Abstract
Size sexual dimorphism occurs in almost all mammals. In Portuguese Water Dogs, much of the difference in skeletal size between females and males is due to the interaction between a Quantitative Trait Locus (QTL) on the X-chromosome and a QTL linked to Insulin-like Growth Factor 1 (IGF-1) on the CFA 15 autosome. In females, the haplotype of CFA 15 resulting in small size is dominant. In males, the haplotype for large size is dominant. Females, homozygous at the CHM marker on the X chromosome and homozygous for the large size CFA 15 haplotype are, on average, as large as large males. However, all females that are heterozygous at the CHM marker are small, regardless of their CFA 15 genotype. This interaction suggests a genetic mechanism that in turn leads to a scenario for the evolution of size sexual dimorphism consistent with a proposal of Lande that sexual dimorphism can evolve because females secondarily become smaller than males as a consequence of natural selection for optimal size. Our results also can explain Rensch's Rule, which states that size is often positively correlated with the level of size sexual dimorphism.
Since Darwin (1875), sexual selection for dimorphism in size has been a subject of intense interest to biologists. A difference in size between the sexes (sexual dimorphism) is observed in almost all mammals (Abouheif and Fairbairn 1997), including dogs (Canis familiaris). The mechanisms for maintaining sexual dimorphism in a population are not completely understood, but may involve interaction between the sex chromosomes and autosomes. For example, the Sry locus on the Y chromosome plays an important role in sex determination and dimorphism (McLaren 1990). The X chromosome has been shown to harbor an excess of genes related to sex and reproduction, mental functions, and skeletal muscle (Bortoluzzi et al. 1998; Saifi and Chandra 1999; Wang et al. 2001; Zechner et al. 2001; Qiu et al. 2002; Vallender and Lahn 2004). Several autosomal loci are expressed differentially in males and females with concomitant effects on sexual dimorphism (Udy et al. 1997; Vaughn et al. 1999; McMahon et al. 2003; Salih et al. 2005).
We are analyzing the genetic basis for skeletal morphology in Portuguese Water Dogs (Chase et al. 1999, 2002, 2004). Portuguese Water Dogs (PW dogs) are a recent breed of dog, whose descent from a few founders is documented by accurate and complete pedigree records (Molinari 1993). They are descended from two major founding kennels that disagreed on an appropriate size standard, resulting in considerable size variation in the founding population. The population structure of the breed is favorable for detailed genetic analysis of quantitative phenotypes (Chase et al. 1999) and variation in size and shape is still encountered within the current population. This is, in part, due to a fairly lax size standard for the breed, although a different average standard is maintained for males and females. Moreover, any pure-bred PW dog can compete in “performance events” (obedience, agility, waterwork, etc.) regardless of size.
Insulin-like Growth Factor 1 (IGF-1) regulates postnatal skeletal growth in mammals (Baker et al. 1993). Here we present evidence that an autosomal genetic marker, linked to IGF1, is associated with size sexual dimorphism in PW dogs. This locus interacts with another locus on the X chromosome to regulate differences in size between the female and male populations.
Results
Female PW dogs are, on average, 85% the size of males. Based on 42 metrics from the pelvis, fore- and hind-limbs, we have estimated size as the first Principal Component (PC1) of skeletal variation (Chase et al. 2002). (PC1 is significantly correlated with body mass (r = 0.7) and represents an averaging over each dog's normalized skeletal metrics. It is less influenced by environmental factors such as diet and exercise.) This trait is highly heritable (h2 = 0.45) and we have associated PC1 variation in PW dogs with quantitative trait loci (QTLs) on four autosomes and the X chromosome (approximate locations on the dog genome are presented in Fig. 3 of Carrier et al. [2005]).
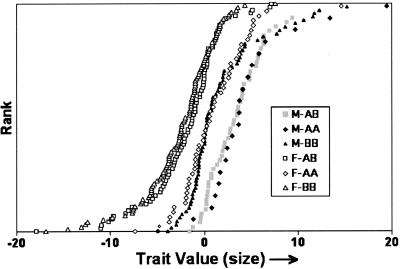
Figure 3.
