Abstract
Objective:
To study the incidence of gastroesophageal reflux (GER)related complications after correction of esophageal atresia (EA).
Summary Background Data:
The association of EA and GER in children is well known. However, little is known about the prevalence of GER and its potential complications in adults who have undergone correction of EA as a child.
Methods:
Prospective analysis of the prevalence of GER and its complications over 28 years after correction of EA by means of a questionnaire, esophagogastroscopy, and histologic evaluation of esophageal biopsies.
Results:
The questionnaire was returned by 38 (95%) of 40 patients. A quarter of the patients had no complaints. Swallowing solid food was a problem for 13 patients (34%), and mashed foods for 2 (5%). Heartburn was experienced by 7 patients (18%), retrosternal pain by 8 (21%). However, none of the patients were using antireflux medication. Twenty-three patients (61%) agreed to undergo esophagogastroscopy, which showed macroscopic Barrett esophagus in 1 patient, which was confirmed by histology. One patient developed complaints of dysphagia at the end of the study. A squamous cell esophageal carcinoma was diagnosed and treated by transthoracic subtotal esophagectomy.
Conclusions:
This study shows a high incidence of GER-related complications after correction of EA, but it is still very disputable if all EA patients should be screened at an adult age.
The prevalence of gastroesophageal reflux was assessed in adults who underwent correction of esophageal atresia in childhood. One third of the patients had some degree of dysphagia, 20 of 21 biopsies showed some degree of esophagitis, and 1 patient was diagnosed with squamous cell esophageal carcinoma.
It is well known that the prevalence of gastroesophageal reflux (GER) is increased in children treated for esophageal atresia (EA).1–3 If untreated, GER can result in esophagitis and intestinal metaplasia (Barrett esophagus), which is a known precursor of adenocarcinoma4,5. In our center, EA patients are nowadays prospectively screened for GER 3 months after surgical correction of their EA. However, in adults who have undergone correction of EA as a child, little is known about the prevalence of GER and its potential complications.
There are only 3 studies that prospectively investigated the prevalence of GER and its sequelae after correction of EA by means of esophagoscopy and histologic evaluation of biopsies.6–8 Lindahl et al and Somppi et al found esophagitis and gastric metaplasia, which in those days was also called Barrett’s esophagus. Only Krug et al found intestinal metaplasia, Barrett esophagus according to modern definitions.9 The longest follow-up period in these studies is 26 years. In the present study, the prevalence of GER and its complications over 28 years after correction of EA was analyzed.
PATIENTS AND METHODS
Between September 1947 and September 1972, 105 patients were treated for EA and/or tracheoesophageal fistula (TEF) at the Pediatric Surgical Center of Amsterdam. Medical charts, operative reports, and office notes were used to assess the medical history of each patient and to collect data. Sixty-three patients (60%), mostly from the early years of the series, died before the start of the study and were excluded. The causes of death of these patients are listed in Table 1. All but 1 of these patients died before the age of 3 years (the other patient died at age 29 from a car accident). Two patients were lost to follow-up, due to emigration in one, and due to adoption in the other. Forty patients could finally be included.
TABLE 1. Causes of Death in 63 Patients Who Died Before the Start of the Study

After approval of the protocol by the Medical Ethical Committee, a questionnaire was sent to the patients to assess their symptoms. In an accompanying letter, the patients were offered esophagogastroscopy (EGS) with biopsies. The completed questionnaire was returned by 38 patients (95%), and twenty-three patients (61%) were willing to undergo EGS.
EGS was performed by a gastroenterologist, under local anesthesia. Findings at EGS were classified according to the modified system of Savary-Miller.10 Biopsies were taken from the anastomotic scar, from the distal esophagus above the Z-line and from macroscopically abnormal regions. All biopsies were judged for esophagitis and metaplasia by a pathologist (who was not aware of clinical and endoscopic findings) and scored according to Ismael-Beigi.11 Barrett esophagus was defined as a change in the esophageal epithelium of any length that could be recognized at EGS and had to be confirmed by biopsy to contain intestinal metaplasia.9 Data were collected in a database and analyzed using SPSS software, version 10.0.1. Differences between groups were analyzed by means of the χ2 test or Fisher exact test. The level of significance was defined as P = 0.05.
RESULTS
Patient Characteristics
The study group consisted of 26 men and 14 women. The mean birth weight had been 2760 g (range, 1960–3600 g), and the mean gestational age 39 weeks (range, 34–42 weeks). Nine patients (23%) had associated congenital malformations. One patient had a long gap EA without TEF (Gross type A); the other 39 patients had EA with a distal fistula (Gross type C). Primary repair was performed in 38 patients, a colon interposition in 2 patients, all without major complications.
One patient underwent an anterior gastropexy according to Boerema at the age of 19 years. The Boerema anterior gastropexy is the standard antireflux procedure for children in our center.12,13 Sixteen patients (40%) underwent more than 3 esophageal dilatations. However, in most of these patients, it was not clear in retrospect whether the dilatations were performed due to complaints and/or symptoms, or as a routine procedure.
