Abstract
Hypoxia causes the accumulation of the transcription factor hypoxia-inducible factor 1 (HIF-1), culminating in the expression of hypoxia-inducible genes such as those for vascular endothelial growth factor (VEGF) and NDRG-1/Cap43. Previously, we have demonstrated that intracellular calcium (Ca2+) is required for the expression of hypoxia-inducible genes. Here we found that, unlike with hypoxia or hypoxia-mimicking conditions, the elevation of intracellular Ca2+ neither induced the HIF-1α protein nor stimulated HIF-1-dependent transcription. Furthermore, the elevation of intracellular Ca2+ induced NDRG-1/Cap43 mRNA in HIF-1α-deficient cells. It also increased levels of c-Jun protein, causing its phosphorylation. The protein kinase inhibitor K252a abolished c-Jun induction and activator protein 1 (AP-1)-dependent reporter expression caused by Ca2+ ionophore or hypoxia. K252a also significantly decreased hypoxia-induced VEGF and NDRG-1/Cap43 gene expression in both human and mouse cells. Using a set of deletion VEGF-Luc promoter constructs, we found that both HIF-1 and two AP-1 sites contribute to hypoxia-mediated induction of transcription. In contrast, only AP-1 sites contributed to Ca2+-mediated VEGF-Luc induction. A dominant-negative AP-1 prevented Ca2+-dependent transcription and partially impaired hypoxia-mediated transcription. In addition, dominant-negative AP-1 diminished the expression of the NDRG-1/Cap43 gene following hypoxia. We conclude that during hypoxia, an increase in intracellular Ca2+ activates a HIF-1-independent signaling pathway that involves AP-1-dependent transcription. Cooperation between the HIF-1 and AP-1 pathways allows fine regulation of gene expression during hypoxia.
Oxygen sensing is an important function of living cells. Under low-oxygen conditions (hypoxia), a cell must respond by coordinated expression of numerous genes to ensure adaptation. Hypoxia-inducible factor 1 (HIF-1), a transcription factor that accumulates during hypoxia, stimulates genes involved in glucose metabolism, angiogenesis, and cell survival (29).
Recent studies indicate that Ca2+ is also involved in the cellular response to hypoxia. Indeed, a significant increase in free intracellular Ca2+ was observed in endothelial cells after 2 h of hypoxia (2). This increase in cytosolic calcium was due to the release of Ca2+ from intracellular stores (13, 24). Elevation of intracellular Ca2+, caused by the Ca2+ ionophore A23187, induced the expression of hypoxic genes, including those for vascular endothelial growth factor (VEGF) and NDRG-1/Cap43 (6, 26). Additionally, chelation of intracellular calcium by 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N-tetraacetic acid-acetoxymethyl ester (BAPTA-AM) abolished hypoxia-inducible expression of both VEGF and NDRG-1/Cap43 (21, 26, 27). The modulation in the concentration of extracellular Ca2+ from 0 to 10 mM did not affect NDRG-1/Cap43 gene induction by the hypoxia-mimicking agent nickel (Ni), indicating an important role of intracellular stores in the induction of hypoxia-inducible genes (26).
Genes for both VEGF and NDRG-1/Cap43, were expressed in response to hypoxia in a manner that depended upon HIF-1 (9, 25, 27, 31). It was unclear whether intracellular Ca2+ mediates its effects via HIF-1-dependent transcription. Here we demonstrate that, unlike hypoxia, intracellular Ca2+ did not cause the accumulation of the HIF-1α protein. In addition, by using a reporter plasmid containing a HIF-1 response element, we found that Ca2+ ionophore did not stimulate HIF-1-dependent transcription. Finally, the Ca2+ ionophore A23187 induced NDRG-1/Cap43 expression in HIF-1α-deficient mouse embryo fibroblasts (MEF), proving that HIF-1 is not involved in Ca2+-dependent activation of hypoxic genes.
Ca2+ ionophore induced c-Jun expression and activated AP-1-dependent transcription of the reporter plasmid. Both the activation of c-Jun expression and the activator protein 1 (AP-1)-dependent transcription of reporter plasmid were sensitive to the protein kinase inhibitor K252a. This inhibitor also suppressed the induction of VEGF and NDRG-1/Cap43 by hypoxia, confirming the involvement of AP-1 in the regulation of the gene. Furthermore, we used a dominant-negative (DN) AP-1 transcription factor to confirm the involvement of AP-1 in the hypoxic response. Given that (i) hypoxia induces intracellular Ca2+ and AP-1, (ii) Ca2+ is required for hypoxia-responsive expression of the VEGF and NDRG-1/Cap43 genes, (iii) promoters of both genes contain HIF-1 and AP-1 binding sites, and (iv) DN AP-1 suppresses gene expression caused by hypoxia, we hypothesize that Ca2+-induced AP-1 may cooperate with HIF-1 in the hypoxic response.
MATERIALS AND METHODS
Materials.
Deferoxamine (DFX) was obtained from Sigma. Cyclosporine and BAPTA-AM were obtained from Biomol. Nickel chloride and cobalt chloride were obtained from Alfa Aesar. Cell culture media, fetal calf serum (FCS), glutamine, and antibiotics were obtained from Gibco-BRL. The most commonly used chemicals were purchased from Sigma.
