Abstract
Objective:
To investigate the effect of a restricted intravenous fluid regimen versus a standard regimen on complications after colorectal resection.
Summary Background Data:
Current fluid administration in major surgery causes a weight increase of 3–6 kg. Complications after colorectal surgery are reported in up to 68% of patients. Associations between postoperative weight gain and poor survival as well as fluid overload and complications have been shown.
Methods:
We did a randomized observer-blinded multicenter trial. After informed consent was obtained, 172 patients were allocated to either a restricted or a standard intraoperative and postoperative intravenous fluid regimen. The restricted regimen aimed at maintaining preoperative body weight; the standard regimen resembled everyday practice. The primary outcome measures were complications; the secondary measures were death and adverse effects.
Results:
The restricted intravenous fluid regimen significantly reduced postoperative complications both by intention-to-treat (33% versus 51%, P = 0.013) and per-protocol (30% versus 56%, P = 0.003) analyses. The numbers of both cardiopulmonary (7% versus 24%, P = 0.007) and tissue-healing complications (16% versus 31%, P = 0.04) were significantly reduced. No patients died in the restricted group compared with 4 deaths in the standard group (0% versus 4.7%, P = 0.12). No harmful adverse effects were observed.
Conclusion:
The restricted perioperative intravenous fluid regimen aiming at unchanged body weight reduces complications after elective colorectal resection.
A restricted intraoperative and postoperative intravenous fluid regimen was tested against a standard regimen representing current practice. The restricted regimen significantly reduced postoperative complications, with the most distinct effect on cardiopulmonary complications.
Little is known about the influence of perioperatively administered intravenous fluid volume on the outcome of surgery. We found 5 randomized trials evaluating effects of intraoperative fluid volume on recovery time and well-being after outpatient surgery,1–5 and 4 randomized trials showing oral postoperative hydration to be safe.6–9 No trials were designed to evaluate the effects of the combined intraoperative and postoperative intravenous fluid volume on surgical complications or death. Current fluid therapy in major surgery causes a weight increase of 3–6 kg.10–12 Intravenous fluid overload during or after surgery has been shown to decrease muscular oxygen tension13 and delay recovery of gastrointestinal function.10 Furthermore, postoperative weight gain and intraoperative fluid overload have been associated with poor survival14 and complications.15,16 We hypothesized that fluid overload may cause general edema, impeding tissue healing and cardiopulmonary function.
The aim of this study was to compare the effect of a restricted perioperative intravenous fluid regimen to a standard regimen on complications after colorectal resection.
MATERIALS AND METHODS
We conducted a randomized, observer-blinded clinical trial at 8 Danish hospitals.
Patients
Adult patients admitted for elective colorectal resection were considered eligible if they had no life-threatening systemic diseases (ASA groups 1–3) and did not meet the following exclusion criteria: pregnancy, lactation, mental disorders, language problems, alcohol consumption of more than 35 drinks/wk, diabetes mellitus, renal insufficiency, disseminated cancer, secondary cancers, inflammatory bowel disease, or diseases hindering epidural analgesia. The presence of both the investigating anesthesiologist and surgeon was mandatory for inclusion, and patients were not screened for eligibility in periods of absence of either one of them. A minimum of 16 patients was required from each center. After giving both oral and written consent, patients were randomized preoperatively to either a restricted (R) or a standard (S) perioperative intravenous fluid regimen. A computer generated the randomization sequence into blocks of 4, and persons otherwise not involved prepared sealed, opaque, consecutively numbered envelopes for each center. The randomization was stratified for colon/rectum surgery and for the centers. The coordinating investigator controlled the randomization sequence at all centers after completion of the study. Two errors were identified. Due to miscommunication between the investigators, 2 patients were allocated the same number; 1 was excluded due to disseminated cancer, and 1 completed the trial. Both are included in the analysis. At another center, an envelope was overlooked and the following assigned. The seal was unbroken and deliberate act was not suspected. No other violations were observed.
