Abstract
Objective:
The purpose of the study was to compare in prospective randomized fashion a manually sutured esophagogastric anastomosis in the neck and a stapled in the chest after esophageal resection and gastric tube reconstruction.
Summary Background Data:
Despite the fact that all reconstructions after esophagectomy will result in a cervical or a thoracic anastomosis, controversy still exists as to the optimal site for the anastomosis. In uncontrolled studies, both neck and chest anastomoses have been advocated. The only reported randomized study is difficult to evaluate because of varying routes of the substitute and different anastomotic techniques within the groups. The reported high failure rate of stapled anastomoses in the neck and the fact that most surgeons prefer to suture cervical anastomoses made us choose this technique for anastomosis in the neck. Our routine and the preference of most surgeons to staple high thoracic anastomoses became decisive for type of thoracic anastomoses.
Methods:
Between May 9, 1990 and February 5, 1996, 83 patients undergoing esophageal resection were prospectively randomized to receive an esophagogastric anastomosis in the neck (41 patients) or an esophagogastric anastomosis in the chest (42 patients). To evaluate selection bias, patients undergoing esophageal resection during the same period but not randomized (n = 29) were also followed and compared with those in the study (n = 83). Objective measurements of anastomotic level and diameter were assessed with an endoscope and balloon catheter 3, 6, and 12 months after surgery. The long-term survival rates were compared with the log-rank test.
Results:
Two patients (1.8%) died in hospital, and the remaining 110 patients were followed until death or for a minimum of 60 months. The genuine 5-year survival rate was 29% for chest anastomoses and 30% for neck anastomoses. The overall leakage rate was 1.8% (2 cases of 112) with no relation to mortality or anastomotic method. All patients in the randomized group had tumor-free proximal and distal resection lines, but 1 patient in the nonrandomized group had tumor infiltrates in the proximal resection margin. At 3, 6, and 12 months after operation, there was no difference in anastomotic diameter between the esophagogastric anastomosis in the neck and in the thorax (P = 0.771), and both increased with time (P = 0.004, ANOVA repeated measures). Body weight development was the same in the two groups. With similar results in randomized and nonrandomized patients, study bias was eliminated.
Conclusions:
When performed in a standardized way, neck and chest anastomoses after esophageal resection are equally safe. The additional esophageal resection of 5 cm in the neck group did not increase tumor removal and survival; on the other hand, it did not adversely influence morbidity, anastomotic diameter, or eating as reflected by body weight development.
Controversy still exists concerning the optimal site for an esophagogastric anastomosis. This trial compares in a prospective randomized fashion two types of esophagogastric anastomosis: one in the neck and the other in the right chest. We conclude that neck and chest anastomoses after esophageal resection are equally safe and provide equal morbidity, mortality, anastomotic diameter, body weight development, and long-term survival.
The organ most used for reconstruction after esophagectomy is the stomach.1,2 Advantages include ease of construction and the prospect to achieve a substitute of sufficient length. Proximal gastric necrosis, with subsequent gastroduodenal content contaminating the anastomotic area, is a disastrous complication, however, and bears a high mortality rate irrespective of whether the anastomosis is in the neck or in the thorax.3,4 The ideal anastomotic localization has been the subject of several clinical investigations1,3,4,5 but has not been adequately evaluated. Some authors favor cervical esophagogastric anastomoses despite an increased incidence of leakage,5 stricture formation,6 and damage to the recurrent laryngeal nerve7 because of better tumor eradication5 and reduced mortality and morbidity associated with an anastomotic breakdown.1,2,4 Others deny a difference in leakage rates,3 stricture frequency,3,5 and the innocence of dehiscence in cervical anastomoses3,5,8 or a better long-term survival.5 The variation in the results might be explained by different methods of preparing, localizing, and anastomosing the esophageal substitute.
The quality of an anastomosis is infrequently expressed as anastomotic diameter but more often is judged by the frequency of stricture during follow-up. This may be misleading because there is no accepted definition of a stricture. Nor is dysphagia a good marker because it is well known that patients can experience dysphagia with a wide anastomosis and, vice versa, patients may not seek medical attention because of dysphagia until the anastomosis is only a couple of millimeters wide.9 Moreover, it is not reported the length of the additional esophageal resection necessary for a neck anastomosis. The consequence of this on anastomotic width and postoperative weight development has not been studied.
This trial was designed to compare in a prospective randomized fashion two methods of esophagogastric anastomosis, one in the neck and the other in the apex of the right chest, in a group of patients not affected by perioperative radiochemotherapy. The number of patients required in the study to reach 80% power was based on the high radiologic leakage frequency of about 20% for neck anastomoses reported at study start5 and the hope to improve it to the more acceptable level of 2% for intrathoracic anastomoses.10 Hospital mortality and morbidity, anastomotic level and diameter, body weight development, and long-term survival were the endpoints.
PATIENTS AND METHODS
Between May 9, 1990, and February 5, 1996, 100 patients referred for esophageal resection to the Department of Surgery, Lund University, Lund, Sweden, were randomized preoperatively to receive a manually sutured esophagogastric anastomosis in the neck or an anastomosis stapled in the right chest. Seventeen patients were excluded because of nonresectable tumor (n = 14) or operated with gastrectomy or esophageal bypass procedures (n = 3). To evaluate selection bias, patients undergoing esophageal resection during the same period but not randomized (n = 29) were similarly followed and compared with those in the study (n = 83). All patients were assessed preoperatively by endoscopy and chest radiography, and postoperatively the anastomosis was consistently checked radiographically for leakage using water-soluble contrast medium.
Criteria for inclusion in the study were benign or malignant esophageal disease for which a tube gastroplasty with anastomotic site in the proximal chest or in the neck was deemed suitable. Patients with tumors were judged to have sufficient distance from the proximal end of the tumor to the anastomotic site either in the proximal chest or in the neck. Exclusion criteria included previous gastric resection, tumor high in the thoracic esophagus, preoperative chemoradiotherapy for esophageal cancer, or unwillingness to participate in the randomization or postoperative follow-up program. One hundred blinded randomization envelopes containing information about the anastomotic site were prepared, sealed, and mixed before study start. The randomization was done immediately before the operation, after completion of the clinical evaluation and obtaining informed consent.
