Abstract
Objective:
To determine the feasibility of sentinel lymph node mapping in local and in-transit recurrent melanoma.
Summary Background Data:
The accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy (LM/SL) for identification of occult lymph node metastases is well established in primary melanoma. We hypothesized that LM/SL could be useful to detect regional node metastases in patients with isolated local and in-transit recurrent melanoma (RM).
Methods:
Review of our prospective melanoma database of 1600 LM/SL patients identified 30 patients who underwent LM/SL for RM. Patients with tumor-positive sentinel nodes (SNs) were considered for completion lymph node dissection.
Results:
Of the 30 patients, 17 were men and 13 were women; their median age was 57 years (range, 29–86 years). Primary lesions were more often on the extremities (40%) than the head and neck (33%) or the trunk (8%). At least 1 SN was identified in each lymph node basin that drained an RM. Of the 14 (47%) patients with tumor-positive SNs, 11 (78%) underwent complete lymph node dissection; 4 had tumor-positive non-SNs. The median disease-free survival after LM/SL was 16 months (range, 1–108 months) when an SN was positive and 36 months (range, 6–132 months) when SNs were negative. At a median follow-up of 20 months (range, 2–48 months), there were no dissected basin recurrences after a tumor-negative SNs.
Conclusions:
LM/SL can accurately identify SNs draining an RM, and the high rate of SN metastases and associated poor disease-free survival for patients with tumor-positive SN suggests that LM/SL should be routinely considered in the management of patients with isolated RM.
The utility of lymphatic mapping and sentinel lymphadenectomy for 30 patients with local and in-transit recurrent melanoma was studied. A sentinel node was identified in all 30 patients, and at least one sentinel node was positive for metastases in 14 of the patients.
Although local recurrence in melanoma has an estimated incidence of only 3% to 7%,1–7 it is often considered a manifestation of systemic disease and thus carries a dismal prognosis. Likewise, in-transit recurrence, although uncommon, often precedes systemic disease. Urist et al3 reported a median survival of 3 years in 95 patients with local recurrence, with only 20% surviving 10 years. Roses et al5 found that 90% of patients with local metastases and 71% of patients with in-transit metastases developed systemic metastases. Median follow-up was 45 months, and systemic metastases appeared at an average of 9.7 months after local recurrence. Soong et al8 examined 1085 patients with recurrent melanoma and reported a 42% rate of 5-year survival for those with isolated local recurrence. Karakousis et al7 found that 28 of 742 patients with intermediate thickness melanoma developed local recurrence. Of these 28 patients, 82% died of progression of disease; median follow-up was 91 months. Wong et al9 reported a median survival of 19 months for 95 patients with in-transit melanoma.
The wide range of survival results makes it difficult to determine the best treatment approaches and clearly indicates the need for better definition of prognostic groups. Unfortunately, there is not much data on the incidence of nodal metastasis from locally recurrent melanoma. Dong et al1 found that 178 of 648 patients (27%) who had local recurrence of melanoma as a first event subsequently developed in-transit or lymph node metastases. Their rate of survival was only 34% at a median follow-up of 40 months.
Lymphatic mapping and sentinel lymphadenectomy (LM/SL) is a relatively simple surgical staging technique with minimal morbidity. It is central in the management of primary melanoma and may prove useful to detect occult lymph node metastases from isolated local and in-transit recurrent melanoma (RM). This study was undertaken to determine the utility of LM/SL for patients with RM.
MATERIALS AND METHODS
The prospectively collected, computer-assisted melanoma database consisting of 1600 LM/SL patients at the John Wayne Cancer Institute was reviewed to identify all patients who underwent LM/SL for RM. RM was defined as isolated dermal or subcutaneous metastases that recurred within 2 cm of the previous wide excision or outside of 2 cm but along the lymphatic drainage pathway of the primary. LM/SL for RM was undertaken only in patients who had single recurrent lesions based on clinical examination and no evidence of regional adenopathy or distant metastases. The absence of distant disease was confirmed by a complete staging workup that included magnetic resonance imaging of the brain and computed tomographic scanning of the chest, abdomen, and pelvis.
