Abstract
Objective:
To evaluate the protective effects of ischemic preconditioning in a prospective randomized study involving a large population of unselected patients and to identify factors affecting the protective effects.
Summary Background Data:
Ischemic preconditioning is an effective protective strategy in several animal models. Protection has also been suggested in a small series of patients undergoing a hemihepatectomy with 30 minutes of inflow occlusion. Whether preconditioning confers protection in other types of liver resection and longer periods of ischemia is unknown. Therefore, we conducted a prospective randomized study to evaluate the impact of ischemic preconditioning in liver surgery.
Methods:
A total of 100 unselected patients undergoing major liver resection (> bisegmentectomy) under inflow occlusion for at least 30 minutes were randomized during surgery to either receive or not receive an ischemic preconditioning protocol (10 minutes of ischemia followed by 10 minutes of reperfusion). Univariate and multivariate analyses were performed to identify independent factors affecting the protective effects of ischemic preconditioning. ATP contents in liver were measured as a possible mechanism of protection.
Results:
Both groups (n = 50 in each) were comparable regarding age, gender, duration of inflow occlusion, and resected liver volumes. Postoperative serum transaminase levels were significantly lower in preconditioned than in control patients (median peak AST 364 U/L vs. 520 U/L, P = 0.028; ALT 406 vs. 519 U/L, P = 0.049). Regression multivariate analysis revealed an increased benefit of ischemic preconditioning in younger patients, in patients with longer duration of inflow occlusion (up to 60 minutes), and in cases of lower resected liver volume (<50%). Patients with steatosis were also particularly protected by ischemic preconditioning. ATP content in liver tissue was preserved by ischemic preconditioning in young but not older patients.
Conclusions:
This study establishes ischemic preconditioning as a protective strategy against hepatic ischemia in humans. The strategy is particularly effective in young patients requiring a prolonged period of inflow occlusion, and in the presence of steatosis, and is possibly related to preservation of ATP content in liver tissue. Other strategies are needed in older patients.
Ischemic preconditioning has been shown in various animal models and in a pilot study in humans to confer protection against ischemic injury to the liver. We conducted a prospective randomized trial in 100 consecutive unselected patients to establish the protective effects of ischemic preconditioning and showed maximal effects in young patients and patients with steatosis.
Blood loss and transfusions during liver resection are associated with unfavorable short- and long-term outcomes.1-4 Inflow occlusion through clamping of the portal triad (Pringle maneuver) in combination with a low central venous pressure is routinely used in many centers to prevent blood loss during transection of the liver parenchyma. The Pringle, maneuver, however causes ischemic injury to the remaining liver with a risk of poor postoperative outcome.2,5 Diseased livers with steatosis or fibrosis poorly tolerate reperfusion injury and can develop liver failure even after short periods of ischemia.6
Intermittent clamping of the portal triad has been used as an effective strategy to minimize ischemic injury during liver surgery despite long periods of ischemia.7 The protective effects of intermittent clamping against ischemic injury have been demonstrated in several clinical8-10 and experimental studies.11,12 A drawback inherent to intermittent clamping is the blood loss during each period of reperfusion and the increased operative time.10 An alternative to intermittent clamping is ischemic preconditioning, in which a brief period of ischemia and reperfusion is applied prior to the prolonged ischemic insult. Several studies in rodent models have shown significant protection from ischemic preconditioning, including increased animal survival after prolonged periods of hepatic ischemia.13-17 For example, in a study in mice, we found that ischemic preconditioning was as effective as intermittent clamping against ischemic injury with up to 75 minutes of inflow occlusion.13 We also performed a pilot nonrandomized study in 24 patients undergoing an anatomic hemihepatectomy under exactly 30 minutes of inflow occlusion.18 Patients pretreated with ischemic preconditioning (10 minutes of ischemia and 10 minutes of reperfusion) had less postoperative hepatic injury as indicated by lower transaminase levels and endothelial cell injury. Of note was the observation that patients with a steatotic liver were particularly protected by the ischemic preconditioning protocol. However, a randomized study demonstrating the protective effects of ischemic preconditioning in an unselected group of patients is not available.
Therefore, we designed a prospective randomized study in 100 consecutive patients undergoing major liver resection, in which the only exclusion criterion was cirrhosis or impaired preoperative function. The aim of the study was to evaluate the protective effects of ischemic preconditioning with particular attention to identifying factors that may impact on this effect in humans. A secondary aim was to assess potential mechanisms of protection, such as restoration of adenosine triphosphate (ATP) in liver tissue after reperfusion.
METHODS
Experimental Design
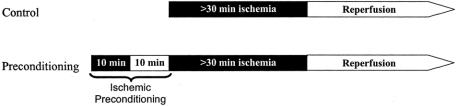
Between April 1999 and December 2001, 100 consecutive patients undergoing major liver resection under continuous inflow occlusion (Pringle maneuver) were randomized to receive ischemic preconditioning prior to continuous inflow occlusion and liver resection. Ischemic preconditioning was performed through an inflow occlusion (Pringle maneuver) of 10 minutes followed by 10 minutes of reperfusion prior to the prolonged ischemic insult, as reported previously 18 (Fig. 1). Each patient was operated under the supervision of the same hepatobiliary surgeon (P.-A.C.). The time of continuous inflow occlusion was adapted to the need but had to exceed 30 minutes, as this is the minimal ischemic time associated with detectable post-reperfusion injury. Cirrhotic patients and patients receiving additional ablation therapies (cryosurgery or radiofrequency) were excluded from the study. Patients requiring total hepatic exclusion, reconstruction of the cava, or a Roux-en-Y hepaticojejunostomy were included. No patient dropout occurred after randomization.
FIGURE 1. Treatment protocol of the preconditioning and the control groups. Preconditioning patients received 10 minutes of ischemia and 10 minutes of reperfusion (ischemic preconditioning) prior to the prolonged ischemic insult.