Anastomotic leakage was defined as extravasation of water-soluble contrast medium and/or clinical symptoms of leakage. Anastomotic stricture was defined as an anastomotic narrowing that did not allow a standard fiber endoscope with a diameter of 9 mm to pass without resistance, and this was an indication for dilatation. Hospital mortality was defined as death during hospitalization for the esophageal resection.
Operative Procedures
Anastomosis in the Neck
For the patients randomized to receive a neck anastomosis, the operation was started with a right posterolateral thoracotomy in the fifth interspace. In the malignant patients, the mediastinum was dissected from the diaphragm to the apex of the chest, along the aorta, left pleura, and pericardium and upwards along the superior vena cava and trachea, with removal of fat, nodes, esophagus, azygos vein, and the thoracic duct en bloc. The subcarinal nodes and the aortopulmonary window were included in the dissection. The thorax was drained and closed and the patient placed in the supine position. The abdomen was explored through a midline incision, and fat and nodes along the upper part of the abdominal aorta and along the celiac trunk were dissected. The duodenum and head of pancreas were mobilized to allow the pylorus to reach the hiatus. To avoid reflux of duodenal content, pyloroplasty was performed only when a stenotic pylorus due to previous ulcer disease was at hand. The spleen was not removed. A gastric tube was created (Fig. 1A). The right gastric and the right gastroepiploic arteries provided the vascular supply of the tube. To prevent subsequent vascular compromise of the substitute, a phrenotomy of the hiatus was done, allowing passage of four fingers into the mediastinum. A cervical incision was made parallel to the medial part of the left sternocleidomastoid muscle. The prepared gastric tube was gently pushed from below through the mediastinum and delivered to the neck. After adjustment of the length, the gastric tube and the esophagus were divided and the anastomosis sutured (Fig. 1B).
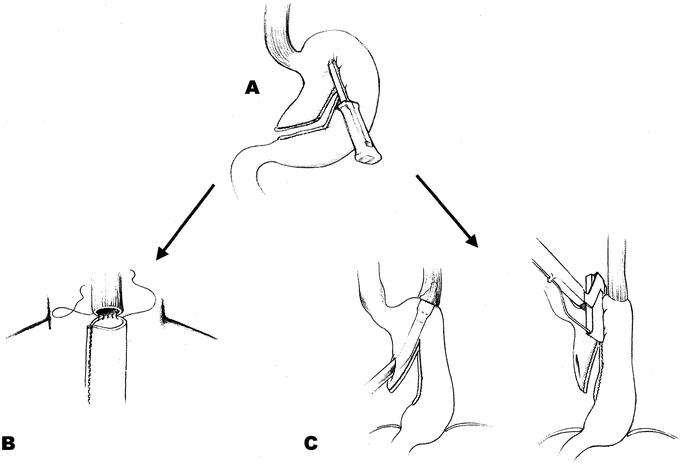
FIGURE 1. The gastric tube substituting the esophagus was created by serial applications of a linear cutting stapling device, TLC 55 (Ethicon, Stockholm, Sweden) parallel to and at a distance of 6 cm from the greater curvature, starting approximately 8 cm proximal to the pylorus at the Crow’s foot (A). When the patients were randomized to receive a neck anastomosis, a running, single-layer end-to-end technique with 4–0 Polydioxanone (PDS II, Ethicon, Sweden) was used through all the layers (B). When the patients were randomized to chest anastomosis (C), the esophagogastrostomy was performed, end-to-greater curvature, by insertion of a circular stapling device (Premium CEEA or Premium CEEA Plus, Autosuture, Sweden) through the subsequently resected (TLH 90 or TL 60, Ethicon, Sweden) lesser curvature. By this technique, everting staple lines in the proximal part of the substitute, the circulation in the most critical part could be evaluated. Care was taken to insert the subsequent stapler in the angle of the previous staple row. The crossings of the staple lines were oversewn; otherwise, no form of reinforcing sutures were used.
Anastomosis in the Chest
When the patients were randomized to receive a stapled intrathoracic anastomosis, the operation was started with the gastric mobilization and finished with the anastomosis in the right chest. The dissection and the handling of the stomach and the esophagus were consistent with that of the neck group. Circular stapling devices (Premium CEEA or Premium CEEA Plus, Autosuture, Stockholm, Sweden) cartridge size 25, 28, or 31 mm were used to construct the esophagogastric anastomosis in the right apex of the chest (Fig. 1C). The stomach was resected with a linear stapling device not closer than 2 cm to the circular anastomosis (Fig. 1C). The 2 “doughnuts” from the circular stapler were checked for completeness. With the anastomosis under saline, inflating air through the nasogastric tube checked its integrity. If a leak were present, or an anastomotic ring were incomplete, the defect was oversewn with an absorbable 4–0 suture.
Irrespective of approach, all gastric tubes were placed in the posterior mediastinum. The mediastinal pleura was drained with two chest tubes, Charrière 28 (Argyle thoracic catheter, Beiersdorf, Kungsbacka, Sweden). No drain was left in the abdomen or neck.
Postoperative Regimen
The nasogastric tube was left with its tip just above the pylorus, and the patient was fed intravenously for 5 days, after which the anastomosis was checked for leakage by x-ray films with water-soluble contrast medium. If no leakage were observed and the nasogastric tube delivered <200 mL, the patients started to drink fluids, followed by a soft diet. On the ninth postoperative day, a regular diet was served. No form of supporting enteral nutrition was provided.
Follow-up
All patients were offered postoperative endoscopy and evaluation for dysphagia after 3, 6, and 12 months. Some patients underwent endoscopy prior to the 3-month control, and some beyond 12 months. The level of the anastomoses was reckoned as the distance between the incisor teeth and the anastomosis measured by endoscopy. The anastomotic diameter was calculated with a balloon and an endoscope, (Fig. 2).
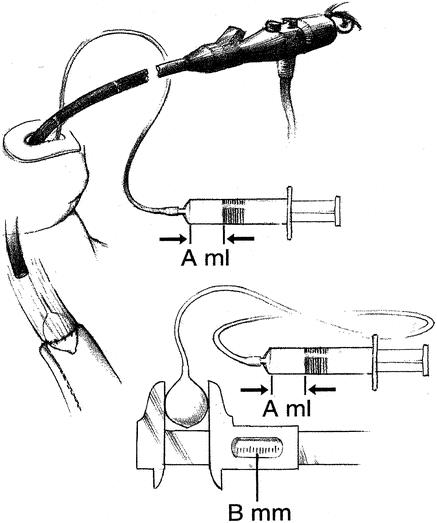
FIGURE 2. The width of the anastomoses was calculated by inserting an aortic occlusion catheter (Fogarty occlusion catheter, model 62-080-8/22F, Bentley-Edwards, Irvine, CA) along with the endoscope. With a syringe we inflated the balloon exactly to the width of the anastomosis (A ml, arrows). After deflating the balloon, the catheter was withdrawn without disconnecting it from the syringe. Outside the patient, the balloon was reinflated (A ml, arrows) and the diameter was measured with a vernier caliper (B mm).
