Abstract
Background:
Hematopoietic failure has been observed in experimental animals following shock and injury. In humans, bone marrow dysfunction has been observed in the red cell component and characterized by a persistent anemia, low reticulocyte counts, and the need for repeated transfusions despite adequate iron stores. While a quantitative defect in white blood cell count has not been noted, an alteration in white blood cell function manifesting as an increased susceptibility to infection is well established. Since the etiology of this anemia remains unknown and the bone marrow has been rarely studied following injury, we measured various parameters of hematopoiesis directly using bone marrow from trauma patients and tested the hypothesis that trauma results in profound bone marrow dysfunction, which could explain both the persistent anemia and the alteration in white blood cell function.
Methods:
Bone marrow aspirates and peripheral blood were obtained between day 1 and 7 following injury from 45 multiple trauma patients. Normal volunteers served as controls. Peripheral blood was assayed for hemoglobin concentration, reticulocyte count, erythropoietin levels, white blood cell count, and differential. Peripheral blood and bone marrow were cultured for hematopoietic progenitors (CFU-GM, BFU-E, and CFU-E colonies).
Results:
Bone marrow CFU-GM, BFU-E, and CFU-E colony formation was significantly reduced while peripheral blood CFU-GM, BFU-E, and CFU-E was increased in the trauma patients compared with normal volunteers. Bone marrow stroma failed to grow to confluence by day 14 in >90% of trauma patients. In contrast, bone marrow stroma from volunteers always reached confluence between days 10 and 14 in culture. The mean hemoglobin concentration and reticulocyte counts of the trauma patients were 8.6 ± 1.0 g/dL and 2.75 ± 0.7% respectively, while their plasma erythropoietin levels were 2 to 10 times greater than control values.
Conclusions:
Release of immature white blood cells into the circulation may also contribute to a failure to clear infection and an increased propensity to organ failure. Concomitantly, profound changes occur within the bone marrow, which include the increased release of erythroid and myeloid progenitors into the circulation, a decrease in progenitor cell growth within the bone marrow, and an impaired growth of the bone marrow stroma. Erythropoietin levels are preserved following trauma, implying that the persistent anemia of injury is related to the failure of the bone marrow to respond to erythropoietin.
Hematopoietic failure has been observed in experimental animals following shock and injury. Bone marrow aspirates were obtained in 45 multiply injured patients. Suppression of growth in the hematopoietic precursor cell compartment and in the bone marrow stroma was observed in all patients.
Trauma remains a significant public health issue and is the leading cause of death in persons younger than 40 years.1 Improvements in trauma systems and trauma centers have improved the early survival in severely injured patients sustaining profound hemorrhagic shock. Late death following these injuries is usually associated with infection, sepsis, and the development of the multiple organ dysfunction syndrome.2 Bone marrow failure is one facet of the multiple organ dysfunction syndrome and is commonly seen in patients recovering from severe trauma and hemorrhagic shock.3 Overt and clinically obvious bone marrow failure is most often observed in the erythroid compartment and is manifested as a persistent anemia associated with a low reticulocyte count, despite adequate iron stores and plasma erythropoietin levels.4,5 Blood replacement in these patients is often necessary, and trauma is one of the most common indications for transfusion. Consequently, these patients often require repeated blood transfusions, which exacerbate injury-induced immune suppression, are costly, carry the risk of infectious disease transmission, and have been associated with a worsening of organ failure.6,7
Injury-associated anemia has long been thought to be due to external events such as blood loss from operative procedures, repeated phlebotomies, the reduced half-life of banked blood, and iatrogenic nutritional deficiencies. Accelerated red blood cell destruction has also been noted following thermal injury.8 The need for ongoing transfusion in the trauma patient is common and occurs long after the acute injury and resuscitation have ended. For example, Corwin et al9 reported that >85% of patients who are in the ICU for more than 1 week required weekly transfusions, and Groeger et al10 found that on any given day 14% of all ICU patients receive transfusions. Most of these transfusions are not associated with any discernible blood loss. These patients undergo no operative procedures and futher suggests that post traumatic anaemia is due to more than just blood loss.5 Corroborating these data, we found that in an internal review in our Surgical Trauma Intensive Care Unit >80% of the trauma patients received weekly blood transfusions despite a current practice of accepting lower hemoglobin levels.
