Abstract
Object:
To determine if a 6-month regimen of prophylactic ursodeoxycholic acid is effective in the prevention of gallstones.
Summary Background Data:
Rapid weight loss after surgery for the treatment of morbid obesity is associated with a high incidence of gallstone formation.
Methods:
Patients with vertical banded gastroplasty (VBG) and adjustable gastric banding (AGB) were enrolled in this study. A single-center, randomized, double-blind, prospective trial evaluated 500 mg of ursodeoxycholic acid versus placebo, beginning within 3 days after surgery and continuing for 6 months or until gallstone development, for patients with morbid obesity. Transabdominal sonography or abdominal CT scan was obtained preoperatively at 3, 6, 12, and 24 months after surgery or until gallstone formation.
Results:
From March 1997 to April 2000, 262 patients were submitted to surgery. Seventy-seven patients refused to participate in the study; 43 patients with previous gallstone operation or verified gallstones preoperatively were excluded. Of 152 patients, 76 were randomized to placebo and 76 to 500 mg of ursodeoxycholic acid daily. Preoperative age, sex, weight, BMI, and postoperative weight loss were not significantly different between groups. Gallstone formation was significantly less (P = 0.0018, Fisher exact test) frequent with ursodeoxycholic acid than with placebo at 12 months, 3% versus 22%, and 8% versus 30% (P = 0.0022) at 24 months, cholecystectomy in 4.7% versus 12%, respectively (P < 0,02, Fisher exact test).
Conclusion:
A daily dose of 500 mg of ursodeoxycholic acid for 6 months is effective prophylaxis for gallstone formation following gastric restrictive procedures.
This long-term follow-up study was designed to determine if a 6-month regimen of prophylactic ursodeoxycholic acid is effective in the prevention of gallstones after restrictive bariatric surgery. Patients with vertical banded gastroplasty and adjustable gastric banding were enrolled in this study. A daily dose of 500 mg of ursodeoxycholic acid for 6 months is effective prophylaxis for gallstone formation after gastric restrictive procedures.
The development of cholesterol gallstones is associated with certain well-defined risk factors.1–4 The risk for developing gallstones during active weight reduction is well accepted.5–11 Between 10% and 25% of persons having lost weight through very low-calorie dieting (VLCD) develop gallstones.12,13 In addition, 35–38% of patients with morbid obesity develop gallstones as they lose weight after bariatric surgery.5,14,15 A routine synchronous cholecystectomy during bariatric surgery is recommended by some centers.5,16 Concomitant cholecystectomy in bariatric surgery can be a very difficult procedure with a higher incidence of complications.17,18 Therefore, a preventive therapy for gallstone formation is recommended in several studies. Ursodeoxycholic acid administered during VLCD seems to inhibit the development of biliary cholesterol crystals.8 Ursodeoxycholic acid (600 mg/d) is highly effective in preventing gallstone formation in patients undergoing dietary-induced weight reduction.19 In prospective randomized studies, the prophylactic administration of ursodeoxycholic acid has been shown to be effective in gastric bypass procedures20–22, as well as in a small series with vertical banded gastroplasty.23
This randomized double-blind, prospective trial was designed to determine the safety and efficacy of 500 mg/d ursodeoxycholic acid (Ursofalk; Falk Corporation, Dortmund, Germany) versus placebo for reducing the incidence of gallstones in morbidly obese patients undergoing adjustable gastric banding and vertical banded gastroplasty surgery for morbid obesity and the effect of long-term follow-up.
