Abstract
Objective:
To compare the clinicopathologic characteristics and outcomes after resection of patients with hepatocellular carcinoma (HCC) treated in the United States, France, and Japan.
Summary Background Data:
Some epidemiologic data suggests that HCC in different regions of the world may represent different forms of the disease.
Methods:
We compared the patient and tumor characteristics, underlying liver damage, and surgical outcomes of 586 patients who underwent resection of HCC from a multi-institutional database.
Results:
A total of 169 patients were treated in the United States, 187 in France, and 230 in Japan. The median tumor size for patients treated in the United States was 8 cm, compared with 6 cm and 3.5 cm in France and Japan, respectively (P < 0.001); 20%, 38%, and 74% of patients in the United States, France, and Japan, respectively, had positive hepatitis C serology (P < 0.001). In addition, 65% of patients in Japan had severe fibrosis/cirrhosis in the adjacent liver compared with 52% and 23% of patients in France and the United States, respectively (P < 0.001). There was no association between site of treatment and 30-day (P = 0.4) or 1-year mortality (P = 0.3). The 5-year survival of patients treated in United States, France, and Japan was not statistically different (31% vs. 31% vs. 41%, respectively; P = 0.3).
Conclusions:
Although the etiology of HCC and clinicopathologic characteristics of patients treated at western and eastern centers vary widely, postresection 5-year survival is similar when controlling for these factors. Future studies should account for histopathologic differences using uniform criteria to allow better comparison of results.
Some epidemiologic data suggests that hepatocellular carcinoma in different regions of the world may represent different forms of the disease. We compared the patient and tumor characteristics, underlying liver damage, and surgical outcomes of 586 patients who underwent resection of hepatocellular carcinoma between 1980 and 1998 at four centers in the United States, France, and Japan. Despite differences in etiology, tumor factors, and underlying liver damage, perioperative outcomes and survival were similar across countries.
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in men and the ninth most common malignancy in women, accounting for 500,000 to 1 million cancer cases annually worldwide.1,2 There is marked geographic variation in the incidence of HCC, which ranges from 2.8 new cases per 100,000 persons per year in the United States to more than 30 new cases per 100,000 persons per year in Hong Kong and Japan.3,4 Although hepatitis B virus (HBV) infection is the leading cause of HCC worldwide, recent studies suggest that chronic infection with hepatitis C virus (HCV) and alcohol abuse are becoming increasingly important etiologic factors, particularly at western centers.5–7
Because of geographic variations in incidence and etiology, several authors have suggested that HCC in different regions of the world may represent different forms of the disease.8–10 However, it is not clear whether the reported geographic differences in tumor characteristics and outcome are due to different types of HCC or, rather, differences in etiology, presentation, or underlying liver damage. In this study, we compared the clinicopathologic characteristics and outcomes after resection in patients with HCC treated at centers in the United States, France, and Japan.
METHODS
Patients and Clinicopathologic Variables
We identified and reviewed the records of 591 patients who underwent attempted curative resection for HCC between 1980 and 1998 at 4 major hepatobiliary centers: University of Texas M.D. Anderson Cancer Center (Houston, TX), Mayo Clinic (Rochester, MN), Hôpital Beaujon (Paris, France), and Kyoto University Graduate School of Medicine (Kyoto, Japan). Clinical data were reviewed on site by 2 of the investigators (J.-N.V. and D.M.N.). Because we were also interested in comparing outcomes across countries, 5 patients with incomplete survival data were excluded, leaving 586 patients in the final study cohort.
Serologic presence of any hepatitis B antigen or antibody was considered as positive evidence of hepatitis B serology. Serologic presence of hepatitis C antibody was considered as positive evidence of hepatitis C serology. The type of hepatic resection performed was classified according to the scheme recently proposed by the terminology committee of the International Hepato-Pancreato-Biliary Association.11,12 Partial hepatectomy was defined as wedge resection or segmentectomy, hemihepatectomy was defined as contiguous resection of segments 2 through 4 or segments 5 through 8, and extended hemihepatectomy was defined as hemihepatectomy with resection of additional contralateral lobe segments (ie, “trisegmentectomy”).