The indication for dilatation of an anastomosis was the presence of a lumen requiring dilatation for adequate passage of the endoscope. Repeated dilatations were made until the narrowing disappeared; in such patients, the reported anastomotic width was the one prior to dilatation.
Statistical Methods
The number of patients enrolled was based on the hope of improving the high radiologic leakage frequency of about 20% for neck anastomoses reported at study start5 but also in recently published series11 to a more acceptable level of 2% for intrathoracic anastomoses.10 With a power of the test of 80% (1 – β) and acceptance of an α=0.05 a total of 86 patients, 43 in each branch, was needed. To evaluate the survival benefit of a neck anastomosis with that number of patients and a median survival prolongation of 10 months, all patients had to be followed at least 5 years (through 2002) or until death, leaving no censored data when calculating the 5-year survival rate.12 The better clearance of all the mediastinum, achievable with neck anastomosis, we calculated, should at least increase the survival with that of the median survival time of a newly diagnosed esophageal carcinoma at that time, that is, 10 months.13 Knowing that about 15% to 20% of the patients might be excluded, we randomized 100 patients. To evaluate selection bias, all not enrolled patients undergoing esophageal resections during study period were followed as those in the study.
Nominal data were calculated with the χ2 test. For quantitative data, the Mann-Whitney U test and the Kruskal-Wallis one-way analysis of variance where appropriate were used to test the differences between unrelated groups. For related groups, the parametric ANOVA (repeated measures model) was used. The survival curves were computed by the Kaplan-Meier method and compared using the log-rank test. A P value of <0.05 was regarded as significant. The SPSS statistical package 8.0 basic and advanced modules (SPSS Inc., Chicago, IL) were used for the statistical testing.
RESULTS
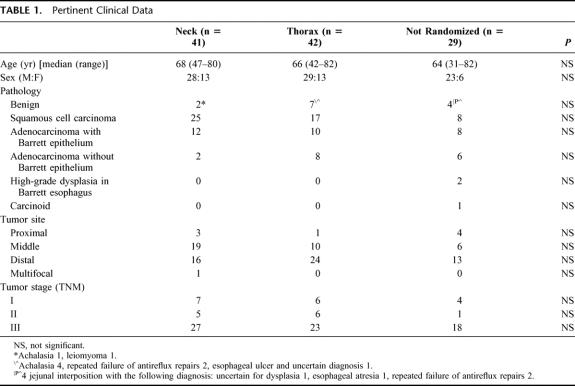
The pertinent characteristics of the 112 patients are listed in Table 1. There were no differences in the age, gender distribution, distribution of disease, tumor stage or site, and the indication for operation between the three groups. Two patients (1.8%) died in hospital, and the remaining 110 patients were followed until death or for a minimum of 60 months.
TABLE 1. Pertinent Clinical Data
All patients in the randomized group (n = 83) and 21 of 29 in the nonrandomized group had gastric tubes as esophageal substitutes. The remaining nonrandomized patients had a jejunal interposition (n = 7) or a colon interposition (n = 1). The reasons for not randomizing 29 patients were high tumor (n = 8), early tumor or benign disease where in suitable patients our first choice is the more demanding procedure, interposition (n = 8), the patient’s refusal to be included (n = 6), obvious palliative intent (n = 4), redo esophagectomy (n = 2), and resection performed by the senior surgeon (B.W.) at another hospital (n = 1).
In-Hospital Period
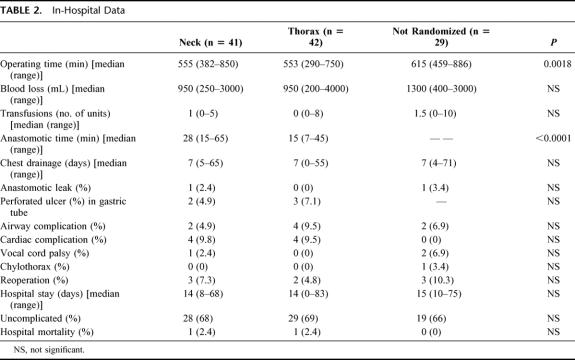
In Table 2, the clinical data of the hospital period are listed. No differences in blood loss, transfusions, duration of chest drainage, hospital stay, morbidity, and mortality were noted between groups. No difference was seen in operating time between the two randomized groups, and both had shorter times than the nonrandomized group (P = 0.0018). The time to perform the anastomoses included in the manually sutured group the hemostasis of the two bowel ends before suturing. In the stapled intrathoracic group, the anastomotic time comprised the circular stapled esophagogastrostomy, completion of the stomach resection with the linear stapling device, and the time required for any reinforcing sutures. The time for the anastomoses in the nonrandomized group varied widely and was not measured.
TABLE 2. In-Hospital Data
We used the 25-mm cartridge size in 24 patients, the 28-mm in 14 patients, and 31-mm in 4 patients for the chest anastomoses in the randomized group. There was no correlation between cartridge size, need for dilatation, and the number of dilatations (χ2 test, P = 0.1079).
The overall leak rate was 1.8% (2 of 112) with no relation to mortality or anastomotic method. All the leaks were treated conservatively. A subclinical esophagojejunal anastomotic leakage shown on contrast study developed in only one of the nonrandomized patients, who received a jejunal interposition. The patient with the leaking anastomosis in the neck complained of odynophagia on the seventh postoperative day but was completely unaffected otherwise. A fluctuating gurgling mass was found elevating the incision. The treatment consisted of complete opening of the wound and daily irrigation and packing. The patient left the hospital in stable condition after 2 months but was readmitted 4 months later with an eventually fatal mediastinal abscess.
Two patients with neck anastomoses and 3 with chest anastomoses in the randomized group had perforations in their substitutes. These perforations, about 5 mm in diameter, were all situated in the serially stapled lesser curvature about 5 to 10 cm below the anastomosis. After replacing the blue cartridges (staple height = 3.85 mm) of the linear stapling device TLC 55 with green (staple height = 4.5 mm) cartridges, this complication was not seen in the subsequent 33 patients reconstructed with gastric tubes.