MATERIALS AND METHODS
Study Design
Patients admitted to vertical banded gastroplasty (VBG) and adjustable gastric banding (AGB) for the treatment of morbid obesity were enrolled in this study. Eligible patients were over 18 years of age and had a body mass index (BMI) ≥ 40 kg/m2. Patients with a BMI ≥ 35 kg/m2 and comorbidities according to the guidelines of the National Institutes of Health Consensus Development Conference Statement March 25–27, 1991, were also enrolled in the study.24
Exclusion criteria for the study included prior cholecystectomy, presence of gallstones, use of other investigational drugs, pregnancy or inadequate use of contraception, refusal, or inability to sign informed consent. Patients agreed to take the trial medication twice semi-daily for 6 months. They were also excluded if during the trial, they continuously used nonsteroidal antiinflammatory drugs, antihyperlipidemics such as cholestyramine or statins, or hepatotoxic drugs. Patients with no concomitant medication were claimed by the ethical committee. Transabdominal sonography or, if difficult to evaluate, abdominal CT scan was obtained preoperatively at 3, 6, 12, and 24 months after surgery or until gallstone formation. The restrictive operative procedures for morbid obesity were performed using either VBG or AGB, by 2 surgeons related to the experience in a nonrandomized fashion. The surgical technique of the procedures were published by the authors elsewhere.25,26
Study Protocol
The study was approved by the ethical committee of the Landesregierung Salzburg, Austria, a government institution. Informed consent was obtained from each patient before the procedure and before initiation of the study. No financial support, grant, or donation was submitted by the Falk Corporation. Investigation site was blinded from Falk Corporation, FRG to the drug codes, except in case of emergency. A single-center, randomized, double-blind, prospective trial evaluated 250 mg of ursodeoxycholic acid twice a day (total dosage of 500 mg/d) versus placebo, beginning within 3 days after surgery and continuing for 6 months or until gallstone development. Medication compliance was monitored at every visit. If an ultrasound scan or a CT scan revealed gallstones, the patient was taken off the study agent and removed from the study. Adverse experiences of the medication were tabulated as mild, moderate, or severe. Weight loss was recorded in all patients. Complications were classified by physicians as either not related, unlikely, possibly, probably, or highly probably related to the study agent.
Statistical Methods
With 76 patients in each treatment group, a difference in proportions of -0.2 (20%) is required to achieve a beta of 0.2 and a sigma of 0.52 on the basis of a two-sided test with a significance level of 0.05. Due to the possible role of concomitant medications, differences < 0.2 were judged to be of doubtful clinical importance. All data were further analyzed by use of a Compaq Pentium III personal computer using the software programs from IDV-Versuchsplanung und Datenanalyse, Gauting, Munich, F.R.G. In each group, median, standard deviation, standard error, range, upper and lower quartile, and total mean values were calculated. Univariate analyses were performed using the Wilcoxon-Mann-Whitney-U test for continuous variables and by using a χ2 test on 2 × 2 table for binary variables (Fisher exact test). The P values are those computed for each comparison, and statistically significant variables are those at the 0.05 level (two-tailed). Odds ratios for categorical variables with respect to gallstone formation were obtained with 95% confidence interval. Odds ratios are significant at P < 0.05 if 95% confidence limits do not include the value of one.
RESULTS
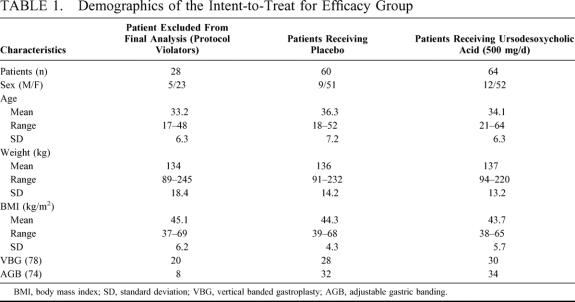
From March 1997 to April 2000, 262 patients were submitted to surgery. In the study, 76 were randomized to placebo and 76 to 500 mg/d ursodeoxycholic acid. One hundred twenty-four patients completed follow-up gallbladder sonography and were eligible for inclusion in the intent-to-treat for efficacy group. One hundred thirty patients refused to participate in the study or did not meet the inclusion criteria. Ultrasonography investigation of the gallbladder was performed in all patients. Additionally, 5 patients in the placebo and 4 patients in the ursodeoxycholic group had CT scan due to inability of gallbladder exposure in the sonography, 8.3% versus 6.25. The demographics are shown in Table 1. There were no significant differences with respect to age, sex, preoperative weight, or BMI between those receiving medication or placebo in any of the patient groups. No patient was withdrawn from the study because of a serious adverse drug reaction. Patients in the placebo and treatment groups dropped out for same reasons at similar rates.
TABLE 1. Demographics of the Intent-to-Treat for Efficacy Group
Compliance with the trial drug regimen was similar across the operated subsets and averaged 79% (60 of 76) for placebo and 84% (64 of 76) for ursodeoxycholic acid. No severe side effects from medication were observed. Mild and moderate side effects such as nausea and constipation were equivalent in both groups: 4 patients in the placebo (6.6%) and 6 patients in the treatment group (9.3%). Mild and moderate side effects did not lead to discontinuation of the study. In the group of protocol violators, 24 patients were unable to swallow the medication (85.7%).