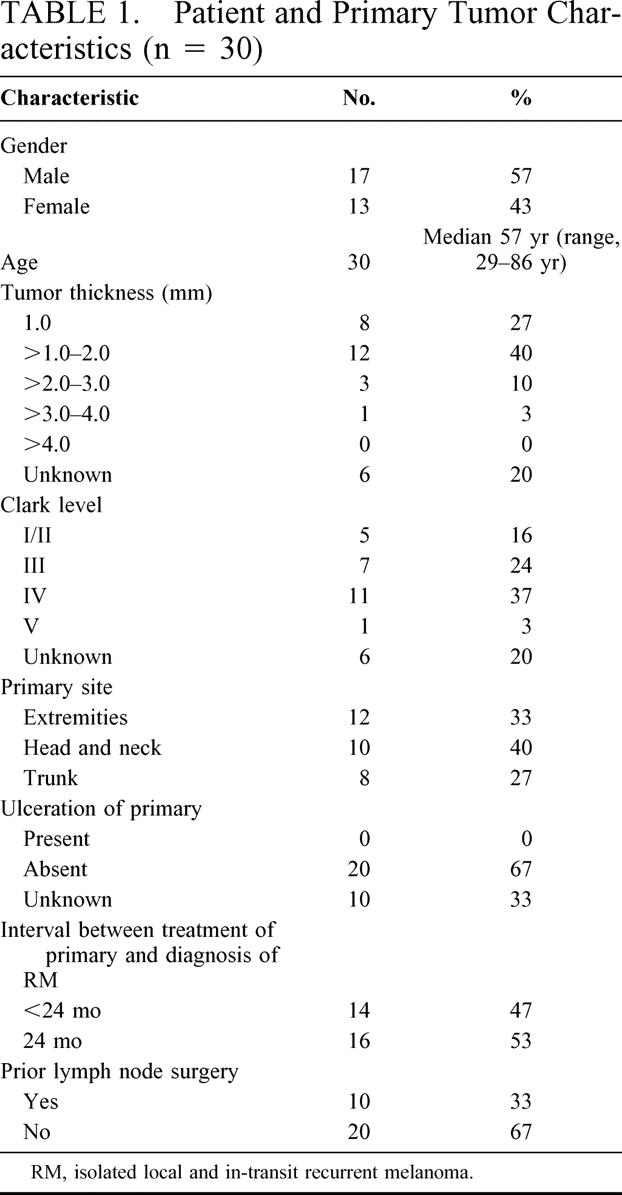
Patient age and gender, characteristics of the primary tumor (Breslow thickness, Clark level of invasion, anatomic site, and presence of ulceration), and surgical management of primary melanoma were noted from our database. The interval between treatment of the primary and diagnosis of RM was determined. The results of LM/SL for RM were recorded as the number of lymphatic drainage basins, the number of SNs, and the rate of SN positivity.
Disease-free survival was defined as the interval between treatment of RM and diagnosis of a subsequent recurrence at any site. The pattern of second recurrence was categorized as either local-regional or distant. Overall survival was defined as the interval between treatment of the primary melanoma and the most recent follow-up visit or death.
LM/SL Technique
LM/SL was performed as previously described.10–12 Briefly, all patients underwent preoperative cutaneous lymphoscintigraphy of the RM with an intradermally injected radiopharmaceutical (0.5–1.0 mCi of filtered technetium-99m sulfur colloid, injected 1–4 hours before surgery) to identify lymphatic drainage patterns and establish those basins at risk for metastasis. LM/SL was then performed with an intradermal injection of 1 to 3 mL of 1% isosulfan blue dye (Lymphazurin; Tyco International, Norwalk, CT) around the RM. A handheld gamma counter (Neoprobe 1000, 1500, or 2000; Neoprobe Corporation, Dublin, OH) was used during LM/SL to facilitate location and verification of SNs.
Each SN was excised and submitted for permanent pathologic analysis. Ten serial sections were removed. Sections 1, 3, 5, and 10 were stained by hematoxylin and eosin, section 2 was stained for S-100 protein, and section 4 was stained for HMB-45. Sections 6 and 7 were negative controls for the immunoperoxidase studies, and sections 8 and 9 were used to repeat any study that was technically unsatisfactory or for additional immunohistochemistry. Only SN-positive patients were considered for complete lymph node dissection.