Size variation (values of PC1) subdivided according to sex and FH2017 genotypes. The FH2017 genotype data in Figure 2 were further subdivided into the male and female subpopulations.
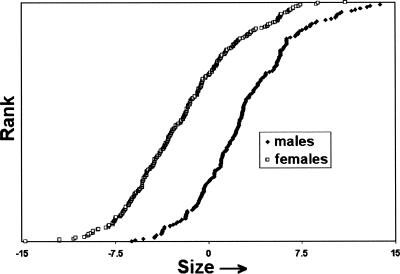
Figure 1 presents size distributions of PC1 for male and female PW dogs. Sexual dimorphism is evident as a shift in population means, but the ranges of size overlap extensively and several females are within the size range of the largest 10% of the male population.
Figure 1.
Size sexual dimorphism in the PW Dog. Forty two skeletal metrics were corrected to a mean of 0 and a standard deviation of 1. A matrix composed of the corrected skeletal metrics of 463 dogs was used to calculate the Principal Components of skeletal variation. Size is represented by values of PC1 calculated using the Pearson correlation option of XLSTAT (http://www.xlstat.com) for 42 skeletal metrics (males♦; females □).
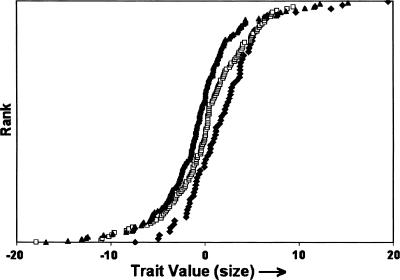
A QTL regulating size variation was associated (P ≤ 0.028) with tetra nucleotide repeat marker FH2017 on autosome 15 (CFA 15; see Carrier et al. 2005). Two major FH2017 alleles (A and B) account for >90% of FH2017 genotypes. On the canine genetic map (Guyon et al. 2003), this marker is linked to canine IGF-1 (Chase et al. 2002). The variation in PC1 associated with genotypes of FH2017 is shown in Figure 2. The data are consistent with an additive mode of inheritance in which genotypic means are ranked: BB(small) < AB < AA(large). This interpretation changes, however, when the population is further subdivided according to sex (Fig. 3). In males, haplotype A (large-size phenotype) is dominant (AA = AB > BB), whereas Haplotype B (small size phenotype) is dominant in females (BB = AB < AA). This difference provides a genetic basis that explains part of the sexual size dimorphism observed between the male and female populations.
Figure 2.
PC1 variation subdivided according to FH2017 genotypes (AA♦; AB□; BB▴). Dogs are ranked on the y-axis according to pedigree-corrected values of PC1 on the x-axis (Chase et al. 2002, 2004). Pedigree effects were removed using BLUP (Lynch and Walsh 1998; Chase et al. 2004).
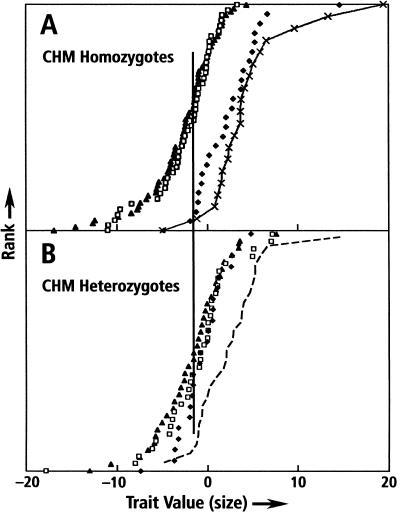
We deconstructed PC1 by comparing the effects of PC1 QTLs on individual bone metrics. With one exception, no significant correlation could be established between the individual QTLs. However, variation associated with the CFA 15 locus (FH2017) was significantly correlated with trait variation explained by the X chromosome PC1 QTL. This suggested the existence of an interaction between CFA 15 and X chromosome PC1 QTLs. This was confirmed when we searched for an interaction between loci on the X chromosome and the FH2017 autosomal locus: A QTL on the X chromosome, associated with the SSR marker CHM, interacts significantly (P ≤ 0.001) with the FH2017 QTL. CHM is located in a region of the X chromosome that has been shown in humans to escape X inactivation (Carrel and Willard 1993, 2005). Similar to FH2017, CHM has only two haplotypes, α (51%) and β (49%) identified by two alleles of an SSR marker in an intron of this gene. In females homozygous at CHM (e.g. αα or ββ), the size of FH2017 AA genotypes approach values found in FH2017 AA males (top, Fig. 4). In contrast, CHM heterozygous females (e.g., αβ) are small and FH2017 genotypes show no segregation for size (Fig. 4, bottom). Hemizygous males, α and β, each do not differ significantly from the pattern of FH2017 size segregation observed for males in Figure 3. This interactive effect, confined to the CHM region of the X, reduces the average size of female PW dogs, but maintains a small fraction of very large females in the population (alternatively, the interaction increases the size of a small portion of female dogs).