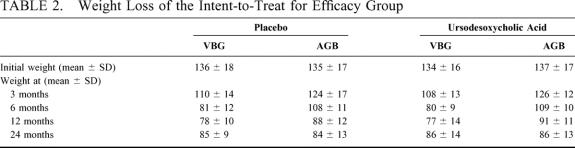
Weight loss was equivalent between the placebo and the ursodeoxycholic acid group (Table 2). In the VBG group, patients showed a noticeably more rapid weight loss, but no significant incident development of gallstone formation was noticed.
TABLE 2. Weight Loss of the Intent-to-Treat for Efficacy Group
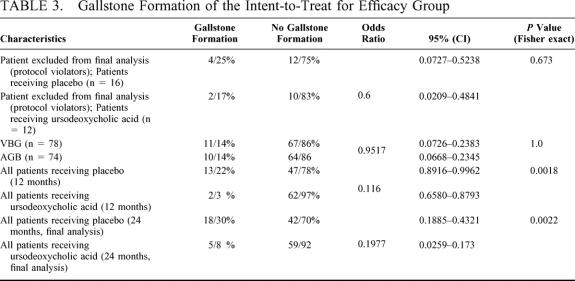
The primary efficacy variable was the proportion of patients developing gallstones in the intent-to-treat for efficacy group. Out of this point of view, gallstone formation was significantly less (P = 0.0018, Fisher exact test) frequent with ursodeoxycholic acid than with placebo at 12 months: 3% versus 22%, respectively, and at 24 months, 8% versus 30% (P = 0,0022, Fisher exact test) (Table 3).
TABLE 3. Gallstone Formation of the Intent-to-Treat for Efficacy Group
In the 24-month follow-up period, 15 cholecystectomies were performed (mean 14.9 ± 4.3 months after bariatric surgery) in patients with symptomatic cholelithiasis: 3 patients in the ursodeoxycholic group and 12 patients in the placebo group, 12% versus 4.7%, respectively (P < 0,02, Fisher exact test).
DISCUSSION
Cholelithiasis is the primary expression of obesity in the hepatobiliary system. In obese subjects, the risk of developing gallstones is increased due to a higher cholesterol saturation of gallbladder bile.8 During weight reduction with VLCD, the incidence of gallstones increases,7 but the mechanism for gallstone formation is not completely understood, and several pathogenetic mechanisms have been suggested: increased saturation of bile, increased gall-bladder secretion of mucin and calcium, and increased presence of prostaglandins and arachidonic acid.8,27,28 Gallstone formation is a well-recognized complication of rapid weight loss.1–4 Therefore, a preventive therapy for gallstone formation in bariatric surgery should be the logical consequence. Synchronous cholecystectomy, medical concomitant therapy, or relatively high-fat diet could be the options. Festi et al8,29 reported that patients with low-fat diet develop asymptomatic gallstones in 54%, and a relatively high-fat intake could prevent gallstone formation, probably by maintaining an adequate gallbladder emptying, which could counterbalance lithogenic mechanisms acting during weight loss. Gallstones (asymptomatic) developed in 6 of 11 (54.5%) (P < 0.01) subjects following the lower-fat diet, but in none with the higher-fat regimen. Similar findings were reported by Erlinger6 and Gebhard et al.30 However, patients with morbid obesity should have an excessive weight loss of 50% minimum for having a significant benefit from the operation, which is one of the criteria for success of the operation.31,32 A higher-fat regimen could lead to failure of the surgical procedure with inadequate weight loss.34,35 In addition, it is necessary to properly study dietary and other environmental factors that may affect the gallstone formation risk. Controversy exists if the gallbladder should be removed when performing gastric restrictive procedures such as VBG and AGB. In 1985, Ameral and Thompson59 recommended in one of the earliest papers a routine cholecystectomy at the time of bariatric surgery. In contrast to the opinions favoring routine cholecystectomy, other surgeons only recommend the removal of the gallbladder in the presence of gallstones or intraoperative evidence of disease, such as cholesterolosis or chronic inflammation.14,37,38 Nevertheless, they mentioned the potential complications of cholecystectomy: intraoperative difficulties in massively obese patients, prolonged operative time, and the relatively low incidence of symptomatic gallstones. Using another approach, Deitel et al39 favored laparoscopic cholecystectomy for cholelithiasis after VBG. Synchronous cholecystectomy in bariatric surgery can be a very difficult procedure with a higher incidence of complications, especially in the field of laparoscopic surgery.17,18 Angrisani et al reported that laparoscopic cholecystectomy in obese patients was technically more difficult, and required a significantly longer operating time.18
Ursodeoxycholic acid administered during VLCD and weight loss after bariatric surgery is effective in preventing gallstone formation19–23
The optimum dose for gallstone prophylaxis appears to be 600 mg/d, or 4 to 5 mg/kg.19,21 This is approximately one half of the dose used for the dissolution of cholesterol gallstones.40,41,42 The results of the present study show an effective prophylaxis with a dosage of 500 mg/d.