The pathologic resection specimens from all patients were reviewed on site by 1 of the investigators (G.Y.L.). At all 4 centers, hematoxylin-eosin was the stain of choice. A total of 2286 sections of HCC were available. One to 21 sections per tumor were reviewed (mean, 4.3 sections per tumor). Tumor size was defined as the largest diameter of the tumor specimen. Microscopic vascular invasion was defined as the presence of tumor emboli within the central vein, the portal vein, or large capsular vessels. Minor vascular invasion was defined as either gross or microscopic involvement of the lobar or segmental branches of the portal vein or the hepatic veins. For the purposes of our analysis, minor vascular invasion and microscopic vascular invasion were grouped as 1 category called “microvascular invasion.” Major vascular invasion was defined as gross invasion of the right or left main branches of the portal vein or the hepatic veins.13 Tumor grade was assessed using the scheme outlined by Edmondson and Steiner14 and was based on the area showing the highest grade. The degree of fibrosis of the surrounding liver parenchyma was graded according to the classification of Ishak et al.15 Grading of fibrosis was performed using trichrome-stained slides, available in most cases, and using sections distant from the tumor to avoid secondary changes due to mass effect.
Statistical Analysis
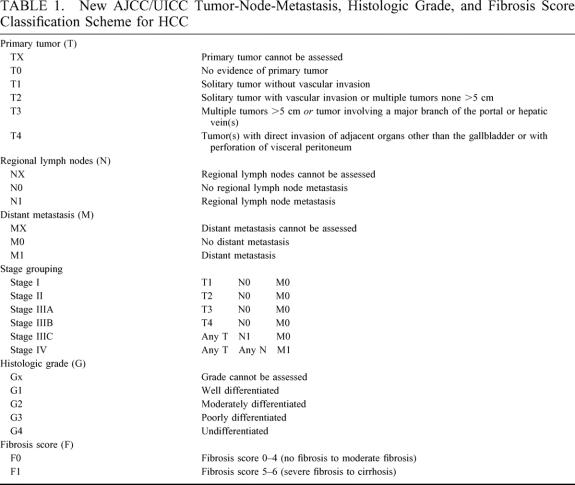
Age, α-fetoprotein level, and tumor size were treated as both continuous and dichotomous variables, using their respective medians as the breakpoints for the statistical analyses. Because of sample-size limitations, patients with poorly differentiated and undifferentiated tumors were combined into a single group. Survival was measured from the time of resection until death or last follow-up. Survival curves were constructed using the Kaplan-Meier product limit method and compared using log-rank tests.16,17 To control for potential confounding due to differences in clinicopathologic variables among centers, patients were also stratified according to the new American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) primary tumor (T) classification system (Table 1). 18 Statistical significance was defined as a P value of ≤ 0.05. The SPSS 10.0 software package (SPSS Inc., Chicago, IL) was used for the statistical analyses.
TABLE 1. New AJCC/UICC Tumor-Node-Metastasis, Histologic Grade, and Fibrosis Score Classification Scheme for HCC
RESULTS
Patient and Tumor Characteristics
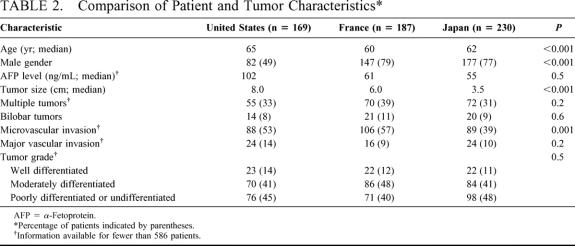
The demographic and clinicopathologic characteristics of the 586 patients in the study cohort are shown in Table 2. A total of 169 patients were treated in the United States, 187 patients were treated in France, and 230 patients were treated in Japan. The median age of patients treated in France and Japan was slightly younger than that of patients treated in the United States (P < 0.001). Fewer than half of the patients treated in the United States were male, while more than 3 quarters of those treated in France and Japan were male (P < 0.001).