One not enrolled patient with a proximal tumor and an anastomosis in the neck had a severe bleed, which ruptured the neck wound and was secondary to erosion of the right subclavian artery by an abscess. The anastomosis was taken down to control the bleeding and subsequently resutured. Healing was uneventful.
One patient was randomized to an anastomosis in the neck, but after the thoracic dissection was finished and the abdomen was entered, the operation was discontinued due to peritonitis from perforated diverticulitis. The esophagus was successfully resected through an abdominal and right thoracotomy approach 2 months later. This was the sole patient who was resected in contrast to randomization. He was followed up as the other patients.
All the patients in the randomized group had tumor-free proximal and distal resection lines, but 1 patient in the nonrandomized group had tumor in proximal margin of resection.
Anastomotic Quality During Follow-up
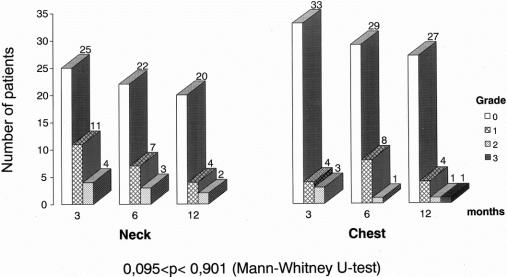
Three, 6, and 12 months after operation, there were no differences in anastomotic diameter between the esophagogastric anastomosis in the neck and in the thorax (P = 0.771), but in both the diameter increased with time (P = 0.004, ANOVA repeated measures) (Fig. 3). There were no differences in experience of dysphagia between groups (P = 0.901), (Fig. 4). The anastomoses in the neck were localized 20 cm (median, range 15–26 cm) from the incisors, while the distance to chest anastomoses was 25 cm (median, range 21–28 cm) (P < 0.0001).
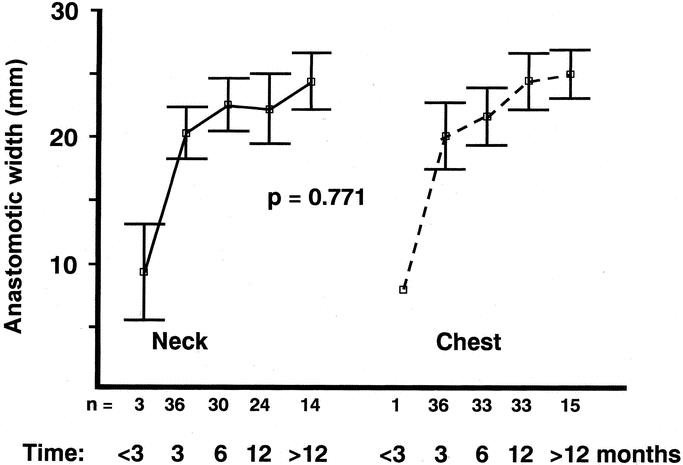
FIGURE 3. Anastomotic diameters in the randomized patients who volunteered for serial measurements 3, 6, and 12 months and occasionally at less than 3 months and more than 12 months after surgery. Number of patients (n), mean, and 95% confidence interval are shown. ANOVA (repeated measures model) was used to test for differences. The few patients, 3 in the neck group and 1 in the chest group, that sought medical attention prior to the scheduled follow-up (<3 months) were measured, dilated, but not included in the ANOVA calculations because of small numbers.
FIGURE 4. Dysphagia score in patients randomized to neck or chest anastomosis. Grade 0 = no dysphagia; Grade 1 = occasional episodes; Grade 2 = required liquids to clear; Grade 3 = food impaction requiring medical treatment. There were no differences between groups or within groups from 3 to 12 months.
Tumor recurred in the anastomosis in one patient in the cervical group, none in the thoracic group, and in 5 nonrandomized patients including the solitary patient with tumor in the proximal margin of resection. In the cervical group, 8 patients (8 of 41, 19.5%) were dilated 17 times, all within the first year. In the stapled thoracic group, 12 patients (12 of 42, 28.6%) required 22 dilatations, but only 1 patient required dilatation after the first year (Fisher exact test, P = 0.443). The median number of dilatations required in the cervical and thoracic groups was 2, range (1–5) and (1–4), respectively.
All patients lost weight during the first 3 postoperative months (P < 0.001) but not later in the first postoperative year. There was no correlation to anastomotic level (P = 0.883, ANOVA repeated measures) (Fig. 5).
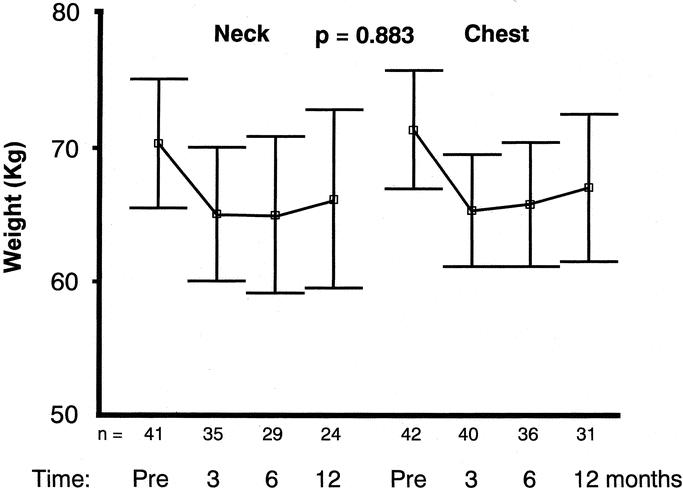
FIGURE 5. The body weight (kg) development following gastric pull-up esophagectomy during the first postoperative year was similar for patients randomized to neck or chest anastomoses (P = 0.883, ANOVA repeated measures model). Mean and 95% confidence interval are shown.
