (C) Serum glucose levels in +/+ (n = 7), +/- (n = 6) and Δ/+ littermates (n = 7) in ad lib. fed 10-wk-old mice. (D) Relative pancreatic endocrine area, 10-wk-old mice, as a percentage relative to the whole pancreas area (means ± SE, n = 3). (E) Serum insulin levels in +/+ (n = 10), +/- (n = 6), and Δ/+ (n = 10) adults (8-11 wk) in overnight fasting and ad lib. fed conditions. (F) Serum glucagon levels in +/+ (n = 10), +/- (n = 7), and Δ/+ (n = 10) adults (8-11 wk old) in overnight fasting and ad lib. fed conditions. (G,H) Total extractable pancreatic insulin and glucagon levels in +/+ (n = 6), +/- (n = 5), and Δ/+ (n = 7) adults (8-11 wk old) during ad lib. feeding.
Discussion
Our experiments reveal an essential in vivo function in foregut organogenesis for the conserved upstream Area I-II-III cis-regulatory region of Pdx1. A major finding from these studies is that the various organs and tissue derivatives of the posterior foregut (caudal stomach, pylorus, rostral duodenum, and pancreas) are differentially sensitive to the quality of Pdx1 expression—i.e., the spatiotemporal and level characteristics—which is substantially altered when Area I-II-III is deleted. Moreover, there is a differential requirement for Pdx1 in specifying the dorsal or ventral pancreatic bud, and our results suggest that the dorsal bud contains different classes of progenitors with respect to their ability to move through the pancreatic differentiation program. These findings set the stage for the future iterative dissection in vivo of specific subregions and selected motifs within this ∼1-kb region, which contains highly clustered binding sites for a variety of well-known endoderm and pancreas transcription factors. Based upon the level of analysis compared with other pancreas genes, Pdx1 could be considered an important reference gene, as it may represent a nexus for the complex signaling and transcriptional inputs that orchestrate proliferation, outgrowth, and differentiation of this organ. It will be particularly relevant to determine how regionalized intercellular signals lead to the dynamic level and timing of Pdx1 expression in specific cell types through the assembly of transcriptional complexes at the various motifs in Area I-II-III, and how these control lineage diversification decisions or, later, affect the maintenance of the mature cell state. Clearly, a substantial hurdle to overcome in such studies will be the current paucity of markers that define critical progenitor subtypes in the pancreatic endocrine differentiation program. Information on the networks orchestrating endodermal progenitor cell differentiation could have a large impact on protocols for directed tissue differentiation leading toward cell-based therapies for diabetes.
Dosage effects of Pdx1 on foregut development and pancreas organogenesis
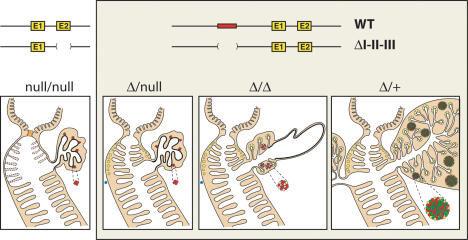
Figure 9 summarizes how foregut development is affected by the various combinations of Pdx1ΔI-II-III, Pdx1-, and wild-type alleles. Notably, removing Area I-II-III does not inactivate the gene, but instead generates a hypomorphic allele in terms of its level and spatiotemporal expression characteristics. This finding is consistent with the recent characterization of cell-type-specific, orientation-independent enhancer activities within Areas I and II (Van Velkinburgh et al. 2005). In several tissues, the level of nuclear Pdx1 produced from the Pdx1ΔI-II-III allele was notably lower than from a wild-type allele. In addition, the high-level expression that is normally present in subsets of the progenitor-harboring pancreatic epithelium during the secondary transition stages of pancreatic organogenesis was absent in the Pdx1ΔI-II-III/ΔI-II-III state. Our preliminary data (data not shown) suggest that Pdx1 expression from the Pdx1ΔI-II-III allele within the foregut epithelium at approximately E9.5 is delayed substantially compared with wild type. Thus, both the spatiotemporal and level characteristics of Pdx1 are affected by removing Area I-II-III.
Figure 9.
Schematic summary: differential effects of Pdx1 dosage on pancreas and foregut epithelial development. The previous protein-null (Offield et al. 1996) and ΔI-II-III alleles are represented. Homozygous protein-null embryos frequently lack villi in the rostral-most duodenum (their variable presence indicated by lighter shading and dotted lines), with severe pyloric dysmorphology (light-orange plug). Brunner's glands are absent from the duodenal collar region adjacent to the pylorus. Not represented here is the enteroendocrine cell deficiency in stomach and duodenum (see text). One copy of the partially functional ΔI-II-III allele restores differentiation of the pylorus, Brunner's glands, and rostral duodenal villi, but the pancreas remains highly abrogated (Figs. 3, 4). Embryos homozygous for the ΔI-II-III allele exhibit quasi-pancreatic differentiation of a dorsal bud-derived pancreatic rudiment proximal to the gut tube, and a distal cystic epithelium (Fig. 4). (Blue dots) Lack of ventral bud specification as marked by Ptf1a-Cre;R26R lineage tracing (Fig. 3). The Δ/+ combination allows full-sized outgrowth of both dorsal and ventral buds, but the islets are highly abnormal (Fig. 7). (Yellow) Acini and Brunner's glands; (red) glucagon-producing α cells; (green) insulin-producing β cells.