Deitel and Petrov reported 13% of patients requiring cholecystectomy after VBG, but the reminder of the subjects were not screened.14 At that time, it was believed that pure gastric restriction procedures had a lesser risk of gallstone formation than malabsorptive procedures.
Ursodeoxycholic acid has been proven to be effective in reducing the risk for gallstones after restrictive bariatric surgery, namely in vertical banded gastroplasty. A small Canadian double-blind study on 29 patients reported that 6 (43%) of the 14 subjects on placebo developed stones 3 months after the operation.23
The question remains of how long ursodeoxycholic acid should be taken and how effective this drug is in long-term weight loss. Randomized studies ranged from 2 months to 6 months.9,10,11,19,21 Sugerman et al could report in their study the results of 12 months, so far the study with the longest follow-up.21 This period may be also insufficient, because in the investigation of Deitel and Petrov, the peak incidence of symptomatic gallstones was at 16 months after surgery. This finding is strikingly similar to the peak frequency at 16 months reported by Amaral et al after gastric bypass. [5,14,]. In our study, cholecystectomy was performed in symptomatic gallstone disease an average of 14.9 ± 4.3 months after bariatric surgery.
It is generally accepted that gallstone formation is correlated with weight loss. Shiffman et al could show that bile cholesterol normalized when the weight stabilized27 24 months after gastric bypass. The authors of the present study could demonstrate in another prospective study on more than 1000 restrictive procedures that a weight stabilization phase is reached not before 24 months.43 Our data from this study show a significantly reduced gallstone formation rate of 8% after 24 months compared with 30% in the placebo group.
Summarizing the available data on gallstone prevention at the time of rapid weight loss, it is clear that UDCA is effective and has few adverse drug reactions, but its use has not been very common yet.
Some authors believe that the large size of the capsules may cause problems in the small outlet of VBGs and AGBs.14 It could be possible that the size of the capsules is one of the reasons for the high drop out rate of 28 patients (18%) in our study. However, among patients with restrictive procedures, those in the VBG group had markedly lower compliance (20 patients, 26%) than those in the AGB Group (8 patients, 10%). Since 2001, the ursodeoxycholic acid suspension is available; therefore, the problem with the drug and the small outlet should be solved.
Due to increasing frequency of extreme obesity in western countries, the demand for bariatric surgery is emphasized.44 Morbid obesity has a high incidence of gallstone formation, and it should be routinely ruled out preoperatively with ultrasonography. Postoperatively, the gallstone formation is correlated with rapid weight loss in a high incidence.
In conclusion, a daily dose of 500 mg of ursodeoxycholic acid in divided doses semi-daily for 6 months is an effective prophylaxis for gallstone formation after gastric restrictive procedures and avoids simultaneous cholecystectomy in morbid obese patients.
Footnotes
Reprints: Karl Miller, MD, Head of the Surgical Department, Krankenhaus Hallein, Buergermeisterstr. 48, A-5400 Hallein, Austria. E-mail: Miller@Eunet.at.