Survival Rate
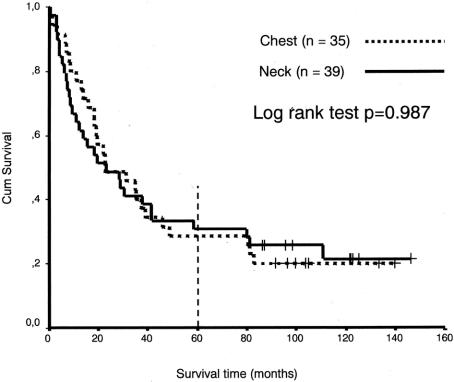
The mean survival time for patients with chest anastomoses was 65.21months, (95% confidence interval, 47.39–83.04 months), and for neck anastomoses 54.62 months, (95% confidence interval 37.34–71.90 months), which was not a significant difference (P = 0.3803, log-rank test). The difference between the groups was still not significant after exclusion of the benign cases (P = 0.9870, log-rank test), with a mean survival time for cancer patients with chest anastomoses (n = 35) of 49.4 months, (95% confidence interval, 33.0–65.8 months), and for cancer patients with neck anastomoses (n= 39), 51.8 months, (95% confidence interval 34.2–69.3 months). A follow up of more than 5 years resulted in a genuine 5-year survival rate for cancer patients of 29% for chest anastomoses and 30% for neck anastomoses (Fig. 6). The only significant factor affecting survival was the stage of disease.
FIGURE 6. Cumulative survival rates (Kaplan-Meier) by anastomotic site in 74 randomized patients with esophageal cancer. It is seen from the censored data (living patients), marked with plus signs (+), that all patients are followed until death or >5 years, resulting in a genuine 5-year survival rate of 29% (chest anastomoses) and 30% (neck anastomoses), respectively. Median survival time for neck anastomoses was 23.1 months (95% confidence interval, 6.9–39.4 months), and for chest anastomoses 23.0 months (95% confidenceinterval, 4.9–41.0 months).
DISCUSSION
Whether the anastomosis should be performed in the neck, allowing larger margins of resection, more common but less dangerous leakage,2,4 and increased risk of injury to the recurrent laryngeal nerve,7 or whether anastomosis in the thorax is to be preferred, with less esophagus removed but decreased margins5 and a lower but more ominous leakage rate,2,4 is still a highly controversial issue in reconstruction after esophagectomy. It is also a pivotal issue because all reconstructions after esophagectomy will result in either a cervical or a thoracic anastomosis. There is only one randomized study addressing the subject.5 This study from 1989 confirms the higher leakage rate in cervical anastomoses but is difficult to evaluate because 8 patients in the cervical group (n = 43) had the gastric tube placed substernally and another 7 in the same group had the anastomoses constructed by hand, while the rest were stapled. The substernal route is longer,14 which might have resulted in more tension in the anastomoses, an adverse factor in all wound healing and potentially lethal in esophagogastric anastomoses.15 Debatably, leakage rates and mortality have both been higher following retrosternal placement of the gastric tube16–19 in prospective randomized studies. In our study, all gastric tubes were positioned in a uniform manner in the posterior mediastinum irrespective of anastomotic site, and all anastomoses in the neck were performed manually, whereas all chest anastomoses were stapled. The main endpoints in our study were the influence of a neck anastomosis on morbidity, mortality, and long-time survival. Therefore, a consistent strategy of resection had to be applied in the two arms. This involved fat and lymph node dissection in the upper abdomen and “clearance” of the mediastinum in the malignant cases. Transhiatal esophagectomy was consequently not considered.
Although cervical esophagogastrostomies can be performed with circular stapling devices both transorally20 and by transitioning the stapler through the subsequent pyloroplasty site and pushing the stomach up to the cervicotomy,21 most surgeons prefer to suture cervical anastomoses.1 The reported high failure rate of attempted stapled anastomoses in the neck5 and the fact that cervical anastomoses can be readily performed manually in a highly standardized way made us choose this technique for anastomosis in the neck.
For hand-sewn anastomoses, almost every suturing technique imaginable has been described. We agree with Zieren et al that given comparable leakage rates, one-layer esophagogastric anastomosis in the neck must be considered superior to the two-layer procedure because of the lower incidence of nonmalignant stricture formation.17 We have reported in a highly standardized experimental model that the one-layer anastomoses were faster to construct, wider after 1 week, and equal to the two-layer anastomoses in regard to breaking strength.22 These were the circumstances that made us choose the one-layer continuous technique for the cervical anastomoses and it was successful in all instances.
Performing an anastomosis at the apex of the pleural cavity has been facilitated with the use of a circular stapling device with a detachable head. In fact, the detachable anvil is a prerequisite for an esophagogastric anastomosis with only a couple of centimeters free esophagus at the apex of the chest.2 This and the circumstance that most surgeons prefer to staple high thoracic anastomoses1 became decisive for us concerning type of thoracic anastomoses to perform in the randomized study.
The manually performed esophagogastrostomy in the neck (28 minutes, range 15–65 minutes) took twice the time of the stapled (15 minutes, range 7–45 minutes), P < 0.0001, to perform but was at least equal to the stapled in regard to stricture formation. The superiority of a manually sutured thoracic anastomosis on stricture frequency23 has been questioned.24 Our study cannot be used because of different location of the anastomosis, for a proper comparison between stapled and sutured esophagogastric anastomoses, and this was not the aim of the study, but the more important to find an optimal location of the anastomosis, that correlates much more to morbidity. The difference in stricture rate between stapled and manually sutured anastomoses is also of less importance compared with leakage rate because strictures are readily managed with balloon dilatation. This is underlined by the fact that authors who found manually performed esophagogastric anastomosis to be superior to stapled concerning stricture rate justify the use of staplers because they are less operator dependent and have a similar postoperative leakage frequency.23 In our series as in others,23 nearly all strictures were remedied within a year after two dilatations on average. The very severe strictures after catastrophic anastomotic complications are of ischemic origin and are not addressed by different suturing techniques but rather by vascularization of the substitute.25 The longer route for the cervical anastomoses, 5 cm on average, did not result in more ischemic substitutes, probably due to the extensive gastroduodenal mobilization and expansion of the hiatus.
The shorter anastomotic time for stapled anastomoses in our series was not reflected in a shorter operation time, as reported by Craig et al.24 This is probably due to the time-consuming two-field dissection in the abdomen and in the thorax and the fact that in every operation trainees under supervision of the senior surgeon (B.W.) performed half of the procedure, the thoracic or the abdominal part.
The high stricture rate for stapled intrathoracic esophagogastric anastomoses, in this series 28.6%, is also reported by Law et al (40%) in a prospective randomized trial.23 The anastomotic narrowing is presumably explained by wound retraction in the annular incision effected by the circular knife of the stapler that cuts through the anastomotic tissue. The accurate mucosa-to-mucosa apposition, considered important for good anastomotic healing,10 was achieved only in the manually sutured neck anastomosis.