TABLE 2. Comparison of Patient and Tumor Characteristics
The median tumor size for patients treated in the United States was 8 cm, compared with 6 cm for patients treated in France and 3.5 cm for patients treated in Japan (P < 0.001). However, there was no difference in tumor size between countries when patients were stratified according to HCV status or presence of severe fibrosis or cirrhosis in the adjacent liver.
There was a direct relationship between increasing tumor size and likelihood of microvascular invasion. Thirty-seven percent of patients with tumors measuring 5 cm or less had evidence of microvascular invasion, compared with 61% of patients with tumors >5 cm (P < 0.001). Only 39% of patients treated in Japan had tumors with microvascular invasion, compared with 53% and 57% of patients treated in the United States and France, respectively (P < 0.001). However, this difference failed to reach statistical significance when patients were stratified by tumor size.
Despite differences in tumor size, the proportions of patients with multiple or bilobar tumors and major vascular invasion were similar across countries. Histopathologic tumor grade was also similar across countries.
Hepatitis Infection, Hepatitis Grade, and Fibrosis Score
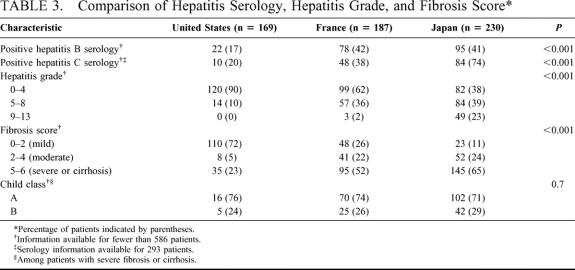
The seroprevalence of HBV and HCV and the extent of inflammatory activity or fibrosis in the adjacent noncancerous liver varied widely between countries (Table 3). Only 17% of patients in the United States were seropositive for HBV, compared with 42% and 41% of patients treated in France and Japan, respectively (P < 0.001).
TABLE 3. Comparison of Hepatitis Serology, Hepatitis Grade, and Fibrosis Score
HCV serology was available for 293 patients: 51 (30%) of the patients treated in the United States, 128 (68%) of the patients treated in France, and 114 (50%) of the patients treated in Japan. Among these patients, only 20% of those treated in the United States and 38% of those treated in France were seropositive for HCV, compared with 74% of patients treated in Japan (P < 0.001). In addition, fewer than one fourth of patients treated in the United States had severe fibrosis or cirrhosis, compared with more than half of patients treated in France and Japan (P < 0.001). The majority of patients with severe fibrosis or cirrhosis in the study cohort were classified as Child class A, while the remainder were classified as Child class B. The Child class distribution did not vary significantly between countries.
Surgical Outcomes and Predictors of Long-Term Survival
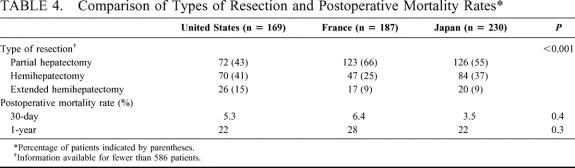
The types of hepatic resections performed reflected the relative rates of underlying liver disease and cirrhosis in the different countries (Table 4). Sixty-six percent of patients treated in France and 55% of those treated in Japan underwent partial hepatectomy, compared with 43% of patients treated in the United States. In contrast, 15% of patients treated in the United States underwent extended hemihepatectomy, compared with fewer than 10% of patients treated in France and Japan (P < 0.001). When controlling for severe fibrosis or cirrhosis in the noncancerous liver, however, the proportion of patients treated with partial hepatectomy, hemihepatectomy, or extended hemihepatectomy did not vary significantly between countries. The perioperative (within 30 days after resection) and early (within 1 year after resection) mortality rates did not differ significantly between countries (P = 0.4 and P = 0.3, respectively).
