Abstract
Objective:
To define the technical factors that might contribute to hospital mortality of recipients of right lobe live donor liver transplantation (LDLT) so as to perfect the design of the operation.
Summary Background Data:
Right lobe LDLT has been accepted as one of the treatments for patients with terminal hepatic failure, but the design and results of the reported series vary and the technical factors affecting hospital mortality have not been known.
Methods:
The data of 100 adult-to-adult right lobe LDLT performed between 1996 and 2002 were prospectively collected and retrospectively analyzed. All grafts except one contained the middle hepatic vein, which was anastomosed to the recipient middle/left hepatic vein in the first 84 recipients and directly into the inferior vena cava (with the right hepatic vein in form of venoplasty) in the subsequent 15 patients. Venovenous bypass was used routinely in the first 29 patients but not subsequently.
Results:
Eight patients died within the same hospital admission for liver transplantation. There was no hospital mortality in the last 53 recipients. Comparison of data of patients with or without hospital mortality showed that graft weight/body weight ratio, graft weight/estimated standard liver weight ratio, technical error resulting in occlusion/absence of the middle hepatic vein, use of venovenous bypass, the lowest body temperature recorded during surgery, the volume of intraoperative blood transfusion, fresh frozen plasma, and platelet infusion were significantly different between the two groups. However, the pretransplant intensive care unit status of the recipients, cold and warm ischemic time of the graft, and occurrence of biliary complications were not. By multivariate analysis, low body temperature recorded during operation, low graft weight/estimated standard liver weight ratio (≤0.35), and the middle hepatic vein occlusion were independent significant factors in determining hospital mortality.
Conclusions:
To achieve a uniformly successful right lobe LDLT, the right lobe graft must contain a patent middle hepatic vein. With a completely patent middle hepatic vein, a graft size of >35% of the estimated standard graft weight may be sufficient for recipient survival. Hypothermia, which predisposes to coagulopathy and is enhanced by the use of venovenous bypass and massive blood, and blood product transfusion must be avoided.
In a consecutive series of 100 right lobe live donor liver transplantation, there were 8 hospital deaths. Lack of a patent middle hepatic vein, graft size of ≤35% of the estimated standard liver weight, and hypothermia during surgery were identified by multivariate analysis as determinants of hospital mortality.
Right lobe live donor liver transplantation (LDLT) has been emerged as one of the treatments for adult patients with terminal hepatic failure.1 However, the design of the operation and outcome of the recipients vary among the reported series.2-9 Up to now, a consensus opinion about the surgical technique has not been reached and the technical factors that affect the outcome have not exactly been known. In this study, we attempt to define the technical factors that might contribute to the hospital mortality of recipients of right lobe LDLT, so as to perfect the design of the operation.
PATIENTS AND METHODS
From June 1996 to June 2002, 100 right lobe LDLTs were performed at the University of Hong Kong Medical Centre, Queen Mary Hospital, Hong Kong. The donors were selected based on routine blood tests, clinical psychologist evaluation, and computed tomography (CT) scan, and volumetry.10 Donors with right lobe volume >40% of the estimated standard liver weight of the recipient11 and left lobe volume >30% of the total liver volume were allowed to undergo the donor operation.12 All right lobe grafts except one (donor 85) contained the middle hepatic vein. The reason that one right lobe graft did not contain the middle hepatic vein was that the middle hepatic vein received a large segment III trunk high above its junction with the segment VIII hepatic vein and inferior vena cava (IVC). Division of the middle hepatic vein proximal to segment III hepatic vein might result in too short middle hepatic vein for anastomosis. In most of the donors, the site of division of the middle hepatic vein in the donor