Like Craig et al,24 we did not perform pyloroplasty. This probably reduced the duodenal reflux suspected of inducing stricture formation, especially in secondary healing that always occurs in circular stapled anastomoses.26 On the other hand, in the study of Chasseray et al,5 with 36 of 43 neck anastomoses stapled and all patients receiving pyloromyotomy or pyloroplasty, the stricture frequency (14%) was not different from the present study (19.5%), where all the anastomoses in the neck were manually sutured.
In the present series, there was no difference in leakage rate between neck and chest anastomoses. This is in contrast to the single previously reported randomized study by Chasseray et al,5 and many retrospective studies that have shown higher rates of leakage in neck anastomoses. The explanation is probably to be found in the standardization of the technique, with consistent positioning of the substitutes, all orthotopic, and with use of an optimal anastomotic technique for the two sites. Second, resection of a cuff of the diaphragm surrounding the hiatus left enough space both for the substitute and the gastroepiploic and right gastric arteries.
The technique used for transforming the stomach into a gastric tube (Fig. 1A) resulted automatically in resection of the least vascularized gastric region, the fundus, and the lesser curvature.27 The latter was in the malignant cases resected together with the left gastric artery and fat and nodes along the celiac axis in an attempt to attain locoregional tumor control. It might be argued that this resection also divided many collateral vessels and probably was responsible for the perforations seen in the longitudinal staple row. This serious complication has not been experienced by others,5 and it disappeared after the blue magazine was replaced by the green in the TLC 55 stapler (Ethicon, Sweden), so that a wider gap probably resulted in fewer necrotic spots.
The left recurrent laryngeal nerve courses about the aortic arch and is at risk in the thoracic phase of the operation during dissection of the aortopulmonary window. In the neck, the recurrent nerve is at risk on the side of incision,28 so that we chose the left side for the anastomosis to avoid the risk of bilateral vocal cord paralysis. The low rate of injury to the recurrent laryngeal nerve in our patients with either neck (n = 1) and chest (n = 0) anastomoses was presumably underestimated because the only indication for laryngoscopy was postoperative hoarseness.29 With the low incidence of severe postoperative lung complications (neck = 4.8%, thorax = 9.5%), defined as more than 1 week of support in the ventilator, pneumonia or pleural effusion necessitating insertion of extra pleural drainage we probably avoided bilateral nerve injuries.
The postoperative hospital stay of 2 weeks, similar in all groups, accorded well with other recent reports16,28 and was not related to operative time. We have in a previous report shown that postoperative length of stay was correlated to a high degree only with postoperative complications and with age to a much lesser degree but not to operative time.30 Advanced age has not been reported to predict length of hospital stay or survival.31
We have shown the subsequent anastomotic widening in both the neck and chest anastomoses earlier in esophagogastric anastomoses.26 This proves that despite the presence of many staples in the anastomosis, it will enlarge if appropriately stimulated, and staples do not constitute an obstacle for widening. This contrasts with the opinions of Law et al.23 The lack of correlation between dysphagia and anastomotic diameter is suspected clinically by others9 and shown in this series. Differences in dysphagia, an experience parameter, might be explained by differences in scar formation in the surrounding tissues or early recurrence but not by differences in anastomotic level according to this study. In this study, no prevention against acid production was given until esophagogastric strictures occurred or the patients suffered from reflux symptoms. The impact of reflux on anastomotic diameter remains to be investigated. The group of patients, however, that had anastomotic dilatations and/or had acid reducing drugs also ended up with the narrowest anastomoses.
Despite a longer operative time, the hospital mortality of 1.8% (2 of 112) with no difference between groups compares well with that of less extensive procedures.23,32 This is presumably an effect of the dedicated and intensive postoperative respiratory care and anesthesiologically supported relief of pain, which reduces postoperative mortality after esophagectomy.33
The 5-year survival rate of patients with chest (29%) and neck anastomoses (30%) compares favorably with transhiatal32 and less extensive transthoracic procedures23 and equals those published with preoperative radiochemotherapy, except for lesser postoperative mortality.34 It approaches the results of the Japanese two-field esophagectomy35 and the Western en bloc resection36,37 but not the results of the three-field esophagectomy.35
We have reported 100% of the patients undergoing esophageal resection during the period of study. With similar results in the randomized and in the nonrandomized group, except for a longer operative time in the nonrandomized group due to more rereconstructions and interpositions, we feel confident that selection bias did not occur.
CONCLUSION
When performed in a standardized fashion, neck and chest anastomoses after esophageal resection are equally safe. The additional esophageal resection of 5 cm in the neck group did not increase tumor removal and survival, but on the other hand did not adversely influence morbidity, anastomotic diameter, or eating as reflected by body weight changes.
Discussion
Dr. A. Peracchia: I would like to congratulate the authors for such an excellent presentation.
Indeed, this topic has been a matter of controversy in esophageal surgery for many years. Dr. Walther and coworkers have attempted a prospective and randomized trial to answer the question of which anastomosis is safer. They have compared the outcome of surgery in patients undergoing an intrathoracic or a cervical anastomosis and have measured the caliber of the anastomosis. This provides a more objective means to assess the functional outcomes. The recent introduction of a semi-mechanical anastomosis in the neck has significantly reduced the incidence of stricture formation, as you can see from my experience and from the Orringer and Collard series.
As far as concerns the volume of esophagectomy, I think that the gain in esophageal length by doing a cervical anastomosis is probably around 2 cm, as it has been also shown by John Wong. This is especially so when you perform a really high intrathoracic anastomosis at the level of thoracic inlet.
Finally, I noticed that you do not use neoadjuvant therapy even in Stage III disease. Is this a rule in your institution or do you have ongoing trials including chemotherapy and radiotherapy?
Dr. B. Walther: Thank you for your comment and questions.
First, about the localization of the anastomosis and the choice to do different types of suturing in the two locations. I consider location of the esophagogastric anastomoses a more relevant issue to address than suturing technique because it correlates much more to morbidity. The difference in stricture rate between stapled and manually sutured anastomoses is of less importance because strictures due to wound retraction during healing phase are readily managed with balloon dilatation. Furthermore, there are at least two good randomized studies comparing equally located stapled and manually sutured esophagogastrostomies. In the study of Simon Law and John Wong, the stricture frequency was higher in the stapled group. In spite of that, the authors justify the use of staplers because they are less operator dependent and have a similar postoperative leakage frequency. In our series, as in theirs, nearly all strictures were remedied within a year after two dilatations on average, indicating a minor problem. The very severe strictures after catastrophic anastomotic complications are of ischemic origin and are not addressed by different suturing techniques, but rather by vascularization of the substitute. In the randomized series of Craig, the superiority of manually sutured thoracic anastomoses on stricture frequency was questioned.
