Abstract
Objective:
To compare duration and rates of underestimation and complete excision for nonpalpable breast lesions using either intraoperative ultrasonographically guided excisioned biopsy (IUGE) or directional vacuum-assisted biopsy (DVAB).
Summary Background Data:
Percutaneous ultrasonography-guided core needle biopsy is preferable to stereotactic biopsy for treatment of nonpalpable breast lesions; however, underestimation and false-negative results can occur, and rebiopsy may be required. To date, however, there has been no comparison of these two procedures in terms of diagnostic accuracy and duration.
Methods:
For 4 consecutive years, IUGE was performed for 104 nonpalpable breast lesions and DVAB for 128 lesions at Chang Gung Memorial Hospital. Of the DVAB cases, the handheld mammotome was used for 53 procedures, with all lesions removed as completely as possible. The duration of the two procedures was calculated from initial skin incision until completion of wound closure. Most of the patients with benign pathology underwent ultrasonographic examination at 3 months after surgery, with a follow-up examination at 1 year. Surgery was performed subsequently for all of the malignancy cases.
Results:
The average ages and mean tumor sizes for patients undergoing IUGE or DVAB were 46 and 47 years and 1.1 and 1.0 cm, respectively. The average IUGE and DVAB surgery durations for 88 benign tumors and 117 benign lesions were 44.3 and 21.5 minutes, respectively (P < 0.001), and 43.5 and 20.6 minutes for the malignant tumors (n = 16 and n = 11), respectively (P = 0.036). The IUGE and DVAB surgery durations for tumors <1 cm in diameter were 43.5 and 20.6 minutes, respectively, and 44.2 and 23.6 minutes for tumors over that size (P < 0.001). An older-model mammotome was used for 75 patients, with an average duration of 24 minutes in comparison to 18 minutes for the handheld variant (P < 0.001). No false-negative results were noted and, except in the case of the malignant tumors, there was no need for reexcisional biopsy. Further, there were no underestimates of the disease for the 4 cases of atypical ductal hyperplasia and the 12 of noninvasive carcinoma. No further ultrasonographic evidence of tumors was noted for 95% of the benign pathologies, with no residual abnormality detected for 13 of the 27 malignant tumors after IUGE or DVAB.
Conclusions:
For treatment of nonpalpable breast lesions, both IUGE and DVAB eliminate false-negative results, underestimates, and the requirement for reexcisional biopsies. In comparison to IUGE, DVAB is more convenient and time efficient for excisional biopsy of nonpalpable breast lesions.
Directional vacuum-assisted core needle biopsy is preferable to ultrasonography-guided biopsy because there are no underestimates or false-negative results and there are significant time savings associated with the procedure.
Over the last 10 years, percutaneous imaging-guided biopsy has become widely adopted for diagnosis of nonpalpable breast lesions, and it is generally accepted as an alternative to open surgical biopsy.1–5 For patients presenting with breast masses, ultrasonography (US)-guided biopsy is preferable to the stereotactic variant, not only because it is more comfortable for the subject and there is no exposure to ionizing radiation but also because it is more cost-effective.6,7 US-guided biopsy for nonpalpable breast lesions can be performed rapidly using fine needle-aspiration cytology; however, its use is usually associated with a high percentage of inadequate specimens.8,9 From a review of the literature, it appears that US-guided core needle biopsy can resolve the problem of inadequate specimens; however, the false-negative rate ranges from 3.6% to 10.9%10,11 and the procedure is associated with underestimation and the need for reexcisional biopsy.2 Intraoperative US-guided excisional biopsy (IUGE) is feasible for treatment of nonpalpable breast lesions, providing an accurate diagnosis and obviating wire-localization breast biopsy.12–15 Recently, directional vacuum-assisted breast biopsy (DVAB) (with an 11-/14-gauge needle) has been recommended for the diagnosis of small lesions.16–18 The aim of this study was to compare the diagnostic accuracy, efficacy, and duration of IUGE and DVAB for nonpalpable breast tumors.