Figure 4.
Size (PC1) distributions of FH2017 genotypes in CHM females: (A) homozygous (αα and ββ); (B) heterozygous (αβ). The data represent genotypic subpopulations of the data in Figure 2. As in Figure 2, dogs are ranked by pedigree-corrected PC1 values on the x-axis. FH2017 genotypes are shown (AA♦; AB□; BB▴). For comparison, male FH2017 AA genotypes (-x-) also are presented. The 50% rankings of BB genotypes (▴) are almost the same (vertical line between A and B). For purposes of comparison, we have added a dashed line to B, reproducing the distribution of AA females from A.
The effects of dominance reversal and the interaction between CHM and FH2017 are significant for each of the individual traits contributing to variation in PC1, but we lack sufficient statistical power to determine whether some traits are affected more than others. The overall effect of dominance reversal and the interaction between CHM and FH2017 is responsible for ∼50% of the size sexual dimorphism observed in the breed.
Finally, we have analyzed parental PW dog phenotypes and genotypes, but failed to find evidence for imprinting. There was no observed effect of FH2017 on size in the female CHM-αβ population regardless of which CHM allele was inherited from the sire. Similarly, there is no significant parent of origin effect for FH2017 alleles (e.g., FH2017-AB females who received the A allele from the sire are no different from those that received the A allele from the dam).
Discussion
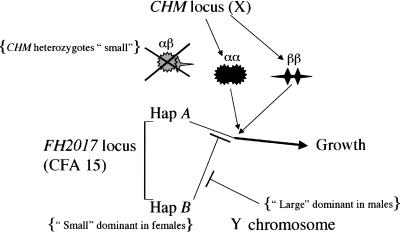
These data present two sex-related phenomena demanding explanation (as shown in Fig. 5), the reversal of dominance at the FH2017 QTL, and the heterozygote-specific interaction between the CHM locus and the FH2017 QTL. The first phenomenon requires the existence of another sex-specific factor. For example, the FH2017 QTL might contain two distinct genes associated with two haplotypes; FH2017 haplotype A acts in both males and females to up-regulate size, while FH2017 haplotype B not only does not up-regulate size, but contains another gene that suppresses this up-regulator. It is this suppressor that is itself suppressed by an unknown factor in males, perhaps derived from the Y chromosome.
Figure 5.
An interactive network between the X (CHM), Y, and CFA 15 chromosomes suggested by the data in Figures 1, 2, 3, 4.
To explain the second phenomenon, we propose that activation of the haplotype A up-regulator requires interaction with a CHM-associated multimeric product, either αα or ββ. The failure of such activation by CHM heterozygotes would occur if a mixed multimeric product (αβ) were inactive (spoiler effect) (Kadouri et al. 1978). Males, being hemizygous, are never heterozygotes and cannot produce the proposed mixed multimer. The difference between heterozygotes and homozygotes at the CHM locus is consistent with the observation that this region of the X chromosome escapes inactivation (both copies of the X are active) (Carrel and Willard 1993, 2005), a phenomenon that has been observed at other X-linked loci (Brown and Greally 2003).