The overall picture that emerges is that the morphogenesis of the caudal stomach, pylorus, and rostral duodenum (including its villi and Brunner's glands) exhibit the lowest sensitivity to reducing the quality of Pdx1 gene expression. Within the foregut region gut epithelium, however, there remains a requirement for normal Pdx1 expression for the differentiation of GIP- and gastrin-producing enteroendocrine cells. In contrast to gut morphogenesis, pancreas development is remarkably sensitive to interference with Pdx1 function. The ventral bud is not specified in the Pdx1-/-, Pdx1ΔI-II-III/-, and Pdx1ΔI-II-III/ΔI-II-III conditions, and while the gut-proximal region of the Pdx1ΔI-II-III/ΔI-II-III dorsal bud forms abnormal pancreatic tissue, the distal region only forms a duct-like cystic epithelium.
Dorsal versus ventral pancreas specification programs and progenitor classes
Our lineage-tracing experiments showed that it is not just ventral pancreatic bud outgrowth that is abrogated in Pdx1ΔI-II-III/ΔI-II-III animals, but that its initial specification is blocked, as evaluated via the Ptf1a-Cre/R26R method. The failure to activate Ptf1a expression shows that ventral bud specification has a more stringent threshold requirement for Pdx1, because the delayed and reduced expression in the Pdx1ΔI-II-III/ΔI-II-III configuration at least initiates the dorsal bud pancreatic program. Differences in the genetic pathways of dorsal versus ventral pancreas differentiation are also known for other genes, such as Isl1, Hlxb9, and Flk1 (Ahlgren et al. 1997; Harrison et al. 1999; Li et al. 1999; Yoshitomi and Zaret 2004), but our approach is different in using lineage labeling to assess the initial tissue specification process.
In Pdx1ΔI-II-III/ΔI-II-III animals, the cystic epithelium seen in gut-distal regions of the dorsal pancreas contrasted the more differentiated gut-proximal pancreatic region. It is possible that the distinct differentiation of the two regions arises through interactions with surrounding tissues (intestine, mesenchyme), and/or that initially intermixed progenitor populations become spatially separated during bud outgrowth. In either case, the differential response of dorsal pancreatic progenitors to reduced quality of Pdx1 expression suggests that the normal pancreatic buds harbor qualitatively different progenitors. The particular response of distinct progenitor populations to the titer or timing of Pdx1 expression may be relevant to methods of enforcing pancreatic differentiation from surrogate cells. ES cell differentiation in the presence of activin can produce endoderm, and allow formation of a small proportion of pancreas-like cells (Kubo et al. 2004; Tada et al. 2005). More efficient commitment of cells to the pancreatic fate may require careful control of the level of Pdx1 expression at precise stages of differentiation.
Pdx1 regulation in enteroendocrine and gut epithelial cell differentiation
Our previous studies (Wu et al. 1997; Gerrish et al. 2000, 2001; Gannon et al. 2001) in transgenic mice and in vitro cell culture focused on pancreatic enhancer-like activities of Area I-II-III and not whether this region regulates Pdx1 in gut enteroendocrine cells. Gastrin-producing cells in the antral stomach-pyloric mucosa and GIP-producing cells in the rostral duodenum strictly depend on the presence of Area I-II-III, and are thus similar to pancreatic β cells. This result strongly suggests that overlapping sets of upstream transcriptional regulators for Pdx1 drive endocrine differentiation in the pancreas and gut. There is evidence that transcriptional regulators of endocrine pancreas development, such as Ngn3, NeuroD, Nkx6.1, Pax6, and Pax4, are essential for enteroendocrine differentiation (Naya et al. 1997; Larsson et al. 1998; Larsson 2000; Jenny et al. 2002; Lee et al. 2002). Of these factors, null mutants for Pax6 have a characteristic phenotype in the enteroendocrine lineages—elimination of duodenal GIP cells and gastrin cells in the antral stomach—that is reminiscent of Pdx1-null mutants (Larsson et al. 1998). Pax6 can bind to Area II in the Pdx1 promoter (Samaras et al. 2002), suggesting a fundamental role in the endocrine-specific regulation of the Pdx1 gene in both pancreas and gut.
