Abstract
Objective:
The purpose of this study was to evaluate prognostic factors in patients with recurrence after curative resection of hepatocellular carcinoma (HCC) and to identify selection criteria for repeat resection.
Summary Background Data:
Recent studies have demonstrated that repeat hepatectomy is effective for treating intrahepatic recurrent HCC in selected patients. However, the prognostic factors in these patients have not been fully evaluated.
Methods:
From October 1994 to December 2000, 334 patients underwent primary resection for HCC, and 67 received a 2nd hepatectomy for recurrent HCC. The survival results in these 67 patients were analyzed, and prognostic factors were determined using 38 clinicopathological variables. The prognosis and operative risk in 11 and 6 patients who received a 3rd and 4th resection were also evaluated.
Results:
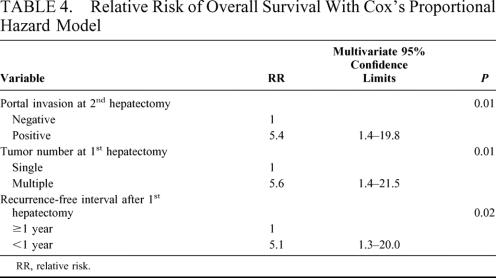
The overall 1-, 3-, and 5-year survival rates of the 334 patients after primary hepatectomy were 94%, 75%, and 56%, while those of the 67 patients after a 2nd resection were 93%, 70%, and 56%, respectively. There was no difference in survival (P = 0.64). All of the patients who underwent a 3rd or 4th are currently alive at a median follow-up of 2.5 and 1.4 years, respectively. The operative time and blood loss in the 2nd resection in patients who underwent a major primary resection were not different from those in patients who underwent minor hepatectomy at the 1st resection, and there were also no differences in these variables among the 2nd, 3rd, and 4th resections. In a multivariate analysis, absence of portal invasion at the 2nd resection (P = 0.01), single HCC at primary hepatectomy (P = 0.01), and a disease-free interval of 1 year or more after primary hepatectomy (P = 0.02) were independent prognostic factors after the 2nd resection. Twenty-nine patients with all 3 of these factors showed 3- and 5-year survival rates of 100% and 86%, respectively, after the 2nd resection.
Conclusions:
Repeat hepatic resection is the treatment of choice for patients who have previously undergone resection of a single HCC at the primary resection and in whom recurrence developed after a disease-free interval of 1 year or more and the recurrent tumor had no portal invasion.
Favorable prognostic factors at repeat resection for recurrent hepatocellular carcinoma were solitary nodule at the first resection, recurrence after 1 year or more, and absence of portal invasion at repeat resection. Twenty-nine patients who had all of these factors had 3- and 5-year survival rates of 100%, and 86%, respectively, after repeat resection. Our criteria can be recommended as surgical indications for repeated hepatectomy in recurrent hepatocellular carcinoma.
Hepatic resection has been established as a curative treatment of hepatocellular carcinoma (HCC).1,2 Surgical indications have been extended toward advanced cases, such as HCC with portal vein tumor thrombus3 and bilateral multiple HCC.4 However, the long-term prognosis after curative resection remains unsatisfactory because of a high rate of recurrence: 5-year cumulative recurrence rates of 77% to 100% have been reported, and 80% to 95% of such recurrence was confined to the liver.5–11 Liver transplantation has been beneficial only in limited patients.12 In this context, the role of hepatic resection and the treatment of recurrent HCC may become more important.
The most widely used treatment of intrahepatic recurrence is transarterial chemoembolization (TACE). The 5-year survival rate has ranged from 0% to 27% in patients with postresectional recurrence, even with repeated TACE.13–16 It is questionable whether this procedure actually enhances survival in such cases.17 Repeat resection for recurrent HCC has been reported to be a highly effective treatment in selected patients. The 5-year survival rate after repeat resection has been reported to be from 37% to 70%.15,18–25 In this study, we retrospectively evaluated 38 clinicopathological prognostic factors after 2nd hepatectomy. We tried to clarify the selection criteria for repeat resection and evaluated the significance of a 3rd and 4th hepatic resection.