REFERENCES
- 1.Bennion LJ, Grundy SM. Risk factors for the development of cholelithiasis in man (second of two parts). N Engl J Med. 1992;307:798–800. [DOI] [PubMed] [Google Scholar]
- 2.Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20:1–19. [PubMed] [Google Scholar]
- 3.Jorgensen T. Gallstones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut. 1989;30:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodama H, Kono S, Todoroki I, et al. Gallstone disease risk in relation to body mass index and waist-to-hip ratio in Japanese men. Int J Obes Relat Metab Disord. 1999;23:211–216. [DOI] [PubMed] [Google Scholar]
- 5.Amaral JF, Thompson WR. Gallbladder disease in morbidly obese. Am J Surg. 1985;149:551–557. [DOI] [PubMed] [Google Scholar]
- 6.Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12:1347–1352. [DOI] [PubMed] [Google Scholar]
- 7.Everhart JE. Contributions of obesity and weight loss to gallstone disease. Ann Intern Med. 1993;119:1029–1035. [DOI] [PubMed] [Google Scholar]
- 8.Festi D, Colecchia A, Larocca A, et al. Review: low caloric intake and gall-bladder motor function. Aliment Pharmacol Ther. 2000;14(Suppl 2):51–53. [DOI] [PubMed] [Google Scholar]
- 9.Liddle RA, Goldstein RB, Saxoton J. Gallstone formation during weight reduction dieting. Arch Intern Med. 1989;149:1750–1753. [PubMed] [Google Scholar]
- 10.Spirt BA, Graves LW, Weinstock R, et al. Gallstone formation in obese women treated by a low-calorie diet. Int J Obes Relat Metab Disord. 1995;19:593–595. [PubMed] [Google Scholar]
- 11.Vezina WC, Grace DM, Hutton LC, et al. Similarity in gallstone formation from 900 kcal/day diets containing 16 g vs 30 g of daily fat: evidence that fat restriction is not the main culprit of cholelithiasis during rapid weight reduction. Dig Dis Sci. 1998;43:554–561. [DOI] [PubMed] [Google Scholar]
- 12.Yang H., Peterson GM., Roth MP, et al. Risk factors for gallstone formation during rapid weight loss. Dig Dis Sci. 1992;37:912–918. [DOI] [PubMed] [Google Scholar]
- 13.Zapata R, Severin C, Manriquez M, et al. Gallbladder motility and lithogenesis in obese patients during diet-induced weight loss. Dig Dis Sci. 2000;45:421–428. [DOI] [PubMed] [Google Scholar]
- 14.Deitel M, Petrov I. Incidence of symptomatic gallstones after bariatric operations. Surg Gynecol Obstet. 1987;164:549–552. [PubMed] [Google Scholar]
- 15.Shiffman ML, Sugerman HJ, Kellum JM, et al. Changes in gallbladder bile composition following gallstone formation and weight reduction. Gastroenterology. 1992;103:214–221. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt JH, Hocking MP, Rout WR, et al. The case for prophylactic cholecystectomy concomitant with gastric restriction for morbid obesity. Am Surg. 1988;54:269–272. [PubMed] [Google Scholar]
- 17.Ammori BJ, Vezakis A, Davides D, et al. Laparoscopic cholecystectomy in morbidly obese patients. Surg Endosc. 2001;15:1336–1339. [DOI] [PubMed] [Google Scholar]
- 18.Angrisani L, Lorenzo M, De Palma G, et al. Laparoscopic cholecystectomy in obese patients compared with nonobese patients. Surg Laparosc Endosc. 1995;5:197–201. [PubMed] [Google Scholar]
- 19.Shiffman ML, Kaplan GD, Brinkman-Kaplan V, et al. Prophylaxis against gallstone formation with ursodeoxycholic acid in patients participating in a very-low-calorie diet program. Ann Intern Med. 1995;122:899–905. [DOI] [PubMed] [Google Scholar]
- 20.Hood KA, Gleeson D, Ruppin DC, Dowling RH. Gall stone recurrence and its prevention: the British/Belgian Gall Stone Study Group’s post-dissolution trial. Gut. 1993;34:1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugerman HJ, Brewer WH, Shiffman ML, et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg. 1995;169:91–96. [DOI] [PubMed] [Google Scholar]
- 22.Wudel LJ Jr, Wright JK, Debelak JP, et al. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res. 2002;102:50–56. [DOI] [PubMed] [Google Scholar]