Surgical Procedure
The protocol was approved by the ethics committees of both Duke University Medical Center (Durham, NC), where the study was initiated, and the University Hospital of Zurich, where the majority of patients were included. An informed consent was obtained from each patient prior to surgery. Each patient was randomized and included in the study in the operating room after intraoperative ultrasound was performed using sealed envelopes (50 controls and 50 ischemic preconditioning). After assigning the patient to either control or preconditioning group, liver resection was performed with continuous clamping of the portal triad for at least 30 minutes. All types of liver resection requiring resection of more than 2 segments were included in the study. The need for a Roux-en-Y hepaticojejunostomy, vena cava resection, or technique of total vascular exclusion was not an exclusion criteria. Clamping of the portal triad (Pringle maneuver) was performed with the tourniquet technique using a 4-mm mersilene tape. A low central venous pressure (0–5 mm Hg) was routinely maintained during the transection period to avoid backflow from the suprahepatic veins. Central venous pressure was kept higher in cases involving total vascular occlusion of the liver, where an additional tourniquet was placed on the infrahepatic cava and a large vascular clamp on the suprahepatic cava. Immediately after clamping of the portal triad, the parenchymal transection was initiated with the Kelly clamp crushing technique under vascular inflow occlusion. Small vessels were occluded with the bipolar forceps. Larger vessels or bile ducts were occluded with metallic clips or ligated with 2–0 silk. Tru cut liver biopsies were obtained prior to resection and 30 minutes after reperfusion.
Data regarding duration of surgery, intraoperative blood loss and transfusion, and ICU and hospital stays were prospectively collected. Each negative intraoperative and postoperative event was also recorded. The percentage of resected liver volume was evaluated intraoperatively by a single surgeon (P.-A.C.) according to the proportion of resected liver parenchyma. The degree of ischemic injury of the liver was determined by serial postoperative serum bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels measured daily until discharge. Prothrombin time was also recorded daily. Each biopsy was evaluated by a pathologist (W.J.) in a blinded fashion to identify underlying disorders such as steatosis, fibrosis, and other types of injury.
Measurement of ATP
Liver tissue for ATP measurement was collected prior to ischemia (baseline) and 30 minutes after reperfusion. ATP in liver tissue was determined by a bioluminescent assay (ATP Assay kit, Calbiochem). Small pieces of liver (∼10 mg) were homogenized using a Teflon homogenizer in HEPES buffer provided in the kit. The proteins were precipitated by the addition of one fifth volume of trichloroacetic acid solution (6%) and of one tenth volume of somatic cell ATP releasing reagent. The samples were centrifuged at 3000 rpm, 4°C for 15 minutes. The supernatant was removed and neutralized with 0.1 N NaOH to establish a pH between 7 and 8. For analysis, the samples were diluted 1:25 in HEPES buffer. Immediately prior to measurements, ATP standards were prepared (10-6 to 10-12 M). A total of 100 μL of standard or sample was added to a white microplate (Fluronunc Maxisorp) and equilibrated to room temperature, followed by the addition of 50 μL of freshly dissolved luciferase. Luminescence was measured in a 96-well Perkin Elmer Bioassay reader. Background luminescence (cells and HEPES without luciferin-luciferase reagent) was insignificant. To ensure that luminescence was derived from tissue ATP, apyrase (U/mL) was added to some test samples, which resulted in an almost complete reduction of luminescence. ATP was calculated and expressed as nmol ATP per mg total protein. For analysis of the data, ATP contents of liver tissue before surgery were subtracted from those after reperfusion.
Statistical Analysis
The patients were evaluated as an intention-to-treat analysis. Summary statistics are expressed as median ± SD. The sample size was determined from the following statement: The assumption was made that the difference between the two groups was such that the probability for a better outcome for one patient in the preconditioning group versus the patient in the control group was at least 0.7. Applying the Mann-Whitney U test to a sample size of n = 44 patients per group will lead to a significant result in at least 90% of the cases.
The Mann-Whitney test was performed to test whether the two groups differed with respect to a continuous variable such as age or AST peak. To test whether the two groups differed with respect to gender, we performed a Fisher exact test. We then compared both groups with respect to AST peak in each of the following 10 subpopulations: men and women, patients younger than 60 years and older than 60 years, patients with resected liver volume <50% and >50%, patients with ischemia time <40 minutes and >40 minutes, and patients with degree of steatosis <25% and >25%.
To formally test for the influence of gender, age, resected liver volume, ischemia time, and degree of steatosis on the difference of the AST peak between the two groups, we computed multiple regression models, which included an interaction between the group and one of the covariates above as explanatory variable, and with the logarithm of AST peak as the response. The logarithm was taken to be closer to a normal distribution and to reduce the influence of possible outliers. The same issue was also tackled as follows. Patients of the two groups were matched for age, ischemia time, and volume. We thus defined 50 “pairs of patients” and we were able to correlate age, ischemia time, and volume with the difference in AST peak between the two groups. A Spearman test was then performed.
RESULTS
Are the Ischemic Preconditioning and the Control Groups Comparable?
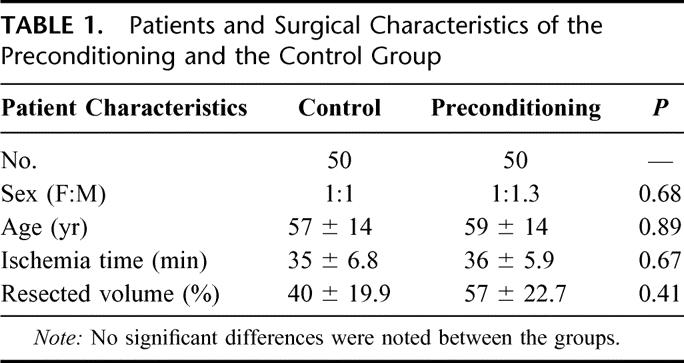
Fifty-seven men and 43 women with a median age of 57 years (range 20–84 years) were included in the study. The median resected liver volume was 45% (range 20%-80%) of the total liver volume with a median Pringle time of 37 minutes (range 30–60 minutes). There was no significant difference regarding the patient age, gender, ischemia time, resected liver volume, or degree of steatosis between patients randomized in the preconditioning versus control group (Table 1).