PATIENTS AND METHODS
The results of IUGE and DVAB for 232 nonpalpable breast lesions treated at Chang Gung Memorial Hospital were retrospectively reviewed for the 4-year period from January 1998 to June 2001. The average ages for the IUGE and DVAB groups were 47 and 46 years, respectively. For Chinese women, US was usually the primary workup examination for young patients, those with small breasts, and as an alternative to x-ray mammography for screening of high-risk patients. The ALOKA SSD 2000 or SSD 5500 system (ALOKA, Tokyo, Japan) was used for US, with a 7.5 or 10.0 MHZ liner array transducer. The results were recorded in a computer database according to the salient sonographic features of Ap/width ratio (anterior-posterior diameter:width), shape, margin, internal echogenicity, internal echotexture, posterior acoustic detail, and presence of bilateral refraction sign, as previously described.19 Indications for biopsy were patient preference, clinical concerns, or presence of suspicious lesion on x-ray mammography or US. As DVAB was not available in the first year of the study, 72 patients with nonpalpable lesions underwent IUGE during that period. During the following 3 years, 32 patients underwent IUGE and 128 DVAB. The IUGE procedures are described elsewhere,12 with the lesions completely and grossly excised. DVAB was performed under local anesthesia using a mammotome (Ethicon Endo-surgery, Cincinnati, OH) and an 11-gauge needle. One year after commencement of the study, a new, handheld mammotome replaced the older model.
The lesions were removed as completely as possible, with no further evidence of tumor detected sonographically. The number of samples obtained was dependent on the size and the position of the needle relative to the lesion as it preceded the probe.
All IUGE and DVAB procedures were performed by an experienced surgeon (S.-C.C). Duration was measured from commencement of the initial skin incision to wound closure or needle withdrawal for the IUGE and DVAB groups, respectively.
Patients whose tumors proved to be benign were asked to undergo US 3 months and 1 year postsurgery; all the patients with premalignant or malignant pathology underwent subsequent surgery (wide excision, partial mastectomy, or modified radical mastectomy).
Comparative data were analyzed to derive statistical significance using a two-sample t test for comparison of continuous variables.
RESULTS
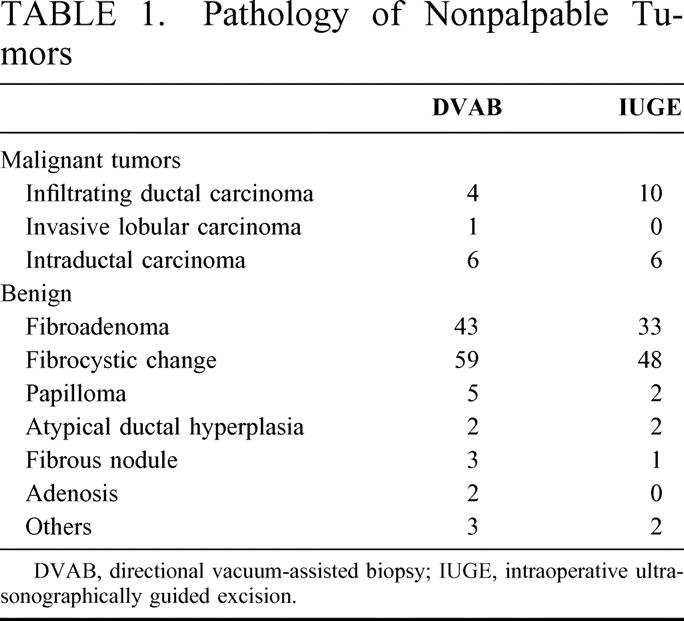
In total, 104 patients underwent IUGE and 128 patients DVAB. The average sizes of the tumor were 1.1 cm (range 0.5–2.5 cm) and 1.0 cm (range 0.4–2.1 cm), respectively, with 90% of the tumors <1.5 cm. For the DVAB group, 11 of the 128 lesions (8.6%) were proved malignant (Table 1); these consisted of 6 intraductal and 4 infiltrating ductal carcinomas, and 1 invasive lobular variant. Tumors for 16 of the 104 patients (15.4%) in the IUGE group proved to be malignant, with 6 intraductal and 10 infiltrating ductal carcinomas. Benign tumors for the DVAB group included 43 fibroadenomas, 59 fibrocystic changes, 5 papillomas, 2 atypical ductal hyperplasias, and 8 other; with 33 fibroadenomas, 48 fibrocystic changes, 2 papillomas, 2 atypical ductal hyperplasias, and 3 other for the IUGE group.