One hundred seventy-two patients were enrolled from November 1999 to August 2001, 86 allocated to each regimen. However, 1 center ended participation after inclusion of 4 patients. An additional 25 patients were excluded because colorectal resection was not performed (n = 7), surgery was not radical (n = 6), epidural catheter could not be placed (n = 4), diabetes was newly discovered (n = 2), alcohol abuse was admitted (n = 3), or the investigating anesthesiologist was unavoidably detained from attending the operation (n = 3). One patient was excluded because a hospital fire the night before surgery made special anesthesia necessary; and 1 patient withdrew from data collection. A total of 141 patients completed the trial, 69 in the R-group and 72 in the S-group. No patients were excluded due to deviations from the planned fluid therapy.
Before the trial, we estimated that a sample of 140 patients completing the trial was required to detect a reduction in complication frequency of 20%, with 80% power at a significance level of 0.05. An interim analysis performed after inclusion of 48 patients confirmed the safety of the R-regimen and the planned number. Inclusion was planned to stop after completion of 140 patients. No other analyses were performed during patient inclusion.
Intraoperative fluid regimens are shown in Table 1. In the S-regimen, 500 mL of Hydroxyethyl starch 6% in normal saline (HAES) preloaded the epidural analgesia, and saline 0.9% replaced loss to third space. These replacements were omitted in the R-regimen. External losses were replaced in both regimens. Operative blood loss was estimated by weighing sponges and measuring the volume collected in suction bottles and drains. In the R-regimen, HAES 6% replaced lost blood on a volume-to-volume basis with an allowance of 500 mL extra. In the S-regimen, 1000–1500 mL of saline 0.9% replaced lost blood up to 500 mL, and HAES 6% replaced additional loss. In both regimens, blood component therapy began when estimated blood loss approximated 1500 mL. The goal was a hematocrit of 25–35%, highest if cardiovascular disease was present. If colloid was needed but the maximum recommended dose of HAES had been reached (33 mL/kg/d), albumin 5% was administered. Diuresis was not replaced.
TABLE 1. Intraoperative Fluid Therapy
Postoperative Fluid Regimens
In the R-regimen, 1000 mL of glucose 5% (with potassium if needed) was planned for the rest of the day of operation, and loss through drains was replaced volume-to-volume with HAES 6%. Department routine, typically recommending 1000–2000 mL of crystalloid, was followed in the S-regimen. In the surgical ward, fluid therapy in the R-group was guided by body weight changes employing the following principles: oral intake was preferred, if inadequate, intravenous fluids were administered. A weight increase exceeding 1 kg was treated with furosemide. However, in cases of prolonged intestinal paralysis, an estimation of accumulated fluid was considered when prescribing intravenous fluids or diuretics. The recommendations from the surgical department guided fluid therapy in the S-group.
Problem Solving
Ephedrine and/or dopamine were administered in both groups to achieve a mean arterial blood pressure above 60 mm Hg during operation. Cases of postoperative hypotension or low urinary output (<0.5 mL/kg/h) were always examined and the cause treated. Bleeding initiated administration of intravenous fluids as previously described. Other external losses (aspiration, vomitus, diarrhea, etc.) were replaced with appropriate intravenous fluids in both regimens. Hypotension or low diuresis without loss of volume could initiate several actions: adjustment of epidural analgesic dose, adjustment of habitual antihypertensive medication, administration of pressor substances, and/or administration of intravenous fluids. If reoperation or intensive care was necessary, allocation was disregarded and treatment followed the routine of the department.
Standardization of Treatment
All patients were allowed to drink clear fluids until 2 hours before surgery. Combined thoracic epidural and general anesthesia were employed. The epidural catheter was tested, and analgesia was maintained with bupivacaine and morphine following the routine of the centers. General anesthesia was induced by thiopental (propofol in 7 cases), fentanyl and rocuronium (cisatracurium in 21 cases), and maintained with additional sevoflurane. Antibiotic- and antithrombotic prophylaxes were administered according to department routine. A nasoduodenal or nasojejunal feeding tube was placed before closure of the abdomen, and feeding commenced 4 hours postoperatively: 500 mL Nutriconcentrated 75 (Nutricia, the Netherlands) on the day of operation and 1000 mL daily on the subsequent 3 days. All patients were additionally encouraged to eat and drink from 4 hours after surgery. Continuous epidural analgesia with a mixture of bupivacaine (2.5 mg/ml) and morphine (50 μg/ml) in adequate dose were used for postoperative pain treatment, supplemented with paracetamol, nonsteroidal antiinflammatory drugs, or systemic morphine if needed.