TABLE 4. Comparison of Types of Resection and Postoperative Mortality Rates
The median follow-up time for all patients was 33 months (range, 1–209 months). The median follow-up time for patients still alive was 66 months, compared with 22 months for patients who had died. The majority of patients in the series (410; 70%) were dead by the end of the follow-up period. The median survival time in our study cohort was 45 months, and the 5- and 10-year survival rates were 36% ± 2% and 14% ± 2%, respectively. The 5-year survival rates for patients treated in the United States, France, and Japan were not significantly different: 31% ± 4%, 31% ± 4%, and 41% ± 3%, respectively (P = 0.3; Fig. 1).
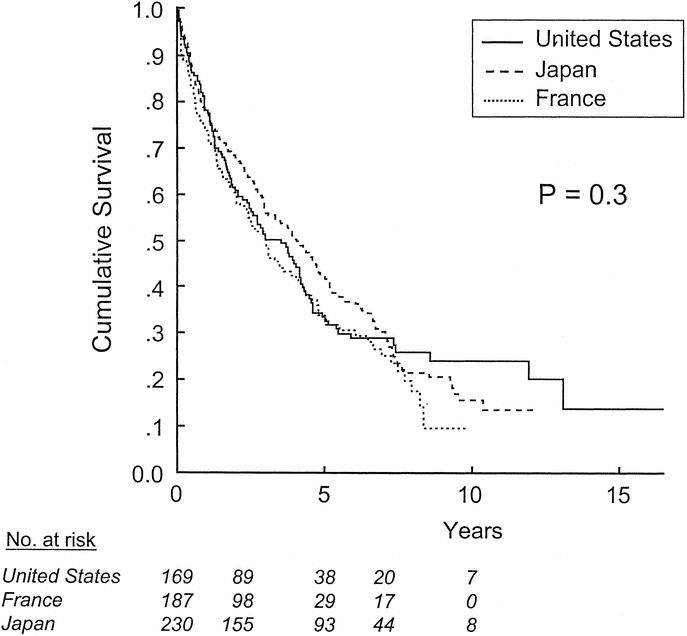
FIGURE 1. Survival after resection of HCC, stratified by site of treatment.
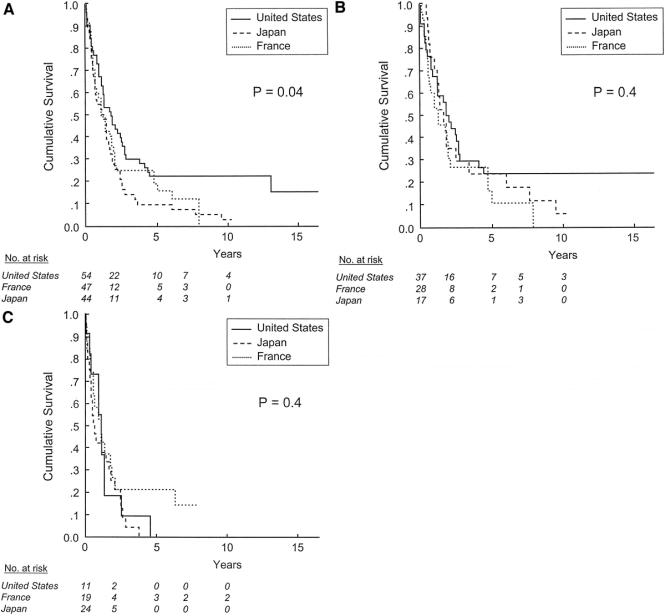
When patients were stratified according to the new AJCC/UICC primary tumor classification system (Table 1), survival did not differ by country in patients with T1 or T2 tumors (Fig. 2). In contrast, among patients with T3 tumors [ie, multiple tumors, any larger than 5 cm, or tumor(s) involving a major branch of the portal vein or the hepatic veins], the 5-year survival rates for patients treated in the United States, France, and Japan were 22% ± 6%, 15% ± 6%, and 9% ± 4%, respectively (P = 0.04, Fig. 3a). However, there was no difference in survival across countries when patients with T3 tumors were stratified according to the presence of severe fibrosis or cirrhosis in the noncancerous liver (Figs. 3b, c). Because only 12 patients in our cohort had T4 tumors, the effect of country of treatment on outcome within this subgroup could not be analyzed.