TABLE 1. Pathology of Nonpalpable Tumors
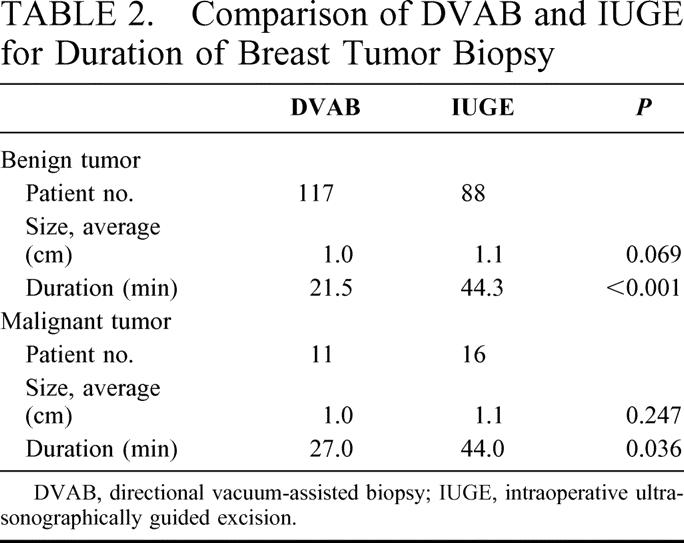
Of the benign tumor cases, 88 patients underwent IUGE and 117 DVAB, with average sizes of 1.1 and 1.0 cm and average durations of 44.3 and 21.5 minutes, respectively (P < 0.001). Average durations for the 16 patients with malignant tumors who underwent IUGE and the 11 DVAB-treated individuals were 44.0 and 27.0 minutes, respectively (P = 0.036; Table 2).
TABLE 2. Comparison of DVAB and IUGE for Duration of Breast Tumor Biopsy
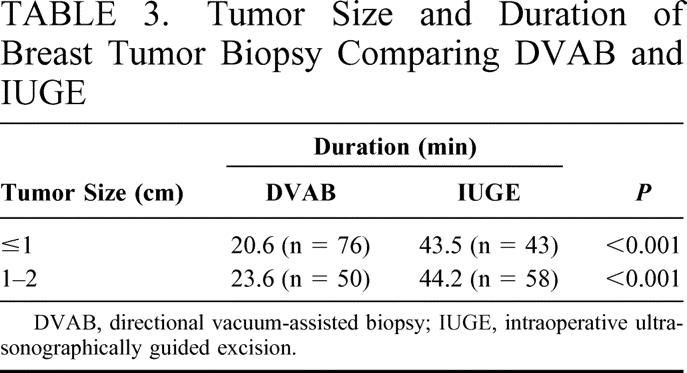
The durations for IUGE and DVAB where tumors were <1 cm in diameter were 43.5 and 20.6 minutes (P < 0.001) and 44.2 and 23.6 minutes for 1- to 2-cm tumors (P < 0.001; Table 3), respectively.
TABLE 3. Tumor Size and Duration of Breast Tumor Biopsy Comparing DVAB and IUGE
In the first year of the study, 75 procedures were performed using the older-model mammotome, with the handheld variant used for 53 procedures in the second year. The average sizes of the excised tumors were 1.0 and 1.1 cm for the old and new equipment, respectively (P = 0.069), with an average of 13 and 14 core needle samples taken per patient (P = 0.910), and average durations of 24 and 18 minutes (P < 0.001), respectively.
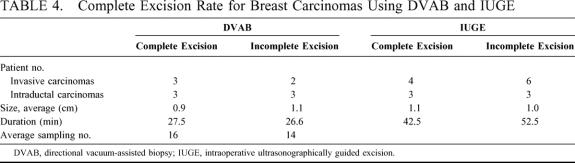
All 4 patients with pathology of atypical ductal hyperplasia subsequently underwent surgery, with no residual abnormality found (Table 4). Of the 11 cases of malignant tumor treated using DVAB, subsequent surgery revealed no evidence of residual tumor for 6 patients. For the 6 cases of intraductal carcinoma, histologic analysis of subsequent surgical specimens revealed no evidence of residual tumors for 3 patients, with only residual intraductal carcinoma noted for the other 3 patients and no underestimation revealed. For the IUGE group, no evidence of residual tumor was noted from examination of subsequent surgical specimens for 7 of the 16 patients. For the benign tumor cases in the DVAB group, no sonographic evidence of residual tumor was noted at the 3-month and 1-year follow-up examinations for 89% of the patients treated using the older-model mammotome and 95% of those where the handheld variant was used.