Data Collection and Outcome Measures
Fluid loss and administration were registered from the beginning of fasting to the sixth postoperative day. The patients were weighed on admission, on the morning of operation, and every morning on the subsequent 6 days. Physiological changes were monitored both intraoperatively and postoperatively, arterial blood was sampled by protocol and by demand, and venous blood was sampled daily until discharge or the sixth postoperative day. The primary outcome was complications registered 30 days postsurgery. The secondary outcomes were death and adverse effects, including impairment of renal function and postoperative hypotensive episodes. The investigating surgeon from each center registered outcomes unblinded (clinically). Four participating surgeons performed an additional blinded assessment. Medical records censured for information on patients’ identification, allocation group, fluid therapy, and weight were evaluated for complications and adverse effects. Three assessors evaluated two thirds of the records, ensuring double evaluation of all patients, but precluding evaluation of patients known to the assessor. A fourth blinded assessor settled cases of disagreement.
Statistical Analyses
All randomized patients were analyzed by intention-to-treat, and patients completing the trial were analyzed per-protocol. Complications were compared by the χ2 test, but mortality was compared by the Fisher exact test because of the few observed cases. Risk was calculated as absolute risk reduction and number needed to treat. Dose-response correlation was analyzed by the χ2 test for trend. Continuous data were analyzed by the Student t test of independent samples or the Mann-Whitney U test depending on the presence of normality. P < 0.05 were accepted as significant. All P-values reported are two-tailed. SPSS 10.0 software was used for analyses.
Ethics
The Scientific Ethics Committee of Copenhagen and Frederiksberg County and the committees representing the hospitals outside Copenhagen approved the protocol (J. no. (KF) 01–227/98).
RESULTS
The clinical characteristics of the patients, level of anastomoses, blood loss, etc. are shown in Table 2. Fluid administration and weight changes are shown in Figure 1. Administered intravenous fluid volume on the day of operation was significantly less in the R-group (R versus S: median, 2740 mL [range, 1100–8050] versus 5388 mL [range, 2700–11083]; P < 0.0005). This was due to administration of less saline 0.9%, more glucose 5% in water, but similar volumes of HAES 6%. Antibiotics were dissolved in saline and included in the saline volume. A difference in administered intravenous fluid volume was also seen on the first postoperative day (R versus S: median: 500 mL [range, 0–5000] versus 1500 mL [range, 0–6000], P = 0.003). The body weight of the patients in the S-group was significantly increased from the day of operation to end of measurement 6 days later. Deviations from the planned fluid regimens on the day of operation were observed: 15% of patients in the R-group received more fluid, while 24% of patients in the S-group received less fluid than planned by protocol.