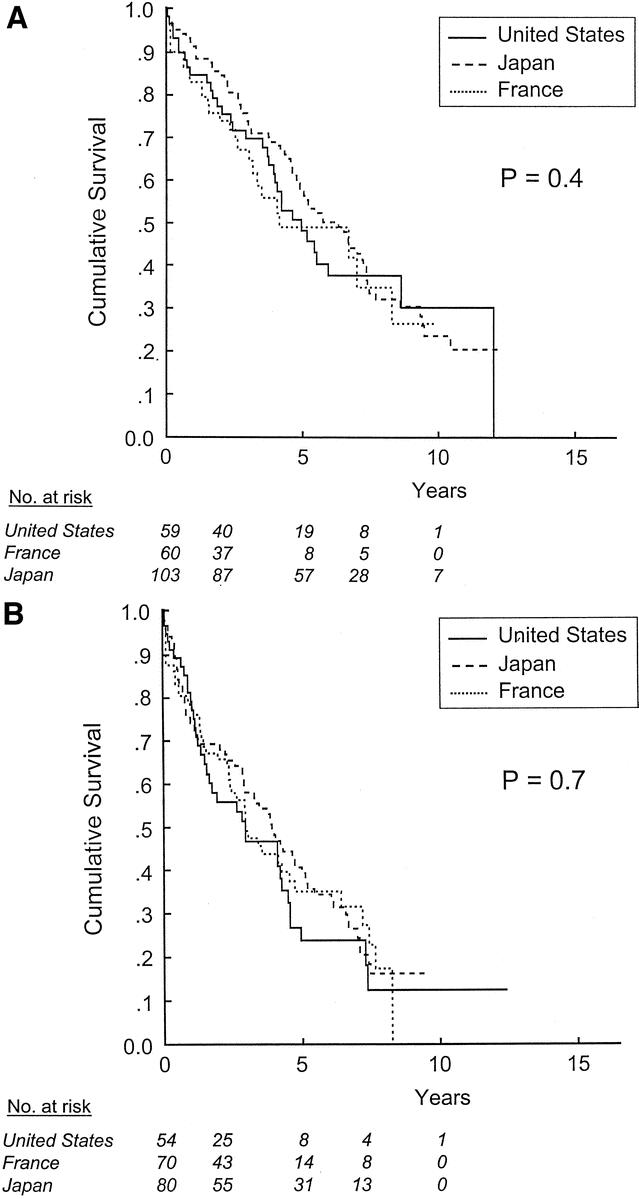
FIGURE 2. Survival after resection of HCC in patients with T1 (a) and T2 (b) tumors, stratified by site of treatment.
FIGURE 3. Survival after resection of HCC in patients with T3 (a) tumors, stratified by site of treatment. Effect of site of treatment in patients with T3 tumors without (b) and with (c) severe fibrosis or cirrhosis, stratified by site of treatment.
DISCUSSION
Previous studies from North America, Europe, and Asia have analyzed outcomes and predictors of survival after resection in patients with HCC.8,19–23 Because of geographic differences in clinicopathologic tumor characteristics and the prevalence of underlying liver disease, these studies have resulted in several different classification systems for HCC, including the Barcelona Clinic Liver Cancer staging classification,24 the Cancer of the Liver Italian Program score,25 the Okuda staging system,26 and the Classification of Primary Liver Cancer of the Liver Cancer Study Group of Japan.10 Recently, our group performed a multivariate analysis and identified common predictors of outcome after resection of HCC.27 The independent predictors of death (tumor size >5 cm, multiple (ie, ≥ 2) tumors, presence of microvascular invasion, presence of major vascular invasion, and severe fibrosis or cirrhosis in the noncancerous liver) were combined to create a simplified staging system, which has since been adopted as the new tumor-node-metastasis staging system of the AJCC/UICC (Table 1).