Cell culture.
A549 (CCL 185) cells were maintained in F12K media supplemented with 10% FCS, and 293 (CRL-1573) cells were maintained in Dulbecco modified Eagle medium supplemented with 10% FCS. Both cell types were purchased from the American Type Culture Collection (Manassas, Va.). MEF and MEF with the HIF-1α gene knocked out (MEF-HIF-1−/−) were obtained from R. Johnson (University of California, San Diego) and have been described previously (25, 27). All cells were maintained at 37°C as monolayers in a humidified atmosphere containing 5% CO2. Cell exposure to hypoxia has been described previously (27).
Plasmids and transient transfection.
The HIF-responsive HRE-Luc plasmid was described previously (28). The AP-1-Luc plasmid was purchased from Clontech (Palo Alto, Calif.). VEGF luciferase reporter plasmid constructs, described previously (30), were as follows: pGL3-V2274, containing the full-length VEGF promoter; pGL3-V1012, containing the full-length promoter minus two AP-1 sites; pGL3-V789, minus the HIF-1 site; and pGL3-V267, minus a third AP-1 site. The plasmid containing a DN AP-1 described elsewhere (16) was provided by C. Vinson (National Institutes of Health).
For the analysis of the expression of reporter plasmids, 50,000 cells were plated in 24-well plates and then transfected the following day with plasmids by using TransFast transfection reagent (Promega) according to the manufacturer's recommendations. After 2 to 6 h of incubation with the plasmid-lipid suspension, the medium was changed and cells were grown for an additional 12 h. The cells were incubated with 260 μM DFX, 5 μM A23187, 10 μM BAPTA-AM, or NiCl2 at concentrations indicated in the figure legends or at 1% oxygen (hypoxia). After 10 h, cells were lysed and analyzed for luciferase activity as described previously (28).
Northern blot analysis.
Total RNA was extracted from cells immediately after exposure by using TRIzol reagent (Gibco-BRL) and was separated by electrophoresis with 15 μg of total RNA/lane in 1.0% agarose-formaldehyde gels. NDRG-1/Cap43, VEGF, or actin probes were labeled with [α-32P]dCTP by using a randomly primed-DNA labeling kit (Promega). Cloning of the NDRG-1/Cap43 gene was described previously (35). The VEGF probe was a kind gift from K. Claffey (University of Connecticut).
Immunoblot analysis.
Proteins were harvested in TNESVF buffer (50 mM Tris HCl [pH 7.5], 2 mM EDTA, 100 mM NaCl, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1% NP-40) with protease inhibitors. Equal quantities of proteins were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE). Western blotting was performed with anti-NDRG-1/Cap43 antibodies. Anti-NDRG-1/Cap43 antibody production has been described elsewhere (23). Anti-c-Jun and anti-phospho-c-Jun antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). HIF-1α and p53 analysis was conducted as described previously (1).
RESULTS
HIF-independent induction of the expression of hypoxic genes by intracellular calcium.
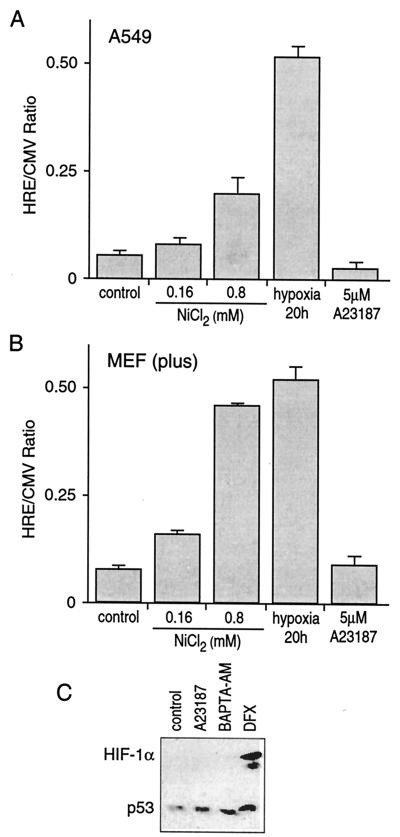
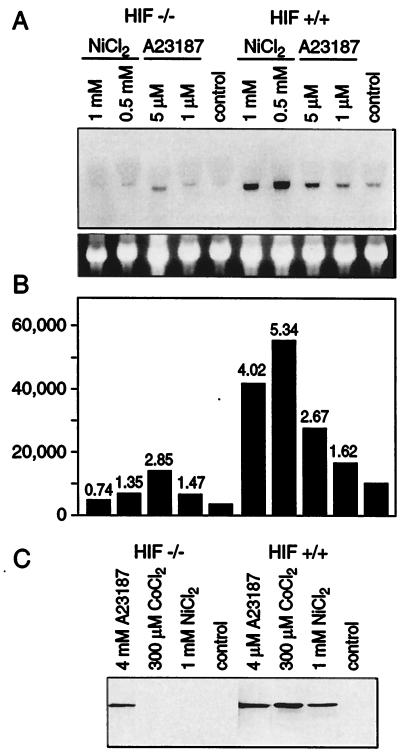
It has been shown that the elevation of intracellular Ca2+ induces hypoxic genes, including those for NDRG-1/Cap43 and VEGF (6, 26, 27). Here, we investigated the effects of Ca2+ on HIF-1-inducible transcription. As shown in Fig. 1A, hypoxia and, to a lesser extent, 0.8 mM NiCl2 induced transcription of HIF-1-dependent promoter construct in human A549 cells. In contrast, a 5 μM concentration of Ca2+ ionophore A23187 (a concentration that induced NDRG-1/Cap43) did not induce the HIF-dependent reporter. Next, we tested MEF and MEF lacking the HIF-1α gene (MEF-HIF-1−/−). As expected, hypoxia and nickel induced the HRE-Luc reporter in parental cells (Fig. 1B) but not in the HIF-deficient cells (not shown). Ca2+ ionophore did not induce the