PATIENTS AND METHODS
From October 1994 to December 2000, 334 patients underwent curative hepatic resection for nonfibrolamellar HCC at the Department of Hepato-Biliary-Pancreatic Surgery, Graduate School of Medicine, University of Tokyo. All of the patients had been followed for recurrence. Serum α-fetoprotein and des-γ-carboxy prothrombin levels were analyzed every month, and abdominal ultrasonography and CT scan were performed every 2 and 6 months, respectively. Extrahepatic recurrence was also checked by chest radiograph, chest CT, and bone scintigram. Suspected intrahepatic recurrence was confirmed by hepatic angiography and CT, which was performed 2 weeks after the injection of an iodized oil (Lipiodol; Guerbet Laboratories, Aulnay Sous Bois, France). Among the 334 patients, 183 developed intrahepatic recurrence and 56 actually underwent a 2nd hepatectomy (rate of repeat resection, 31%). During the same period, 11 additional patients also underwent a 2nd hepatectomy; 4 had undergone primary resection at our institute before October 1994 and 7 had undergone primary hepatectomy at other hospitals. Overall, 67 patients were included in this study. The study population consisted of 56 men and 11 women with a mean age of 62.7 years (range, 37–84 years). Nineteen patients (28%) were positive for the hepatitis B surface antigen and 42 (63%) were positive for the hepatitis C antibody. Seven patients were negative for both viruses, and 1 was positive for both.
In the same period, 11 and 6 patients received a 3rd and 4th hepatic resection for a 2nd and 3rd recurrence.
Treatment Strategy
Repeat hepatectomy for recurrent HCC was considered the treatment of choice for resectable recurrent tumors. Our selection criteria for repeat resection were the same as those for primary resection: ascites was either not detected or was controllable by diuretics, and the serum total bilirubin (TB) level was < 2.0 mg/mL. The resection volume was determined based on TB and the Indocyanine green retention rate at 15 minutes (ICG-R15). Patients with a TB level between 1.1 and 1.9 mg/mL, or those with ICG-R15 >/ = 30% were selected for limited resection or enucleation. In patients with TB </ = 1.0 mg/mL, two thirds of nontumorous liver parenchyma could be removed if ICG-R15 was </ = 10% and less than one third could be resected if this was 10% to 19%, while those with ICG-R15 of 20% to 29% underwent Couinaud’s single segmentectomy or less.26 Within these limitations, if oncologically radical operation was possible, the patients were selected for hepatectomy. Patients with resectable extrahepatic invasion or distant metastases were also selected for resection.
Surgical Management
A total of 25 patients underwent anatomic resection (hemihepatectomy or extended hemihepatectomy in 4, sectoriectomy in 2, and segmentectomy of Couinaud in 19), whereas 42 had limited resections in 1 to 4 locations. All resections were performed under the guidance of intraoperative ultrasonography using total or hemihepatic inflow occlusion. Seven patients developed extrahepatic lesions between the primary and 2nd hepatectomy (lung metastases in 2, localized peritoneal dissemination in 1, lymph node metastasis in 3, and tumor thrombus in the inferior vena cava in 1), which were radically resected. Four patients with gross portal vein tumor thrombus underwent transcatheter arterial chemoembolization 2 weeks prior to the 2nd hepatic resection to improve the prognosis.3
Statistical Analysis
Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Factors that were found to significantly influence survival or tumor recurrence were then used in the Cox proportional regression model for a multivariate analysis. Blood loss and operative time in the 2nd resection were compared using the Mann-Whitney U test, and those in the 2nd, 3rd, and 4th operation were compared by the Kruskal-Wallis test. Significance was specified as P < 0.05.
RESULTS
There was no in-hospital death among the 334, 67, 11, and 6 patients who underwent primary, 2nd, 3rd, and 4th resections, respectively. The median operative duration in the 2nd, 3rd, and 4th hepatectomies was 6 hours 5 minutes (range, 2:35–14:30), 7:35 (5:05–9:25), and 6:50 (3:45–11:20), respectively (P = 0.26). The median blood loss in the 2nd, 3rd, and 4th resections was 535 mL (range: 50–3600 mL), 685 mL (161–1800 mL), and 857 mL (250–1385 mL), respectively (P = 0.62). Three patients received intraoperative blood transfusion in the 2nd hepatectomy. These 2 factors in the 2nd resection were assessed according to the type of primary operation and were almost the same between the 25 patients who received segmentectomy of Couinaud or greater anatomic resection (major hepatectomy) and the 42 patients who underwent limited resection (minor hepatectomy) (Table 1). In a total of 84 repeat hepatectomies, 1 patient experienced a major complication of intestinal bleeding, which required a reoperation.