TABLE 4. Complete Excision Rate for Breast Carcinomas Using DVAB and IUGE
Except for two hematomas, which occurred the day after DVAB, no major complications were noted in the study and, apart from local manual compression, no further surgical intervention was necessary. Wound infection developed in one IUGE patient who recovered after treatment with systemic antibiotics.
DISCUSSION
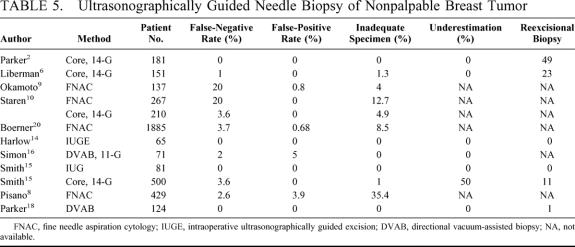
In comparison to stereotactic biopsy, the US-guided percutaneous biopsy has several advantages for treatment of nonpalpable breast lesions. These advantages include increased patient comfort, reduced incidence of vasovagal reactions,16 higher complete-excision rate (because the dimensions of the mass are more evident and the needle position can be visualized real time), reduced cost, no requirement for ionizing radiation, and rapid execution.6,7 The techniques used for US-guided biopsy for diagnosis of nonpalpable breast lesions, which include fine needle aspiration biopsy (FNAB),8,9,20 large-core needle biopsy (LCNB),10,11,21–23 DVAB,16–18 and open surgical biopsy12–15,24,25 were reviewed (Table 5). This comparison clearly demonstrates that DVAB, with completely excisional biopsy as possible, is the best diagnostic techniques for treatment of nonpalpable breast lesions.
TABLE 5. Ultrasonographically Guided Needle Biopsy of Nonpalpable Breast Tumor
FNAB has some diagnostic advantages for palpable lesions, including greater convenience and less trauma, and it is considered a major part of the triple diagnosis for breast tumors. However, the efficacy of FNAB diagnosis has been questioned because of the relatively high percentage of inadequate specimens (4%-35.4%) and false-negative rate (2.6%-20%).8,20 Even though a favorable accuracy rate has been reported in a well-designed, National Cancer Institute-supported study,20 8.5% of the specimens were nondiagnostic, with 33.9% of these finally proved malignant. In a recent multicenter clinical trial,8 it was demonstrated that FNAB was of limited value for diagnosis of nonpalpable breast lesions because of the relatively high rate of insufficient samples. Although LCNB is now widely used for evaluation of nonpalpable breast lesions because the accuracy is similar to that of surgical excision,11 there are a number of potential pitfalls, including difficulty of accurate insertion of the needle into small lesions and the need for multiple insertions, which causes greater epithelial displacement. Further, the number of false-negative results and underestimates cannot be completely eliminated using LCNB, with reexcisional biopsy often required. Smith et al have reported a large LCNB study of 446 women, with 9 underestimates and 50 reexcisional biopsies greater.11 Liberman et al studied the cost-effectiveness of US-guided core biopsy, noting only 1 false-negative result; however, a relatively high reexcisional biopsy rate (15%) and 5 malignant tumors were diagnosed for 23 women after rebiopsy.6
For both IUGE and DVAB procedures in this series, complete removal of the mass as determined from sonographic evidence was attempted to reduce the drawbacks of LCNB. No residual abnormality was noted in subsequent operations for the 4 patients with atypical ductal hyperplasia, and no residual tumor was detected for nearly half of those with malignant tumors. There were no false-negative results, no underestimates of the disease, and no requirement for reexcisional biopsy. Five core samples are routinely obtained using LCNB or mammotome biopsy, and a certain number of reexcisional biopsies are needed to eliminate underestimates and false-negative results.11,26,27 Liberman et al have reported that infiltrating ductal carcinoma may be completely excised if 14 or more core samples are obtained,28 and Parker et al have suggested that 9 core samples can entirely remove a breast mass <1.5 cm.18 In our study, the average number of tissue samplings per lesion was 16 in cases of complete excision, with an average duration of 28 minutes. The average duration for all patients was 22 minutes (18 minutes using handheld mammotome), with no statistically significant difference demonstrated in comparison to LCNB of others.2 Therefore, we agree with Parker et al and recommend complete as removal possible of the sonographic evidence of small breast lesions, which can reduce sampling error, false-negative diagnosis, and rebiopsy.18
Potential advantages of IUGE include avoidance of the complications associated with LCNB and reduced incidence of false-negative results.14 Rahusen et al .13 and Smith et al15 have reported that, using IUGE, adequate margins can be obtained for nearly 90% of malignant tumors. Thus, it seems reasonable to suggest that the role of IUGE appears not only to include tissue diagnosis but also assessment of the surgical margin of the carcinoma. However, there are several limitations to US in diagnosis of breast tumors, including poor visualization of intraductal spread29 and faint microcalcifications without hypoechoic mass,30 and significant difficulty determining the margin for those lesions composed of ductal carcinoma in situ.31 Thus, in the final analysis, IUGE should be limited to tissue diagnosis, and not used for assessment of the surgical margin after curative resection.