Questionnaire Results
The questionnaire was completed and returned by 38 (95%) of 40 patients. The mean age of the responders was 34 years (range, 28–45 years). All had families and normal jobs. None of the offspring had EA, but 1 child had an isolated (Gross type E) TEF.
Ten patients (26%) did not have any complaints. Thirteen patients (34%) experienced problems swallowing solid food; 2 (5%) had problems swallowing mashed food. Four patients (11%) experienced limitations of type of food; 15 (40%) needed to wash down their food with drinks. Most problems were encountered while eating meat or rice. Recurrent respiratory problems occurred 5 patients (13%); heartburn was experienced by 7 patients (18%), retrosternal pain by 8 (21%), and 5 patients (13%) reported both. None of the patients were under medical care for their complaints, and none were using antireflux medication at the time of the questionnaire.
Esophagogastroscopy and Histology
Twenty-three patients (61%) underwent EGS. As many patients with complaints were willing to undergo EGS as patients without complaints (17 of 28 versus 6 of 10, P = 0.627).
EGS showed a macroscopically normal esophagus in 19 patients (82%). Grade I esophagitis was found in 2 patients (9%), and macroscopic Barrett esophagus (Grade V esophagitis) was found in 2 patients (9%). Hiatal hernia was seen in 13 patients. Patients with hiatal hernia did not have more complaints or worse findings at EGS and histology (data not shown).
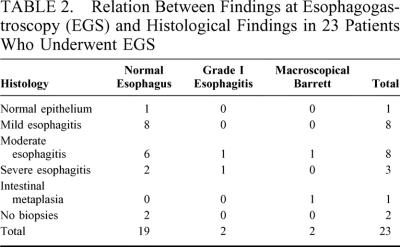
In 21 of 23 patients, biopsies were taken: in 1 patient, EGS was performed in another hospital and no biopsies were taken. In another patient with Ehlers-Danlos, no biopsies were taken because of a slightly increased risk of bleeding. Histology showed normal esophageal squamous epithelium in 1 patient (5%), mild esophagitis in 8 patients (38%), moderate esophagitis in 8 patients (38%), and severe esophagitis in 3 patients (14%). Intestinal metaplasia (Barrett esophagus) was found in 1 of the 2 patients with a macroscopical Barrett at EGS (5%). Table 2 shows the relation between findings at EGS and histology. We found no correlation between complaints and findings at EGS or histology.
TABLE 2. Relation Between Findings at Esophagogastroscopy (EGS) and Histological Findings in 23 Patients Who Underwent EGS
Worth mentioning is the patient who underwent anterior gastropexy in the past, who had a normal EGS and normal esophageal squamous epithelium at histologic evaluation.
One of the patients developed complaints of dysphagia some time after completion of the questionnaire. He underwent EGS at which a squamous cell carcinoma of the esophagus was diagnosed (T3N0M0). A transthoracic subtotal esophagectomy was performed, with gastric tube reconstruction. There are no signs of recurrent disease after more than 3 years of follow-up.14
DISCUSSION
This study analyzes the prevalence of GER, and its complications, over 25 years after correction of EA by means of a questionnaire, EGS, and histologic evaluation of esophageal biopsies.
The 95% response to the questionnaire was high. All seemed to function normally in daily life. Three quarters of the patients had complaints, but they did not seem to be hampered by these complaints in their daily activities. A third of the patients experienced problems swallowing solid food, but most of them could eat everything they wanted. It has been described that after correction of EA, the motility of the esophagus is hampered,15 and it is not clear whether the swallowing complaints were caused by GER or by disturbed motility.
All patients were offered EGS with biopsies, which 61% agreed to undergo. Unfortunately, there is a clear decline in the number of patients willing to cooperate with the several parts of the study. The most logical explanation for this selection is that patients with complaints are more willing to undergo EGS. However, this hypothesis proved to be wrong: patients with complaints did not appear to be more willing to undergo EGS, as compared with those without complaints (17 of 28 versus 6 of 10, P = 0.627). The true reason for this selection must be that it is easier to participate in a questionnaire than to be subject to an invasive investigation. However, even if all patients that did not agree to undergo EGS or biopsies would have had normal histology, the prevalence of histologically proven abnormalities would still be 63% (24 of 38, defining mild esophagitis as normal).
The findings of this study are comparable to those of Krug et al.8 They also found a high number of patients with complaints (77%), and a high prevalence of esophagitis (53%). Similar to our study, they found a striking discrepancy between complaints and abnormalities at EGS and histology. There are several other publications reporting this phenomenon,16–18 but none have offered an explanation.
The methods used in our study focused especially on assessing the sequelae of GER, like esophagitis and Barrett esophagus. It is possible that pH-measurements may help to identify the real number of patients with GER, although its additional value can be disputed in patients who underwent esophageal biopsies. Our next study, combining pH-measurements with manometry, will give more insight in the real prevalence of GER and/or motility problems.