TABLE 1. Time and Blood Loss at 2nd Hepatectomy
The overall 1-, 3-, and 5-year cumulative survival rates of the 334 patients after primary resection were 94%, 75%, and 56%, respectively, and those of the 67 patients after 2nd resection were 93%, 70%, and 56% (P = 0.64)(Fig. 1). The 1-, 3-, and 5-year recurrence-free survival rates of the 334 patients after the primary hepatectomy were 65%, 33%, and 15%, whereas those of the 67 patients after the 2nd resection were 50%, 21%, and 17%, respectively (P = 0.06) (Fig. 2).
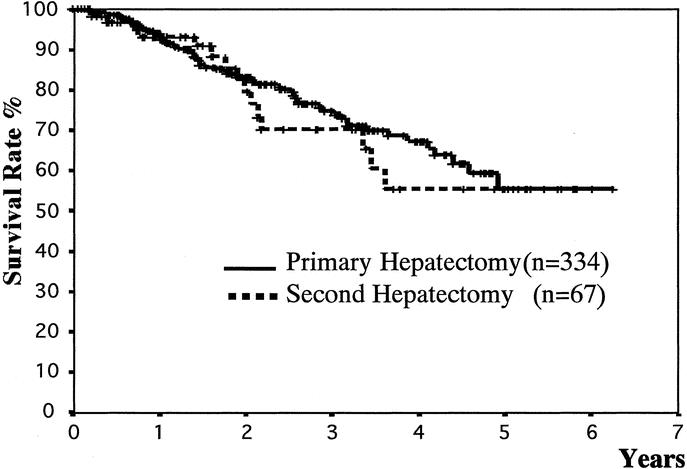
FIGURE 1. Comparison of overall survival rates between primary and 2nd hepatic resections, calculated from the primary and 2nd hepatectomies. There was no significant difference (P = 0.64; log-rank test).
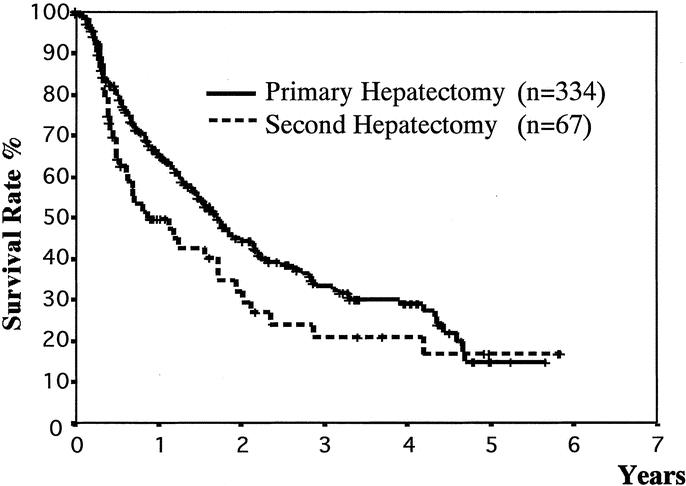
FIGURE 2. Comparison of disease-free survival rates between primary and 2nd hepatic resections, calculated from the primary and 2nd hepatectomies. There was no significant difference (P = 0.06; log-rank test).