TABLE 1. Patients and Surgical Characteristics of the Preconditioning and the Control Group
Forty-three patients were operated for liver metastases from a colorectal cancer, 16 for hepatocellular carcinoma, 4 for cholangiocellular carcinoma, and 8 patients for various kinds of metastatic diseases. In 29 patients, liver resection was performed for benign diseases, such as liver adenoma, focal nodular hyperplasia, or benign bile duct stricture. The different diagnoses were distributed homogeneously between the preconditioning and control groups.
An extended right hemihepatectomy (SIV-VIII) was performed in 22 patients, while 6 patients underwent an extended left liver resection (>SI-IV). A formal left (SI-IV) or right (SV-VIII) hemihepatectomy was performed in 22 and 25 patients, respectively. Twenty-five patients received a segmental resection involving at least 2 segments, including 5 patients with a formal SII-III bisegmentectomy. The type and number of procedures were comparable between both groups.
Does Ischemic Preconditioning Affect Blood Loss, the Need for Transfusion, and Operating Time?
The overall median operation time was 220 minutes (range 140–440 minutes) with a median blood loss of 250 mL (range 50–5000 mL). One patient with a history of major abdominal trauma had a considerable blood loss of 5 L during surgery. There was no difference between the preconditioning and control groups regarding the length of surgery (225 ± 73 minutes vs. 240 ± 92 minutes) or blood loss (250 ± 290 mL vs. 225 ± 325 mL). Only 6 patients in the entire study received blood transfusion during surgery and were equally distributed with 3 in the control and 3 in the preconditioning group, respectively.
Does Ischemic Preconditioning Affect the Intensive Care and Hospital Stays, and Postoperative Complications?
No patient death occurred in this series. No difference was observed between the control and preconditioning group regarding hospital (median 7 days, range 3–54 days) or intensive care unit (median 1 day, range 0–16 days) stays. Four patients (2 in each group) experienced major complications, including 1 postoperative stroke, 1 temporary encephalopathy requiring ICU care, 1 biloma, and 1 large pleural effusion requiring drainage. Other more minor complications included 6 wound infections, 3 urinary tract infections, and 2 cases of urinary retention, resulting in an overall complication rate of 15%.
Does Preconditioning Prevent Postoperative Liver Injury?
The degree of ischemia and reperfusion injury of the liver was assessed by postoperative peak serum AST and ALT levels. The transaminase peak occurred between 12 hours and the third postoperative day in all cases. In most patients, AST and ALT levels returned to normal values within 7 days. Patients treated with ischemic preconditioning had a significantly lower peak AST value when compared with the control group (364 vs. 520 U/L, P = 0.028) (Fig. 2). Similarly, peak ALT values were significantly reduced in the preconditioning group when compared with patients receiving portal clamping alone (406 vs. 519 U/L, P = 0.049). In contrast, there were no differences between the control and the preconditioning group regarding postoperative prothrombin times (76% ± 18% vs. 81% ± 14%), bilirubin (46 ± 43 μmol/L vs. 56 ± 51 μmol/L), alkaline phosphatase (124 ± 80 U/l vs. 174 ± 142 U/L), and creatinine levels (101 ± 70 μmol/L vs. 74 ± 14 μmol/L).
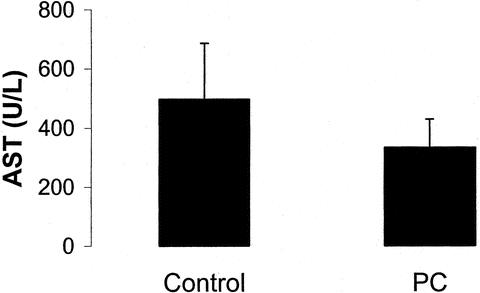
FIGURE 2. Peak AST levels of the preconditioning and the control groups after liver resection. Ischemic preconditioning resulted in decreased AST levels when compared with the control (n = 50 in each group, P = 0.028).
Which Factors Influence the Effects of Preconditioning in the Multivariate Analysis?
Univariate and multivariate analyses were performed to identify factors related to the characteristics of the patients and surgery affecting the protective effects of preconditioning. Two factors were found to be statistically significant in the multivariate analysis, including age of the patients and the amount of resected tissue. Age of the patients was found to be the most significant parameter influencing the effects of ischemic preconditioning. The protective effects were maximal in younger patients and decreased with rising age of the patients (Fig. 3). For example, when compared with the control group, preconditioning halved postoperative transaminase levels in patients younger than 60 years (Fig. 4). This effect was lost in patients older than 60 years of age with similar transaminase levels in the preconditioning and the control groups.
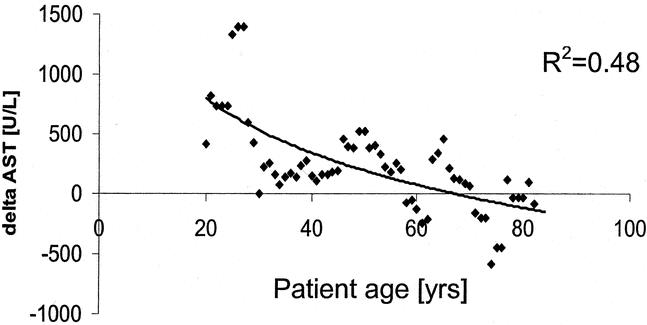
FIGURE 3. The impact of age on postoperative transaminase levels was evaluated in a match pair analysis. Each dot represents a pair of patients, one without preconditioning (control) and one from the preconditioning group. Both patients were matched for age, Pringle time, and resected volume. The y-axis represents the difference in peak AST levels in each pair (delta AST = peak-ASTcontrol – peak-ASTpreconditioning). Higher delta AST values indicate protection from ischemic preconditioning. Younger patients are maximally protected, whereas patients older than 70 years appear to have negative effects.