was determined by the pattern of venous drainage around the IVC. In most instances, the segment IVb hepatic vein was preserved in the donor to maintain venous drainage of segment IV.13 In the recipient operation, after total hepatectomy, the IVC was cross-clamped in all patients except one to facilitate hepatic vein anastomoses. In the first 29 recipients, venovenous bypass was used during cross-clamping of the IVC and portal vein. In the subsequent 71 recipients, venovenous bypass was not performed.14 The right and middle hepatic veins of the donor graft were anastomosed to the recipient’s right and middle or left hepatic veins, respectively, in an end-to-end manner in the first 84 patients. Patient 85 did not have the middle hepatic vein in the graft. For the subsequent 15 recipients, the right and middle hepatic veins of the donor graft were joined to form a common hepatic vein orifice, which was anastomosed to a matched-sized triangular opening in the right and anterior walls of the recipient IVC.15 The right portal vein anastomosis was done in an end-to-end manner. The right hepatic artery was reconstructed using the microvascular surgery technique in all patients. Intraoperative Doppler ultrasonography was performed immediately after the vascular reconstruction to assess the anastomotic patency. Patient 1 did not have the middle hepatic vein anastomosed before graft reperfusion by the portal vein because we wished to reduce the duration of warm ischemic time of the graft. The graft was grossly swollen but became normal in consistency after the middle hepatic vein was reconstructed.16 Seven other patients had occlusion of the middle hepatic vein because of technical errors (twisting of anastomosis, n = 3; folding of middle hepatic vein, n = 1; stenotic anastomosis, n = 2; short graft middle hepatic vein stump leading to tension, n = 1). Together with patients 1 and 85, 9 patients had absent middle hepatic vein drainage in the graft after portal vein reperfusion. Biliary reconstruction was by hepaticojejunostomy in 70 patients, choledocho-choledochal end-to-end anastomosis in 29 patients, and a combination of two techniques in 1 patient.17 During the operation, the body temperature, volume of exogenous blood and blood product transfusion, and duration of cold and warm ischemic time of the liver graft were recorded prospectively.
In this study, the graft was weighed after portal vein flushing by University of Wisconsin solution, and the graft weight was expressed in terms of the body weight of the recipient or the estimated standard liver weight (ESLW) of the recipient using the Urata formula.11 The body temperature of the recipient was monitored continuously by a thermometer placed in the nasopharynx. All blood and blood products were warmed through a heat exchanger before transfusion. Occlusion of the middle hepatic vein was defined by the absence of a flow signal on Doppler ultrasonography in the middle hepatic vein after the portal vein reperfusion of the graft. Biliary complications were either leakage or stenosis of the biliary anastomosis that required surgical or radiologic intervention. All other complications that affected the hospital course were recorded. Hospital mortality was defined as death within the same hospital admission for the transplant operation. All preoperative, intraoperative, and postoperative data were recorded prospectively by a designated research assistant. The data were expressed as median (range). Discrete variables were compared by χ2 test (with Yates’ correction for cell numbers <10). The continuous variables were compared by Mann-Whitney U test. Logistic regression analysis was used to identify independent risk factors that predicted hospital mortality. The right lobe volume determined by CT was correlated with the weight of liver graft measured at the backtable using the Pearson correlation test. P ≤ 0.05 was considered statistically significant.
RESULTS
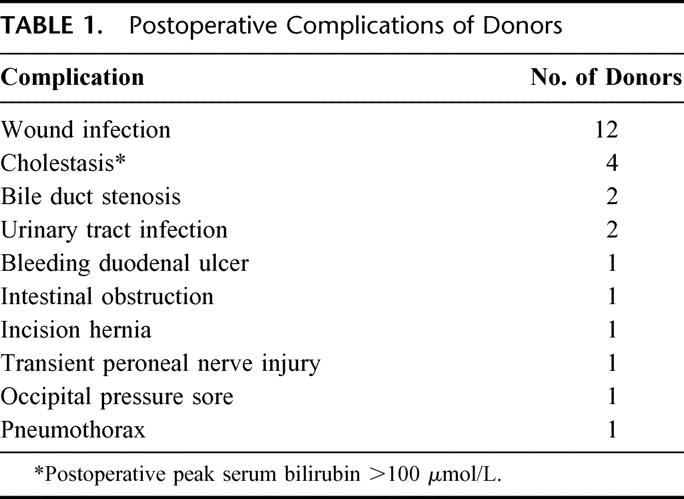
The median age of the donors was 37.5 years (range 18–57 years). Only four grafts showed fatty change of 20% to 30%. Two donors had intraoperative complications: left hepatic vein occlusion and portal vein thrombosis. They were well after the corrective treatment. Twenty-five donors developed postoperative complications (Table 1). There was no donor mortality.