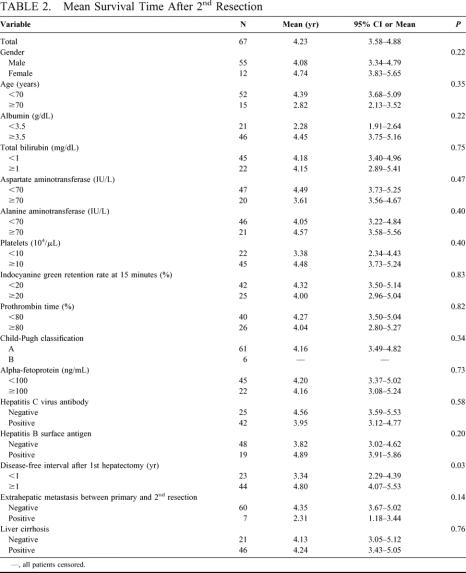
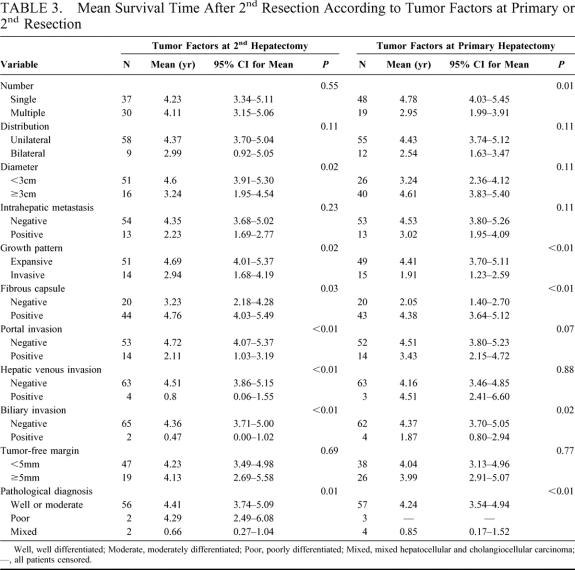
All of the patients who underwent a 3rd or 4th resection are still alive at a median follow-up 2.5 years and 1.4 years, respectively. But 6 of the 11 patients who underwent 3rd resection and 2 of 6 instances who received 4th hepatectomy have recurred. The mean survival times after the 2nd hepatic resection according to the patients’ characteristics are summarized in Table 2, and those stratified according to tumor factors at the primary and 2nd hepatectomy are shown in Table 3. The presence of fibrosis in the underlying liver of those 67 patients at 2nd hepatectomy was graded from 0 to 4 according to the classification of Knodell et al.27 Accordingly, 2 (3%) patients had a normal liver without fibrosis (grade 0), 9 (13%) had fibrous portal expansion (grade 1), 10 (15%) had bridging fibrosis (grade 3), and 46 (69%) had cirrhosis (grade 4). The grade of fibrosis in the underlying liver resected at primary hepatectomy was as follows: grade 0, 1; grade 1, 5; grade 3, 11; grade 4, 39; and that of 11 patients was unknown.
TABLE 2. Mean Survival Time After 2nd Resection
TABLE 3. Mean Survival Time After 2nd Resection According to Tumor Factors at Primary or 2nd Resection
The factors at the 2nd hepatectomy, which significantly influenced the prognosis after the 2nd hepatectomy, were HCC <3 cm in diameter (P = 0.02), expansive pattern of growth (P = 0.02), positive fibrous capsule (P = 0.03), absence of portal invasion (P < 0.01), hepatic venous invasion (P < 0.01), biliary invasion (P < 0.01), and mixed type of HCC (P < 0.01). Favorable factors at the primary resection were single HCC (P = 0.01), expansive pattern of growth (P < 0.01), positive fibrous capsule (P < 0.01), absence of biliary invasion (P = 0.02), and mixed type of HCC (P < 0.01). A disease-free interval of 1 year or more after the primary resection (P = 0.03) was also a favorable prognostic factor. These 13 significant factors were analyzed by a multivariate Cox proportional hazard model. The favorable factors that influenced overall survival after the 2nd resection were absence of portal invasion at the 2nd resection (P = 0.01), single HCC at the primary hepatectomy (P = 0.01), and a disease-free interval of 1 year or more after the primary resection (P = 0.02) (Table 4). The overall 3- and 5-year survival rates after the 2nd resection in the 29 patients who had all 3 of these favorable prognostic factors were 100% and 86%, respectively (Fig. 3).