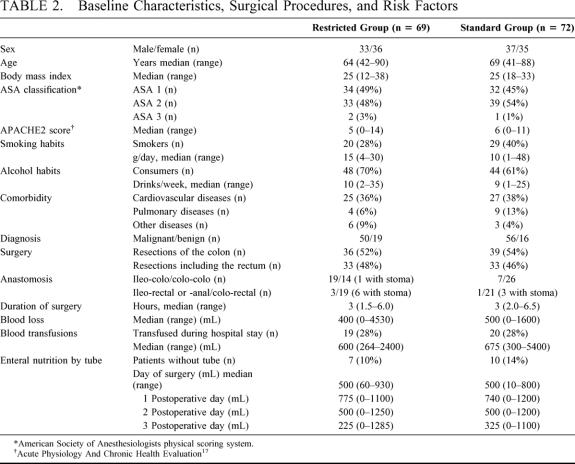
TABLE 2. Baseline Characteristics, Surgical Procedures, and Risk Factors
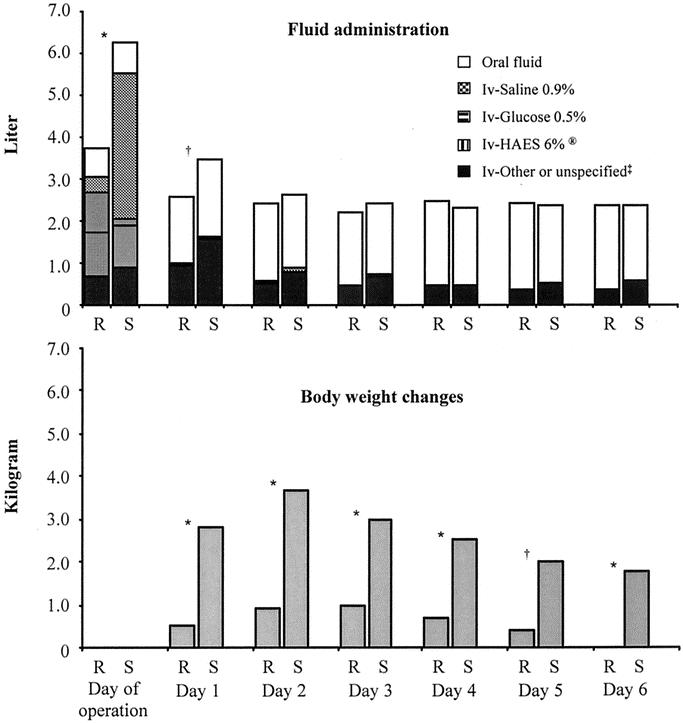
FIGURE 1. Administered fluid and body weight changes. R marks the restricted group and S the standard group. Fluids are presented as summation of means. * P < 0.001. † P < 0.01. ‡ “Other or unspecified” represents blood, albumin, and/or fresh frozen plasma on the day of operation. Type of intravenous fluids was not specified on postoperative day 1 to 6. Weight changes are compared with the weight the morning of operation.
Outcome
The median follow-up time was 34 days. The number of patients with postoperative complications was significantly reduced in the R-group compared with the S-group. Intention-to-treat analyses showed 28 patients (33%) with complications in the R-group versus 44 (51%) in the S-group (P = 0.013) with blinded assessment. When assessed unblinded, the corresponding numbers were 27 (31%) versus 47 (55%) (P = 0.002). Results of the per-protocol analyses are shown in Table 3. Calculated from the result of the blinded assessment, the number of patients needed to treat (NNT) to avoid a complication was 4 for overall complications, 7 for major complications, 4 for minor complications, 7 for tissue-healing complications, and 6 for cardiopulmonary complications. Requirements for acceptance of complications and all complications registered by the blinded assessment are shown in Table 4. Patients with complications had an average of 1.2 complications in the R-group versus 2.1 in the S-group (P = 0.032).
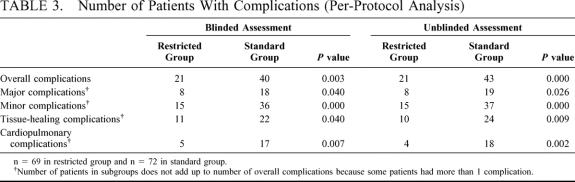
TABLE 3. Number of Patients With Complications (Per-Protocol Analysis)
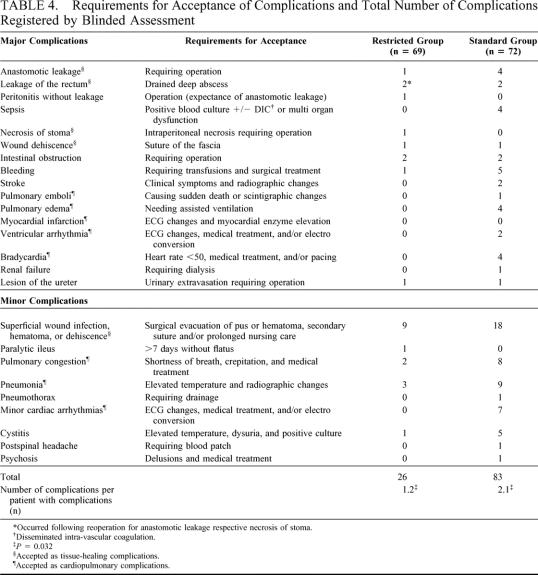
TABLE 4. Requirements for Acceptance of Complications and Total Number of Complications Registered by Blinded Assessment
A dose-response relation between complications and increasing volumes of intravenous fluid (P < 0.001) as well as increasing body weight (P < 0.001) on the day of operation was found independent of allocation group (Fig. 2).