RESULTS
Of the more than 1600 patients who have undergone LM/SL at the John Wayne Cancer Institute, 30 (<0.2%) underwent LM/SL of isolated RM. As shown in Table 1, most of the primary melanomas in these patients were no thicker than 2.0 mm and free of ulceration. Surgical management of the primary melanoma included not only wide local excision of the primary but also a lymph node procedure in 10 patients. Of these 10 patients, 8 underwent LM/SL and 2 underwent complete lymph node dissection (no LM/SL was done previously on these 2 patients). Nonetheless, all 10 of these patients mapped to an SN when they underwent LM/SL of their recurrence. Of the 8 patients who underwent prior LM/SL, 1 patient had a tumor-positive SN when the recurrence was mapped. Of the 2 patients who underwent prior complete lymph node dissection, 1 had 14 nodes removed and the other had 19 nodes removed. One of these 2 patients had a tumor-positive SN when the recurrence was mapped. About half of the study group developed RM within 24 months after diagnosis of primary melanoma (Table 1). Nineteen patients had recurrences within 2 cm of the previous wide excision and 11 patients had recurrences beyond 2 cm but along the expected lymphatic drainage pathway of the primary.
TABLE 1. Patient and Primary Tumor Characteristics (n = 30)
In all 30 patients, LM/SL of the RM identified at least 1 SN in each drainage basin; 18 patients (60%) had more than 1 SN per drainage basin (Table 2). At least 1 SN was positive for metastatic melanoma in 14 (47%) patients, including 1 of the 8 patients who had undergone LM/SL of the primary melanoma and 1 of the 2 patients who had undergone complete lymph node dissection of the primary melanoma. All tumor deposits in these 14 SNs were identified by hematoxylin and eosin staining. Tumor-positive SNs from RM were identified in 58% of extremity primary melanomas, 40% of head and neck primaries, and 38% of truncal primaries.
TABLE 2. Results for LM/SL of RM in 30 patients
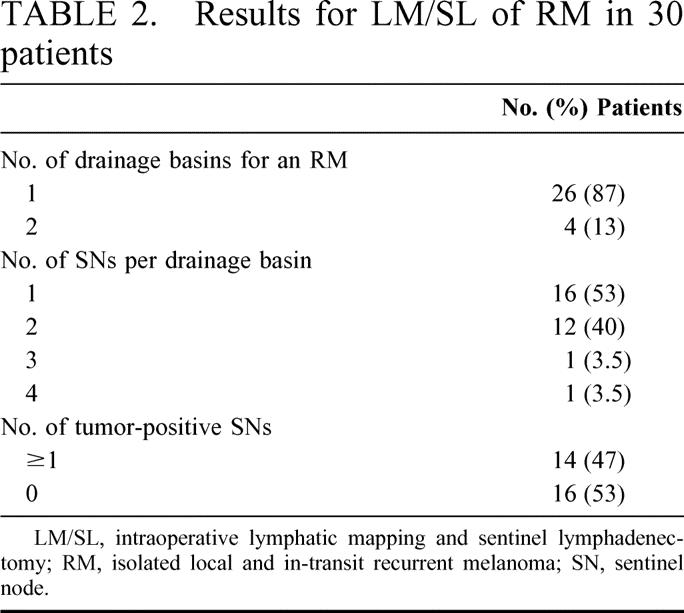
Of the 14 patients (47%) who had at least 1 tumor-positive SN, 11 underwent complete lymph node dissection and 3 patients declined further surgery. Of the 11 patients (36%) who underwent complete lymph node dissection, 4 had tumor-positive non-SNs: 1 patient had 1 positive node, 2 patients had 2 positive nodes, and 1 patient had 8 positive nodes.
The median interval between surgical treatment of the primary melanoma and diagnosis of RM was 36 months (range, 4–96 months) in the group with tumor-positive SNs and 54 months (range, 5–240 months) in the group with tumor-negative SNs (P = 0.3320) (Fig. 1). Median follow-up after treatment of RM was 20 months. Median disease-free survival was significantly shorter after tumor-positive than tumor-negative LM/SL (16 months vs. 36 months, P = 0.0251) (Fig. 2). Of the 14 patients who had a tumor-positive SN, 2 developed further local recurrence, 2 developed distant recurrence, and 10 remained free of disease. Among the 16 patients with tumor-negative SNs, 2 had local recurrences, 3 had distant metastases, and 10 remained disease free. None of the patients with tumor-negative SNs developed a recurrence in the dissected basin. With our relatively short follow-up period, there appears to be no difference in overall survival between the tumor-positive and tumor-negative patient groups.