The enterocytes and Brunner's gland cells, which are affected in Pdx1-null mice, underwent normal differentiation in Pdx1ΔI-II-III/ΔI-II-III or Pdx1ΔI-II-III/- animals. Other elements must therefore regulate the basal transcription of Pdx1 or generate enough cis-activation for the normal differentiation of the caudal stomach, pylorus, bile duct, and duodenum. Currently, we hypothesize that distal 5′ regions, such as the phylogenetically conserved Area IV (Sharma et al. 1997; Gerrish et al. 2004), or more distributed cis-regulatory motifs, are involved in driving normal expression in the duodenum and stomach (D. Boyer and C. Wright, unpubl.). Future discoveries may reveal that potentiation of Area I-II-III activity by these elements (Gerrish et al. 2004) fine-tunes Pdx1 expression in the pancreatic endocrine lineage.
Etiology of the diabetic phenotype in Pdx1ΔI-II-III/+ mutants
By several measurements—serum insulin and glucose levels under ad lib. feeding or glucose challenge, abnormal islet architecture—Pdx1ΔI-II-III/+ mice are more severely affected than their Pdx1+/- littermates, despite similar pancreatic insulin content. One major contributor to the phenotype may be the lack of Glut2 on the β cells of Pdx1ΔI-II-III/+ islets compared with the moderate reduction in Pdx1+/- mice (Fig. 7). Glut2 is required for glucose-stimulated insulin secretion in mice (Guillam et al. 1997) and is a β-cell gene target of Pdx1 (Waeber et al. 1996). Quantitative Western blot analysis (data not shown) detected a similar overall Pdx1 level in Pdx1ΔI-II-III/+ and Pdx1+/- pancreata, and Pdx1ΔI-II-III/+ islets faithfully expressed Pdx1 in β cells (Fig. 5G,H), suggesting that it is not lower Pdx1 levels or inconsistent expression that lead to the lack of Glut2. We hypothesize that an underlying defect in islet genesis and organization is responsible for a profound disruption of the glucose-sensing machinery. Other mouse models in which peripheral islet cells mix into the islet core, such as enforced expression of HNF6 or dominant-negative HNF1α (Gannon et al. 2000; Yamagata et al. 2002), also show absent or largely reduced Glut2 expression. Mixing of peripheral cell types with β cells may disrupt the gap junctional or other coupling that is involved in a concerted, efficient insulin release (Caton et al. 2002).
Heterozygous enhancer deletion and the mechanism of disruption of islet morphology
The mixed islet phenotype and abnormally high α, δ, and PP cell numbers in Pdx1ΔI-II-III/+ mice compared with wild-type or Pdx1+/- mice (Supplementary Fig. 3) suggests that loss of one copy of the Area I-II-III region leads to defective lineage allocation from the endocrine progenitors. At the current time, mostly related to a lack of knowledge on how to mark specific progenitor classes, this result cannot be explained mechanistically. However, we consider it unlikely that the difference is attributable to genetic background differences between the various mutant combinations. All comparisons between wild-type, Pdx1ΔI-II-III/+, and Pdx1+/- were between siblings, and the results were consistent among multiple litters. Furthermore, analysis of mice bearing the floxed allele from which the Pdx1ΔI-II-III allele was derived showed no defects in islet architecture in Pdx1floxI-II-III/+, Pdx1floxI-II-III/floxI-II-III, or Pdx1floxI-II-III/- pancreata (data not shown). One potential reason that heterozygosity for Area I-II-III is more severe than heterozygosity for the protein-coding region could be that its enhancer activity also affects the expression of another gene involved in islet progenitor differentiation. Candidate genes are the homeobox genes flanking Pdx1 in the “Parahox cluster”, Gsh1 and Cdx2. Both genes may be expressed in the developing pancreas and mature islets (Rosanas-Urgell et al. 2005), and Cdx2 can transcriptionally activate Glucagon in pancreatic cell lines (Andersen et al. 1999). It is also possible that deletion of Area I-II-III eliminates a repressor element resulting in ectopic expression of Pdx1; however, no ectopic expression was observed in any tissues at any of the stages examined. Another possibility is that Area I-II-III heterozygosity blunts a large-scale fold increase in Pdx1 expression that is linked to the channeling of cells from a common endocrine precursor pool toward the β-cell fate. Lineage-tracing experiments show that all pancreatic endocrine precursors express Pdx1 (Gu et al. 2002), and in the mature organ Pdx1 is found at the highest levels in β cells (Guz et al. 1995). The Pdx1ΔI-II-III allele produces a substantial baseline amount of Pdx1 in immature endoderm (Figs. 2L, 5B), but is deficient for high-level expression (Fig. 5D-F), consistent with the idea (see above) that Area I-II-III trans-activators promote high-level β-cell-selective expression. It is therefore plausible that the fold increase between baseline and maximum expression levels would be reduced in Pdx1ΔI-II-III/+ mice compared with wild type, while the active allele in +/- mice would still achieve a fold-increase similar to wild type. If the efficiency of converting common endocrine precursors to committed β-cell progenitors depends on the degree of up-regulation of Pdx1 expression at a specific lineage commitment point, then the selective impairment to high-level expression could lead to cells defaulting to the other endocrine fates.