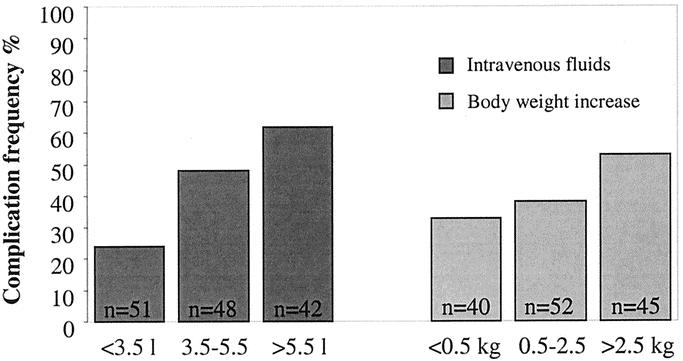
FIGURE 2. Complication frequency related to intravenous fluid administration and body weight increase on the day of operation. P < 0.001 both for increasing intravenous fluid volume and increasing body weight (χ2 test for trend).
Four patients (4.7%) died in the S-group, but no deaths occurred in the R-group (P = 0.12; absolute risk reduction, 5.6% [95% CI, 0.3–10.9%]). The causes of death were pulmonary edema (two cases), pneumonia with septicemia, and pulmonary embolism.
The number of patients with a postoperative hypotensive episode was similar in the 2 groups (R versus S: 28% versus 22%, P = 0.465). More patients in the R-group had low urinary output (<0.5 mL/kg/h) with smaller urinary volumes the day of operation (12% versus 3%, P = 0.008; volume median, 1125 mL [range, 400–3319] versus 1670 mL [range, 250–3885], P < 0.0005). No significant differences in urinary output were observed on days 1 to 6. A lower serum concentration of creatinine was observed on arrival to the recovery room in the S-group (R versus S: mean: 86.0 M/L [SD, 17.6] versus 75.8 M/l [SD, 19.2], P = 0.002), but no significant differences were observed during the subsequent days. No significant differences were observed for serum urea at any time. One case of renal failure was observed in the S-group after sepsis.
DISCUSSION
Arterial blood pressure decreases after induction of epidural analgesia. Volume preloading is expected to counteract this, but the effectiveness is not convincing.18,19 Loss to third space is usually replaced according to algorithms.20,21 However, clinical randomized trials have not been performed to investigate possible effects of replacement of these internal losses on outcome. The results of our trial show that omission of these fluid replacements substantially reduced complications after elective colorectal surgery. Associations between pulmonary congestion and fluid overload have been reported, but a causal relationship has not been previously shown. The reduction of cardiopulmonary complications in the restricted group was pronounced. Subclinical edema in lungs and other tissues may cause decreased tissue oxygenation,13 and may explain our finding of significantly more tissue-healing complications in the standard group. The dose-response correlation between complications and intravenous fluid overload supports this. Being the first trial addressing this problem, all complications were registered to avoid overlooking adverse effects and to generate new hypotheses if unexpected findings appeared, ie, relations have been reported between fluid overload and thrombosis22 and prolongation of intestinal paralysis.10 A reduction in fluid volume was not the only difference between the 2 study groups. Patients in the standard group received more intravenous sodium and chloride (mean, 489 mmol), but less glucose (mean, 41 g) the day of operation compared with the restricted group. New trials are needed to determine any possible importance of this. The complication frequency of our material may appear high, but complication frequencies have been reported as high as 68%.23 Comparing studies implies several difficulties.24 Low incidence of anastomotic leakage is regarded as an indicator for good surgical technique and is reported in 3–23% of anastomoses in nonrandomized studies25–27 and 2.3–12% in patients undergoing elective colorectal surgery in randomized clinical trials.28–30 Lowest frequencies are reported in the trials with few (19%)29 or no rectal anastomoses.25 In our trial, 47% of the patients had rectal surgery and the median age of the patients was 66 years, with preoperative comorbidity in 52%. We had an overall leakage of 4.5%, with 1 (1.5%) leakage of colon anastomosis and 4 (9%) leaks of rectal anastomoses.