Does this give us enough evidence to advocate screening of all EA patients at an adult age? The intended effect of screening would be to detect intestinal metaplasia, to start treatment early and to improve survival. However, before starting a screening program, we should first have to be informed about the incidence per age group of intestinal metaplasia after repair of EA and make an estimate of the effect of EGS and histologic evaluation on improved survival. In such an estimate, we would also have to take into account the duration of the preclinical detectable phase, the effect of false-positive test results, and the side effects of treatment. Furthermore, cost-effectiveness and aspects concerning the implementation of a screening program should be taken into consideration. Moreover, healthy ex-patients are asked to undergo invasive investigations, which makes them a patient again. It is also important to realize that it still is not clear if any treatment of Barrett esophagus, be it medical, ablative, or surgical, will have any effect on the natural history of the disease.19 So far, an increased incidence of esophageal cancer in EA-patients has not been reported in the literature.
In summary, this study shows a high prevalence of esophagitis, probably due to chronic GER after correction of EA, and a high frequency of complications, but no correlation between subjective and objective findings. It is still very disputable if all EA patients should be screened at an adult age.
Footnotes
Reprints: D. C. Aronson, M.D. Ph.D., Pediatric Surgical Center of Amsterdam, Emma Children’s Hospital AMC, PO box 22660, 1100 DD Amsterdam, The Netherlands. E-mail: d.c.aronson@amc.uva.nl.
REFERENCES
- 1.Orringer MB, Kirsh MM, Sloan H. Long-term esophageal function following repair of esophageal atresia. Ann Surg. 1977;186:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manning PB, Morgan RA, Coran AG, et al. Fifty years’ experience with esophageal atresia and tracheoesophageal fistula. Beginning with Cameron Haight’s first operation in 1935. Ann Surg. 1986;204:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engum SA, Grosfeld JL, West KW, et al. Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Arch Surg. 1995;130:502–508. [DOI] [PubMed] [Google Scholar]
- 4.Hameeteman W, Tytgat GNJ, Houthoff HJ, et al. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. [DOI] [PubMed] [Google Scholar]
- 5.Johnston BT, Carre IJ, Thomas PS, et al. Twenty to 40 year follow up of infantile hiatal hernia. Gut. 1995;36:809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindahl H, Rintala R, Sariola H. Chronic esophagitis and gastric metaplasia are frequent late complications of esophageal atresia. J Pediatr Surg. 1993;28:1178–1180. [DOI] [PubMed] [Google Scholar]
- 7.Somppi E, Tammela O, Ruuska T, et al. Outcome of patients operated on for esophageal atresia: 30 years’ experience. J Pediatr Surg. 1998;33:1341–1346. [DOI] [PubMed] [Google Scholar]
- 8.Krug E, Bergmeijer JH, Dees J, et al. Gastroesophageal reflux and Barrett’s esophagus in adults born with esophageal atresia. Am J Gastroenterol. 1999;94:2825–2828. [DOI] [PubMed] [Google Scholar]
- 9.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–1032. [DOI] [PubMed] [Google Scholar]
- 10.Ollyo JB, Lang F, Fontollet Ch, et al. Savary’s new endoscopic grading of reflux oesophagitis: a simple, reproducible, logical, complete and useful classification. Gastroenterology. 1990;98:A100. [Google Scholar]
- 11.Ismail-Beigi F, Pope CE. Distribution of the histological changes of gastroesophageal reflux in the distal esophagus of man. Gastroenterology. 1974;66:1109–1113. [PubMed] [Google Scholar]
- 12.Boerema I. Anterior gastropexy: a simple operation for hiatus hernia. Aust N Z J Surg. 1969;39:173–175. [DOI] [PubMed] [Google Scholar]
- 13.Heij HA, Vos A. Long-term results of anterior gastropexy for gastroesophageal reflux in children. Ped Surg Int. 1988;4:256–259. [Google Scholar]
- 14.Deurloo JA, van Lanschot JJ, Drillenburg P, et al. Esophageal squamous cell carcinoma 38 years after primary repair of esophageal atresia. J Pediatr Surg. 2001;36:629–630. [DOI] [PubMed] [Google Scholar]
- 15.Tovar JA, Diez-Pardo JA, Murcia J, et al. Ambulatory 24-hour manometric and pH metric evidence of permanent impairment of clearance capacity in patients with esophageal atresia. J Pediatr Surg. 1995;30:1224–1231. [DOI] [PubMed] [Google Scholar]
- 16.Leape LL, Bhan I, Ramenofsky ML. Esophageal biopsy in the diagnosis of reflux esophagitis. J Pediatr Surg. 1981;16:379–384. [DOI] [PubMed] [Google Scholar]
- 17.Biller JA, Winter HS, Grand RJ, et al. Are endoscopic changes predictive of histologic esophagitis in children? J Pediatr. 1983;103:215–218. [DOI] [PubMed] [Google Scholar]
- 18.Black DD, Haggitt RC, Orenstein SR, et al. Esophagitis in infants. Morphometric histological diagnosis and correlation with measures of gastroesophageal reflux. Gastroenterology. 1990;98:1408–1414. [PubMed] [Google Scholar]
- 19.Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569–1591. [DOI] [PubMed] [Google Scholar]