TABLE 1. Postoperative Complications of Donors
Eight (8%) patients died within the same hospital admission for liver transplantation (median 40 days, range 11–98 days from liver transplantation). The causes of hospital mortality were fungal infection (n = 2), hepatic veins stenosis (n = 1), thrombotic thrombocytopenic purpura (n = 1), biliary leakage (n = 1), acute pancreatitis (n = 1), primary graft nonfunction (n = 1), and Legionnaire’s disease (n = 1). Five patients died 9.6 months (range, 5.2–19.4 months) after discharge from hospital. The causes of death included complications related to biliary stenosis (n = 2),17 empyema thoracis (n = 1), dissecting aortic aneurysm (n = 1), and recurrence of hepatocellular carcinoma (n = 1). The 6-month and 1-year graft and patient survival rates were both 91% and 88%, respectively.
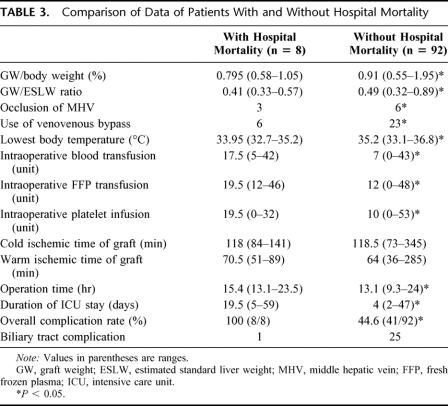
The preoperative data of patients with or without hospital mortality were comparable (Table 2). Comparison of the intraoperative data between patients with or without hospital mortality showed that the graft weight (GW)/body weight ratio, GW/ESLW ratio, occlusion of the middle hepatic vein, the use of venovenous bypass, operation duration, lowest body temperature recorded, and units of blood, fresh frozen plasma, and platelet infusion were significantly different between the two groups (Table 3). The pretransplant intensive care unit status, the model for end-stage liver disease score (Table 1),18 duration of cold and warm ischemic time of graft, and incidence of biliary tract complication (Table 3) did not affect the hospital mortality rate. Patients who died in hospital had a significantly higher complication rate and longer intensive care unit stay.
TABLE 2. Comparison of Preoperative Data of Patients With and Without Hospital Mortality
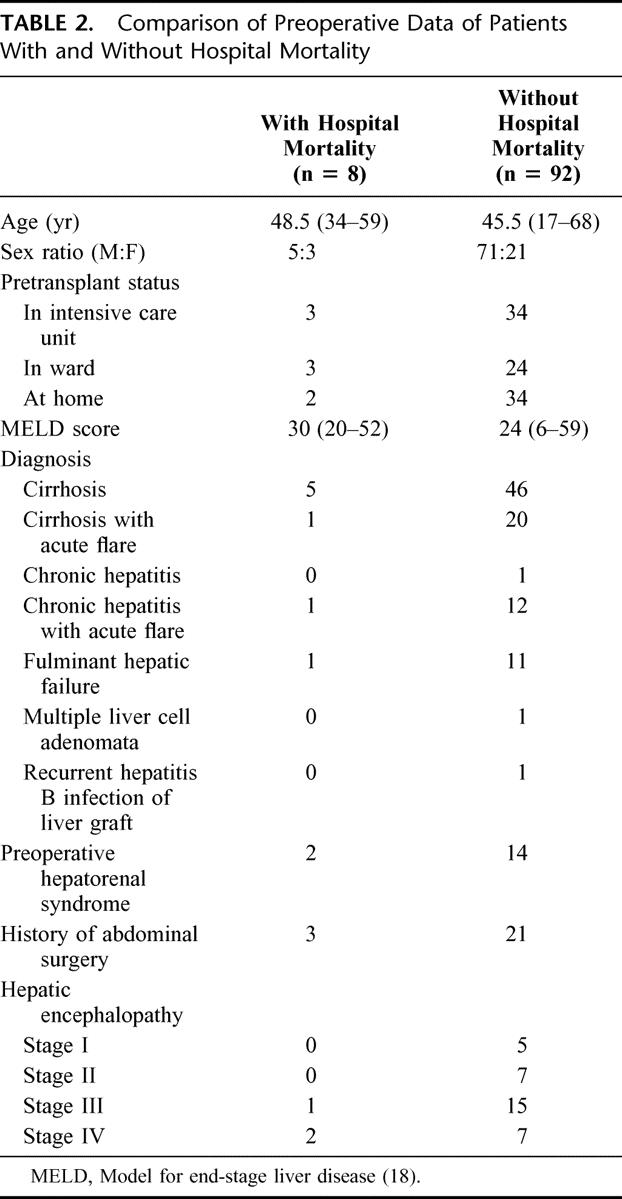
TABLE 3. Comparison of Data of Patients With and Without Hospital Mortality