HIF-dependent reporter either in parental or in HIF-1-deficient MEF. These data suggested that HIF-1 transcription factor was not involved in the Ca2+-mediated response. To confirm this conclusion, the HIF-1α protein level was measured in the nuclear extract of A549 cells under conditions that modulated the level of intracellular Ca2+. Neither Ca2+ ionophore A23187 nor BAPTA-AM induced HIF-1α, whereas treatment with the iron chelator DFX, a known inducer of the HIF-1α protein, increased its levels (Fig. 1C). Next, we compared levels of NDRG-1/Cap43 expression in parental and HIF-deficient MEF. Nickel, a hypoxia-mimicking metal, induced NDRG-1/Cap43 mRNA in parental cells but not in HIF-1-deficient cells. In contrast, Ca2+ ionophore stimulated NDRG-1/Cap43 mRNA in both cell lines (Fig. 2A), proving that the HIF-1 transcription factor is not required for Ca2+-mediated transcription. Lower levels of NDRG-1/Cap43 in HIF-1-deficient cells than in parental cells may be explained by lower basal levels of this gene in HIF-1-deficient cells. The levels of inducibility of NDRG-1/Cap43 by Ca2+ ionophore, calculated as the ratio of the induced expression to the basal expression, were similar in both cell lines (Fig. 2B). This result was also confirmed at the protein level (Fig. 2C). Neither nickel nor cobalt, both hypoxia-mimicking metals, induced NDRG-1/Cap43 protein in HIF-deficient cells, indicating that this induction is HIF-1 dependent. In contrast, Ca2+ ionophore induced the NDRG-1/Cap43 protein in HIF-1-deficient cells, indicating that factors other than the HIF-1 transcription factor are involved in gene and protein expression (Fig. 2C).
FIG. 1.
Calcium ionophore A23187 does not induce HIF-1-dependent transcription in MEF or in A549 cells. (A) HIF-1-dependent transcription was not affected by the calcium ionophore in A549 cells. Cells were transfected with 1 μg of HRE-Luc or 1 μg of plasmid CMV-Luc by using TransFast transfection reagent. On the day after transfection, cells were treated in some instances with 0.16 or 0.8 mM nickel chloride, hypoxia (1% O2), or 5 μM A23187 for 20 h. Cells were lysed and luciferase activity was determined as described previously (28). The data are the ratios of luciferase expression on HRE-Luc to that on CMV-Luc (HRE/CMV Ratio). (B) HIF-1-dependent transcription was not affected by calcium ionophore in MEF. The exposure conditions were similar to those described for panel A. (C) The HIF-1α protein level was not affected by calcium ionophore. Sixty micrograms of nuclear extracts from A549 cells untreated or treated with 5 μM A23187, 10 μM BAPTA-AM, or 260 μM DFX for 20 h was resolved by SDS-8% PAGE and subjected to Western blot analysis for detection of HIF-1α and p53 as described in Materials and Methods. The detection of another nuclear protein, p53, in the same samples was conducted to confirm protein loading in the cells.
FIG. 2.
Calcium ionophore A23187 stimulated expression of NDRG-1/Cap43 in a HIF-1-independent manner. (A) Northern blot analysis of NDRG-1/Cap43 expression in HIF-proficient and HIF-deficient fibroblasts. Cells were exposed to 0.5 or 1 mM nickel chloride or 1 or 5 μM A23187 for 20 h. Total RNA was isolated and subjected to Northern blot analysis. NDRG-1/Cap43 cDNA was used as a probe. The bottom panel shows ethidium bromide staining of the same gel. (B) Quantitation of NDRG-1/Cap43 expression with a PhosphorImager. The labeling is identical to that described for panel A. The multiple of induction over the control level is shown on top of each bar. (C) Western blot analysis of NDRG-1/Cap43 protein levels in HIF-proficient and HIF-deficient fibroblasts. Cells were exposed to 1 mM nickel chloride, 300 μM cobalt chloride, or 4 μM A23187 for 20 h. Cells were lysed and extracts were resolved by SDS-10% PAGE.