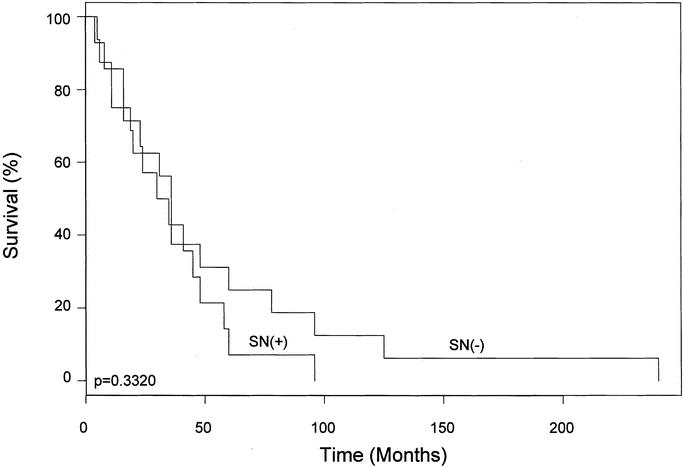
FIGURE 1. Interval between surgical treatment of primary melanoma and diagnosis of RM, according to the tumor status of the SN draining the recurrence. RM, isolated local and in-transit recurrent melanoma; SN, sentinel node.
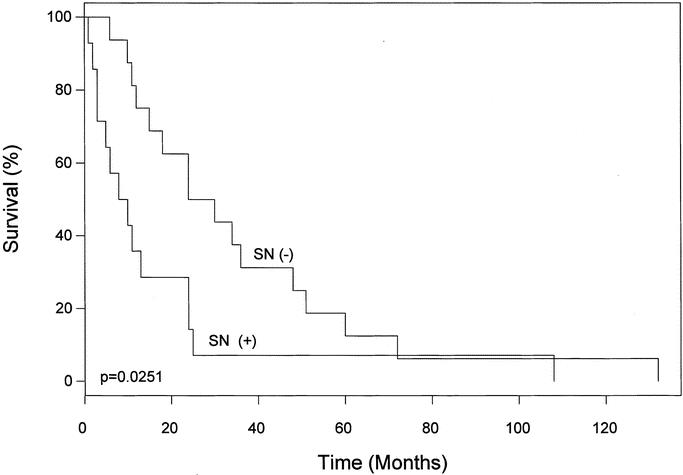
FIGURE 2. Disease-free survival after LM/SL for RM, according to the tumor status of the SN draining the recurrence. LM/SL, intraoperative lymphatic mapping and sentinel lymphadenectomy; RM, isolated local and in-transit recurrent melanoma; SN, sentinel node.
DISCUSSION
The staging accuracy of LM/SL has been well studied in primary melanoma, but its accuracy and utility for recurrent disease is largely unknown. Previous studies of LM/SL excluded patients who had undergone prior surgery in a lymph node basin,14–16 based on the assumption that prior surgery might complicate identification of the SN or disrupt lymphatic channels from the recurrent site. Studies have shown that previous wide skin excision (not including rotational flaps) does not affect the ability to map an SN, but there are no studies examining the effect of previous lymph node basin surgery on sentinel lymph node mapping.17,18 However, our results indicate that neither wide excision of the primary melanoma nor prior surgery on the same lymph node basin hinders lymphatic mapping from RM. We were able to identify a blue and/or radioactive SN in all 30 patients, including the 10 patients who had undergone prior lymph node sampling of the same drainage basin, 2 of whom had a complete lymph node dissection and 8 of whom had LM/SL. However, the fact that RM metastasized to nodes in the same basin that drained the primary melanoma confirms the tendency of melanoma to spread initially via the lymphatic system and underscores the importance of accurately staging the regional lymph nodes. Although 85% of melanomas spread via lymphatics, approximately 15% of melanomas will disseminate using the hematogenous route to seed organ sites. In the scenario of hematogenous spread, LM/SL would not be helpful. Although 53% of our patients had tumor-negative SNs, the false-negative rate of LM/SL was zero.