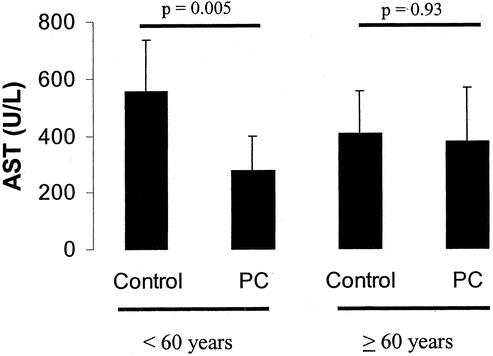
FIGURE 4. Ischemic preconditioning in patients younger than 60 years versus patients 60 years of age or older. Preconditioning resulted in a significantly reduced AST level in young patients. In contrast, older patients were not protected by ischemic preconditioning with similar AST values when compared with the control.
The protective effects of ischemic preconditioning inversely correlated with the percentage of resected tissue. Preconditioning was associated with a more than half reduction of peak transaminase levels in patients undergoing resections involving <50% of the total liver volume. In contrast, the effects of preconditioning was lost in patients undergoing major tissue loss (>50%) (Fig. 5).
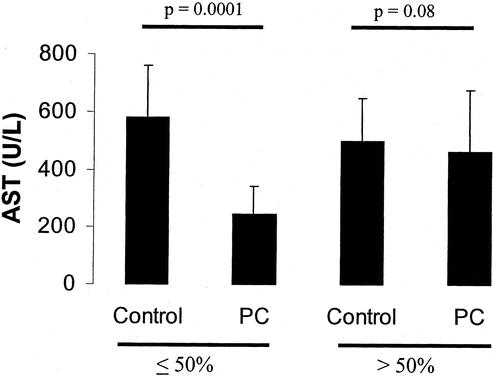
FIGURE 5. Ischemic preconditioning was particularly effective in patients receiving liver resections of <50% liver volume. In contrast, no statistical difference was detected between the preconditioning and the control group if >50% liver volume were resected.
Finally, the protective effects of ischemic preconditioning were also more apparent in patients with increasing ischemic times. While only moderate protection of preconditioning was observed in patients with clamping times <40 minutes, patients with longer ischemic times demonstrated more pronounced protective effects (Fig. 6). Although a 50% decrease of AST was present in patients with prolonged ischemia times, the value did not reach statistical significance due to a high standard deviation (P = 0.086).
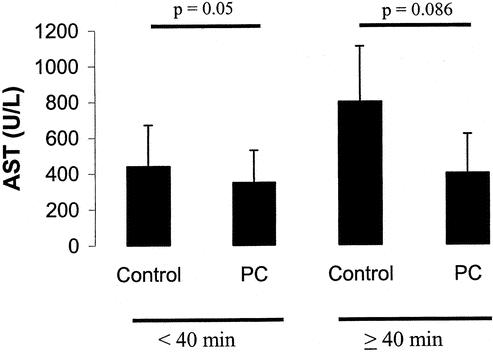
FIGURE 6. Effects of ischemia time on ischemic preconditioning. Ischemic preconditioning resulted in a 50% decrease in AST levels when >40 minutes inflow occlusion was used during liver resection. If the inflow occlusion were <40 minutes, only a moderate decrease of AST levels was achieved by preconditioning.
Did the Presence of Steatosis or Fibrosis Influence the Protective Effects of Ischemic Preconditioning?
Particular attention was paid to the presence of steatosis in the baseline biopsy. Although steatosis was not found to be significant in the multivariate analysis, probably due to the small sample size (13 patients), a univariate analysis was performed to assess the effects of preconditioning in this population of patients. Six patients in the control group and 7 in the ischemic preconditioning group were found to have fat deposition in >25% of the hepatocytes in the baseline biopsy.
In steatotic patients, ischemic preconditioning demonstrated particularly strong protective effects in terms of postoperative serum peak AST levels (363 vs. 602 U/L P = 0.049) (Fig. 7). To assess the influence of steatosis on the protective effects of ischemic preconditioning in the analysis of the whole study population, a new analysis was performed excluding patients with steatosis. In this population, including only patients (n = 87) with normal liver, the protective effects of ischemic preconditioning remained significant when compared with the control group (AST levels: 364 vs. 471 U/L, P = 0.046) (Fig. 7).
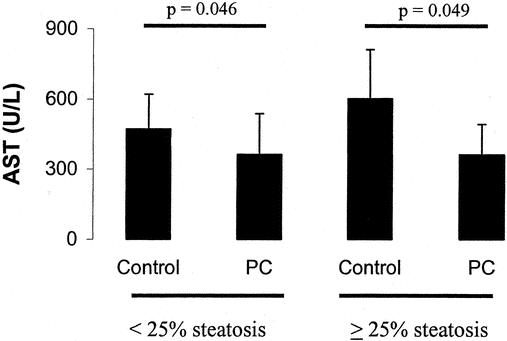
FIGURE 7. Ischemic preconditioning was particularly effective in steatotic livers with a 40% decrease of postoperative peak AST levels. In contrast, a smaller AST reduction was achieved by preconditioning in nonsteatotic livers when compared with the control.
Although cirrhosis was an exclusion criterion, 5 patients in the control group and 4 patients in the preconditioning group were found to have mild portal fibrosis on the baseline biopsy. Preconditioning had comparable protective effects in patients with mild fibrosis as in patients with normal livers with a significant reduction in postoperative peak AST levels (279 vs. 362 U/L, P = 0.04).
Is the Protective Effect of Preconditioning Mediated by Intrahepatic ATP Levels?