- 23.Williams C, Gowan R, Perey BJ. A double-blind placebo-controlled trial of ursodeoxycholic acid in the prevention of gallstones during weight loss after vertical banded gastroplasty. Obes Surg. 1993;3:257–259. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health. Gastrointestinal Surgery for Severe Obesity. NIH Consens Statement. 1991;9:1–20. [PubMed] [Google Scholar]
- 25.Hell E, Miller K. Long term results with vertical banded gastroplasty in the treatment of morbid obesity. Obes Surg. 1998;8:373. [Google Scholar]
- 26.Miller K, Hell E. Laparoscopic adjustable gastric banding—a prospective 4-year follow up study. Obes Surg. 1999;2:183–187. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman ML, Shamburek RD, Schwartz CC, et al. Gallbladder mucin, arachidonic acid, and bile lipids in patients who develop gallstones during weight reduction. Gastroenterology. 1993;105:1200–1208. [DOI] [PubMed] [Google Scholar]
- 28.Shiffman ML, Sugerman HJ, Kellum JH, et al. Gallstones in patients with morbid obesity. Relationship to body weight, weight loss and gallbladder bile cholesterol solubility. Int J Obes Relat Metab Disord. 1993;17:153–158. [PubMed] [Google Scholar]
- 29.Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord. 1998;22:592–600. [DOI] [PubMed] [Google Scholar]
- 30.Gebhard RL, Prigge WF, Ansel HJ, et al. The role of gallbladder emptying in gallstone formation during diet-induced rapid weight loss. Hepatology. 1996;24:544–548. [DOI] [PubMed] [Google Scholar]
- 31.Oria HE, Moorehead MK. Bariatric Analysis and Reporting Outcome System (BAROS). Obes Surg. 1998;8:487–499. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AM, Falcone AR, Kortner B, et al. BAROS: an effective system to evaluate the results of patients after bariatric surgery. Obes Surg. 2000;10:445–450. [DOI] [PubMed] [Google Scholar]
- 33.Greenstein RJ, Rabner JG, Taler T. Bariatric surgery vs. conventional dieting in the morbidly obese. Obes Surg. 1994;4:16–23. [DOI] [PubMed] [Google Scholar]
- 34.Hsu LK, Sullivan SP, Benotti PN. Eating disturbances and outcome of gastric bypass surgery: a pilot study. Int J Eat Disord. 1997;21:385–390. [DOI] [PubMed] [Google Scholar]
- 35.Deitel M. Avoidance of weight regain after gastric bypass. Obes Surg. 2001;11:474. [DOI] [PubMed] [Google Scholar]
- 36.Leitzmann MF, Willett WC, Rimm EB, et al. A prospective study of coffee consumption and the risk of symptomatic gallstone disease in men. JAMA. 1999;281:2106–2112. [DOI] [PubMed] [Google Scholar]
- 37.Fakhry SM, Herbst CA, Buckwalter JA. Cholecystectomy in morbidly obese patients. Am Surg. 1987;53:26–28. [PubMed] [Google Scholar]
- 38.Jones KB Jr. Simultaneous cholecystectomy: to be or not to be. Obes Surg. 1995;5:52–54. [DOI] [PubMed] [Google Scholar]
- 39.Deitel M, Smith L, Harmantas A. Laparoscopic cholecystectomy after vertical banded gastroplasty. Obes Surg. 1994;4:13–15. [DOI] [PubMed] [Google Scholar]
- 40.Maton PN, Murphy GM, Dowling RH. Ursodeoxycholic acid treatment of gallstones. Dose-response study and possible mechanism of action. Lancet. 1977;2:1297–1301. [DOI] [PubMed] [Google Scholar]
- 41.Salen G, Colalillo A, Verga D, et al. Effect of high and low doses of ursodeoxycholic acid on gallstone dissolution in humans. Gastroenterology. 1980;78:1412–1418. [PubMed] [Google Scholar]
- 42.Tint GS, Salen G, Colalillo A, et al. Ursodeoxycholic acid: a safe and effective agent for dissolving cholesterol gallstones. Ann Intern Med. 1982;97:351–356. [DOI] [PubMed] [Google Scholar]
- 43.Miller K, Höller E, Hell E. Restrictive procedures in the treatment of morbid obesity – vertical banded gastroplasty vs. adjustable gastric banding. Zentralbl Chir. 2002;127:1038–1043. [DOI] [PubMed] [Google Scholar]
- 44.Brolin RE. Update: NIH consensus conference. Gastrointestinal surgery for severe obesity. Nutrition. 1996;12:403–404. [DOI] [PubMed] [Google Scholar]