TABLE 4. Relative Risk of Overall Survival With Cox’s Proportional Hazard Model
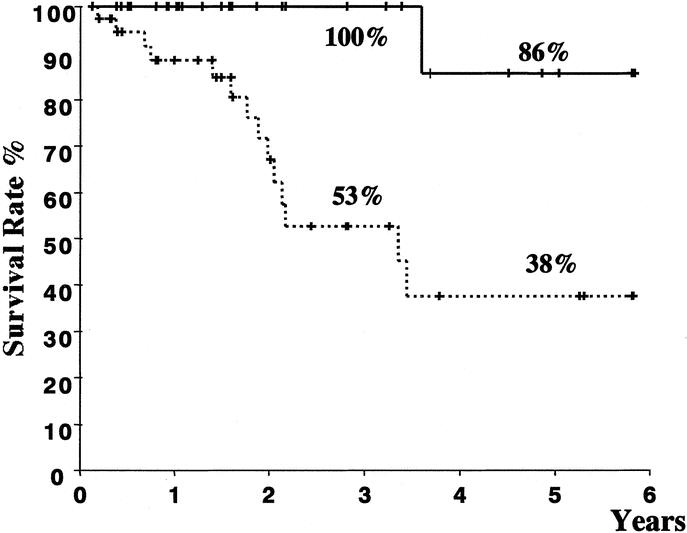
FIGURE 3. Comparison of survival after the 2nd resection between 29 patients with all 3 favorable prognostic factors (thick line) and 38 who lacked at least 1 of these factors (broken line). The median survival time in the former group (5.5 years) (95% confidence interval, 4.9–6.1) was significantly longer than that in the latter (3.4 years) (95% confidence interval, 2.6–4.3) (log-rank test; P < 0.01).
DISCUSSION
The cumulative 5-year recurrence rates after curative resection for HCC have ranged from 77% to 100% in both Eastern and Western centers, and the rates stratified according to hepatitis viruses in a single center have been almost the same.28 Since a high rate of recurrence is the main cause of late death, various efforts have been made to prevent recurrence. Two randomized controlled studies have shown that preoperative TACE is not effective for reducing postoperative recurrence and death.29,30 In patients with HCC accompanied by portal vein tumor thrombus, the prognosis was improved by preoperative TACE followed by hepatectomy.3 The effectiveness of postoperative adjuvant TACE or hepatic arterial chemotherapy has been controversial, and new approaches using an acyclic retinoid (polyprenoic acid), intra-arterial iodine-131- labeled lipiodol, and adoptive immunotherapy have shown a lower rate of recurrence and an improved recurrence-free outcome in randomized controlled trials.31–33 However, since these new adjuvant treatments have not been established as standard therapy, treatment of recurrent HCC still plays an important role.
TACE is a widely used treatment modality for intrahepatic recurrence and has a wide range of indications. Recently, percutaneous ethanol injection, radiofrequency ablation, and microwave coagulation therapy have sometimes been selected for the treatment of recurrent HCC. Llovet et al reported that the incidence of needle tract dissemination after radiofrequency ablation was 12.5%.34 In this context, these treatment modalities should perhaps not be used in patients who may undergo liver transplantation. Based on these results, the first treatment of choice for recurrent HCC appears to be repeated resection. The safety of repeated resection has already been established, with operative mortality rates ranging from 0% to 8.5%.15,18–25 In our series, no operative mortality was observed in the 2nd, 3rd, or 4th resection. Only 3 patients required intraoperative blood transfusion. Furthermore, repeated resection gives the best long-term results, with 5-year survival rates of 37% to 70%.15,18–25 In our series, this value was 57%. However, the favorable results in patients who undergo repeated resection could be due in part to the selection of patients who may have had a good prognosis regardless of the treatment. Without a prospective randomized trial, it is difficult to prove that the better prognosis was not simply a function of selection bias. However, since the overall survival rates after repeat resection were not worse than those after primary resection in our series and other reports, these results may support the use of repeat resection for recurrent HCC.