Stimulation of erythropoiesis requires the production of erythropoietin. Plasma erythropoietin levels have not been well described following blunt and penetrating trauma and remain largely unknown. Erythropoietin levels have been found to be elevated after thermal injury4 and spinal cord injury.5 In contrast, low circulating erythropoietin levels were found in a population of predominantly medical ICU patients.9 Thus, a measurement of circulating erythropoietin levels after major trauma is necessary to adequately evaluate patients with posttraumatic anemia.
The bone marrow is also the source of immune cells; thus, an alteration of bone marrow hematopoiesis will increase the patient’s susceptibility to infection and sepsis following shock and injury. In contrast to a quantitative defect in red blood cell formation, bone marrow failure following injury may be more related to qualitative changes in immune cell maturation. Recently, Santangelo et al reported that the bone marrow is driven to monocytopoieis following thermal injury and infection in mice.11 Additionally, Moore et al demonstrated a decreased ability of peripheral blood mononuclear cells to support bone marrow growth in patients following severe torso trauma.12 Thus, although trauma appears to be associated with bone marrow failure, the mechanisms by which this occurs remain to be fully determined. There exist only a few reports directly examining bone marrow hematopoiesis following mechanical injury.13–15 None of these reports focused solely on trauma patients, nor did they assess bone marrow hematopoiesis in a systematic fashion. Thus, the goals of the current investigations were to assess hematopoiesis in multiply injured patients admitted to the SICU by evaluating the effect of major trauma on the growth of bone marrow erythroid progenitor cells and stromal elements as well as to begin to investigate the mechanisms accounting for bone marrow failure following injury.
PATIENTS AND METHODS
Study Subjects
Patients admitted to the Surgical Intensive Care Unit or the operating room after severe injury were eligible for inclusion. Patients were excluded from consideration if they were likely to leave the SICU or die within 3 days of admission, had a history of hematologic diseases or preexisting anemia, had active HIV infection, or had a history of renal or liver failure. Informed consent was obtained from each donor according to the guidelines of the Institutional Review Board of UMDNJ-New Jersey Medical School. Peripheral blood was assayed for hemoglobin levels and reticulocyte counts and was cultured for hematopoietic progenitor cell growth. Plasma was obtained for determination of circulating erythropoietin levels. Bone marrow aspirates were obtained from the iliac crest and placed into preservative-free heparin. Samples were drawn from patients at various times after initial trauma beginning at 12 hours up to 7 days. The timing of sampling was day 1 in 2 patients, day 2 in 12, day 3 in 5, day 4 in 7, day 5 in 8, day 6 in 3, and day 7 in 6. Only 1 sample was obtained from each patient. The mean and median day of sampling was 4 and 2, respectively. The demographics and the severity of injury for the patients are shown in Table 1. Bone marrow aspirates and peripheral blood from age- and gender-matched normal volunteers (n = 4–8 per experiment) were used as controls. These volunteers are on no medications and have no hematologic diseases.