Cervical esophagogastrostomies can be performed with circular stapling devices both transorally and by transitioning the stapler through the subsequent pyloroplasty site and pushing the stomach up to the cervicotomy. The reported high failure rate of attempted stapled anastomoses in the neck and the fact that cervical anastomoses can be readily performed manually in a highly standardized way made us choose this technique for anastomoses in the neck. The semi-mechanical anastomoses inquired were not described due to lack of suitable staplers at study start and subsequently not considered.
Performing an anastomosis at the apex of the pleural cavity has been facilitated with the use of a circular stapling device with a detachable head. This and the circumstance that most surgeons prefer to staple high thoracic anastomoses became decisive for us concerning type of thoracic anastomoses to perform in the randomized study.
The level of the anastomoses was reckoned as the distance between the incisor teeth and the anastomoses measured by endoscopy. This gives a reproducible measurement of the level of the anastomoses, and to the best of my knowledge, no one has really done this before. The anastomoses in the neck were localized 20 cm (median, range 15–26 cm) from the incisors, while the distance to chest anastomoses was 25 cm (median, range 21–28 cm), a difference of 5 cm in median. The cervical anastomosis was performed as close to the cricopharyngeal muscle as possible for comfortable suturing. This gave us a difference of 5 cm in median between the cervical and thoracic anastomoses. I look forward to the reports of others measuring the level of cervical and thoracic esophagogastric anastomoses.
Finally, to the comment on neoadjuvant therapy, we don’t use this at all in our institution. The background is that so far not a single prospective randomized study shows a benefit of neoadjuvant therapy on genuine 5-year survival rate.
Dr. H. Obertop: Thank you. I enjoyed your paper very much; it is really a very good result. I know how precise you work, I have seen you operate, it takes a long time but the end result is very nice.
I have a question, in the calculation of the sample size you suggested that you have a leakage rate of 20% bringing it down to 2%. I do not think it very realistic, but in your two groups you have a leakage rate of 2%. What is the secret to have such a low leakage rate in the neck anastomosis and also such a low stricture rate? Many surgeons, like ourselves, have stricture rates of 25% doing practically the same sort of anastomosis you do, manually in one layer, also when using the same stapler. What is the secret?
Dr. B. Walther: Thank you for the comments and also thank you, Dr. Obertop, for teaching me this; you were in fact the one who taught me to do this gastric tube, and I am very glad for that.
First, I will address the question about the calculation of sample size. The number of patients enrolled was based on the hope of improving the high radiologic leakage frequency of about 20% for neck anastomoses reported at study start to a more acceptable level of 2%. Chasseray showed in Surgery Gynecology and Obstetrics (1989), the only previously published randomized study, a leakage rate of 26% for neck anastomoses. This is true also in a recently published series, Singh reported in 2001, in the Annals of Thoracic Surgery, a leakage rate of 23% for manually sutured neck anastomoses. The level of 2% leakage was at study start reported for intrathoracic anastomoses by Akiyama and was the results of our own experience in stapled esophageal anastomoses.
Comments about the low leakage rate in neck anastomoses. In the present series, there was no difference in leakage rate between neck and chest anastomoses. This is in contrast to other studies showing higher rates of leakage in neck anastomoses. The explanation is probably to be found in the standardization of the technique, with consistent positioning of the substitutes, all orthotopic, and with use of an optimal anastomotic technique with exact mucosa to mucosa apposition. The longer route for the cervical anastomoses, 5 cm on average, did not result in more ischemic substitutes, probably due to the extensive gastroduodenal mobilization and resection of a cuff of the diaphragm surrounding the hiatus that left enough space both for the substitute and the gastroepiploic and right gastric arteries. This is most likely the explanation for the stricture rate of 19.5% that, however, presumably is not a difference from yours of 25%. Patients with stapled intrathoracic anastomoses developed strictures in 28.6%.
Dr. T. Lerut: Dr. Walther, in the long-term survivors, it seems that there is an increasing number of reports mentioning the occurrence of intestinal metaplasia above the anastomosis. I just wonder whether you have looked into this in your series. If metaplasia did occur, what was the difference between the two groups? What is your policy at home to suppress acid reflux and maybe also biliary reflux that, of course, will be induced by the pyloroplasty?
Dr. B. Walther: In this study we did not look for the development of intestinal metaplasia in the postoperative period. We have however explored this issue in a paper published last year in Annals of Surgery where we found that patients resected for adenocarcinoma in Barrett’s esophagus were more prone to develop metaplastic columnar mucosa proximal to the anastomosis than other patients. Only three patients developed intestinal metaplasia with no correlation to neck or chest anastomosis.
Approximately one third of our patients, similar in both groups, are on proton pump inhibitors after esophagectomy and gastric pull-up esophagoplasty.
We have an ongoing prospective randomized study comparing proton pump inhibitors and no such medication after esophagectomy with the end points anastomotic diameter and need for dilatation besides symptomatic evaluation.
Footnotes
This study has been supported by grants from the Crafoord Foundation, Lund, Sweden, and the Lund University Hospital, Lund, Sweden.
Reprints: Bruno Walther, MD, PhD, Department of Surgery, Lund University Hospital, SE-221 85 Lund, Sweden. E-mail:Bruno.Walther@kir.lu.se.