Conserved Pdx1 enhancers as targets for MODY syndrome
Various transcriptional activators implicated in the human MODY syndrome, or as major regulators of endoderm development, have been shown capable of binding within Area I-II-III: HNF1α, Foxa2/HNF3β, Pdx1 itself, Maf, Pax6, and HNF6 (Gerrish et al. 2000, 2001; Marshak et al. 2000; Ben-Shushan et al. 2001; Samaras et al. 2002, 2003; Jacquemin et al. 2003). In addition, MODY factors have been reported to synergistically regulate Pdx1 expression in β cells in vivo (Shih et al. 2002). These findings suggest that Area I-II-III may be a major site of action for transcription factors implicated in MODY. Consistent with this hypothesis, we found that heterozygous deletion of Area I-II-III results in post-weaning diabetes as early as 4 wk of age. Although mutations in the equivalent region of human IPF1 have not yet been identified, it is possible that even heterozygous Area I-II-III mutations/deletions, or reductions in the level or activity of trans-acting MODY factors that operate through Area I-II-III, could be a significant underlying influence in MODY and type II diabetes.
Among previously characterized mutants for the factors that can bind within Area I-II-III, the Hnf6-null mice (Jacquemin et al. 2003) exhibit delayed and reduced Pdx1 expression similar to that of Pdx1ΔI-II-III/ΔI-II-III mutants. Although the pancreatic phenotype of Hnf6-/- seems less severe overall, both types of mutant have delayed pancreas specification, impaired endocrine differentiation, and blocked ventral bud outgrowth. The phenotypic similarities raise the possibility that HNF6 may be a key regulator of Pdx1 via Area I-II-III during the early phases of pancreas formation. A future goal will be to test rigorously the contribution of individual trans-acting factors to the Pdx1ΔI-II-III/ΔI-II-III phenotype by altering specific binding motifs in Area I-II-III.
Materials and methods
PdxArea I-II-III flox mice
All animal experiments were performed in accordance with the guidelines of the Vanderbilt University Animal Care and Use Committee. Details of the targeting vector construction are available upon request from Y. Fujitani. In brief, a 5′-located 3.4-kb XbaI-PstI fragment (converted to a SalI-ClaI fragment; 5′ arm), a 1.2-kb PCR fragment with added HindIII ends encompassing Area I-II-III, and an ∼10-kb fragment encompassing the Pdx1 gene from a NotI site added by PCR immediately 3′ of Area III to the SacI site ∼3 kb 3′ of the stop codon (3′ arm) were subcloned into pFRT.loxP (vector from M.A. Magnuson), which contains a thymidine kinase cassette, FRT-flanked neomycin resistance cassette, and loxP-flanked multiple cloning site. Linearized targeting vector was electroporated into ES cells (TL1/129S6 strain) and clones selected for homologous integration. Southern analysis of BamHI-digested DNA from doubly resistant ES cell clones revealed a targeting rate of 5.5% (10/182 clones). ES cells were injected into C57BL/6 blastocysts that were transferred into pseudopregnant ICR female mice; male chimeras were bred to Black Swiss females and agouti offspring genotyped by Southern analysis. Heterozygous offspring were bred to ACTB:FLPe mice (see below) to remove the neomycin-resistance cassette.
Other genetically engineered animals
EIIA-cre (H. Westphal, National Institutes of Health, Bethesda, MD) and ACTB:FLPe mice (S.M. Dymecki, Harvard Medical School, Boston, MA) were provided by B. Hogan (Duke University, Durham, NC). PdxlacZko and Ptf1aCRE/+ mice were described (Offield et al. 1996; Kawaguchi et al. 2002). All mice were kept on a C57BL/6 and DBA mixed-inbred background. Genotyping was by PCR or Southern blot with tail DNA from animals staged E16.5 through adult. DNA for genotyping was isolated from heads of E14.5 or earlier embryos. Primer sequences for PCR genotyping are available upon request to Y. Fujitani.
X-gal staining
Embryos or dissected tissues were fixed (ice-cold 4% paraformaldehyde/PBS, 4°C, 60 min) then washed twice in permeabilization solution (2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40 in PBS). β-Galactosidase was detected using X-gal (Wu et al. 1997). Samples were post-fixed and dehydrated for embedding as described below. Photographs were taken on an SZX12 microscope (Olympus) and Spot digital camera.
Histology
Dissected tissues were fixed (ice-cold 4% paraformaldehyde, 4°C, 60 min), dehydrated in an ethanol series, washed into Histo-Clear (National Diagnostics), infiltrated in Histo-Clear/paraffin (1:1 v/v) and two changes of paraffin under vacuum at 56°C, embedded, and cut into 5-μm sections.