The hypothesis in Figure 5 suggests a two-step scenario for the evolution of sexual dimorphism. (1) Male competition results in selection for larger males. This sexual selection acts on many body-size genes on the X and autosomal chromosomes. For example, a dominant haplotype A might arise at the FH2017 locus, driving both males and females to become larger. (2) Natural selection then acts to favor a smaller, optimal body size in females. Modifications such as the reversal of dominance and the interaction with the X chromosome create smaller females and sexual dimorphism. Interestingly, this system maintains sexual size dimorphism only when the alleles involved segregate nearly evenly in the population, as we indeed observe. Our hypothetical evolutionary scenario thus supports Lande's proposal (Lande 1980) that sexual dimorphism evolves when females secondarily return to their natural selection optimum after having undergone correlated evolution due to sexual selection acting on males.
The interaction between the X-chromosome and CFA 15 also maintains size polymorphism in females. Long-term changes in food availability and the strength of competition could favor maintenance of a body-size polymorphism in longlived animals. Thus, large individuals would be most effective in fighting with neighboring social groups and capturing large prey, while smaller individuals can use their higher agility to capture small prey (Peters 1983). Such factors are known to be important for ants (Holldobler and Wilson 1990) and could operate in other social systems including wolves (Canis lupus) from which Canis familiaris is descended (Vila et al. 1997).
If such a genetic system works across species, those species with a major gene (such as that linked to FH2017) of large effect will simultaneously show larger average size and a larger degree of sexual size dimorphism caused by repression of that effect in females. Our data are thus consistent with Rensch's Rule that the level of sexual size dimorphism is often positively correlated with body size (Rensch 1950, 1960; Abouheif and Fairbairn 1997) and female body size in particular. Species in which this correlation holds include primates (Abouheif and Fairbairn 1997; Leutenegger 1978), other mammals (Jarman 1983, 1989; Reiss 1986; Abouheif and Fairbairn 1997), birds (Webster 1992; Abouheif and Fairbairn 1997; Szekely et al. 2004), and snakes (Abouheif and Fairbairn 1997).
Many hypotheses have been proposed to explain this rule (for review, see Webster 1992; Abouheif and Fairbairn 1997). The only hypotheses that can be generalized across taxa attributes the pattern to sexual selection on males (Fairbairn and Preziosi 1994). In one scenario, sexual selection favoring large males produces an increase in male size and a smaller, correlated increase in female size because of the high genetic correlation between the sexes (Leutenegger 1978; Lande 1980; Fairbairn and Preziosi 1994; Aboufeif and Fairbairn 1997).
As we have argued, our data are more consistent with Lande's prediction (1980) that sexual dimorphism evolves because females secondarily become smaller than males as a result of natural selection for optimal size. Reduction of female size relative to that of males through an inhibition of genes that enhance growth, such as that associated with FH2017 on CFA 15, may commonly be related to the evolution of sexual size dimorphism. Such a pattern of inverse dominance, in which small is dominant in females, whereas large is dominant in males, should be relatively easily tested in other species where controlled mating is feasible and hybrid progeny can be examined.
Our data indicate an interactive X chromosome role in the regulation of size sexual dimorphism. We have not identified the specific genes involved in this interaction. While some candidates are suggested by proximity to QTLs (e.g., IGF-1), the actual identification of the phenotype-associated variants will be a challenging process. In order to truly establish cause and effect, a large number of dogs representing a range of well-characterized phenotypes must be sequenced in regions of interest and rigorous statistical methods applied to the resulting data. Given that we are not searching for mutations causing a profound disease, rather a phenotype associated with some variability, it is likely that DNA variants involved in gene expression levels, exonskipping message stability, or protein folding will prove to be important. Such variants, indeed, are likely to be the syntax associated with genes like IGF-1 and others that account for the continuum of phenotypic variation observed in dogs. However, once the causative variants are truly identified, it will be possible to search for this mechanism in other canids, such as the wolf, as well as other mammals including humans, to test whether variants and/or molecular mechanisms are preserved by selection (Garrigan and Hedrick 2003).
Methods
We have increased the number of dogs in the data set previously used for analysis of the full skeleton and focused on metrics of the hip and limb (Fig. 3B,D,E in Chase et al. 2002). We have obtained phenotypes (skeletal metrics from radiographs) and DNA genotypes (alleles of simple sequence repeat (SSR) markers) from 463 PW dogs. Radiographs for skeletal metrics and blood for DNA were obtained from owners of individual dogs. These radiographs, together with the measurements taken from them, have been described in a previous publication (Chase et al. 2002).