The rate of repeat resection has been reported to range from 10.4% to 27.4% in papers with 40 or more recurrent patients.15,18,19,21–25 In our series, this rate was 31%. A possible obstacle to repeated resection may be the idea that it is difficult to perform repeated hepatectomy for patients who have undergone major resection as the primary operation. However, we found that both the operative time and the blood loss in the 2nd resection were almost the same between patients who were divided according to the type of primary resection: major resection or limited resection. In this context, a 2nd resection is a treatment of choice in patients who have undergone major resection as the primary resection. Another obstacle to repeat hepatectomy may be the fact that selection criteria for repeated resection have not been established. This may be due to a lack of knowledge regarding prognostic factors after repeated resection. Until now, only 2 studies on this subject have been reported. Hu et al reported that gender and 4 or more tumors at repeat resection affected a second recurrence and gender, age, and tumor invasiveness were significant factors for survival.24 However, they did not evaluate the disease-free interval after primary resection or factors at the primary resection. Shimada et al reported that a recurrence-free interval of less than 1 year after primary resection, Edmondson and Steiner’s grade 3 at repeat resection, and portal invasion at primary resection significantly predicted a poor prognosis.21
In our series, early recurrence after the primary resection significantly impaired overall survival after the 2nd resection, which is compatible with Shimada’s result. Most intrahepatic metastases from the primary HCC appear within 3 years after the initial hepatectomy, and the incidence of 2nd primary HCC is constant in patients with hepatitis C virus antibody.35 Based on these results, many of the cases of recurrence within 1 year might be intrahepatic metastasis from the primary HCC, and their worse outcome may reflect a lower survival rate in patients who develop intrahepatic metastases.
Portal invasion at repeat resection also significantly influenced survival after repeat resection. However, portal invasion at the primary resection did not influence the prognosis. A possible explanation for this result can be found in our policy for treating HCC accompanied by portal invasion. In patients with portal invasion that is apparent by diagnostic modalities, preoperative TACE followed by hepatic resection is the treatment of choice at our department: a 5-year survival rate of 42% was attained in primary resected cases with gross portal vein tumor thrombosis.3 The same policy was applied to recurrent HCC with portal invasion, but the 5-year survival rate in 14 patients was 16%, which was much lower than the 65% in patients without this condition. Based on these results, portal invasion at repeat resection may have different prognostic significance than that at the primary resection.
Patients from whom a single HCC was removed at the primary resection had a favorable prognosis after the 2nd resection, and multiple HCC at the 2nd resection was not a significant factor. These results suggest that multiple recurrent HCC should be removed if the primary HCC is a single tumor.
We next considered the 3rd and 4th resections for the 2nd and 3rd recurrences. Our series showed that the 3rd and 4th resections were safe, and the operation duration and blood loss was not different from those in the 2nd resection. With regard to the prognosis, 11 and 6 patients who underwent a 3rd and 4th hepatectomy are still alive and the rates of recurrence after these resections were almost the same as those after the primary and 2nd resections. These results suggest that 3rd and 4th resections can be indicated for selected patients. However, in patients with frequent appearance of new nodules as a result of a highly carcinogenic liver, liver transplantation may be a better choice.
With regard to repeated hepatectomy, extrahepatic metastases are a discouraging problem. Of the 67 patients who underwent a 2nd resection, 7 developed extrahepatic metastases between the primary and 2nd resections, which were curatively removed. The mean survival time of these 7 patients (2.31 years) was not significantly different from that in the other 60 patients (4.35 years) (P = 0.14) (Table 2). These results suggest that resection of extrahepatic metastases should be considered if they are localized and resectable and if the hepatic lesion is curable. It is noteworthy that the rate of extrahepatic spread after hepatic resection is low: the incidence of extrahepatic metastases after primary hepatectomy is reportedly 5% to 20%.6,9–11 The rate after the 2nd resection was almost the same as that after the primary resection. This low rate also supports repeated resection.
Our investigation showed that a disease-free interval of more than 1 year after primary hepatectomy, single HCC at primary resection, and negative portal invasion at repeated resection were favorable prognostic factors after repeated resection. Patients with all 3 of these factors showed 3- and 5-year survival rates of 100% and 86%, respectively. These patients should be indicated for repeat resection, even if they have undergone major hepatic resection as the primary hepatectomy. A 3rd and 4th hepatectomy for a 2nd and 3rd recurrence should be considered if liver function can be preserved.
ACKNOWLEDGMENTS
The authors thank Dr. Yoichi Ito and Ms. Makiko Naka, Department of Pharmacoepidemiology, Graduate School of Medicine, University of Tokyo, for their help with the statistical analysis.