The NF-AT transcription factor is not involved in calcium-dependent activation of NDRG-1/Cap43 gene expression.
Since HIF-1-dependent transcription was not activated by Ca2+ ionophore (Fig. 1), we next considered the nuclear factor of activated T cells (NF-AT), which can mediate Ca2+-activated transcription (14), as a potential candidate for transcriptional upregulation of hypoxia-inducible genes. Additionally, using MacVector 6.5 software, we identified the consensus sequence representing the NF-AT binding site in the VEGF and NDRG-1/Cap43 promoters as well as at the 3′ end of the VEGF and NDRG-1/Cap43 genes. NF-AT-dependent transcription is cyclosporine sensitive (14). In our experiments, cyclosporine at concentrations of 1 to 5 μM did not abolish NDRG-1/Cap43 expression that had been induced by Ca2+ ionophore or nickel (Fig. 3A). Similarly, cyclosporine did not affect NDRG-1/Cap43 expression induced by nickel or Ca2+ ionophore in MEF (Fig. 3B). Although cyclosporine may have other cellular targets in addition to NF-AT, none of them appear to be involved in hypoxia- or Ca2+-mediated transcription of NDRG-1/Cap43.
FIG. 3.
Cyclosporine did not suppress NDRG-1/Cap43 gene induction. (A) Northern blot analysis of NDRG-1/Cap43 expression in A549 cells. Cells were exposed to 0.5 or 1 mM nickel chloride or 5 μM A23187 in the absence or presence of cyclosporine for 20 h. Total RNA was isolated and subjected to Northern blot analysis. The bottom panel shows ethidium bromide staining of the same gel. (B) Northern blot analysis of NDRG-1/Cap43 expression in MEF. The exposure was similar to that described for panel A.
AP-1 transcription factor is induced by calcium ionophore.
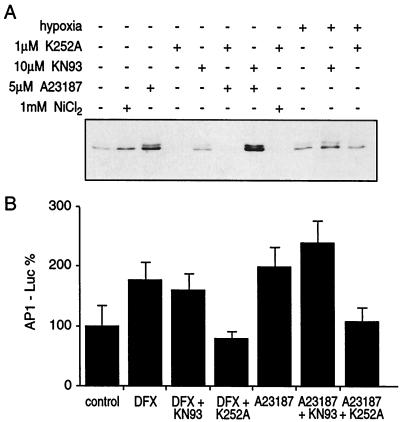
It has been previously reported that the AP-1 transcription factor potentiates the HIF-1-mediated, hypoxia-induced transcriptional activation of VEGF (7). We tested whether Ca2+-dependent activation of hypoxic genes is mediated by AP-1. The AP-1 transcription factor is a heterodimer composed of two proteins, c-Fos and c-Jun or other members of this transcription factor family (34). Immunoblot analysis revealed a strong induction of the c-Jun protein by Ca2+ ionophore and, albeit to a lesser degree, by nickel and hypoxia (Fig. 4A). Furthermore, these stimuli caused c-Jun phosphorylation (data not shown). The induction of c-Jun by all these stimuli was very sensitive to K252a, a serine/threonine protein kinase inhibitor, but not to the calmodulin-dependent kinase II inhibitor KN93. Using the luciferase reporter with an AP-1 binding site, we investigated the ability of Ca2+ ionophore A23187 to stimulate AP-1-dependent transcription. Indeed, Ca2+ ionophore A23187 stimulated AP-1-dependent transcription in a K252a-sensitive manner (Fig. 4B). Next, we investigated the effects of two protein kinase inhibitors, K252a and KN93, on VEGF and NDRG-1/Cap43 gene expression. As shown in Fig. 5, the expression of both the VEGF and the NDRG-1/Cap43 genes that had been caused by either Ca2+ ionophore A23187, hypoxia, or nickel was efficiently inhibited by K252a but not by KN93. Finally, hypoxia caused a 2.1-fold ± 0.18-fold increase in AP-1-Luc expression, which was completely eliminated by the calcium chelator BAPTA-AM. This indicated that hypoxia induces functional AP-1 in a Ca2+-dependent manner. Although results based on inhibitors alone are never conclusive, taken together with the ability of Ca2+ to activate AP-1-dependent transcription and to induce c-Jun protein, the possible involvement of AP-1 in Ca2+-induced transcription was indicated. Therefore, we next carried out a more detailed investigation of the involvement of AP-1 in Ca2+- and hypoxia-mediated transcription.
FIG. 4.
The AP-1 transcription factor was involved in calcium-dependent expression of hypoxic genes. (A) c-Jun protein was induced by calcium ionophore A23187 and was sensitive to the K252a inhibitor. A549 cells were exposed to 1 mM nickel chloride, hypoxia, or 5 μM A23187 in the presence or absence of the KN93 or K252a inhibitor. Forty micrograms of protein extracts from untreated or treated cells was resolved by SDS-10% PAGE and subjected to Western blot analysis for c-Jun protein as described in Materials and Methods. (B) AP-1-dependent transcription was induced by the calcium ionophore and was sensitive to the K252a inhibitor. A549 cells were transfected with 1 μg of the HRE-Luc or 1 μg of the AP-1-Luc reporter plasmid as described in Materials and Methods. The efficiency of transfection has been corrected by using plasmid CMV-Luc.