No patients in the restricted regimen died. The 4 deaths in the standard group were all caused by cardiopulmonary complications. It is important to stress that mortality outcomes must be interpreted with caution in a study including only 172 patients. The finding is, however, supported by the recent Cochrane review31 analyzing the effects of fluid optimization techniques in orthopedic surgery. It was concluded that invasive intraoperative optimization regimens caused increased administration of fluid with a possible increased risk of death. The 4 deaths in our trial occurred at 4 different centers. This reflects our impression of the positive results being generally observed uniformly across the centers. The similar number of patients with postoperative hypotensive episodes was unexpected and suggests an inefficiency of intravenous fluids to prevent hypotension caused by epidural analgesia. The present trial has strengths and weaknesses. The strengths are that we used adequate methods for generation of allocation sequence and concealment. Furthermore, we employed blinded assessment of outcome measures. A double-blinded design of the study was considered, but it was rejected because of the individual adjustment of fluid therapy. The practical and ethical problems in handling patients undergoing major surgery without knowledge of the fluid administered were considered as well. The trial may also have weaknesses. First, the patient sample was not fully consecutive, as the presence of both the anesthesiologist and the surgeon was mandatory for inclusion; second, we encountered 2 violations of the randomization sequence; third, the number of patients was relatively small, and small samples may give rise to unequal distribution of important prognostic factors. In accordance, the number of patients currently smoking and the level of colonic anastomoses both favored the restricted group. We cannot rule out that these factors may have had a beneficial influence. However, the different level of colonic anastomoses may influence the risk of leakage but probably not other complications. Excluding anastomotic leakage from analysis does not change the result. The observed tendency toward intravenous fluid restriction even in the standard group was most likely an effect of the restricted regimen. Additional intravenous fluid to the restricted group may have treated hypotension or reflected accidental standard treatment. The result of these deviations was less difference between treatment groups and should cause less difference in outcome. Nevertheless, we were able to demonstrate a clear difference in postoperative morbidity.
We conclude that perioperative intravenous fluid therapy aiming at unchanged body weight reduces complications after elective colorectal surgery.
ACKNOWLEDGMENTS
We thank Peer Wille Jørgensen, MD, DMSc, and Lars Borly, MD, The Cochrane Colorectal Cancer Group, Bispebjerg University Hospital, with Ann Møller MD, The Cochrane Anesthesia Review Group, Bispebjerg University Hospital, for kind support, recommendations of the study, and many a methodological discussion. We also thank Christian Gluud, MD, DMSc, Copenhagen Trial Unit, Rigshospitalet, for discussions regarding design of the trial and comments on a previous version of the manuscript.
For positive reception of the idea and thorough assistance collecting data we thank the nursing staff at the anaesthesiological and surgical departments.