Immunohistochemistry and confocal image analysis
Hematoxylin-eosin (H&E) staining and periodic acid-Schiff staining were as described (Offield et al. 1996). For immunoperoxidase, Vectastain ABC kit (Vector Labs) was used. Primary antibodies: guinea pig anti-bovine insulin (Linco), 1:10,000; anti-glucagon (Linco), 1:10,000; rabbit anti-somatostatin (Dako), 1:1000; guinea pig anti-pancreatic polypeptide (Linco), 1:2000; rabbit anti-amylase (Sigma), 1:20,000; rabbit anti-Pdx1 (Peshavaria et al. 1994; Kawaguchi et al. 2002), 1:5000. Antibodies for immunofluorescence: guinea pig anti-Pdx1 (Hingorani et al. 2003), 1:5000; rabbit anti-Pdx1, 1:250; rabbit anti-rat Glut2 (Alpha Diagnostic), 1:200. Secondary antibodies: CY2-conjugated donkey anti-guinea pig IgG (Jackson ImmunoReaerch laboratories, for insulin), CY3-conjugated donkey anti-rabbit IgG (for glucagon and Glut2) and CY5-conjugated anti-guinea pig IgG (Pdx1 staining in Fig. 2). Some samples were counterstained with the nuclear dye, YO-PRO-1 (Molecular Probes; 1: 1000 in PBS). Immunofluorescence was imaged on a Zeiss LSM 510 confocal microscope. TIFF images were processed in Adobe Photoshop.
Quantification of gastrin-, GIP-, and serotonin-positive cells
E18.5 foregut sections were immunostained with rabbit GIP antiserum (Research Diagnostics) or mouse serotonin monoclonal antibody (Dako). P7 distal stomach was immunostained with rabbit gastrin antiserum (Research Diagnostics). Sections were subjected to immunoperoxidase staining and light hematoxylin counterstain to reveal nuclei and general morphology. For each animal, cell counting was performed on five nonadjacent longitudinal sections, to avoid double scoring, within an area bounded by a 2:1 ratio rectangle whose short side was similar to the gut diameter (∼900 μm), short side adjacent to the pylorus. For Pdx1-/- mutants, the rectangle was shifted ∼0.3 mm distal to the pylorus to avoid the abnormal cuboidal epithelium in the rostral duodenum. Eight fields (two rows of four nonoverlapping fields [340 μm × 450 μm] running down each side of the rectangle, with the gut wall included at one edge) of each section were photographed (SPOT camera) and printed at 450× magnification to count gastrin-, GIP-, or serotonin-positive cells, and the total number of epithelial cells.
Glucose tolerance tests (IP-GTT)
Following a 16- to 18-h fast, baseline blood glucose levels (milligrams per deciliter) were measured in tail-vein blood by BD Logic glucose monitor (Becton Dickinson). Dextrose (2 mg/g body weight) was injected intraperitoneally and blood glucose measured at 15, 30, 60, 90, and 120 min after injection. For simultaneous measurement of plasma insulin, blood was obtained via the saphenous vein.
Radioimmunoassay
Plasma insulin and glucagon were assayed by radioimmunoassay (RIA; Linco) according to the manufacturer's protocol. Pancreatic insulin and glucagon content was assessed by RIA in acid-ethanol extracts of whole pancreas.
Islet morphometric analysis
Relative endocrine area was measured using NIH Image 1.41 software. For each experimental group, 10-wk-old mice were used. There was no statistical difference in pancreas weight among these mice. A total of nine sections were prepared from three pancreata (three sections per mouse). Each section was separated enough to avoid double scoring the same islet. The complete tissue area over all three sections was determined and percentage represented by islet cells calculated.
Statistics
Data are expressed as mean ± SE. A two-way ANOVA for repeated measures was used to analyze time course differences between groups. When significant changes were obtained over time, post hoc comparisons were made by paired t-test. Pairwise comparison for the number of enteroendocrine cells, blood glucose levels, plasma insulin and glucagon, pancreatic insulin and glucagon, and relative endocrine area was made by unpaired t-test. P values <0.05 were considered statistically significant.
Acknowledgments
We thank A. Means, K. Gerrish, S. Samaras, D. Melton, G. Gu, and members of the Wright, Stein, and Magnuson labs for discussions, and P. Soriano for R26R mice. Support from the Transgenic Mouse/ES Cell Shared Resource, Cell Imaging Core, Sequencing Core, and Histology Core at Vanderbilt University is acknowledged. This work was funded in part by Research Fellowships of the Japan Society for the Promotion of Science and Juvenile Diabetes Research Foundation International (to Y.F.) and NIH Grant U19 DK 042502 (to C.V.E.W.).
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1360106.