FIG. 5.
NDRG-1/Cap43 and VEGF expression was sensitive to the K252a inhibitor. Shown are the results of a Northern blot analysis of NDRG-1/Cap43 (A) and VEGF (B) expression in A549 cells. Cells were exposed to 1 mM nickel chloride, hypoxia, or 5 μM A23187 in the presence or absence of the KN93 or K252a inhibitor. Total RNA was isolated and subjected to Northern blot analysis by using the NDRG-1/Cap43 (A) and VEGF (B) probes. The darker panels show ethidium bromide staining of the same gels.
Regulation of the VEGF promoter.
The VEGF promoter contains three AP-1 sites and one HIF-1 site (30). Hypoxia significantly induced expression of pGL3-V2274, the full-length VEGF-Luc promoter construct (Fig. 6A). Ca2+ ionophore caused a twofold increase in expression of this construct. In order to further understand the role of AP-1 in the regulation of the VEGF promoter, we used the DN AP-1 expression vector. It has been shown previously that this vector expresses mutant A-FOS and completely blocks AP-1-dependent transcription (16). We found that DN AP-1 completely eliminated VEGF-Luc induction caused by Ca2+. In contrast, it only partially impaired hypoxia-mediated induction of VEGF-Luc (Fig. 6A). Next, we used HRE-Luc, the construct lacking AP-1 sites. Consistent with the absence of AP-1 sites in this reporter plasmid, hypoxia, but not Ca2+, induced its expression (Fig. 6B). Moreover, DN AP-1 did not affect HRE-Luc induction by hypoxia (Fig. 6B).
FIG. 6.
Role of AP-1 in the induction of VEGF-Luc by hypoxia or calcium. A549 cells were transfected with 1 μg of the VEGF-Luc (A) or 1 μg of the HRE-Luc (B) reporter plasmid as described in Materials and Methods. + DN, cells cotransfected with 1 μg of DN AP-1. In all other cases, cells were cotransfected with an empty vector. After transfection, cells were either incubated at 1% O2 for 16 h (hypoxia) or treated with a 5 μM concentration of calcium ionophore A23187. Cells were lysed and luciferase activity was determined as described previously (28). Results were calculated as percentages of the control level and are means ± standard deviations.
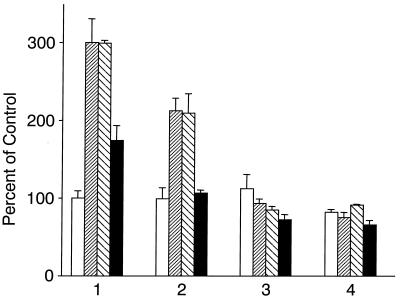
Next, we investigated the effect of deletion of AP-1 or HIF-1 binding sites in the VEGF promoter on HIF-1- or Ca2+-dependent transcription. As expected, full-length VEGF-Luc containing three AP-1 binding sites (pGL3-V2274) was induced by hypoxia, DFX, and Ca2+ (Fig. 7). The construct lacking two AP-1 sites (pGL3-V1012) did not respond to Ca2+, indicating that these AP-1 sites are functional and required for Ca2+ responsiveness. This construct still responded to hypoxia, albeit to a lesser degree than did full-length VEGF-Luc. These differences in levels of responsiveness are in agreement with the functional role of AP-1 during hypoxia. Further deletion of the HIF-1 site made the promoter construct nonresponsive to hypoxia, DFX, and Ca2+. The last AP-1 site was nonfunctional.
FIG. 7.
Role of AP-1 in the induction of VEGF-Luc by hypoxia or calcium. A549 cells were transfected with 1 μg of pGL3-V2274, containing the full-length VEGF promoter (bars labeled 1); pGL3-V1012, containing the full-length promoter minus two AP-1 sites (bars labeled 2); pGL3-V789, minus the HIF-1 site (bars labeled 3); and pGL3-V267, minus a third AP-1 site (bars labeled 4). Cells were either incubated under 1% O2 for 16 h (hypoxia; shaded bars), treated with 260 μM DFX (striped bars), treated with a 5 μM concentration of calcium ionophore (solid bars), or left untreated (control; open bars). Cells were lysed and luciferase activity was determined as described previously (28). Results are expressed as percentages of the control level (the activity of full-length VEGF-Luc left untreated) and are means ± standard deviations.
AP-1 cooperates with HIF-1 during hypoxia.
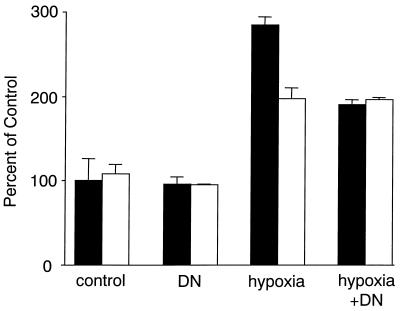
DN AP-1 diminished hypoxia-induced expression of pGL3-V2274, the full-length VEGF-Luc promoter construct (Fig. 8), but did not affect the expression of pGL3-V1012, the promoter construct lacking two AP-1 sites (Fig. 8). In the presence of DN AP-1, a full-length promoter and the promoter lacking two AP-1 sites were induced by hypoxia at the same levels.