Lymphoscintigraphy identified a single drainage basin for most (87%) of the RMs in our study. The lymphatic drainage pathways from RM were predictable: lower and upper extremity lesions drained to the groin and axillary nodes, respectively, whereas head and neck lesions drained to the cervical or preauricular/postauricular areas. Fifty-three percent of RMs drained to 1 SN per basin and 40% drained to 2 SNs per basin. By contrast, studies of LM/SL for primary melanoma11,14 have shown that approximately 70% of primary tumors drain to 1 SN and 20% to 30% drain to 2 SNs. We were quite surprised that LM/SL was very similar in primary and RM. The slightly higher number of SNs identified may relate to the larger subcutaneous metastases in which LM/SL was performed.
In our study, 47% of patients with isolated recurrences already had regional metastases. Fourteen patients had positive SNs, and 11 of these patients underwent complete lymph node dissection. Tumor-positive non-SNs were identified in 4 complete lymph node dissection specimens, but only 1 specimen contained more than 2 positive nodes. This high percentage of patients with occult nodal involvement may help explain why the prognosis for RM is so poor. Indeed, the disease-free survival following treatment of RM was 16 months when the SN contained tumor and 36 months when the SN was tumor free (P = 0.0251). This difference in survival suggests the importance of early, accurate assessment of the regional nodes in patients with RM. In addition, the interval before RM was 36 months in those with a positive SN versus 54 months in those with a negative SN. This difference, although not reaching statistical significance, suggests that SN-positive patients tend to recur sooner and are more likely to have aggressive disease. With LM/SL we can identify those with RM whose prognosis is more favorable and who may benefit from further surgery. Longer follow-up and more patients are needed before we can determine if LM/SL of RM can definitively provide survival benefit.
Another benefit of LM/SL for RM is more accurate staging. In the new classification system, those with in-transit metastases without metastatic nodes are classified as N2, whereas those with metastatic nodes are classified as N3. LM/SL in patients presenting with RM will help classify disease as N2 or N3 according to the new TNM classification system.19 The survival differences between these 2 groups are substantial and further demonstrate the importance of LM/SL in differentiating patients with in-transit disease into favorable and unfavorable prognostic groups.19,20
Several prognostic factors of the primary melanoma have been proposed to predict survival after local recurrence. Soong et al8 found that tumor thickness and lesion location were significant for survival following local recurrence. Dong et al1 demonstrated that Breslow depth, Clark level, and ulceration of the primary were associated with poor prognosis after local recurrence. Our study group was too small for a multivariate analysis of prognostic factors; however, of the primary melanomas we have data on, none were ulcerated and the median Breslow depth associated with a positive SN was 1.48 mm.
Our findings indicate that LM/SL can accurately detect nodal metastases for a single RM, but it also may be feasible for patients with more than 1 RM. This issue deserves particular attention since many patients with in-transit disease present with multiple lesions. We do not have a large experience with lymphatic mapping of multiple in-transit lesions because it is difficult to determine the optimal site for injection of tracer. One strategy might be to inject isosulfan blue dye and radiopharmaceutical tracer at each site, remove the SN, and then proceed to the next lesion. However, we have little data to support this approach. Isolated limb perfusion has been shown to give very effective local-regional control of extensive extremity disease; thus, patients with multiple lesions are probably better candidates for isolated limb perfusion or alternative therapies. Fortunately, LM/SL of primary melanoma carries little morbidity and patients experience few side effects from LM/SL.
Current management of RM includes wide local excision, isolated hyperthermic limb perfusion, local ablation, local immunotherapy, or chemotherapy; however, none of these treatment strategies is associated with an unequivocal survival benefit. At present, the FDA-approved treatment of stage III disease is interferon therapy; however, trials looking at interferon therapy excluded our study group. Of our patients with a tumor-positive SN who underwent complete lymph node dissection, a majority were subsequently enrolled in an experimental immunotherapy protocol at our institute testing a polyvalent allogeneic vaccine. Moreover, they all underwent a metastatic staging workup, which did not reveal any disseminated disease. If immunotherapy fails, then more aggressive therapy in the form of isolated limb perfusion, biologic therapy, or systemic chemotherapy may be appropriate but must be individualized for each patient. The rationale for LM/SL in these patients is to gain control of the local-regional disease without subjecting all the patients to a complete lymph node dissection. The selective approach allows us to spare our patients the morbidity of complete lymph node dissections, which may be significant in this particular group of patients, although many will ultimately have isolated limb perfusion. LM/SL provides a simple and accurate means to detect occult lymph node metastases from RM, and it should be included in the management of these patients.