REFERENCES
- 1.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634–640. [DOI] [PubMed] [Google Scholar]
- 2.Müller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845–857. [DOI] [PubMed] [Google Scholar]
- 3.Lam TCF, Fok M, Cheng SWK, et al. Anastomotic complications after esophagectomy for cancer: a comparison of neck and chest anastomoses. J Thorac Cardiovasc Surg. 1992;104:395–400. [PubMed] [Google Scholar]
- 4.Patil PK, Patel SG, Mistry RC, et al. Cancer of the esophagus: esophagogastric anastomotic leak - a retrospective study of predisposing factors. J Surg Oncol. 1992;49:163–167. [DOI] [PubMed] [Google Scholar]
- 5.Chasseray VM, Kiroff GK, Buard JL, et al. Cervical or thoracic anastomosis for esophagectomy for carcinoma. Surg Gynecol Obstet. 1989;169:55–62. [PubMed] [Google Scholar]
- 6.Dewar L, Gelfand G, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg. 1992;163:484–489. [DOI] [PubMed] [Google Scholar]
- 7.Fok M, Law S, Stipa F, et al. A comparison of transhiatal and transthoracic resection for oesophageal carcinoma. Endoscopy. 1993;25:660–663. [DOI] [PubMed] [Google Scholar]
- 8.Iannettoni MD, Whyte RI, Orringer MB. Catastrophic complications of the cervical esophagogastric anastomosis. J Thorac Cardiovasc Surg. 1995;110:1493–1501. [DOI] [PubMed] [Google Scholar]
- 9.Wong J, Cheung H, Lui R, et al. Esophagogastric anastomosis performed with a stapler: the occurrence of leakage and stricture. Surgery. 1987;101:408–415. [PubMed] [Google Scholar]
- 10.Akiyama H. Esophageal anastomosis. Arch Surg. 1973;107:512–514. [DOI] [PubMed] [Google Scholar]
- 11.Singh D, Maley RH, Santucci T, et al. Experience and technique of stapled mechanical cervical esophagogastric anastomosis. Ann Thorac Surg. 2001;71:419–424. [DOI] [PubMed] [Google Scholar]
- 12.Dupont WD, Plummer WD Jr. Power and sample size calculations: a review and computer program. Controlled Clin Trials. 1990;11:116–128. [DOI] [PubMed] [Google Scholar]
- 13.Herskovic A, Martz K, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. [DOI] [PubMed] [Google Scholar]
- 14.Ngan SYK, Wong J. Lengths of different routes for esophageal replacement. J Thorac Cardiovasc Surg. 1986;91:790–792. [PubMed] [Google Scholar]
- 15.Rahamim J, Cham CW. Oesophagogastrectomy for carcinoma of the oesophagus and cardia. Br J Surg. 1993;80:1305–1309. [DOI] [PubMed] [Google Scholar]
- 16.van Lanschot JJB, van Blankenstein M, Oei HY, et al. Randomized comparison of revertebral and retrosternal gastric tube reconstruction after resection of oesophageal carcinoma. Br J Surg. 1999;86:102–108. [DOI] [PubMed] [Google Scholar]
- 17.Zieren HU, Müller JM, Pichlmaier H. Prospective randomized study of one- or two-layer anastomosis following oesophageal resection and cervical oesophagogastrostomy. Br J Surg. 1993;80:608–611. [DOI] [PubMed] [Google Scholar]
- 18.Bartels H, Thorban S, Siewert JR. Anterior versus posterior reconstruction after transhiatal oesophagectomy: a randomized controlled trial. Br J Surg. 1993;80:1141–1144. [DOI] [PubMed] [Google Scholar]
- 19.Gawad KA, Hosch SB, Bumann D, et al. How important is the route of reconstruction after esophagectomy: a prospective randomized study. Am J Gastroenterol. 1999;94:1490–1496. [DOI] [PubMed] [Google Scholar]
- 20.Ancalmo N, Knabb JL. Transoral cervical esophagogastrostomy using the EEA stapling device. Ann Thorac Surg. 1985;39:387. [DOI] [PubMed] [Google Scholar]
- 21.Sarfati E, Gossot D, Celerier M. Use of circular stapler for the cervical esophagogastric anastomosis in retrosternal gastric esophagoplasty. J Thorac Cardiovasc Surg. 1991;101:745–746. [PubMed] [Google Scholar]
- 22.Graffner H, Andersson L, Löwenhielm P, et al. The healing process of anastomoses of the colon: a comparative study using single, double-layer or stapled anastomosis. Dis Colon Rectum. 1984;27:767–771. [DOI] [PubMed] [Google Scholar]
- 23.Law S, Fok M, Chu K-M, et al. Comparison of handsewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg. 1997;226:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig SR, Walker WS, Cameron EWJ, et al. A prospective randomized study comparing stapled with handsewn oesophagogastric anastomoses. J R Coll Surg Edinb. 1996;41:17–19. [PubMed] [Google Scholar]
- 25.Beitler AL, Urschel JD. Comparison of stapled and hand-sewn esophagogastric anastomoses. Am J Surg. 1998;175:337–340. [DOI] [PubMed] [Google Scholar]
- 26.Johansson J, Zilling T, Staël von Holstein C, et al. Anastomotic diameters and strictures following esophagectomy and total gastrectomy in 256 patients. World J Surg. 2000;24:78–85. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DM, Langford RM, Russell RCG, et al. The anatomical basis for gastric mobilization in total oesophagectomy. Br J Surg. 1979;66:230–233. [DOI] [PubMed] [Google Scholar]
- 28.Hulscher JBF, van Sandick JW, Devriese PP, et al. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg. 1999;86:1583–1587. [DOI] [PubMed] [Google Scholar]
- 29.Johnsson PR, Kanegoaker GS, Bates T. Indirect laryngoscopic evaluation of vocal cord function in patients undergoing transhiatal esophagectomy. J Am Coll Surg. 1994;178:605–608. [PubMed] [Google Scholar]
- 30.Zilling T, Olsén P, Walther B. Prediction of hospital stay after total gastrectomy. Anticancer Res. 1997;17:1355–1360. [PubMed] [Google Scholar]
- 31.Johansson J, Walther B. Clinical outcome and long-term survival rates after esophagectomy are not determined by age over 70 years. J Gastrointest Surg. 2000;4:55–62. [DOI] [PubMed] [Google Scholar]
- 32.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsui SL, Law S, Fok M, et al. Postoperative analgesia reduces mortality and morbidity after esophagectomy. Am J Surg. 1997;173:472–478. [DOI] [PubMed] [Google Scholar]
- 34.Bosset J-F, Gignoux M, Triboulet J-P, et al. Chemo-radiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. [DOI] [PubMed] [Google Scholar]
- 35.Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer: comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg. 1995;222:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altorki NK, Girardi L, Skinner DB. En bloc esophagectomy improves survival for stage III esophageal cancer. J Thorac Cardiovasc Surg. 1997;114:948–956. [DOI] [PubMed] [Google Scholar]
- 37.Lerut T, De Leyn P, Coosemans W, et al. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg. 1992;216:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]