The authors thank our grant supporters: Eastern Danish Health Science Research Forum, University of Copenhagen; Clinical Unit of Preventive Medicine and Health Promotion, Bispebjerg University Hospital; The Copenhagen Hospital Corporation, Council of Research; The Danish Hospital Foundation for Medical Research, Region of Copenhagen, The Faeroe Islands and Greenland; The Danish Medical Association Research Found; The Northern Jutland Council of Research; The Research and Development Council, Vejle County; “Olga Bryde Nielsen’s Fund;” “Hans and Nora Buchards Fund;” “Inge and Jorgen Larsen’s Mindelegat;” “Grosserer Valdemar Foersom and wife Thyra Foersoms fund.” Nutricia A/S and N.C. Nielsen Hospital equipment A/S kindly donated enteral nutrition and feeding tubes.
Footnotes
Reprints: Birgitte Brandstrup, MD, PhD, Clinical Unit of Preventive Medicine and Health Promotion, H:S Bispebjerg University Hospital, Bispebjerg Bakke 23, DK-2400 Copenhagen NV, Denmark. E-mail: bbrandstrup@hotmail.com.
This study was funded by: Eastern Danish Health Science Research Forum, University of Copenhagen; Clinical Unit of Preventive Medicine and Health Promotion, H:S Bispebjerg University Hospital; The Copenhagen Hospital Corporation, Council of Research; The Danish Hospital Foundation for Medical Research, Region of Copenhagen, The Faeroe Islands and Greenland; The Danish Medical Association Research Found; The Northern Jutland Council of Research; The Research and Development Council, Vejle County; Olga Bryde Nielsens Fund; Hans and Nora Buchards Fund; Inge and Jørgen Larsen’s Mindelegat; Grosserer Valdemar Foersom and wife Thyra Foersoms Fund. Nutricia A/S and N.C. Nielsen Hospital equipment A/S kindly donated enteral nutrition and feeding tubes.
REFERENCES
- 1.Bennett J, McDonald T, Lieblich S, Piecuch J. Perioperative rehydration in ambulatory anesthesia for dentoalveolar surgery. Oral Surg Oral Med Oral Pathol. 1999;88:279-284. [DOI] [PubMed] [Google Scholar]
- 2.Cook R, Anderson S, Riseborough M, Blogg CE. Intravenous fluid load and recovery. A double-blind comparison in gynaecological patients who had day-case laparoscopy. Anaesthesia. 1990;45:826-830. [DOI] [PubMed] [Google Scholar]
- 3.Keane PW, Murray PF. Intravenous fluids in minor surgery. Their effect on recovery from anaesthesia. Anaesthesia. 1986;41:635-637. [DOI] [PubMed] [Google Scholar]
- 4.Spencer EM. Intravenous fluids in minor gynaecological surgery. Their effect on postoperative morbidity. Anaesthesia. 1988;43:1050-1051. [DOI] [PubMed] [Google Scholar]
- 5.Yogendran S, Asokumar B, Cheng DC, et al. A prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesth Analg. 1995;80:682-686. [DOI] [PubMed] [Google Scholar]
- 6.Cook JA, Fraser IA, Sandhu D, et al. A randomised comparison of two postoperative fluid regimens. Ann R Coll Surg Engl. 1989;71:67-69. [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings AG, Hamer-Hodges DW, Ferguson A. Fluid replacement after appendectomy or cholecystectomy. A comparison of oral glucose-electrolyte solution with intravenous fluids. J R Coll Surg Edinb. 1987;32:281-284. [PubMed] [Google Scholar]
- 8.Robertson GSM, Bundred NJ, Bullen BR, et al. A drip is unnecessary after cholecystectomy. J R Coll Surg Edinb. 1992;37:244-246. [PubMed] [Google Scholar]
- 9.Salim AS. Duration of intravenous fluid replacement after abdominal surgery: a prospective randomised study. Ann R Coll Surg Engl. 1991;73:119-123. [PMC free article] [PubMed] [Google Scholar]
- 10.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812-1818. [DOI] [PubMed] [Google Scholar]