To our knowledge, the current study represents the first effort to compare outcomes after resection of HCC between western and eastern countries using uniform clinical and pathologic criteria for both tumor factors (eg, microvascular invasion, major vascular invasion, and histopathologic grade) and liver-disease factors (ie, hepatitis activity and fibrosis score). Our results suggest that there are important differences in etiology, clinicopathologic factors, and prevalence of underlying liver damage among patients treated in the United States, France, and Japan. Despite these marked differences, however, perioperative and long-term outcomes were similar between countries overall and for subgroups of patients when patients were stratified according to the new AJCC/UICC primary tumor classification system, which controls for several known predictors of survival.
There was a marked difference in the gender distribution of patients treated in the various countries. Fewer than 50% of the patients treated in the United States were male, compared with more than three fourths of the patients treated in France and Japan. Other investigators have previously noted an association between male gender and severe fibrosis or cirrhosis.28,29 In a series reported by Bismuth et al, the male:female ratio among patients with cirrhosis was 8:1, compared with 1.8:1 among patients without cirrhosis.29 Although the relationship between male gender and HCC has been largely attributed to the higher frequency of viral hepatitis and alcoholic cirrhosis among men, some increased risk persists even after controlling for these confounders, suggesting that other reasons for this association may exist.3
We also found a highly significant difference in tumor size at presentation among patients treated in the United States, France, and Japan. In addition, there was an inverse relationship between the prevalence of HBV and HCV infection and tumor size in the 3 countries, suggesting that the observed differences in tumor size may have resulted from periodic radiologic screening of chronically infected patients and lead-time bias.
Patients treated in Japan had the smallest median tumor size and the lowest rates of microvascular invasion. Tsai et al recently noted an association between tumor size and increasing rates of both microscopic and macroscopic vascular invasion.30 A recent report analyzing the predictors of microvascular invasion in patients with HCC who were candidates for orthotopic liver transplantation showed a similar association: approximately 25% of the patients with tumors smaller than 2 cm had microvascular invasion, compared with 31% of patients with tumors between 2 and 4 cm, and 50% of patients with tumors larger than 4 cm.31 Several recent articles have emphasized the need for systematic review of microvascular invasion in resected specimens,30,32 and its presence is used to stratify patients with single tumors in the new AJCC/UICC T classification scheme (Table 1). Unlike microvascular invasion, which cannot be detected prior to resection or transplantation, invasion of the major branches of the portal vein or the hepatic veins can be detected by imaging in 81% to 95% of cases.33–35 Not surprisingly, the rate of major vascular invasion in our series was similar across the 3 countries.
More than 40% of the patients treated in France and Japan had evidence of HBV infection, compared with only 17% of the patients treated in the United States. In addition, 38% of the patients treated in France and 74% of the patients treated in Japan had evidence of HCV infection, compared with less than 20% of the patients treated in the United States. As a result, a higher proportion of patients treated in France and Japan had moderate to severe necroinflammatory activity and fibrosis in the adjacent, noncancerous liver. The types of hepatic resections performed reflected the relative rates of liver damage in the different countries: partial hepatectomies were more common in France and Japan, while fewer than 10% of the patients treated in France and Japan underwent extended resections. Despite these differences, however, the perioperative outcomes in the 3 countries were similar, likely due to modifications in patient selection, surgical technique, and perioperative care at the various centers.