Methods for identifying QTLs and for estimating the genotypic means of markers near a QTL in an unstructured population have been published (Lynch and Walsh 1998; Chase et al. 2002, 2004). Briefly, QTLs are identified using an allele-sharing method. This method compares pedigree-corrected allele-sharing values at a marker with phenotypic similarity to establish an association between marker and phenotype. Monte Carlo simulations of a random trait with heritability equal to the target phenotype are used to estimate the NULL distribution of the marker-phenotype association. This NULL distribution is used to estimate the significance of the association between the marker and the target phenotype. Marker P-values are adjusted for the ca. 700 markers tested. Marker genotypic means are estimated using a mixed model in which the marker genotypes are treated as fixed effects and the additive genetic background and error deviations are treated as random effects. Infrequent genotypes are bulked for this analysis.
Interaction between FH2017 and CHM was evaluated using females only. The variation explained by FH2017 in the CHM αα, αβ, or ββ subpopulations is 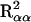 ,
, 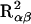 , and
, and 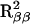 , respectively. The Variance (VR) among these R2 values was used as a test statistic for the interaction between the two loci. We used permutation tests to establish the significance of this interaction. For each permutation test, the genotypes for CHM were permuted with respect to the individuals and VR recalculated. Each permutation test created a random assortment of CHM genotypes, but maintained the relationship for the FH2017 genotypes, allowing us to fit the animal for FH2017 in each sub group. We used 5000 permutation tests to establish the null distribution for the VR statistic.
, respectively. The Variance (VR) among these R2 values was used as a test statistic for the interaction between the two loci. We used permutation tests to establish the significance of this interaction. For each permutation test, the genotypes for CHM were permuted with respect to the individuals and VR recalculated. Each permutation test created a random assortment of CHM genotypes, but maintained the relationship for the FH2017 genotypes, allowing us to fit the animal for FH2017 in each sub group. We used 5000 permutation tests to establish the null distribution for the VR statistic.
We tested for imprinting using parent of origin tests. Heterozygotes at each marker were subdivided according to the parental origin of a specific allele (A or α) and tested for significant differences in QTL effects. We tested for differences between the CHM-αβ (α from sire) and CHM-αβ (α from dam) as well as the FH2017-AB (A from sire) and FH2017-AB (A from dam).
We calculated the amount of size sexual dimorphism explained by these loci and the interaction between them as 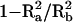 ; where
; where 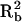 is the amount of variation explained by sex in the raw data and
is the amount of variation explained by sex in the raw data and 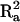 is the amount of variation explained by sex in the residuals after accounting for the two loci and the interaction. Treating males as homozygous at the X locus size was modeled as: size = μ + Ai + Amd + Afd + Xhet + e where μ is the mean, Ai is the allele count for FH2017-A, Amd is a male-specific dominance factor (1 for male FH2017-AB and 0 for all others), Afd is a female-specific dominance factor (1 for female FH2017-AB and 0 for all others), and Xhet is a factor for heterozygotes at the CHM locus.
is the amount of variation explained by sex in the residuals after accounting for the two loci and the interaction. Treating males as homozygous at the X locus size was modeled as: size = μ + Ai + Amd + Afd + Xhet + e where μ is the mean, Ai is the allele count for FH2017-A, Amd is a male-specific dominance factor (1 for male FH2017-AB and 0 for all others), Afd is a female-specific dominance factor (1 for female FH2017-AB and 0 for all others), and Xhet is a factor for heterozygotes at the CHM locus.
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation Grant no. NSF IBN-0212141 to David Carrier and the National Institutes of Health/NIGMS Grant no. 63056 to Karl G. Lark, by a gift from the Judith L. Chiara Foundation, and gifts from more than 100 PW dog owners. We thank Makiko Uemera, Kerry Matz, and Tyler Jarvik for technical assistance. Materials (blood and x-rays) were obtained from owners by Deborah Broughton through the Georgie Project, Karen Miller director. Materials for genotypic analysis (blood from which DNA was extracted) and for phenotypic analysis were provided by more than 200 owners of Portuguese Water Dogs.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.3712705.