References
- Ahlgren, U., Pfaff, S.L., Jessell, T.M., Edlund, T., and Edlund, H. 1997. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 385: 257-260. [DOI] [PubMed] [Google Scholar]
- Ahlgren, U., Jonsson, J., Jonsson, L., Simu, K., and Edlund, H. 1998. β-Cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes & Dev. 12: 1763-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, F.G., Heller, R.S., Petersen, H.V., Jensen, J., Madsen, O.D., and Serup, P. 1999. Pax6 and Cdx2/3 form a functional complex on the rat glucagon gene promoter G1-element. FEBS Lett. 445: 306-310. [DOI] [PubMed] [Google Scholar]
- Bell, G.I. and Polonsky, K.S. 2001. Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414: 788-791. [DOI] [PubMed] [Google Scholar]
- Ben-Shushan, E., Marshak, S., Shoshkes, M., Cerasi, E., and Melloul, D. 2001. A pancreatic β-cell-specific enhancer in the human PDX-1 gene is regulated by hepatocyte nuclear factor 3β (HNF-3β), HNF-1α, and SPs transcription factors. J. Biol. Chem. 276: 17533-17540. [DOI] [PubMed] [Google Scholar]
- Brissova, M., Shiota, M., Nicholson, W.E., Gannon, M., Knobel, S.M., Piston, D.W., Wright, C.V., and Powers, A.C. 2002. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 277: 11225-11232. [DOI] [PubMed] [Google Scholar]
- Caton, D., Calabrese, A., Mas, C., Serre-Beinier, V., Wonkam, A., and Meda, P. 2002. β-cell crosstalk: A further dimension in the stimulus-secretion coupling of glucose-induced insulin release. Diabetes Metab. 28: 3S45-3S53; discussion 3S108-3S112. [PubMed] [Google Scholar]
- Dutta, S., Bonner-Weir, S., Montminy, M., and Wright, C. 1998. Regulatory factor linked to late-onset diabetes? Nature 392: 560. [DOI] [PubMed] [Google Scholar]
- Gannon, M., Ray, M.K., Van Zee, K., Rausa, F., Costa, R.H., and Wright, C.V. 2000. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of β cell function. Development 127: 2883-2895. [DOI] [PubMed] [Google Scholar]
- Gannon, M., Gamer, L.W., and Wright, C.V. 2001. Regulatory regions driving developmental and tissue-specific expression of the essential pancreatic gene pdx1. Dev. Biol. 238: 185-201. [DOI] [PubMed] [Google Scholar]
- Gerrish, K., Gannon, M., Shih, D., Henderson, E., Stoffel, M., Wright, C.V., and Stein, R. 2000. Pancreatic β cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3β sites. J. Biol. Chem. 275: 3485-3492. [DOI] [PubMed] [Google Scholar]
- Gerrish, K., Cissell, M.A., and Stein, R. 2001. The role of hepatic nuclear factor 1α and PDX-1 in transcriptional regulation of the pdx-1 gene. J. Biol. Chem. 276: 47775-47784. [DOI] [PubMed] [Google Scholar]
- Gerrish, K., Van Velkinburg, J.C., and Stein, R. 2004. Conserved transcriptional regulatory domains of the pdx-1 gene. Mol. Endocrinol. 18: 533-548. [DOI] [PubMed] [Google Scholar]
- Gu, G., Dubauskaite, J., and Melton, D.A. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447-2457. [DOI] [PubMed] [Google Scholar]
- Guillam, M.T., Hummler, E., Schaerer, E., Yeh, J.I., Birnbaum, M.J., Beermann, F., Schmidt, A., Deriaz, N., and Thorens, B. 1997. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat. Genet. 17: 327-330. [DOI] [PubMed] [Google Scholar]
- Guz, Y., Montminy, M.R., Stein, R., Leonard, J., Gamer, L.W., Wright, C.V., and Teitelman, G. 1995. Expression of murine STF-1, a putative insulin gene transcription factor, in β cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development 121: 11-18. [DOI] [PubMed] [Google Scholar]
- Harrison, K.A., Thaler, J., Pfaff, S.L., Gu, H., and Kehrl, J.H. 1999. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat. Genet. 23: 71-75. [DOI] [PubMed] [Google Scholar]
- Hebrok, M., Kim, S.K., and Melton, D.A. 1998. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes & Dev. 12: 1705-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani, S.R., Petricoin, E.F., Maitra, A., Rajapakse, V., King, C., Jacobetz, M.A., Ross, S., Conrads, T.P., Veenstra, T.D., Hitt, B.A., et al. 2003. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4: 437-450. [DOI] [PubMed] [Google Scholar]
- Holland, A.M., Hale, M.A., Kagami, H., Hammer, R.E., and MacDonald, R.J. 2002. Experimental control of pancreatic development and maintenance. Proc. Natl. Acad. Sci. 99: 12236-12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin, P., Lemaigre, F.P., and Rousseau, G.G. 2003. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev. Biol. 258: 105-116. [DOI] [PubMed] [Google Scholar]