- 11.Perco MJ, Jarnvig I, Højgaard-Rasmussen N, et al. Electric impedance for evaluation of body fluid balance in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001;15:44-48. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen LA, Rosenberg J, Crawford ME, et al. [Perioperative regimes for fluid and transfusion therapy]. Ugeskr Laeger. 1996;158:5286-5290. [PubMed] [Google Scholar]
- 13.Lang K, Boldt J, Suttner S, et al. Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg. 2001;93:405-409. [DOI] [PubMed] [Google Scholar]
- 14.Lowell JA, Schifferdecker C, Driscoll DF, et al. Postoperative fluid overload: not a benign problem. Crit Care Med. 1990;18:728-733. [DOI] [PubMed] [Google Scholar]
- 15.Møller AM, Pedersen T, Svendsen P-E, et al. Perioperative risk factors in elective pneumonectomy: the impact of excess fluid balance. Eur J Anaesthesiol. 2002;19:57-62. [DOI] [PubMed] [Google Scholar]
- 16.Bennett-Guerrero E, Feierman DE, Barclay GR, et al. Preoperative and intraoperative predictors of postoperative morbidity, poor graft function, and early rejection in 190 patients undergoing liver transplantation. Arch Surg. 2001;136:1177-1183. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] [Google Scholar]
- 18.Kinsella SM, Pirlet M, Mills MS, et al. Randomized study of intravenous fluid preload before epidural analgesia during labour. Br J Anaesth. 2000;85:311-313. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura N, Kajimoto Y, Kabe T, et al. The effects of volume loading during epidural analgesia. Resuscitation. 1985;13:31-40. [DOI] [PubMed] [Google Scholar]
- 20.Hurford WE, Bailin MT, Davison JK, et al. Clinical anesthesia procedures of the Massachusetts General Hospital. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2000:229. [Google Scholar]
- 21.Miller RD, Cucchiara RF, Miller JE, et al. Anesthesia. 5th ed. Philadelphia, PA: Churchill Livingstone 2000:1606-1607. [Google Scholar]
- 22.Janvrin SB, Davies G, Greenhalgh RM. Postoperative deep vein thrombosis caused by intravenous fluids during surgery. Br J Surg. 1980;67:690-693. [DOI] [PubMed] [Google Scholar]
- 23.Wise WE Jr, Padmanabhan A, Meesig DM, et al. Abdominal colon and rectal operations in the elderly. Dis Colon Rectum. 1991;34:959-963. [DOI] [PubMed] [Google Scholar]
- 24.Isbister WH. Study populations and casemix: influence on analysis of postoperative outcomes. Aust N Z J Surg. 2000;70:279-284. [DOI] [PubMed] [Google Scholar]
- 25.Basse L, Hjort JD, Billesbolle P, et al. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf W, Glimelius B, Bergstrom R, et al. Complications after double and single stapling in rectal surgery. Eur J Surg. 1991;157:543-547. [PubMed] [Google Scholar]
- 27.Vignali A, Fazio VW, Lavery IC, et al. Factors associated with the occurrence of leaks in stapled rectal anastomosis: a review of 1014 patients. J Am Coll Surg. 1997;185:105-113. [DOI] [PubMed] [Google Scholar]
- 28.Gottrup F, Diederich P, Sorensen K, et al. Prophylaxis with whole gut irrigation and antimicrobials in colorectal surgery. A prospective, randomized double-blind clinical trial. Am J Surg. 1985;149:317-322. [DOI] [PubMed] [Google Scholar]
- 29.Panton ON, Atkinson KG, Crichton EP, et al. Mechanical preparation of the large bowel for elective surgery. Comparison of whole-gut lavage with the conventional enema and purgative technique. Am J Surg. 1985;149:615-619. [DOI] [PubMed] [Google Scholar]
- 30.Titlestad IL, Ebbesen LS, Ainsworth AP, et al. Leukocyte-depletion of blood components does not significantly reduce the risk of infectious complications. Results of a double-blinded, randomized study. Int J Colorectal Dis. 2001;16:147-153. [DOI] [PubMed] [Google Scholar]
- 31.Price J, Sear J, Venn R. Perioperative fluid volume optimization following proximal femoral fracture (Cochrane review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. www.cochrane.org. [DOI] [PubMed]