References
- Abouheif, E. and Fairbairn, D.J. 1997. A comparative analysis of allometry for sexual size dimorphism: Assessing Rensch's rule. Am. Naturalist 149: 540-562. [Google Scholar]
- Baker, J., Liu, J.P., Robertson, E.J., and Efstratiadis, A. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75: 73-82. [PubMed] [Google Scholar]
- Bortoluzzi, S., Rampoldi, L., Simionati, B., Zimbello, R., Barbon, A., d'Alessi, F., Tiso, N., Pallavicini, A., Toppo, S., Cannata, N., et al. 1998. A comprehensive, high-resolution genomic transcript map of human skeletal muscle. Genome Res. 8: 817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, C.J. and Greally, J.M. 2003. A stain upon the silence: Genes escaping X inactivation. Trends Genet. 19: 432-438. [DOI] [PubMed] [Google Scholar]
- Carrel, L. and Willard, H.F. 1993. The X-linked choroideremia gene escapes X chromosome inactivation. (Abstract) Am. J. Hum. Genet. 53: A42. [Google Scholar]
- ———. 2005. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434: 400-404. [DOI] [PubMed] [Google Scholar]
- Carrier, D.R., Chase, K., and Lark, K.G. 2005. Genetics of canid skeletal variation: Size and shape of the pelvis. Genome Res. (this issue). [DOI] [PMC free article] [PubMed]
- Chase, K., Adler, F.R., Miller-Stebbings, K., and Lark, K.G. 1999. Teaching a new dog old tricks: Identifying Quantitative Trait Loci [in dogs] using lessons from plants. J. Heredity 90: 43-51. [DOI] [PubMed] [Google Scholar]
- Chase, K., Carrier, D.R., Adler, F.R., Jarvik, T., Ostrander, E.A., and Lark, K.G. 2002. Genetic basis for systems of skeletal quantitative traits: Principal component analysis of the canid skeleton. Proc. Natl. Acad. Sci. 99: 9930-9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, K., Lawler, D., Adler, F., Ostrander, E., and Lark, K.G. 2004. Bilaterally asymmetric effects of quantitative trait loci (QTLs): QTLs that affect laxity in the right versus left coxofemoral (hip) joints of the dog (Canis familiaris). Am. J. Med. Genet. 124A: 239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C. 1875. The descent of man, and selection in relation to sex. D. Appleton and Company, New York.
- Fairbairn, D.J. and Preziosi, R.F. 1994. Sexual selection and the evolution of allometry for sexual size dimorphism in the waterstrider, Aquarius remigis. Am. Naturalist 144: 101-118. [Google Scholar]
- Garrigan, D. and Hedrick, P.W. 2003. Perspective: Detecting adaptive molecular polymorphism: Lessons from the MHC. Evolution 57: 1707-1722. [DOI] [PubMed] [Google Scholar]
- Guyon, R., Lorentzen, T.D., Hitte, C., Kim, L., Cadieu, E., Parker, H.G., Quignon, P., Lowe, J.K., Renier, C., Gelfenbeyn, B., et al. 2003. A 1 Mb resolution radiation hybrid map of the canine genome. Proc. Natl. Acad. Sci. 100: 5296-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holldobler, B. and Wilson, E.O. 1990. The ants. The Belknap Press of Harvard University Press, Cambridge, MA.
- Jarman, P. 1983. Mating system and sexual dimorphism in large, terrestrial, mammalian herbivores. Biol. Rev. 58: 485-520. [Google Scholar]
- ———. 1989. Sexual dimorphism in Macropodoidea. In Kangaroos, Wallabies, and Rat-Kangaroos (eds. G. Grigg, P. Jarman, and I. Hume), pp. 433-447. Surrway Beatty & Sons, New South Wales.
- Kadouri, A., Kunce, J.J., and Lark, K.G. 1978. Evidence for dominant mutations reducing HGPRT activity. Nature 274: 256-259. [DOI] [PubMed] [Google Scholar]
- Lande, R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34: 292-305. [DOI] [PubMed] [Google Scholar]
- Leutenegger, W. 1978. Scaling of sexual dimorphism in body size and breeding system in primates. Nature 272: 610-611. [DOI] [PubMed] [Google Scholar]
- Lynch, M. and Walsh, B. 1998. Genetics and analysis of quantitative traits, pp. 745-751. Sinauer Associates, Inc., Sunderland, MA.