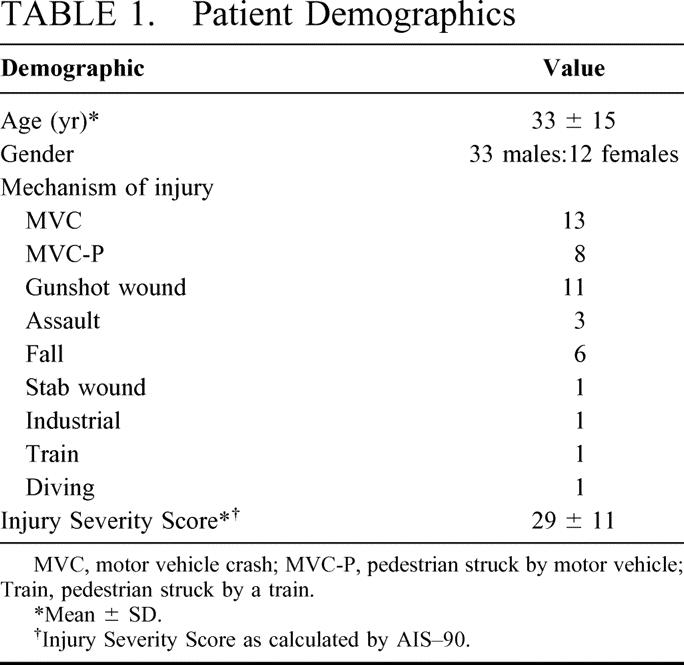
TABLE 1. Patient Demographics
Erythropoietin Levels
Erythropoietin levels in plasma were determined in duplicate using a commercial ELISA kit (R&D systems, Minneapolis, MN). Briefly, 100 μL of assay diluent was added to each well of the ELISA plate (precoated with murine monoclonal antihuman erythropoietin antibodies) followed by the addition of 100 μL of the standard, control, or patient plasma in duplicate. The plates were incubated at room temperature for 2 hours, and following decanting and washing, the antigens were incubated for 2 hours with polyclonal antihuman erythropoietin antibodies conjugated to peroxidase. After multiple washings, 200 μL of substrate solution (tetramethylbenzidine and hydrogen peroxide) was added to each well and the optical density measured at wavelength of 450 nm using an EL 311s microplate reader (Bio-Tek Instruments Inc., Winooski, VT).
Bone Marrow Histology
Bone marrow smears were prepared from the initial 0.1 mL of bone marrow aspirate. Slides were allowed to air dray and then stained with Wright-Giemsa. Slides were examined and bone marrow cells morphology was determined a single observer (V.C.) blinded to the patient’s history or other culture data.
Hematopoietic Progenitor Cell Cultures
Low density bone marrow or peripheral blood mononuclear cells mononuclear cells were separated by Ficoll-Hypaque density gradient (Pharmacia LKB Biotechnology, Piscataway, NJ) and then resuspended in RPMI 1640 (Sigma) containing 10% fetal calf serum (Hyclone Laboratories, Logan, UT). Peripheral blood or bone marrow mononuclear cells (1 × 105) were plated in duplicate in Iscoves media containing 30% fetal calf serum, 2% bovine serum albumin, 1% methylcellulose, 2 × 10-4 mol/L 2-ME and glutamine (Cellgro; Mediatech, Herndon, VA) supplemented with 1.3 U/mL rhEpo and 6 U/mL rhIL-3 (Genetics Institute, Cambridge, MA) for BFU-E/CFU-E or 3 U/mL rhGM-CSF for CFU-GM. Cultures were incubated at 37°C in 5% CO2. CFU-E colonies with >20 cells were enumerated at day 7; CFU-GM and BFU-E colonies were counted at day 15 by an observer who was blinded to the origin of the samples.
Bone Marrow Stromal Cultures
Unseparated bone marrow cells (1 × 107) were suspended in 5 mL of stromal media and added to 25 cm2 Falcon 3109 tissue culture flasks (Becton Dickinson, Franklin Lakes, NJ). Stomal medium consisted of: α-MEM (Life Technologies, Grand Island, NY), 12.5% fetal calf serum, 12.5% horse serum (Hyclone Laboratories), 0.1 μmol/L hydrocortisone, 0.1 mmol/L 2-ME, and 1.6 mmol/L glutamine. Cultures were incubated at 33°C for 3 days. On day 3, mononuclear cells were isolated from the nonadherent cell population by Ficoll-Hypaque density gradient and then replaced into the original culture flasks. Stromal cultures were reincubated until the adherent cells were confluent. Throughout the culture period, 50% of the stromal media were replaced at weekly intervals. Bone marrow stromal growth was assessed as the percent confluence on days 14, 21, and 40.