Although the impact of seropositivity for HBV or HCV on survival following resection of HCC remains controversial,36–39 several studies have documented an association between cirrhosis and tumor recurrence that is presumably due to continued carcinogenesis in the affected liver remnant.19,40–42 Kosuge et al reported an unfavorable effect of liver disease, particularly cirrhosis, on disease-free survival that became more pronounced over time.19 In another analysis of 159 patients who survived 5 or more years after resection of HCC, the presence of moderate to severe fibrosis or cirrhosis was the most important predictor of death,42 overshadowing all other tumor or liver factors. Our group recently analyzed the impact of fibrosis score on survival after resection of HCC. Five-year survival rates were significantly higher for patients without than for patients with severe fibrosis or cirrhosis among patients with T1 tumors (64% vs., 49%, P = 0.01), T2 tumors (46% vs. 30%, P = 0.01), and T3 tumors (17% vs. 9%, P = 0.005).27 Like microvascular invasion, the presence of severe fibrosis or cirrhosis in the adjacent liver has been incorporated into the new AJCC/UICC T classification scheme for HCC (Table 1). Use of the fibrosis scores defined by Ishak et al15 is recommended, and disease is classified as being associated with none to moderate fibrosis (F0) or with severe fibrosis or cirrhosis (F1). According to this scheme, fibrosis score can thus be used to further stratify patients once they have been categorized by tumor number and degree of vascular invasion.
The current study is based on a detailed pathologic review of resected specimens at several hepatobiliary centers worldwide and encompasses the broad spectrum of tumor and liver-disease factors associated with prognosis in HCC. Of particular importance is the systematic pathologic evaluation of microscopic vascular invasion and fibrosis in the underlying liver, both of which have been shown to be independent predictors of death following resection of HCC.27 Microscopic and minor vascular invasion can be easily assessed by examination of hematoxylin-eosin-stained sections, while liver fibrosis can be assessed using trichrome-stained slides and a previously validated fibrosis staging system.15
In conclusion, this study showed that differences in HCV positivity and liver damage in the United States, France, and Japan resulted in marked differences in tumor size and rates of microvascular invasion at presentation. These factors have been shown to be independent predictors of survival and, when combined with the degree of fibrosis or cirrhosis in the underlying liver, help determine long-term survival after resection of HCC. Future studies should account for these clinicopathologic and liver-disease factors using uniform criteria to allow more meaningful interpretation and comparison of results.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of S. Fujita, MD, for translation of clinical data and A. Encarnacion, RN, for data management. They also wish to thank Ruth J. Haynes for secretarial assistance.
Footnotes
Supported by a T-32 Surgical Oncology Training Grant from the National Institute of Health.
Reprints: Jean-Nicolas Vauthey, MD, Department of Surgical Oncology, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 444, Houston, TX 77030. jvauthey@mdanderson.org.
Presented at the 5th World Congress of the International Hepato-Pancreato-Biliary Association, Tokyo, Japan, April 2002.
REFERENCES
- 1.Bosch FX. Global epidemiology of hepatocellular carcinoma. In: Okuda K, Tabor E, eds. Liver Cancer. New York: Churchill Livingstone; 1997:13–28. [Google Scholar]
- 2.Di Bisceglie AM. Malignant tumors of the liver. In: Schiff ER, Sorrell MF, Maddrey WC, eds. Schiff’s Diseases of the Liver, 8th ed. Philadelphia: Lippincott-Raven; 1999:1281–1304. [Google Scholar]
- 3.El-Serag H. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. [DOI] [PubMed] [Google Scholar]
- 4.Falk H. Liver. In: Schottenfeld D, Fraumeni J, eds. Cancer Epidemiology and Prevention. Philadelphia: Saunders; 1982:668–682.
- 5.Ezaki T, Stansby G, Hobbs K. Regional differences in hepatocellular carcinoma and its surgical treatment. HPB Surg. 1991;4:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonetti R, Camma C, Fiorello F, et al. Hepatocellular carcinoma: a worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–972. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag H, Mason A. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. [DOI] [PubMed] [Google Scholar]
- 8.Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 9.Azii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology. 2000;32:1224–1229. [DOI] [PubMed] [Google Scholar]
- 10.Liver Cancer Study Group of Japan. Classification of Primary Liver Cancer. Tokyo: Kanehara; 1997. [Google Scholar]
- 11.Couinaud C. Plaidoyer pour une segmentation hepatique exacte et une technique anatomique de resection reglee du foie. Presse Med. 1966;74:2849–2852. [PubMed] [Google Scholar]
- 12.Strasberg SM. The Brisbane 2000 terminology of hepatic anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- 13.Hermanek P, Henson DE, Hutter RVP, et al., eds. TNM Supplement. Berlin: Springer-Verlag; 1993.