DVAB with attempt to complete removal of the US-evident tumor has similar advantages to IUGE, with no false-negative results, no underestimates, and no requirement for rebiopsy. Moreover, the procedure is more comfortable for the patient and less costly. In our study, it was demonstrated that DVAB was more time efficient whether benign or malignant tumors (Table 2) and tumor-size subgroups (Table 3). Further, using the handheld mammotome, most of the procedures can be completed within 20 minutes, offering significant time savings than in comparison with the older-model mammotome or IUGE.
Many women with solid tumors who undergo biopsy prefer complete removal rather than follow-up at regular intervals, especially where the diagnosed lesions are BI-RADS category >3. The DVAB procedure will satisfy the requirement for complete removal. In this study, the average number of samplings was 14 for the old-model mammotome and 13 for the handheld variant; with a duration of only 18 minutes for the latter. Thus, DVAB should be considered the most convenient and effective diagnostic tool for nonpalpable breast lesions.
Footnotes
Reprints: Shin-Cheh Chen, MD, Department of Surgery, Chang Gung Memorial Hospital, 199 Tung Hwa North Road, Taipei, Taiwan, 105. E-mail: Chensc@adm.cgmh.org.tw.
REFERENCES
- 1.Jackman RJ, Nowel KW, Shepard MJ, et al. Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology. 1994;193:359–364. [DOI] [PubMed] [Google Scholar]
- 2.Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187:507–511. [DOI] [PubMed] [Google Scholar]
- 3.Verkooijen HM, Peeters PHM, Buskens E, et al. Diagnostic accuracy of large-core needle biopsy for nonpalpable breast disease: a meta-analysis. Br J Cancer. 2000;82:1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elvecrog EL, Lechner MC, Nelson MT. Nonpalpable breast lesions: correlation of stereotaxic large-core needle biopsy and surgical biopsy results. Radiology. 1993;188:453–455. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrman GM, Cederbom GJ, Bolton JS, et al. Image-guided core-needle breast biopsy is an accurate technique to evaluate patients with nonpalpable imaging abnormalities. Ann Surg. 1998;227:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberman L, Feng TL, Dershaw DD, et al. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208:717–723. [DOI] [PubMed] [Google Scholar]
- 7.Rubin E, Mennemeyer ST, Desmond RA, et al. Reducing the cost of diagnosis of breast carcinoma: impact of ultrasound and imaging-guided biopsies on a clinical breast practice. Cancer. 2001;91:324–332. [DOI] [PubMed] [Google Scholar]
- 8.Pisano ED, Fajardo LL, Caudry DJ, et al. Fine-needle aspiration biopsy of nonpalpable breast lesions in a multicenter clinical trial: results from the radiologic diagnostic oncology group V. Radiology. 2001;219:785–792. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto H, Ogawara T, Inoue S, et al. Clinical management of nonpalpable or small breast masses by fine-needle aspiration biopsy (FNAB) under ultrasound guidance. J Surg Oncol. 1998;67:246–250. [DOI] [PubMed] [Google Scholar]
- 10.Staren ED, O’Neill TP. Ultrasound-guided needle biopsy of the breast. Surgery. 1999;126:629–634. [PubMed] [Google Scholar]
- 11.Smith DN, Rosenfield Darling ML, Meyer JE, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med. 2001;20:43–49. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Hwang TL, Chen MF. Intraoperative ultrasound-guided excisional biopsy for nonpalpable breast lesions. J Surg Assoc ROC. 1997;30:389–393. [Google Scholar]
- 13.Rahusen FD, Taets van Amerongen AH, van Diest PJ, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancers: a feasibility study looking at the accuracy of obtained margins. J Surg Oncol Suppl. 1999;72:72–76. [DOI] [PubMed] [Google Scholar]