Statistical Analysis
All data are expressed as mean ± SD. Statistical analyses were performed using a Student’s t test or analysis of variance and Tukey-Kramer multiple comparisons test where appropriate. A P value of <0.05 was considered significant.
RESULTS
Histology
Examination of the bone marrow aspirates revealed moderate to severely decreased cellularity in 65% of trauma patients studied. In addition, there was a general shift of the marrow to more immature forms. The myeloid to erythroid ratio, which was close to normal (between 1 and 2) on postinjury day 2, increased markedly over the first week following injury (Fig. 1).
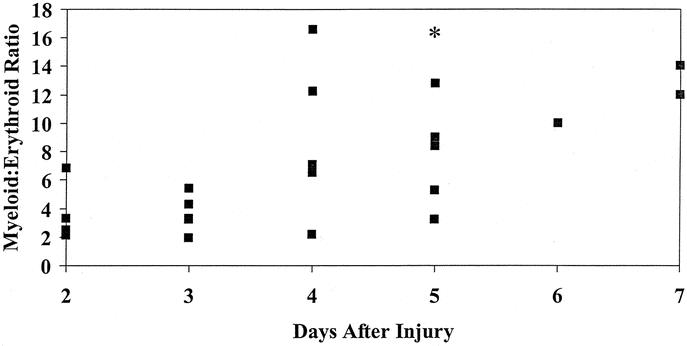
FIGURE 1. Bone marrow myeloid to erythroid (M:E) ratio plotted against the day after injury (n = 22). There is a marked increase from control values (between 1 and 2) as the time progresses after injury. *M:E ratio of 45 for an additional patient.
Hematopoietic Progenitor Growth
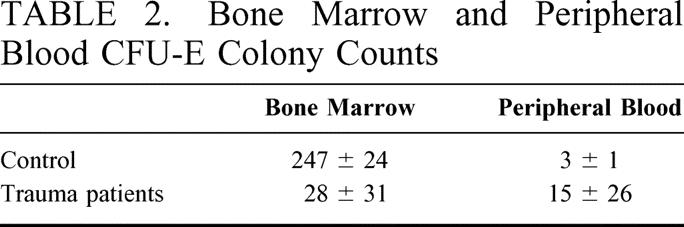
The data for bone marrow and peripheral blood BFU-E and CFU-GM growth are shown in Figure 2. These figures clearly demonstrate that severe injury results in a significant decrease in bone marrow progenitor growth compared with normal volunteers. In addition, there is a concomitant elevation in peripheral blood BFU-E and CFU-GM. The differences in bone marrow CFU-E compared with BFU-E growth in the trauma patients and controls were even more striking (Table 2). Bone marrow CFU-E growth was 34 ± 53 colonies per plate in the trauma patients compared with 229 ± 25 colonies per plate in normal controls (P < 0.05). In the peripheral blood of the trauma patients, there were 19 ± 20 CFU-E per plate compared with 0 to 5 colonies/plate in the control population (P < 0.05). These data suggest that the decreased progenitor cell growth within the bone marrow compartment is associated with progenitor cell released from the bone marrow into the peripheral circulation after trauma. These changes appear to become more profound as the cells reach the state of terminal differentiation, since the more mature CFU-E progenitor cells are inhibited to a greater extent than the BFU-E.

FIGURE 2. Bone marrow and peripheral blood BFU-E and CFU-GM colony formation from trauma patients (n = 45) and normal volunteers (n = 12). All trauma patients were admitted to the SICU and the mean ISS was 29. The mean and median day of sampling was 4 days and 2 days, respectively. Volunteers were matched to age and gender. *P < 0.05 versus control values.