- 14.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481.
- 17.Peto R, Peto J. Asymptomatically efficient rant invariant test procedure. J R Stat Soc. 1972;135:185–206. [Google Scholar]
- 18.Liver (including intrahepatic bile ducts). In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC Cancer Staging Manual, 6th ed. Philadelphia: JB Lippincott; 2002:131–144.
- 19.Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328–332. [PubMed] [Google Scholar]
- 20.Izumi R, Shimizu K, Ii Tohru II, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. [DOI] [PubMed] [Google Scholar]
- 21.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28–34. [DOI] [PubMed] [Google Scholar]
- 22.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a western center. Ann Surg. 1998;229:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Center of the Liver Italian Program (CLIP) investigators. Hepatology. 2000;28:751–755. [DOI] [PubMed]
- 24.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. [DOI] [PubMed] [Google Scholar]
- 25.Cancer of the Italian Liver Program Investigators.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000;31:840–845. [DOI] [PubMed] [Google Scholar]
- 26.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56:918–928. [DOI] [PubMed] [Google Scholar]
- 27.Vauthey JN, Lauwers GY, Esnaola NF, et al. A simplified staging system for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. [DOI] [PubMed] [Google Scholar]
- 28.Smalley SR, Moertel CG, Hilton JF, et al. Hepatoma in the noncirrhotic liver. Cancer. 1988;62:1414–1424. [DOI] [PubMed] [Google Scholar]
- 29.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World J Surg. 1995;19:35–41. [DOI] [PubMed] [Google Scholar]
- 30.Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. [DOI] [PubMed] [Google Scholar]
- 31.Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232. [DOI] [PubMed] [Google Scholar]
- 32.Vauthey JN, Sobin LH. On the uniform use of the AJCC/UICC staging system for hepatocellular carcinoma. Surgery. 2000;128:870. [DOI] [PubMed] [Google Scholar]
- 33.Nelson RC, Chezmar JL, Sugarbaker PH, et al. Preoperative localization of focal liver lesions to specific liver segments: utility of CT during portography. Radiology. 1990;176:89–94. [DOI] [PubMed] [Google Scholar]
- 34.Bach AM, Hann LE, Brown KT, et al. Portal vein evaluation with United States: comparison to angiography combined with CT arterial portography. Radiology. 1996;201:149–154. [DOI] [PubMed] [Google Scholar]
- 35.Hann LE, Schwartz LH, Panicek DM, et al. Tumor involvement in hepatic veins: comparison of MR imaging and United States for preoperative assessment. Radiology. 1998;206:651–656. [DOI] [PubMed] [Google Scholar]
- 36.Takenaka K, Yamamoto K, Taketomi A, et al. A comparison of the surgical results in patients with hepatitis B versus hepatitis C-related hepatocellular carcinoma. Hepatology. 1995;22:20–24. [PubMed] [Google Scholar]
- 37.Yamanaka N, Tanaka T, Tanaka W, et al. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer. 1997;79:1509–1515. [DOI] [PubMed] [Google Scholar]
- 38.Chen MF, Jeng LB, Lee WC, et al. Surgical results in patients with dual hepatitis B- and C-related hepatocellular carcinoma compared with B- or C-related hepatocellular carcinoma. Surgery. 1998;123:554–559. [DOI] [PubMed] [Google Scholar]
- 39.Kubo S, Nishiguchi S, Hironashi K, et al. Clinical significance of prior hepatitis B virus infection in patients with hepatitis C virus-related hepatocellular carcinoma. Cancer. 1999;86:793–798. [DOI] [PubMed] [Google Scholar]
- 40.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto J, Kosuge T, Takayama T, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219–1222. [PubMed] [Google Scholar]
- 42.Bilimoria MM, Lauwers GY, Doherty DA, et al. Underlying liver disease but not tumor factors predict long-term survival after hepatic resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. [DOI] [PubMed] [Google Scholar]