- McLaren, A. 1990. What makes a man a man? Nature 346: 216. [DOI] [PubMed] [Google Scholar]
- McMahon, C.D., Popovic, L., Jeanplong, F., Oldham, J.M., Kirk, S.P., Osepchook, C.C., Wong, K.W., Sharma, M., Kambadur, R., and Bass, J.J. 2003. Sexual dimorphism is associated with decreased expression of processed myostatin in males. Am. J. Physiol. Endocrinol. Metab. 284: E377-E381. [DOI] [PubMed] [Google Scholar]
- Molinari, C. 1993. The Portuguese Water Dog. ELO-Publicidade, Portugal.
- Peters, R.H. 1983. The ecological implications of body size. Cambridge University Press, Cambridge, UK.
- Qiu, P., Benbow, L., Liu, S., Greene, J.R., and Wang, L. 2002. Analysis of a human brain transcriptome map 2002. BMC Genomics 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, M.J. 1986. Sexual dimorphism in body size: Are larger species more bimophic? J. Theor. Biol. 121: 163-172. [Google Scholar]
- Rensch, B. 1950. Die Abhangigkeit der relativen sexual differenz von der Korpergrosse. Bonner. Zoologische Beitraege 1: 58-69. [Google Scholar]
- ———. 1960. Evolution above the species level. pp. 157-159. Columbia University Press, New York.
- Saifi, G.M. and Chandra, H.S. 1999. An apparent excess of sex- and reproduction-related genes on the human X chromosome. Proc. R. Soc. Lond. B. Biol. Sci. 266: 203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih, D.A., Mohan, S., Kasukawa, Y., Tripathi, G., Lovett, F.A., Anderson, N.F., Carter, E.J., Wergedal, J.E., Baylink, D.J., and Pell, J.M. 2005. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology 146: 931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely, T., Freckleton, R.P., and Reynolds, J.D. 2004. Sexual selection explains Rensch's rule of size dimorphism in shorebirds. Proc. Natl. Acad. Sci. 101: 12224-12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udy, G.B., Towers, R.P., Snell, R.G., Wilkins, R.J., Park, S.H., Ram, P.A., Waxman, D.J., and Davey, H.W. 1997. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. 94: 7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallender, E.J. and Lahn, B.T. 2004. How mammalian sex chromosomes acquired their peculiar gene content. Bioessays 26: 159-169. [DOI] [PubMed] [Google Scholar]
- Vaughn, T.T., Pletscher, L.S., Peripato, A., King-Ellison, K., Adams, E., Erikson, C., and Cheverud, J.M. 1999. Mapping quantitative trait loci for murine growth: A closer look at genetic architecture. Genet. Res. 74: 313-322. [DOI] [PubMed] [Google Scholar]
- Vila, C., Savolainen, P., Maldonado, J.E., Amorim, I.R., Rice, J.E., Honeycutt, R.L., Crandall, K.A., Lundeberg, J., and Wayne, R.K. 1997. Multiple and ancient origins of the domestic dog. Science 276: 1687-1689. [DOI] [PubMed] [Google Scholar]
- Wang, P.J., McCarrey, J.R., Yang, F., and Page, D.C. 2001. An abundance of X-linked genes expressed in spermatogonia. Nat. Genet. 27: 422-426. [DOI] [PubMed] [Google Scholar]
- Webster, M.S. 1992. Sexual dimorphism, mating system and body size in new world blackbirds (Icterinae). Evolution 46: 1621-1641. [DOI] [PubMed] [Google Scholar]
- Zechner, U., Wilda, M., Kehrer-Sawatzki, H., Vogel, W., Fundele, R., and Hameister, H. 2001. A high density of X-linked genes for general cognitive ability: A run-away process shaping human evolution? Trends Genet. 17: 697-701. [DOI] [PubMed] [Google Scholar]
Web site reference
- http://www.xlstat.com; XLSTAT.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.