TABLE 2. Bone Marrow and Peripheral Blood CFU-E Colony Counts
Bone Marrow Stromal Growth
The bone marrow stroma of the control volunteers (n = 10) reached confluence between days 10 and 14. In contrast, trauma severely inhibited bone marrow stroma growth as only 1 of the 45 patients grew to confluence by day 10 and stroma in 6 additional patients achieved confluence between days 16 and 28. Stroma from 7 trauma patients had only minimal growth by day 40. In 5 patients, the stroma became contaminated and was discarded. In the remaining patients (n = 26), the mean percent confluence at days 14, 21, and 40 was 33%, 52%, and 71%, respectively.
Erythropoietin Levels After Injury
The mean erythropoietin level in healthy volunteers controls (n = 8) with hemoglobin concentrations in the normal range (13–15 g/dL) was 12 ± 2 units/mL. Simultaneous measurement of hemoglobin and erythropoietin in the trauma patients demonstrate that erythropoietin levels were significantly increased over control levels after injury (Fig. 3). Although the mean hemoglobin level of the trauma patients was 8.3 ± 1.0 g/dL (range 7.0–10.2 g/dL) and the erythropoietin levels were significantly elevated, the mean reticulocyte count of this patient population was only slightly elevated 2.6% ± 0.7% (normal <2%). This inappropriately low level of reticulocytosis in the face of elevated circulating erythropoietin levels indicates that there is a defect in the production of red blood cells by the bone marrow in these anemic trauma patients.
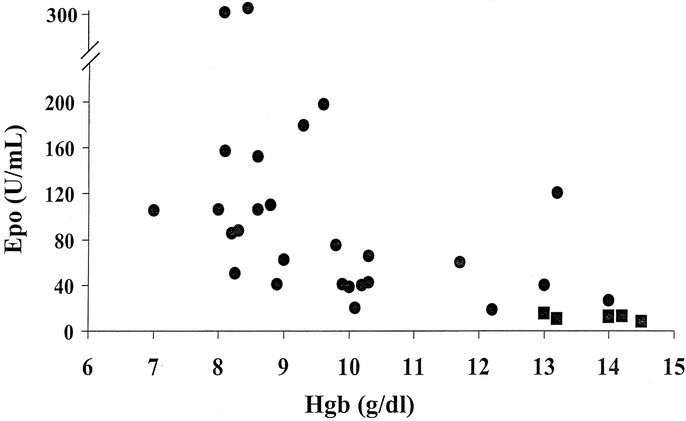
FIGURE 3. Plasma erythropoietin levels plotted against the hemoglobin concentrations. The circles (•) represent values from individual trauma patients and the squares (▪) represents the values from normal volunteers (n = 6).
Exogenous Erythropoietin
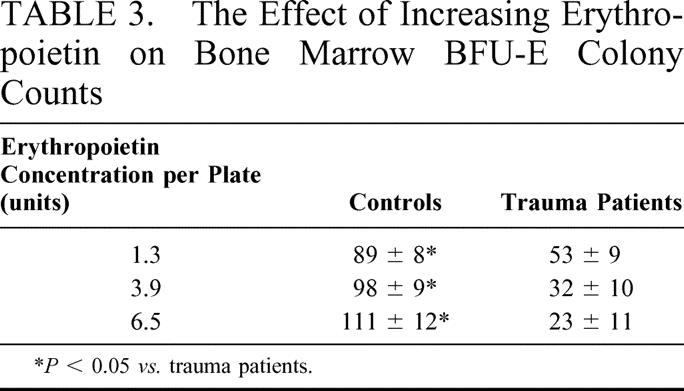
While circulating plasma erythropoietin levels were increased after injury, we investigated whether these increased erythropoietin levels were insufficient to stimulate erythropoiesis after injury, by growing trauma bone marrow in the presence of increasing concentrations of erythropoietin from 1.3 units (standard for the growth of normal bone marrow) to 6.5 units of erythropoietin per plate (n = 6–8). Increasing the concentration of the erythropoietin in the culture media did not increase BFU-E growth and in fact actually resulted in a 38% decline in BFU-E colony numbers at the highest erythropoietin concentration tested (Table 3). In contrast, bone marrow from normal volunteers (n = 4) had an increase in BFU-E colonies as the concentration of erythropoietin increased.