- Jenny, M., Uhl, C., Roche, C., Duluc, I., Guillermin, V., Guillemot, F., Jensen, J., Kedinger, M., and Gradwohl, G. 2002. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21: 6338-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, J. 2004. Gene regulatory factors in pancreatic development. Dev. Dyn. 229: 176-200. [DOI] [PubMed] [Google Scholar]
- Jepeal, L.I., Fujitani, Y., Boylan, M.O., Wilson, C.N., Wright, C.V., and Wolfe, M.M. 2005. Cell-specific expression of glucose-dependent-insulinotropic polypeptide is regulated by the transcription factor PDX-1. Endocrinology 146: 383-391. [DOI] [PubMed] [Google Scholar]
- Johnson, J.D., Ahmed, N.T., Luciani, D.S., Han, Z., Tran, H., Fujita, J., Misler, S., Edlund, H., and Polonsky, K.S. 2003. Increased islet apoptosis in Pdx1+/- mice. J. Clin. Invest. 111: 1147-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson, J., Carlsson, L., Edlund, T., and Edlund, H. 1994. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371: 606-609. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, Y., Cooper, B., Gannon, M., Ray, M., MacDonald, R.J., and Wright, C.V. 2002. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32: 128-134. [DOI] [PubMed] [Google Scholar]
- Kim, S.K. and Hebrok, M. 2001. Intercellular signals regulating pancreas development and function. Genes & Dev. 15: 111-127. [DOI] [PubMed] [Google Scholar]
- Kim, S.K., Hebrok, M., and Melton, D.A. 1997. Notochord to endoderm signaling is required for pancreas development. Development 124: 4243-4252. [DOI] [PubMed] [Google Scholar]
- Kubo, A., Shinozaki, K., Shannon, J.M., Kouskoff, V., Kennedy, M., Woo, S., Fehling, H.J., and Keller, G. 2004. Development of definitive endoderm from embryonic stem cells in culture. Development 131: 1651-1662. [DOI] [PubMed] [Google Scholar]
- Lammert, E., Cleaver, O., and Melton, D. 2001. Induction of pancreatic differentiation by signals from blood vessels. Science 294: 564-567. [DOI] [PubMed] [Google Scholar]
- Larsson, L.I. 2000. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc. Res. Tech. 48: 272-281. [DOI] [PubMed] [Google Scholar]
- Larsson, L.I., Madsen, O.D., Serup, P., Jonsson, J., and Edlund, H. 1996. Pancreatic-duodenal homeobox 1-role in gastric endocrine patterning. Mech. Dev. 60: 175-184. [DOI] [PubMed] [Google Scholar]
- Larsson, L.I., St-Onge, L., Hougaard, D.M., Sosa-Pineda, B., and Gruss, P. 1998. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech. Dev. 79: 153-159. [DOI] [PubMed] [Google Scholar]
- Lee, C.S., Perreault, N., Brestelli, J.E., and Kaestner, K.H. 2002. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes & Dev. 16: 1488-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, J., Peers, B., Johnson, T., Ferreri, K., Lee, S., and Montminy, M.R. 1993. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol. Endocrinol. 7: 1275-1283. [DOI] [PubMed] [Google Scholar]
- Li, H., Arber, S., Jessell, T.M., and Edlund, H. 1999. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat. Genet. 23: 67-70. [DOI] [PubMed] [Google Scholar]
- Marshak, S., Benshushan, E., Shoshkes, M., Havin, L., Cerasi, E., and Melloul, D. 2000. Functional conservation of regulatory elements in the pdx-1 gene: PDX-1 and hepatocyte nuclear factor 3β transcription factors mediate β-cell-specific expression. Mol. Cell. Biol. 20: 7583-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloul, D., Marshak, S., and Cerasi, E. 2002. Regulation of pdx-1 gene expression. Diabetes 51: S320-S325. [DOI] [PubMed] [Google Scholar]
- Miller, C.P., McGehee Jr., R.E., and Habener, J.F. 1994. IDX-1: A new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 13: 1145-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya, F.J., Huang, H.P., Qiu, Y., Mutoh, H., DeMayo, F.J., Leiter, A.B., and Tsai, M.J. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/NeuroD-deficient mice. Genes & Dev. 11: 2323-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield, M.F., Jetton, T.L., Labosky, P.A., Ray, M., Stein, R.W., Magnuson, M.A., Hogan, B.L. and Wright, C.V. 1996. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122: 983-995. [DOI] [PubMed] [Google Scholar]
- Ohlsson, H., Karlsson, K., and Edlund, T. 1993. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 12: 4251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshavaria, M., Gamer, L., Henderson, E., Teitelman, G., Wright, C.V., and Stein, R. 1994. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol. Endocrinol. 8: 806-816. [DOI] [PubMed] [Google Scholar]