Further analysis was made to determine the influence of the graft size on the outcome of the patients. The graft size measured by the CT scan was strongly correlated with the GW measured at the backtable (r = 0.857, P < 0.001). Using a cutoff of the GW/ESLW ratio of 0.40, 3 of 15 patients with GW/ESLW ratio of ≤0.40 died, while 5 of 85 patients with GW/ESLW >0.40 died. The difference in hospital mortality rate was not statistically significant. The cutoff of GW/ESLW ratio was decreased to 0.35. Two of 5 patients with GW/ESLW ≤0.35 died, while 6 of 95 patients with GW/ESLW died (χ2 = 7.323, P = 0.05).
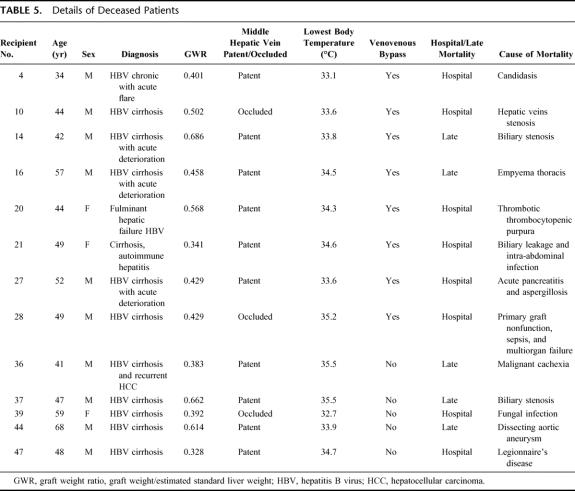
Logistic regression analysis was performed to identify independent significant factors predictive of hospital mortality (Table 3). The factors included in the multivariate analysis were GW/ESLW ratio (≤0.35), incidence of occlusion of the middle hepatic vein, use of venovenous bypass, lowest body temperature recorded during transplantation, intraoperative blood, fresh frozen plasma, platelet infusion, and period of transplantation (first 50 patients vs. second 50 patients). The factors of the lowest body temperature recorded during operation, GW/ESLW ratio (≤0.35), and occlusion of the middle hepatic vein were found to be the independent predictive factors of hospital mortality (Table 4). The adverse predictive factors of the deceased patients are listed in Table 5. The patients with hospital mortality had at least one adverse predictive factor.
TABLE 4. Factors Affecting Hospital Mortality
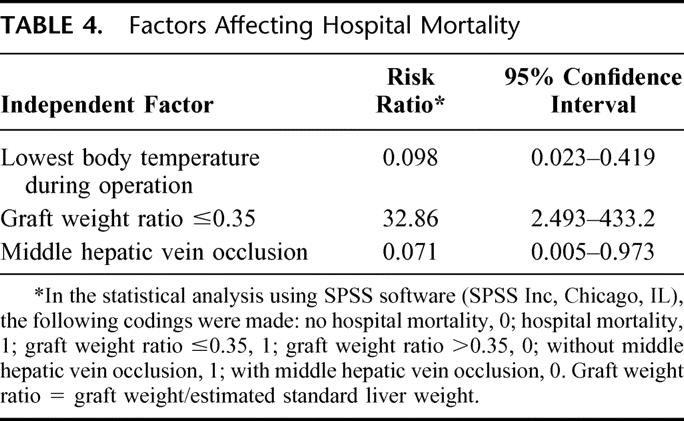
TABLE 5. Details of Deceased Patients
DISCUSSION
The present study establishes that the small graft size, lack of a patent middle hepatic vein at the time of portal vein reperfusion, and hypothermia during surgery are detrimental to the outcome of right lobe LDLT. Most of the hospital deaths in the present series were related to poor graft function, infection, and technical errors. Technical errors lead to impaired liver function, which in turn predisposes the patient to invasive infection. Thus, the risk factors are interrelated (Fig. 1).