We evaluated whether preconditioning protects the liver against ischemic injury by preserving the intrahepatic contents of ATP. As the effects of ischemic preconditioning differed between young and older patients (see above), we hypothesized that ATP contents may account, at least in part, for this observation. To address this hypothesis, we measured ATP levels in the baseline biopsy (at the opening of the abdomen) and the biopsy performed 30 minutes after reperfusion in a selected group of patients younger and older than 60 years, with or without preconditioning. Therefore, four groups were selected, including 5 patients in each group. A matched-paired study analysis was performed for the young and old patients, respectively. Patients were matched for ischemia time, resected volume, and age. Patients younger than 60 years had a significant decrease in intrahepatic ATP levels between baseline and post-reperfusion values. In contrast, preconditioning was associated with higher ATP levels after reperfusion in this population (Fig. 8). In older patients, the results were comparable in the control group, but ischemic preconditioning did not correct the loss of ATP but rather resulted in an additional decrease in ATP levels after reperfusion (Fig. 8).
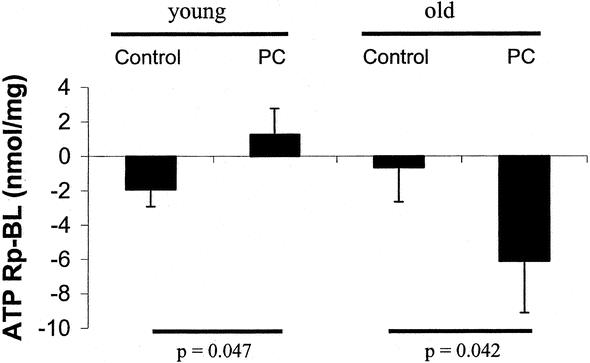
FIGURE 8. ATP levels were determined in the liver tissue from old (≥60 years) and young (<60 years) patients with or without preconditioning. Patients were matched for age, ischemia time, and resected liver volume in the young and old patient group. The results are expressed as ATP content in the post-reperfusion biopsy (Rp) minus the baseline biopsy (BL) per milligram of protein. In the young group, control patients decreased the intrahepatic ATP levels during ischemia and reperfusion, while ischemic preconditioning resulted in an increased intrahepatic ATP content. In the old group, control patients also decreased the ATP level during ischemia and reperfusion. Ischemic preconditioning in old patients resulted in a further decline of post-reperfusion ATP values.
DISCUSSION
This prospective randomized study in an unselected group of patients establishes ischemic preconditioning as a protective strategy against hepatic ischemia in humans. Additionally, the data newly indicate that the protective effects correlate with the age of the patients and the volume of the remaining liver left after resection. The study also confirms previous results suggesting that ischemic preconditioning is particularly effective in diseased liver such as steatosis. Finally, the results indicate preservation of ATP contents in the liver after reperfusion as a protective mechanism of ischemic preconditioning, and may explain the failure of the older liver to respond to preconditioning.
In a previous nonrandomized study, we provided the first evidence that ischemic preconditioning may protect against ischemic injury to the liver in humans.18 In this initial study, including 24 patients, all efforts were made to minimize variability. Each patient underwent a standardized anatomic hemihepatectomy under exactly 30 minutes of inflow exclusion with a low (0–5 mm Hg) central venous pressure. On an alternative basis, half of the patients received the same ischemic preconditioning protocol as in the current randomized study. The results indicated a significant protection with 50% reduction of the postoperative serum transaminases. The protection was even stronger among the 7 patients with mild to moderate steatosis. Additionally, as data from several animal models21,22 have shown that the initial injury following warm ischemia occur in the sinusoidal endothelial cells, we also investigated endothelial cell injury in the pilot study.18 Using special staining such as Tunnel assay for apoptosis and electron microscopy, we found strong protection from ischemic preconditioning against early sinusoidal endothelial cell injury. The purpose of the current randomized study was to evaluate the effectiveness of ischemic preconditioning in a large unselected group of patients and to identify factors affecting the protective effects.
First, considering the entire series, we found statistically significant protection from ischemic preconditioning in terms of postoperative AST and ALT levels. These results highlight the overall important protective effects against reperfusion injury considering the heterogeneous group of patients with ischemic times ranging from 30 minutes to 60 minutes, resection volumes from only 20% to as much as 70% of the total liver volume, the use of additional procedures such as hepaticojejunostomy, and patients with or without hepatic steatosis. The lack of differences regarding morbidity rates, and the ICU and hospital stays should be related to the overall low complication rates and early discharge from the ICU and hospital. For example, only 4 patients in this series experienced major complications requiring a significant prolongation of the ICU or hospital stay. There was also no mortality in this series. However, one can speculate that with a much larger sample size differences will be found as postoperative transaminase levels following ischemia are the best markers of injury.23
Next, another important aim of the study was to identify factors affecting the protective effects of ischemic preconditioning. Using a multivariate analysis, we found that age of the patients was the most significant factor affecting the effectiveness of ischemic preconditioning. For example, the protective effect was completely lost in patients older than 60 years while the effects were maximal in young patients. This is a novel finding with important clinical and scientific significance. Several mechanisms might account for the difference in protection between young and older patients. First, the protective effect of preconditioning might be associated with the preservation of post-reperfusion ATP levels, as suggested in animal studies.24,25 We found that continuous inflow occlusion was associated with a decrease in intrahepatic ATP levels in young and old patients. Ischemic preconditioning prevented the decrease of ATP in young, but not in older, patients. These results are consistent with experimental data in young mice showing that ischemic preconditioning prevents the ATP decrease during hepatic ischemia.24,25 High post-reperfusion ATP contents might be important to withstand reperfusion injury. We can speculate that a high ATP content might enable hepatocytes and sinusoidal endothelial cells to survive, while energy depletion could lead to cell death. Furthermore, apoptosis as the main form of post-reperfusion cell death requires ATP for the activation of the apoptotic cascade. This pathway might fail in the presence of low ATP contents, resulting in an unfavorable necrotic form of cell death.