Footnotes
Presented at the 55th Annual Cancer Symposium of the Society of Surgical Oncology, March 14–17, 2002, Denver, CO.
Supported by grant CA29605 from the National Cancer Institute and by funding from the Harold McAlister Charitable Foundation (Los Angeles, CA) the Amyx Foundation, Inc (Boise, ID), and Mrs. Alice Johnson McKinney.
Reprints: Donald Morton, MD, John Wayne Cancer Institute, 2200 Santa Monica Boulevard, Santa Monica, CA 90404. E-mail: mortond@jwci.org.
REFERENCES
- 1.Dong XD, Tyler D, Johnson JL, et al. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer. 2000;88:1063–1071. [DOI] [PubMed] [Google Scholar]
- 2.Cohn-Cedermark G, Mansson-Brahme E, Rutqvist LE, et al. Outcomes of patients with local recurrence of cutaneous malignant melanoma. Cancer. 1997;80:1418–1425. [PubMed] [Google Scholar]
- 3.Urist MM, Balch CM, Soong S, et al. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985;55:1398–1402. [DOI] [PubMed] [Google Scholar]
- 4.Wildemore JK, Schuchter L, Mick R, et al. Locally recurrent malignant melanoma characteristics and outcomes: a single institution study. Ann Plast Surg. 2001;46:488–494. [DOI] [PubMed] [Google Scholar]
- 5.Roses DF, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision for primary cutaneous malignant melanoma. Ann Surg. 1988;198:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6:315–321. [DOI] [PubMed] [Google Scholar]
- 7.Karakousis CP, Balch CM, Urist MM, et al. Local recurrence in malignant melanoma: long term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol. 1996;3:446–452. [DOI] [PubMed] [Google Scholar]
- 8.Soong S, Harrison RA, McCarthy WH, et al. Factor affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol. 1998;67:228–233. [DOI] [PubMed] [Google Scholar]
- 9.Wong J, Cagle L, Kopald KH, et al. Natural history and selective management of in transit melanoma. J Surg Oncol. 1990;44:146–150. [DOI] [PubMed] [Google Scholar]
- 10.Morton DL, Chan AD. Current status of intraoperative lymphatic mapping and sentinel lymphadenectomy for melanoma: is it standard of care? J Am Coll Surg. 1999;189:214–233. [DOI] [PubMed] [Google Scholar]
- 11.Morton D, Wen D, Wong J, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. [DOI] [PubMed] [Google Scholar]
- 12.Essner R, Bostick PJ, Glass EC, et al. Standardized probe-directed sentinel node dissection in melanoma. Surgery. 2000;127:26–31. [DOI] [PubMed] [Google Scholar]
- 13.Deleted in proof.
- 14.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early stage melanoma. Ann Surg. 1999;230:453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer. N Engl J Med. 1998;339:941–946. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karakousis C, Grigoropoulos P. Sentinel node biopsy before and after wide excision of the primary melanoma. Ann Surg Oncol. 1999;6:785–789. [DOI] [PubMed] [Google Scholar]
- 18.Keleman P, Essner R, Foshag L, et al. Lymphatic mapping and sentinel lymphadenectomy after wide local excision of primary melanoma. J Am Coll Surg. 1999;189:247–252. [DOI] [PubMed] [Google Scholar]
- 19.Balch CM, Buzaid AC, Soong S, et al. Final version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. J Clin Oncol. 2001;19:3635–3648. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Soong S, Gershenwald JE, et al. Prognostic factors analysis of 17, 600 melanoma patients validation of the American Joint Committee on Cancer Melanoma Staging System. J Clin Oncol. 2001;19:3622–3634. [DOI] [PubMed] [Google Scholar]