- Rosanas-Urgell, A., Marfany, G., and Garcia-Fernandez, J. 2005. Pdx1-related homeodomain transcription factors are distinctly expressed in mouse adult pancreatic islets. Mol. Cell. Endocrinol. 237: 59-66. [DOI] [PubMed] [Google Scholar]
- Samaras, S.E., Cissell, M.A., Gerrish, K., Wright, C.V., Gannon, M., and Stein, R. 2002. Conserved sequences in a tissue-specific regulatory region of the pdx-1 gene mediate transcription in pancreatic β cells: Role for hepatocyte nuclear factor 3β and Pax6. Mol. Cell. Biol. 22: 4702-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras, S.E., Zhao, L., Means, A., Henderson, E., Matsuoka, T.A., and Stein, R. 2003. The islet β cell-enriched RIPE3b1/Maf transcription factor regulates pdx-1 expression. J. Biol. Chem. 278: 12263-12270. [DOI] [PubMed] [Google Scholar]
- Servitja, J.M. and Ferrer, J. 2004. Transcriptional networks controlling pancreatic development and β cell function. Diabetologia 47: 597-613. [DOI] [PubMed] [Google Scholar]
- Sharma, S., Jhala, U.S., Johnson, T., Ferreri, K., Leonard, J., and Montminy, M. 1997. Hormonal regulation of an islet-specific enhancer in the pancreatic homeobox gene STF-1. Mol. Cell. Biol. 17: 2598-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, D.Q., Heimesaat, M., Kuwajima, S., Stein, R., Wright, C.V., and Stoffel, M. 2002. Profound defects in pancreatic β-cell function in mice with combined heterozygous mutations in Pdx-1, Hnf-1α, and Hnf-3β. Proc. Natl. Acad. Sci. 99: 3818-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers, D.A., Zinkin, N.T., Stanojevic, V., Clarke, W.L., and Habener, J.F. 1997. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat. Genet. 15: 106-110. [DOI] [PubMed] [Google Scholar]
- Stoffers, D.A., Stanojevic, V., and Habener, J.F. 1998. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J. Clin. Invest. 102: 232-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada, S., Era, T., Furusawa, C., Sakurai, H., Nishikawa, S., Kinoshita, M., Nakao, K., and Chiba, T. 2005. Characterization of mesendoderm: A diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132: 4363-4374. [DOI] [PubMed] [Google Scholar]
- Thorens, B. 2001. GLUT2 in pancreatic and extra-pancreatic gluco-detection (review). Mol. Membr. Biol. 18: 265-273. [DOI] [PubMed] [Google Scholar]
- Van Velkinburgh, J.C., Samaras, S.E., Gerrish, K., Artner, I., and Stein, R. 2005. Interactions between areas I and II direct pdx-1 expression specifically to islet cell types of the mature and developing pancreas. J. Biol. Chem. 280: 38438-38444. [DOI] [PubMed] [Google Scholar]
- Waeber, G., Thompson, N., Nicod, P., and Bonny, C. 1996. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol. Endocrinol. 10: 1327-1334. [DOI] [PubMed] [Google Scholar]
- Watada, H., Kajimoto, Y., Umayahara, Y., Matsuoka, T., Kaneto, H., Fujitani, Y., Kamada, T., Kawamori, R., and Yamasaki, Y. 1996. The human glucokinase gene β-cell-type promoter: An essential role of insulin promoter factor 1/PDX-1 in its activation in HIT-T15 cells. Diabetes 45: 1478-1488. [DOI] [PubMed] [Google Scholar]
- Wilson, M.E., Scheel, D., and German, M.S. 2003. Gene expression cascades in pancreatic development. Mech. Dev. 120: 65-80. [DOI] [PubMed] [Google Scholar]
- Wright, C.V., Schnegelsberg, P., and De Robertis, E.M. 1989. XlHbox 8: A novel Xenopus homeo protein restricted to a narrow band of endoderm. Development 105: 787-794. [DOI] [PubMed] [Google Scholar]
- Wu, K.L., Gannon, M., Peshavaria, M., Offield, M.F., Henderson, E., Ray, M., Marks, A., Gamer, L.W., Wright, C.V., and Stein, R. 1997. Hepatocyte nuclear factor 3β is involved in pancreatic β-cell-specific transcription of the pdx-1 gene. Mol. Cell. Biol. 17: 6002-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata, K., Nammo, T., Moriwaki, M., Ihara, A., Iizuka, K., Yang, Q., Satoh, T., Li, M., Uenaka, R., Okita, K., et al. 2002. Overexpression of dominant-negative mutant hepatocyte nuclear factor-1α in pancreatic β-cells causes abnormal islet architecture with decreased expression of E-cadherin, reduced β-cell proliferation, and diabetes. Diabetes 51: 114-123. [DOI] [PubMed] [Google Scholar]
- Yoshitomi, H. and Zaret, K.S. 2004. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development 131: 807-817. [DOI] [PubMed] [Google Scholar]