TABLE 3. The Effect of Increasing Erythropoietin on Bone Marrow BFU-E Colony Counts
DISCUSSION
The data presented here demonstrate that trauma induces profound alterations in hematopoiesis within the bone marrow. These observations are novel, and the depression of both bone marrow progenitor cell and stromal growth following nonthermal injury has not been previously reported in humans. Plasma erythropoietin levels were significantly elevated compared with control values and were similar to levels observed in patients following thermal injury.4 These plasma levels were also higher than what was reported by Corwin et al in a population of patients from a mixed medical-surgical ICU.9 In that study, only 15% of patients were trauma victims; thus, the lower levels of circulating erythropoietin may be due to the predominance of medical patients with noninjury-related diagnoses. Furthermore, the mean hemoglobin level was considerably higher (10.1 ± 1.3 g/dL) than the values reported here (8.3 ± 1.0 g/dL). While the plasma erythropoietin levels in the trauma patients reported in this series were elevated, especially compared with normal volunteers, the values were below erythropoietin levels that have been described for patients with similar hemoglobin levels secondary to iron deficiency anemia.16 Thus, it remains possible that even higher erythropoietin levels are necessary to drive hematopoiesis following injury. This possibility is unlikely, however, as the addition of exogenous erythropoietin to cultures of trauma bone marrow not only failed to increase BFU-E growth but appeared to have a suppressive effect.
Trauma resulted in a marked rise in a number of erythroid progenitor cells in peripheral blood. A similar rise in myeloid progenitors has been found in the circulation of patients following major torso trauma and surgical trauma.12 This egress of progenitor cells from the bone marrow to the periphery could deplete the bone marrow of a significant population of hematopoietic precursors, which could decrease erythropoietic activity, ie, there are less cells available to mature into erythrocytes. This hypothesis is supported from the histologic examination of the marrow that disclosed moderate to severe decreased cellularity in 65% of patients studied. The myeloid shift in the bone marrow may also explain why there is a greater quantitative defect in circulating red blood cells as compared with white cells.
Bone marrow stromal cells are necessary for successful hematopoiesis, and the data presented demonstrate a marked decrease in stromal growth. One mechanism by which bone marrow stromal cells regulate hematopoiesis includes the production of extracellular matrix proteins. ECM proteins and glycosaminoglycans interact with adhesion molecules expressed on progenitor cells and are important in several facets of erythropoiesis, including the retention of immature hematopoietic cells within the bone marrow, the maintenance of progenitor cell survival, and the support of hematopoiesis.17,18 The failure of the bone marrow stroma from the trauma patients to grow normally could result in an alteration in the extracellular matrix and could have contributed to the egress of cells from the bone marrow into the periphery.