- 14.Harlow SP, Krag DN, Ames SE, et al. Intraoperative ultrasound localization to guide surgical excision of nonpalpable breast carcinoma. J Am Coll Surgeons. 1999;189:241–246. [DOI] [PubMed] [Google Scholar]
- 15.Smith LF, Rubio LT, Henry-Tillman R, et al. Intraoperative ultrasound-guided breast biopsy. Am J Surg. 2000;180:419–423. [DOI] [PubMed] [Google Scholar]
- 16.Simon JR, Kalbhen CL, Cooper RA, et al. Accuracy and complication rates of US-guided vacuum-assisted core breast biopsy: initial results. Radiology. 2000;215:694–697. [DOI] [PubMed] [Google Scholar]
- 17.Brem RF, Schoonjans JM, Goodman SN, et al. Nonpalpable breast cancer: percutaneous diagnosis with 11- and 8-gauge stereotactic vacuum-assisted biopsy devices. Radiology. 2001;219:793–796. [DOI] [PubMed] [Google Scholar]
- 18.Parker SH, Klaus AJ, McWey PJ, et al. Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJRAm J Roentgenol. 2001;177:405–408. [DOI] [PubMed] [Google Scholar]
- 19.Chao TC, Lo YF, Chen SC, et al. Prospective sonographic study of 3093 breast tumors. J Ultrasound Med. 1999;18:363–370. [DOI] [PubMed] [Google Scholar]
- 20.Boerner S, Fornage BD, Singletary E, et al. Ultrasound-guided fine-needle aspiration (FNA) of nonpalpable breast lesions: a review of 1885 FNA cases using the National Cancer Institute-supported recommendations on the uniform approach to breast FNA. Cancer. 1999;87:19–24. [DOI] [PubMed] [Google Scholar]
- 21.Smith DN, Christian R, Meyer JE. Large-core needle biopsy of nonpalpable breast cancers. Arch Surg. 1997;132:256–259. [DOI] [PubMed] [Google Scholar]
- 22.Acheson MB, Patton RG, Howisey RL, et al. Histologic correlation of image-guided core biopsy with excisional biopsy of nonpalpable breast lesions. Arch Surg. 1997;132:815–821. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JE, Smith DN, Lester SC, et al. Large-core needle biopsy of nonpalpable breast lesions. JAMA. 1999;281:1638–1641. [DOI] [PubMed] [Google Scholar]
- 24.di Giorgio A, Arnone P, Canavese A. Ultrasound guided excisional biopsy of non-palpable breast lesions: technique and preliminary results. Eur J Surg. 1998;164:819–824. [DOI] [PubMed] [Google Scholar]
- 25.Snider HC Jr, Morrison DG. Intraoperative ultrasound localization of nonpalpable breast lesions. Ann Surg Oncol. 1999;6:308–314. [DOI] [PubMed] [Google Scholar]
- 26.Maganini RO, Klem DA, Huston BJ, et al. Upgrade rate of core biopsy-determined atypical hyperplasia by open excisional biopsy. Am J Surg. 2001;182:355–358. [DOI] [PubMed] [Google Scholar]
- 27.Adrales G, Turk P, Wallace T, et al. Is surgical excision necessary for atypical ductal hyperplasia of the breast diagnosed by mammotome? Am J Surg. 2000;180:313–315. [DOI] [PubMed] [Google Scholar]
- 28.Liberman L, Zakowski MF, Avery S, et al. Complete percutaneous excision of infiltrating carcinoma at stereotactic breast biopsy: how can tumor size be assessed? AJR Am J Roentgenol. 1999;173:1315–1322. [DOI] [PubMed] [Google Scholar]
- 29.Satake H, Shimamoto K, Sawaki A, et al. Role of ultrasonography in the detection of intraductal spread of breast cancer: correlation with pathologic findings, mammography and MR imaging. Eur Radiol. 2000;10:1726–1732. [DOI] [PubMed] [Google Scholar]
- 30.Teh W, Wilson AR. The role of ultrasound in breast cancer screening: a consensus statement by the European Group for Breast Cancer Screening. Eur J Cancer. 1998;34:449–450. [DOI] [PubMed] [Google Scholar]
- 31.Schoonjans JM, Brem RF. Sonographic appearance of ductal carcinoma in situ diagnosed with ultrasonographically guided large core needle biopsy: correlation with mammographic and pathologic findings. J Ultrasound Med. 2000;19:449–457. [DOI] [PubMed] [Google Scholar]