FIG. 8.
Role of AP-1 sites in the induction of pGL3-V2274 by hypoxia. A549 cells were transfected with 1 μg of VEGF-Luc (solid bars) reporter plasmid or 1 μg of pGL3-V1012 reporter plasmid lacking two AP-1 sites (open bars) as described in Materials and Methods. + DN, cells cotransfected with 1 μg of DN AP-1. Otherwise, cells were cotransfected with an empty vector. Cells were either incubated with 1% O2 for 16 h (hypoxia and hypoxia + DN) or left at normal levels of oxygen (control and DN). Cells were lysed and luciferase activity was determined as described previously (28). Results were calculated as percentages of the control level (the activity of full-length VEGF-Luc under normal oxygen conditions) and are means ± standard deviations.
Finally, we investigated the role of AP-1 in hypoxia-induced expression of the endogenous gene for NDRG-1/Cap43 (Fig. 9). We took advantage of the high transfection efficiency in 293 cells, which reached almost 100%. Cells were transfected with DN AP-1 and then exposed to hypoxia or left at normal levels of oxygen. As shown in Fig. 9, hypoxia dramatically induced NDRG-1/Cap43 mRNA in these cells. DN AP-1 partly diminished this induction, indicating that AP-1 is involved in the hypoxia-mediated response of the endogenous gene.
FIG. 9.
Role of AP-1 in hypoxia-mediated induction of NDRG-1/Cap43. The results of a Northern blot analysis of NDRG-1/Cap43 expression in A549 cells are shown. + DN, cells cotransfected with 10 μg of DN AP-1. Otherwise, cells were cotransfected with an empty vector. Cells were either incubated at 1% O2 for 16 h (hypoxia and hypoxia + DN) or left at normal levels of oxygen (control and DN). Total RNA was isolated and subjected to Northern blot analysis by using the NDRG-1/Cap43 probe. The bottom panel shows ethidium bromide staining of the same gel.
DISCUSSION
Hypoxia induces a variety of genes that act in concert to facilitate the supply of oxygen and nutrients and promote cell survival and growth control (8, 10, 11, 15, 29). The HIF-1 transcription factor, which rapidly accumulates following hypoxia, is a major regulator of gene expression in hypoxic response (15, 29). Hypoxia-dependent genes are also induced by numerous other stimuli, including 12-O-tetradecanoyl-phorbol-13-acetate (TPA), dibutyryl-cyclic AMP, and A23187, a Ca2+ ionophore (3, 4, 12, 17, 21, 22). Two hypoxia-inducible genes, those for VEGF and NDRG-1/Cap43, display similar means of complex regulation at both transcriptional and posttranscriptional levels (4, 18, 19, 27, 32, 33). Their transcriptional upregulation is HIF-1 dependent (9, 15, 18, 27) as well as Ca2+ dependent (6, 21, 26, 27).
Here, we evaluated the involvement of intracellular Ca2+ in HIF-dependent responses. We found that Ca2+ ionophore (i) did not cause HIF-1α protein accumulation and (ii) did not stimulate transcription of HIF-1-responsive reporters. Moreover, the expression of the hypoxia-dependent gene for NDRG-1/Cap43 was stimulated by ionophore in both HIF-proficient and -deficient fibroblasts, thus proving the activation of a HIF-1-independent pathway by Ca2+.
Since the activation of the NF-AT transcription factor is Ca2+ and calcineurin dependent, we first investigated the involvement of NF-AT in the induction of hypoxic genes. Using cyclosporine, an inhibitor of calcineurin, we ruled out the involvement of NF-AT in the transcriptional regulation of the NDRG-1/Cap43 gene by either Ca2+ ionophore, nickel, or hypoxia.
The VEGF promoter contains three AP-1 sites (30, 33). Moreover, TPA, dibutyryl-cyclic AMP, and Ca2+ ionophore induce VEGF transcription, suggesting the presence of a functional AP-1 binding site in the VEGF promoter (4, 6, 7). Both VEGF and NDRG-1/Cap43 can be upregulated by TPA (4, 23, 26). Using computer analysis, we found an AP-1 response element in the 5′ end of the NDRG-1/Cap43 gene. These data suggested that both the VEGF and NDRG-1/Cap43 mRNAs could potentially be induced by Ca2+ via the AP-1 transcription factor and that the elevation of the level of intracellular Ca2+ during hypoxia could lead to the activation of the AP-1 transcription factor. It has been reported that hypoxia induces AP-1, which in turn can activate transcription of VEGF (7). However, the mechanism of AP-1 activation by hypoxia was not understood. We found that the elevation of intracellular Ca2+ caused an increase in c-Jun protein levels. The appearance of functional AP-1 was proved by the stimulation of AP-1-dependent transcription (AP-1-Luc). Using the K252a protein kinase inhibitor, we demonstrated that Ca2+ induced AP-1 transcriptional activity and that the expression of the two hypoxic genes was coupled.