Furthermore, the tolerance of hepatocytes against ischemic injury might be different in young versus older patients. The short period of ischemia during preconditioning might be harmless on hepatocytes in young patients and induce protection, while the same ischemic time may cause significant injury in the elderly. Another possibility might be that aging directly affects the protective mechanisms of ischemic preconditioning, which might no longer be active after 60 years of age. For example, the sublethal oxidative stress triggering the protective mechanisms of ischemic preconditioning26 might not occur in older patients. Finally, ischemic injury in young and old patients might be associated with different mechanisms of reperfusion injury, such as the increased susceptibility to the apoptotic cascade or dysfunction of mediators of protection, such as Bcl-2.
The protective effects of preconditioning were more pronounced with increased ischemic time. In our study, the lower limit of inflow occlusion was set at 30 minutes because this is the minimal ischemia time associated with detectable injury after reperfusion. It is possible that the relatively low degree of reperfusion injury in livers subjected to ischemic times <40 minutes, as highlighted in this series by postoperative transaminase levels, may have somewhat prevented the identification of the protective effects of preconditioning. Of note, in rodent models, we observed only minimal apoptosis after ischemic periods <45 minutes, whereas the number of apoptotic hepatocytes and sinusoidal endothelial cells increased dramatically with longer periods of ischemia.27 As preconditioning mainly prevents hepatocyte and sinusoidal cell apoptosis in these models, it can be expected that the protective effects are mainly apparent when the ischemic injury is sufficient to induce apoptosis in a large number of cells.
Another finding was the decreased protective effects in terms of transaminase levels in patients with larger resection volume. These results should be considered with caution, as small remnant liver masses were associated with lower post-reperfusion serum AST levels than larger residual volumes. Indeed, matching patients for the same ischemic time, we found that patients with extended liver resection (70% resected liver volume) had lower postoperative peak AST levels than patients with smaller resection volumes (<70%) (data not shown). Therefore, this observation is likewise due to a type 2 error (ie, the inability to detect a significant difference due to the sample size). Studies in patients with major liver resection are further warranted, as ischemic preconditioning might be important to avoid postoperative liver failure and death. The current study did not enable us to address this issue.
Our previous pilot study in humans also suggested a higher degree of protection by ischemic preconditioning in the fatty than in the normal liver. In this study, the protective effects in patients with fatty deposition in >25% of the hepatocytes were stronger than in the normal liver. This finding is of particular clinical relevance as the complication rate including postoperative liver failure is high in the presence of steatosis in patients undergoing major liver resection. The strong protective effects of preconditioning in steatotic livers might be associated with the preservation of ATP during ischemia. Other mechanisms in the fatty liver can certainly not be excluded. For example, others have shown that preconditioning of the steatotic mouse liver results in improved post-reperfusion microcirculation.28
Whether ischemic preconditioning should be used routinely during liver surgery performed under inflow occlusion or whether intermittent clamping should rather be used has remained unclear. Data in humans comparing both strategies are not available. In a rodent model, we found that intermittent clamping and ischemic preconditioning confer comparable protective effects against ischemic injury with up to 75 minutes of inflow occlusion.18 For longer periods, intermittent clamping was superior. As almost all liver resections are performed with a clamping time below 75 minutes, we believe that ischemic preconditioning should be preferable. The argument mainly relies on the increased blood loss observed with intermittent clamping when compared with continuous inflow occlusion.10 In our series, blood loss was low with only 6 patients (6%) receiving blood transfusion in the entire series. Additionally, the succession of several periods of ischemia and reperfusion may increase the operating time significantly. In our study, the operating times were similar between patients with versus without ischemic preconditioning as the period of ischemic preconditioning was performed during the preparation of the liver prior to resection.
CONCLUSION
We present the first prospective randomized study establishing the protective effects of ischemic preconditioning during liver resection in humans. We believe that ischemic preconditioning is now justified in young patients, as the protective effects were particularly pronounced in this population, and in patients with steatosis. We would recommend the use of intermittent clamping in patients older than 60 years of age as ischemic preconditioning was ineffective, and in cirrhotic patients, as no data are currently available in this population. Further studies are warranted in these latter two groups to better understand the mechanisms of injury and to design novel protective strategies.
Discussion
Dr. D. Jaeck: Congratulations for this very interesting presentation, which is complementary to your previous experimental and clinical studies on ischemic preconditioning. I would like to make two comments and to raise three questions. First, in the present prospective randomized study, you have compared ischemic preconditioning to continuous clamping; it would probably have been more relevant, for inflow occlusion periods of more than 30 minutes, to choose, for the control group, an intermittent clamping method. Second, as there is a great heterogeneity in your patients regarding age, volume of liver resection, and indication for hepatectomy, it would be interesting to report, for both groups, your data concerning mean blood loss, complication rate, hospital stay, ICU stay, etc.
My first question is about other parameters than serum AST concerning the evaluation of hepatic ischemic injury, such as postoperative bilirubin level or prothrombin time: how was the evolution of these markers in subgroups of the control and ischemic preconditioning groups (eg, in patients undergoing major hepatic resection) in patients with steatosis and in older patients? Second, did you use in some cases any method of outflow occlusion (eg, total vascular exclusion or selective hepatic vein occlusion), and if yes, which effects did you observe? Third, in your previous pilot clinical study, you focused on apoptosis of the sinusoidal endothelial cells and demonstrated that apoptosis of the sinusoidal lining cells was reduced by the ischemic preconditioning. Did you analyze damage of the sinusoidal lining cells in the present study and can you suspect why the ischemic preconditioning was not effective for the older patients and less effective in patients undergoing major hepatic resection (50% or more hepatic volume resection)? Finally, I would like to congratulate again the authors for this remarkable study.