Bone marrow stroma also influence hematopoiesis by the production of regulatory soluble factors that can exert either positive or negative effects on the development of blood cells.19–21 Trauma has been shown to alter the production of numerous regulatory cytokines that have hematopoietic activity.22 As several cytokines such as GM-CSF and IL-3, in addition to erythropoietin, can modulate erythropoiesis, it is possible that bone marrow failure observed after trauma could be due in part to the lack of pro-regulatory cytokines within the bone marrow microenvironment. Moore et al found a decreased production of myeloid colony stimulating factors by monocytes from patients following multiple injury.12 Cytokines inhibitory to hematopoiesis such as TGF-β123,24 have also been reported to be elevated following severe trauma,25,26 and it is possible that the bone marrow failure observed is due to an overexpression of negative hematopoietic regulators. Recently, we have reported that plamsa obtained from trauma patients can induce TGF-β in normal bone marrow stromal cultures.27
The degree of immune suppression following mechanical injury and surgical trauma has been associated with the magnitude of the injury sustained, ie, the greater the injury, the greater the immune suppression.12,28 In this small ample of 45 patients, it is impossible to determine which, if any, factors are most responsible for the observed bone marrow failure. Furthermore, the patient group was all severely injured as measured by ISS. Ninety-one percent were in shock as measured by an elevated lactate or base deficit and 80% required blood transfusions in the first 24 hours. Even so, there appeared some interesting differences between certain of the patients in this series. In 3 patients, bone marrow BFU-E levels were equivalent to control and the peripheral blood BFU-E colony numbers were 0, 6, and 4 colonies per plate, respectively. These 3 patients appeared to sustain lesser degrees of soft tissue injury (isolated severe head injury, near-amputation of an upper extremity, and a C6 incomplete central cord syndrome) without associated shock compared with the other patients who sustained more extensive injuries. While this observation is based only upon 3 patients, it raises the possibility that the extent of the bone marrow failure may also be related to both the magnitude and type of injuries sustained. In addition, while the bone marrow progenitor cell growth was depressed, bone marrow stroma from 5 of the 12 women (2 of the stromal cultures were contaminated) reached confluence on days 16, 17, 24, 28, and 30. This rate and degree of bone marrow growth appear to be greater than what was observed in male patients. These data bring up the possibility that bone marrow function after injury is potentially modulated by gender and sex steroids. Indeed, estrogen have been shown to influence hematopoiesis in other clinical situations.29,30 Further samples from a larger patient population will be required before any statements can be made as to what effect specific injury type, overall injury severity, or gender play on bone marrow failure following trauma.
In summary, the data presented demonstrate that severe trauma results in marked alterations in bone marrow hematopoiesis. These changes appear within 12 hours of admission and persist for at least 1 week following injury. We think that these data represent a unique and novel observation and that bone marrow failure after major injury could explain the persistent anemia of trauma and need for repeated blood transfusions documented in this patient population. The failure of exogenous erythropoietin to improve posttraumatic BFU-E growth in vitro provides a note of caution to studies evaluating the early administration of recombinant erythropoietin to trauma patients to improve hematopoiesis. Ongoing studies into the molecular mechanism involved in posttraumatic hematopoiesis are needed to better target potentially successfully therapeutic strategies and reduce reliance on blood transfusion.
Footnotes
Reprints: David H. Livingston, MD, University Hospital E-245, 150 Bergen Street, Newark, NJ 07103. E-mail:livingst@umdnj.edu.
REFERENCES
- 1.Baker SP. Injuries: the neglected epidemic: Stone Lecture, 1985 American Trauma Society Meeting. J Trauma. 1987;27:343–348. [PubMed] [Google Scholar]
- 2.Moore FA, Haenel JB, Moore EE, et al. Incommensurate oxygen consumption in response to maximal oxygen availability predicts postinjury multiple organ failure. J Trauma. 1992;33:58–67. [DOI] [PubMed] [Google Scholar]
- 3.Heckbert SR, Vedder NB, Hoffman W, et al. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–552. [DOI] [PubMed] [Google Scholar]