Footnotes
Reprints: Masatoshi Makuuchi, MD, Department of Hepato-Biliary-Pancreatic Surgery, Department of Artificial Organ and Transplantation, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113–8655, Japan. makuuchi-tky@umin.ac.jp.
REFERENCES
- 1.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CC, Ho WL, Lin MC, et al. Hepatic resection for bilobar multicentric hepatocellular carcinoma: is it justified? Surgery. 1998;123:270–277. [PubMed] [Google Scholar]
- 5.Poon R, Fan S, Lo C, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 2001;234:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World J Surg. 1995;19:35–41. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States: prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–2222. [DOI] [PubMed] [Google Scholar]
- 9.Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328–332. [PubMed] [Google Scholar]
- 10.Makuuchi M, Takayama T, Kubota K, et al. Hepatic resection for hepatocellular carcinoma: Japanese experience. Hepatogastroenterology. 1998;45:1267–1274. [PubMed] [Google Scholar]
- 11.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki M, Yamasaki S, Ono H, et al. Chemoembolotherapy for recurrent hepatocellular carcinoma in the residual liver after hepatectomy. Hepatogastroenterology. 1993;40:320–323. [PubMed] [Google Scholar]
- 14.Takayasu K, Wakao F, Moriyama N, et al. Postresection recurrence of hepatocellular carcinoma treated by arterial embolization: analysis of prognostic factors. Hepatology. 1992;16:906–911. [DOI] [PubMed] [Google Scholar]
- 15.Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakao N, Kamino K, Miura K, et al. Recurrent hepatocellular carcinoma after partial hepatectomy: value of treatment with transcatheter arterial chemoembolization. AJR Am J Roentgenol. 1991;156:1177–1179. [DOI] [PubMed] [Google Scholar]
- 17.Groupe d’Etude et de Traitement du Carcinome H. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256–1261. [DOI] [PubMed] [Google Scholar]
- 18.Suenaga M, Sugiura H, Kokuba Y, et al. Repeated hepatic resection for recurrent hepatocellular carcinoma in eighteen cases. Surgery. 1994;115:452–457. [PubMed] [Google Scholar]
- 19.Kakazu T, Makuuchi M, Kawasaki S, et al. Repeat hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology. 1993;40:337–341. [PubMed] [Google Scholar]
- 20.Matsuda Y, Ito T, Oguchi Y, et al. Rationale of surgical management for recurrent hepatocellular carcinoma. Ann Surg. 1993;217:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada M, Takenaka K, Taguchi K, et al. Prognostic factors after repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg. 1998;227:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arii S, Monden K, Niwano M, et al. Results of surgical treatment for recurrent hepatocellular carcinoma: comparison of outcome among patients with multicentric carcinogenesis, intrahepatic metastasis, and extrahepatic recurrence. J Hep Bil Pancr Surg. 1998;5:86–92. [DOI] [PubMed] [Google Scholar]
- 23.Farges O, Regimbeau JM, Belghiti J. Aggressive management of recurrence following surgical resection of hepatocellular carcinoma. Hepatogastroenterology. 1998;45:1275–1280. [PubMed] [Google Scholar]
- 24.Hu RH, Lee PH, Yu SC, et al. Surgical resection for recurrent hepatocellular carcinoma: prognosis and analysis of risk factors. Surgery. 1996;120:23–29. [DOI] [PubMed] [Google Scholar]
- 25.Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996;111:720–726. [DOI] [PubMed] [Google Scholar]
- 26.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. [DOI] [PubMed] [Google Scholar]
- 27.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. [DOI] [PubMed] [Google Scholar]
- 28.Miyagawa S, Kawasaki S, Makuuchi M. Comparison of the characteristics of hepatocellular carcinoma between hepatitis B and C viral infection: tumor multicentricity in cirrhotic liver with hepatitis C. Hepatology. 1996;24:307–310. [DOI] [PubMed] [Google Scholar]
- 29.Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma: Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. [DOI] [PubMed] [Google Scholar]
- 32.Lau WY, Leung TW, Ho SK, et al. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353:797–801. [DOI] [PubMed] [Google Scholar]
- 33.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. [DOI] [PubMed] [Google Scholar]
- 35.Kumada T, Nakano S, Takeda I, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. [DOI] [PubMed] [Google Scholar]