Dr. P.A. Clavien: Dr. Jaeck, thank you so much for your kind comments and questions. Regarding your suggestion to use intermittent clamping as the control group, I think we need first to conclusively compare ischemic preconditioning with continuous inflow occlusion, since this is the simplest technique of inflow occlusion. As now we confirm in this study that ischemic preconditioning is superior, I would agree that the next step is to compare it with intermittent clamping. Your first question focuses on parameters of injury. Serum transaminase levels are the best markers of reperfusion injury in human studies and correlate well with survival in animal models. The significant difference in AST levels between the two groups suggests strong protection considering the heterogeneity of the entire series, including different surgeries, resected volumes, and ischemia times. The lack of significant differences regarding other parameters such as blood loss, bilirubin, PT, ICU stay, and complication rates is related to limitations inherent to any human trial. Safety was our primary priority, and as a result, the complication rates were low. For example, we had no mortality and no patient required a reoperation for a complication in this series. Only 6 patients required blood transfusion in the entire series, which also highlights the protective effects of inflow occlusion against blood loss. Regarding your second question, 4 patients underwent a technique of total vascular exclusion; thus, no conclusion can been drawn from this small sample size. We did not use techniques of selective hepatic vein occlusion in this series. Your third question refers to our previous study suggesting protection of ischemic preconditioning against sinusoidal endothelial cell injury. We did not investigate this type of injury in these patients.
Dr. T.E. Starzl: This is a very interesting paper and I have been quite interested in the phenomena that you described before and again today. It is slightly counterintuitive, as has been implied here, but to me the most interesting thing is the 60-year break between the young and the old patients, and I was just wondering whether it would be possible to give us some idea of the numbers below and above 60, because your mean age of the two groups is almost 60 years; it is 57 something in 1 group and 59 in the other, which would suggest that the number of young patients must be very small indeed. Could you tell us how many patients were over 60 and how many were under? (It would be very helpful.)
Dr. P.A. Clavien: Dr. Starzl, thank you so much for this question. The age of patients ranged between 20 and 84 years. In both groups, there were 27 patients below 60 years and 23 above. Figure 3 seems indeed to indicate that the younger the patient, the stronger the effect of ischemic preconditioning.
Dr. A.M.M. Eggermont: You have a very nice overall data set, which is going to lead to further biologic research regarding this age question. Nevertheless, we all wrestle with the same question, as to how you got the number 60.
Can you tell me whether the P value shown and whether the statement that the P values are significant have been corrected for multiple analyses? I ask this because obviously 60 was not predefined; therefore, it was only by a postanalysis that you came to the conclusion that 60 was the cutoff value.
Dr. P.A. Clavien: Professor Eggermont, thank you for pointing out the caution needed in interpreting subgroup analysis in such a data set. This is the reason we look at the effects of age from different angles to convince ourselves that the finding is significant and may have an impact on therapeutic strategies. Both the raw data and various statistical analyses indicated a central role of age on the effects of ischemic preconditioning. Since then, we went back to the laboratory, and in a mouse model of ischemic injury to the liver, we found also that age of animals has an strong impact on the protective effects of preconditioning. We are now looking at the underlying mechanisms. I also agree with you that we need to be careful about our suggested cutoff of 60 years. The data in Figure 3 indicate that ischemic preconditioning appears to be deleterious in patients older than 70 years of age, and for wise practical advise we suggest not using ischemic preconditioning in patients older than 60 years of age at this point.
Dr. M. Makuuchi: Thank you for your beautiful presentation. In the introduction, you said that when you perform intermittent clamping, you had a large amount of bleeding during reperfusion. So, you did not apply the method. But when we perform living donor liver transplantation, the intermittent Pringle maneuver is used routinely in donor hepatectomy. There is no bleeding after declamping. It is mainly depending whether hemostasis of the transecting surface of the removing side of the liver is undergone or not. If hemostasis is not performed on the resecting side, it results in a massive bleeding during declamping. Massive bleeding is depending on the divided portal pedicle, but the hepatic vein is not the cause. We perform a 10-minute occlusion as preconditioning and followed by 5 minutes of perfusion and then after a 15-minute occlusion is repeated. In hemihepatic or selective inflow occlusion, we perform 30-minute occlusion. There is no difference of the transaminase levels after liver resection.
I would like to ask you whether you perform preconditioning and intermittent inflow occlusion in living donor liver transplantation settling. We perform the intermittent Pringle maneuver with preconditioning in liver donor hepatectomy. The posttransplantation ALT value was significantly lower in the intermittent Pringle group. I believe that intermittent inflow occlusion is very safe even in living donor hepatectomy for liver transplantation.
Dr. P.A. Clavien: Thank you very much Prof. Makuuchi for your comments. We did not study intermittent clamping here, but this strategy presents the risk in many hands of bleeding during each reperfusion period. For example, Belghiti et al showed in a paper published in Annals of Surgery (1999) that bleeding was significantly higher during parenchymal dissection when intermittent clamping was used, when compared with continuous occlusion. As Professor Jaeck suggested, I believe a comparison between ischemic preconditioning and intermittent clamping would be very timely. Regarding your second point, we have not used or studied ischemic preconditioning in the setting of living related transplantation or cold ischemia. Finally, I agree with your last comment that the best strategy might be a protocol between ischemic preconditioning and intermittent clamping.
Dr. K.G. Tranberg: We have rarely used the Pringle maneuver in the last years. Still, we have a decent amount of bleeding and rarely have to transfuse the patients. Another reason for having a restrained attitude against intermittent inflow occlusion is that there is some experimental and also clinical evidence that this might detach cells from the periphery of the liver tumor.
Maybe it is a good idea to try intermittent inflow occlusion, or at least to use it only when really needed, from an oncologic point of view.