- 4.Deitch EA, Sittig KM. A serial study of the erythropoietic response to thermal injury. Ann Surg. 1993;217:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaziri N, Eltorai I, Segal J, et al. Erythropoietin profile in spinal cord injured patients. Arch Phys Med Rehabil. 1993;74:65–67. [PubMed] [Google Scholar]
- 6.Greenburg AG. Benefits and risks of blood transfusions in surgical patients. World J Surg. 1996;20:1189. [DOI] [PubMed] [Google Scholar]
- 7.Valeri CR, Crowley JP, Loscalzo J. The red cell transfusion trigger: has the sin of commission now become a sin of omission? Transfusion. 1998;38:602–607. [DOI] [PubMed] [Google Scholar]
- 8.Loebl E, Baxter C, Curreri P. The mechanism of erythrocyte destruction in the early post-burn period. Ann Surg. 1973;178:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corwin H, Gettinger A, Rodriguez R, et al. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346–2350. [DOI] [PubMed] [Google Scholar]
- 10.Groeger J, Guntupalli K, Strosberg M, et al. Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization. Crit Care Med. 1993;21:279–291. [DOI] [PubMed] [Google Scholar]
- 11.Santangelo S, Gamelli R, Shankar A. Myeloid commitment shifts toward monocytopoiesis after thermal injury and sepsis. Ann Surg. 2001;233:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore FA, Peterson VM, Moore EE, et al. Inadequate granulopoiesis after major torso trauma: a hematopoietic regulatory paradox. Surgery. 1990;108:667–675. [PubMed] [Google Scholar]
- 13.Amos R, Deane M, Ferguson C, et al. Observations on the hemopoietic response to critical illness. J Clin Pathol. 1990;43:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andes W, Rogers P, Beason J, et al. The erythropoietin response to the anemia of thermal injury. J Lab Clin Med. 1976;88:584–592. [PubMed] [Google Scholar]
- 15.Lelkes G, Bernat I. Electron microscopical study of the bone marrow of burned patients. Haematologia. 1970;4:295–301. [PubMed] [Google Scholar]
- 16.Rogiers P, Zhang H, Leeman M, et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 1997;23:159–162. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen K, Kravitz J, Kincade P, et al. Adhesion receptors on bone marrow stromal cells: in vivo expression of vascular cell adhesion molecule-1 by reticular cells and sinusoidal endothelium in normal and gamma-irradiated mice. Blood. 1996;87:73–78. [PubMed] [Google Scholar]
- 18.Koopman G, Keehnen R, Lindhout E, et al. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994;152:3760–3764. [PubMed] [Google Scholar]
- 19.Sudo T, Ito M, Ogawa Y, et al. Interleukin 7 production and function is stromal cell-dependent. J Exp Med. 1989;170:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray R, Furlonger C, Williams D, et al. Characterization of thymic stromal-derived lymphopoiesis (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–17. [DOI] [PubMed] [Google Scholar]
- 21.Lisovsky M, Braun S, Ge Y, et al. Flt-3 ligand production by human bone marrow stromal cells. Leukemia. 1996;10:1012–1016. [PubMed] [Google Scholar]
- 22.Hauser C, Zhou X, Joshi P, et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J Trauma. 1997;42:895–904. [DOI] [PubMed] [Google Scholar]
- 23.Murohashi I, Endho K, Nishida S, et al. Differential effects of TGF-beta 1 on normal and leukemic human hematopoietic cell proliferation. Exp Hematol. 1995;23:970–979. [PubMed] [Google Scholar]
- 24.Dybedal I, Jacobsen S. Transforming growth factor beta (TGF-beta), a potent inhibitor of erythropoiesis: neutralizing TGF-beta antibodies show erythropoietin as a potent stimulator of murine burst-forming unit erythroid colony formation in the absence of a burst-promoting activity. Blood. 1995;86:949–957. [PubMed] [Google Scholar]
- 25.Meert K, Ofenstein J, Genyea C, et al. Elevated transforming growth factor-beta concentration correlates with posttrauma immunosuppression. J Trauma. 1996;40:901–906. [DOI] [PubMed] [Google Scholar]
- 26.Morganit-Kossman M, Hans V, Lenzlinger P, et al. TGF-β is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma. 1999;16:617–628. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Livingston D, Hauser C, et al. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of tgf-beta1 by bone marrow stroma. Ann Surg. 2001;234:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vittimberga FJ, Foley D, Meyers W, et al. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998;227:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mountford J, Bunce C, Hughes S, et al. Estrone potentiates myeloid cell differentiation: a role for 17 beta-hydroxysteroid dehydrogenase in modulating hemopoiesis. Exp Hematol. 1999;27:451–460. [DOI] [PubMed] [Google Scholar]
- 30.Horner S, Pasternak G, Hehlmann R. A statistically significant sex difference in the number of colony-forming cells from human peripheral blood. Ann Hematol. 1997;74:259–263. [DOI] [PubMed] [Google Scholar]