Not only hypoxia but also Ca2+ induced the expression of the full-length VEGF-luciferase promoter construct. Deletion of the first two AP-1 sites completely eliminated transactivation of the VEGF-Luc construct by Ca2+ but merely diminished the response to hypoxia. Similarly, DN AP-1 completely eliminated the pGL3-V2274 induction caused by Ca2+ but only partially impaired the hypoxia-mediated induction of this construct. Therefore, both the deletion of AP-1 binding sites and the expression of DN AP-1 identically inhibited hypoxia-induced transcription. Moreover, by preventing the AP-1-mediated response, DN AP-1 suppressed the induction of endogenous NDRG-1/Cap43 mRNA caused by hypoxia. We conclude that both HIF-1-dependent and Ca2+-dependent (AP-1) pathways regulate expression of hypoxic genes (Fig. 10).
FIG. 10.
Illustration of the regulation of hypoxia-inducible genes. Under hypoxic conditions, the accumulation of HIF-1a was accompanied by an elevation of intercellular calcium. HIF-1 directly transactivates hypoxia-inducible genes. Ca activates the JNK/AP-1 pathway, which contributes to transactivation, and potentially may participate in the stabilization of the mRNA of hypoxia-inducible genes.
The cooperation between HIF-1-dependent and AP-1-dependent transcription has important biological implications. In addition to being regulated by hypoxia, hypoxia-inducible genes are regulated by many other conditions, including those involving mitogens and mitogen-activated signaling such as activated Ras, protein kinase C, Akt, and the JNK pathway (3, 4, 7, 20, 22). These conditions are drastically different from hypoxia and, in many instances, act in a HIF-1-independent manner, initiating the activation of a distinct set of hypoxia-inducible genes. Under hypoxic conditions, HIF-1 is the master regulator of hypoxia-induced gene expression. However, our experiments indicated that Ca2+-dependent induction of c-Jun and activation of AP-1-mediated transcription were also involved in the hypoxic response. It is possible that this pathway allows the fine regulation of a subset of hypoxia-inducible genes that have functional AP-1 sites.
Importantly, a further increase in expression of hypoxia-inducible genes was also achieved by mRNA stabilization (19, 32). Like hypoxia, Ca2+ and protein kinase C can increase the stability of several hypoxia-inducible mRNAs. The JNK pathway can cause stabilization of target mRNA through the 3′ untranslated region (5). It is conceivable that the JNK pathway mediated both AP-1-dependent transactivation and stabilization of mRNA caused by Ca2+ during the hypoxic response.
Acknowledgments
We are grateful to K. Claffey for providing the VEGF probe and to C. Vinson for providing DN AP-1.
This work was supported by grant numbers ES05512, ES00260, and ES10344 from the NIH/NIEHS and grant number CA16087 from the NIH/NCI.
REFERENCES
- 1.An, W. G., M. Kanekal, M. C. Simon, E. Maltepe, M. V. Blagosklonny, and L. M. Neckers. 1998. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392:405-408. [DOI] [PubMed] [Google Scholar]
- 2.Arnould, T., C. Michiels, I. Alexandre, and J. Remacle. 1992. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J. Cell. Physiol. 152:215-221. [DOI] [PubMed] [Google Scholar]
- 3.Berra, E., J. Milanini, D. E. Richard, M. Le Gall, F. Vinals, E. Gothie, D. Roux, G. Pages, and J. Pouyssegur. 2000. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem. Pharmacol. 60:1171-1178. [DOI] [PubMed] [Google Scholar]
- 4.Chabannes, E., S. Fauconnet, S. Bernardini, H. Wallerand, G. Adessi, and H. Bittard. 2001. Protein kinase C signalling pathway is involved in the regulation of vascular endothelial growth factor expression in human bladder transitional carcinoma cells. Cell. Signal. 13:585-591. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. Y., F. Del Gatto-Konczak, Z. Wu, and M. Karin. 1998. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science 280:1945-1949. [DOI] [PubMed] [Google Scholar]
- 6.Claffey, K. P., W. O. Wilkinson, and B. M. Spigelman. 1992. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J. Biol. Chem. 267:16317-16322. [PubMed] [Google Scholar]
- 7.Damert, A., E. Ikeda, and W. Risau. 1997. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 327:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang, C. V., and G. L. Semenza. 1999. Oncogenic alterations of metabolism. Trends Biochem. Sci. 24:68-72. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe, J. A., B.-H. Jiang, N. V. Iyer, F. Agani, S. W. Leung, R. D. Koos, and G. L. Semenza. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freiberg, R. A., L. Susannah, S. L. Green, and A. J. Giaccia. 2001. Hypoxia and cell cycle, p. 143-154. In M. V. Blagosklonny (ed.), Cell cycle checkpoints and cancer. Landes Bioscience, Austin, Tex.