Dr. P.A. Clavien: Here we are dealing with beliefs rather than facts. Whether ischemia and reperfusion injury promotes tumor dissemination remains speculative. Maybe this is the opposite, as this type of injury is associated with the release of anti-tumoral cytokines such as TNFα. I personally enjoy performing my major liver resection under inflow occlusion and low central venous pressure. As seen in this series, with this strategy blood loss is low and complications are rare. Dr. Makuuchi seems to like intermittent clamping even for his living related resection and has excellent results. There is no doubt that the same can be achieved without occlusion, as you do routinely, but with the need for a prolonged period of dissection of the parenchyma. We have the same experience with living related hepatectomy. These differences in practice suggest that we need additional randomized studies to know the truth.
Dr. P.J. Friend: ATP is a sensitive marker of parenchymal injury, but it does not look particularly at other cells. I wondered if you looked at other biochemical markers; of course, there is a range of possiblities.
Dr. P.A. Clavien: No we did not look at specific cells or mechanisms of injury in this series. ATP was assessed because of the recent available data in animal models suggesting that ischemic preconditioning mainly acts through preservation or rapid restoration of ATP after reperfusion. This clinical study is consistent with this finding as ATP was well preserved by ischemic preconditioning in the protected patients (ie, the younger patients) but not in those without a protective effect. This clinical study, as all those related to translational research, has generated further important questions and a need to go back to bench studies.
Footnotes
Reprints: Pierre-A. Clavien, MD, PhD, FACS, Department of Visceral and Transplantation Surgery, University Hospital Zürich, Rämistrasse 100, 8091 Zurich, Switzerland. E-mail: Clavien@chir.unizh.ch.
REFERENCES
- 1.Wobbes T, Bemelmanns B, Kuypers J, et al. Risk of postoperative septic complications after abdominal surgery treatment in relation to perioperative blood transfusion. Surg Gynecol Obstet. 1990;171:1990. [PubMed] [Google Scholar]
- 2.Makuuchi M, Mori T, Gunven P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155-158. [PubMed] [Google Scholar]
- 3.Nagorney DM, van Heerden JA, Ilstrup DM, et al. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;10:740–748; discussion 748—749. [PubMed] [Google Scholar]
- 4.Tsao J, Loftus J, Nagorney D, et al. Trends in morbidity and mortality of hepatic resection for malignancy: a matched comparative analysis. Ann Surg. 1994;220:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454-458. [PubMed] [Google Scholar]
- 6.Ezaki T, Seo Y, Tomoda H, et al. Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg. 1992;79:224-226. [DOI] [PubMed] [Google Scholar]
- 7.Chiappa A, Makuuchi M, Zbar A, et al. Comparison of continuous versus intermittent hepatic pedicel clamping in an experimental model. Hepatogastroenterology. 2001;48:1416-1420. [PubMed] [Google Scholar]
- 8.Horiuchi T, Muraoka R, Tabo T, et al. Optimal cycles of hepatic ischemia and reperfusion for intermittent pedicle clamping during liver surgery. Arch Surg. 1995;130:754-758. [DOI] [PubMed] [Google Scholar]
- 9.Hardy KJ, Tancheroen S, Shulkes A. Comparison of continuous versus intermittent ischaemia-reperfusion during liver resection in an experimental model. Br J Surg. 1995;82:833-836. [DOI] [PubMed] [Google Scholar]
- 10.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isozaki H, Adam R, Gigou M, et al. Experimental study of the protective effect of intermittent hepatic pedicel clamping in the rat. Br J Surg. 1992;79:310-313. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt G, Halliday I, MacCaigue M, et al. Mortality, endotoxaemia and cytokine expression after intermittent and continuous hepatic ischemia. Br J Surg. 1995;82:1424-1426. [DOI] [PubMed] [Google Scholar]
- 13.Rudiger H, Kang K, Sindram D, et al. Comparison of ischemic preconditioning, intermittent and continuous inflow occlusion in the murine liver. Ann Surg. 2002; in press. [DOI] [PMC free article] [PubMed]
- 14.Peralta C, Closa D, Hotter G, et al. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264-270. [DOI] [PubMed] [Google Scholar]
- 15.Peralta C, Fernandez L, Panes J, et al. Preconditioning protects against systemic disorders associated with haptic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100-113. [DOI] [PubMed] [Google Scholar]
- 16.Peralta C, Closa D, Xaus C, et al. Hepatic preconditioning in rats is defined by a balance of adenosine and xanthine. Hepatology. 1998;28:768-773. [DOI] [PubMed] [Google Scholar]
- 17.Yadav S, Sindram D, Perry D, et al. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deleted in proof.
- 20.Deleted in proof.
- 21.Natori S, Selzner M, Valentino K, et al. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89-96. [DOI] [PubMed] [Google Scholar]
- 22.Yadav S, Howell D, Steeber D, et al. P-selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494-1502. [DOI] [PubMed] [Google Scholar]
- 23.Iu S, Harvey P, Makowka L, et al. Markers of allograft viability in the rat: relationship between transplantation viability and liver function in the isolated perfused rat liver. Transplantation. 1987;45:562-569. [DOI] [PubMed] [Google Scholar]
- 24.Peralta C, Bartrons R, Riera L, et al. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol. 2000;279:G163-G171. [DOI] [PubMed] [Google Scholar]
- 25.Selzner M, Selzner N, Rüdiger H, et al. Ischemic preconditioning: a novel strategy to protect the fatty liver against reperfusion injury. Hepatology. 2001;34:273A.11481612 [Google Scholar]
- 26.Rüdiger H, Clavien P. Sublethal oxidative stress triggers the protective effects of ischemic preconditioning in the murine liver. Hepatology. 2003; in press. [DOI] [PubMed]
- 27.Selzner M, Rudiger H, Sindram D, et al. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280-1288. [DOI] [PubMed] [Google Scholar]
- 28.Serafin A, Rosello-Catafau J, Prats N, et al. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587-601. [DOI] [PMC free article] [PubMed] [Google Scholar]