- 11.Gardner, L. B., Q. Li, M. S. Park, W. M. Flanagan, G. L. Semenza, and C. V. Dang. 2001. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 276:7919-7926. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg, M. A., and T. J. Schneider. 1994. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J. Biol. Chem. 269:4355-4359. [PubMed] [Google Scholar]
- 13.Hampl, V., D. N. Cornfield, N. J. Cowan, and S. L. Archer. 1995. Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur. Respir. J. 8: 515-522. [PubMed] [Google Scholar]
- 14.Huang, C., P. Mattjus, W. Ma, M. Rincon, N. Chen, R. E. Brown, and Z. Dong. 2000. Involvement of nuclear factor of activated T cells activation in UV response. Evidence from cell culture and transgenic mice. J. Biol. Chem. 275:9143-9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer, N. V., L. E. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, A. C., B. A. Murphy, C. M. Matelis, Y. Rubinstein, E. C. Piebenga, L. M. Akers, G. Neta, C. Vinson, and M. Birrer. 2000. Activator protein-1 mediates induced but not basal epidermal growth factor receptor gene expression. Mol. Med. 6:17-27. [PMC free article] [PubMed] [Google Scholar]
- 17.Kieser, A., H. A. Weich, G. Brandner, D. Marme, and W. Kolch. 1994. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene 9:963-969. [PubMed] [Google Scholar]
- 18.Levy, A. P., N. S. Levy, S. Wegner, and M. A. Goldberg. 1995. Transcriptional regulation of rat vascular endothelial growth factor gene by hypoxia. J. Biol. Chem. 270:13333-13340. [DOI] [PubMed] [Google Scholar]
- 19.Levy, A. P., N. S. Levy, and M. A. Goldberg. 1996. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J. Biol. Chem. 271:2746-2753. [DOI] [PubMed] [Google Scholar]
- 20.Minet, E., G. Michel, D. Mottet, J. P. Piret, A. Barbieux, M. Raes, and C. Michiels. 2001. C-JUN gene induction and AP-1 activity is regulated by a JNK-dependent pathway in hypoxic HepG2 cells. Exp. Cell Res. 265:114-124. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay, D., and H. I. Akbarali. 1996. Depletion of [Ca2+]i inhibits hypoxia-induced vascular permeability factor (vascular endothelial growth factor) gene expression. Biochem. Biophys. Res. Commun. 229:733-738. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay, D., L. Tsiokas, and V. P. Sukhatme. 1995. Wild-type p53 and v-src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 55:6161-6165. [PubMed] [Google Scholar]
- 23.Piquemal, D., D. Joulia, P. Balaguer, A. Basset, J. Marti, and T. Commes. 1999. Differential expression of the RTP/Drg1/Ndr1 gene product in proliferating and growth arrested cells. Biochim. Biophys. Acta 1450:364-373. [DOI] [PubMed] [Google Scholar]
- 24.Pisani, A., P. Bonsi, D. Centonze, P. Giacomini, and P. Calabresi. 2000. Involvement of intracellular calcium stores during oxygen/glucose deprivation in striatal large aspiny interneurons. J. Cereb. Blood Flow Metab. 20:839-846. [DOI] [PubMed] [Google Scholar]
- 25.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salnikow, K., T. Kluz, and M. Costa. 1999. Role of Ca2+ in the regulation of nickel-inducible Cap43 gene expression. Toxicol. Appl. Pharmacol. 160:127-132. [DOI] [PubMed] [Google Scholar]
- 27.Salnikow, K., M. V. Blagosklonny, H. Ryan, R. Johnson, and M. Costa. 2000. Carcinogenic nickel induces genes involved in hypoxic stress. Cancer Res. 60:38-41. [PubMed] [Google Scholar]
- 28.Salnikow, K., W. G. An, G. Melillo, M. V. Blagosklonny, and M. Costa. 1999. Nickel-induced transformation shifts the balance between HIF-1a and p53 transcription factors. Carcinogenesis 20:1819-1823. [DOI] [PubMed] [Google Scholar]
- 29.Semenza, G. L. 2000. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J. Clin. Investig. 106:809-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi, Q., X. Le, J. L. Abbruzzese, Z. Peng, C.-N. Qian, H. Tang, Q. Xiong, B. Wang, X.-C. Li, and K. Xie. 2001. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 61:4143-4154. [PubMed] [Google Scholar]
- 31.Shweiki, D., A. Itin, D. Soffer, and E. Keshet. 1992. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843-845. [DOI] [PubMed] [Google Scholar]
- 32.Stein, I., M. Neeman, D. Shweiki, A. Itin, and E. Keshet. 1995. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol. Cell. Biol. 15:5363-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tischer, E., R. Mitchell, T. Hartman, M. Silva, D. Gospodarowicz, J. C. Fiddes, and J. A. Abraham. 1991. The human gene for vascular endothelial growth factor. J. Biol. Chem. 266:11947-11954. [PubMed] [Google Scholar]
- 34.Wisdom, R. 1999. AP-1: one switch for many signals. Exp. Cell Res. 253:180-185. [DOI] [PubMed] [Google Scholar]
- 35.Zhou, D., K. Salnikow, and M. Costa. 1998. Cap43, a novel gene specifically induced by Ni2+ compounds. Cancer Res. 58:2182-2189. [PubMed] [Google Scholar]