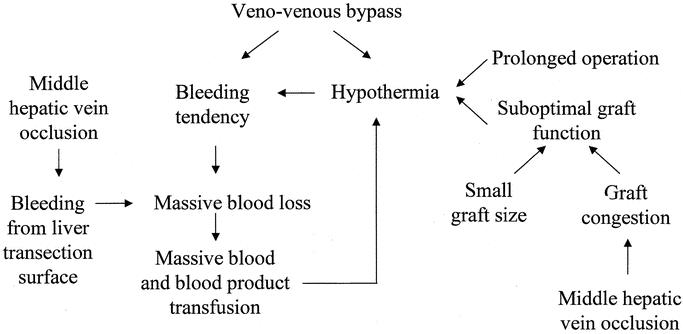
FIGURE 1. Interrelationship of risk factors for hospital mortality in right lobe live donor liver transplantation.
Hypothermia appears to play a pivotal role in determining the outcome. Hypothermia during liver transplantation could be due to the cumulative effect of exposing blood to the extracorporeal circulation in the venovenous bypass, use of excessive amount of banked and saved blood, suboptimal liver function, small graft size, graft congestion, and prolonged operation. To obtain immediate excellent graft function, selection of a graft of sufficient size, perfect portal and hepatic vein reconstruction, avoidance of venovenous bypass, and technical refinement in avoiding massive bleeding and reducing operating time are essential.
Our recent report19 has emphasized the importance of a patent middle hepatic vein in the right lobe graft. With a patent middle hepatic vein, the right anterior sector is adequately drained and the right posterior sector would not sustain serious mechanical injury from a large volume of portal blood flowing into it.20 As a result, the liver function immediately restores and, because of absence of congestion in the right anterior sector, bleeding from the liver transection surface is minimal. However, technical error may occur in end-to-end middle hepatic vein anastomosis leading to outflow obstruction. To ensure a patent middle hepatic vein anastomosis, we have adopted the venoplasty technique,21 which guarantees perfect venous outflow in the right lobe graft and hence liver function.15
The use of venovenous bypass was considered essential to maintain hemodynamic stability during cross-clamping of the portal vein and IVC,22 and cross-clamping of the IVC is considered essential for constructing a perfect venous anastomosis in right lobe LDLT.23 However, as we have demonstrated in a separate report,14 venovenous bypass is probably harmful because it may induce hypothermia,24 coagulopathy,25 and many other complications. In the presence of venovenous bypass, a vicious circle of hypothermia and bleeding tendency is established (Fig. 1) and not broken until the bypass is removed and the liver graft starts to function. In the case that the graft is suboptimal in function because of middle hepatic vein occlusion or relatively small graft size, massive bleeding continues from the liver transection surface, retroperitoneum, and laparotomy wound, and leads to a poor patient outcome.26,27
The minimum volume of the right lobe graft that can sustain survival of an adult patient in LDLT is not yet known. A small-for-size graft might not function after implantation or cannot meet the metabolic demand of the patients especially those with acute on chronic liver failure.28 Makuuchi et al29 and Kawasaki et al30 estimated that the donor liver could regenerate from a volume of 40% to a mass sufficient for the recipient. We also selected the donors based on a similar assumption and demonstrated in a small series that grafts <40% of the required liver weight were associated with a higher mortality rate and graft dysfunction.31 However, in this report, in patients with a GW/ESLW ratio ≤0.40, 80% of the patients could survive the operation. At a lower cutoff ratio of ≤0.35, the survival rate decreased to 60% and the difference in the hospital mortality rate between the group receiving grafts below this ratio and that receiving grafts above this ratio was statistically significant. The implication is that we can use a right lobe graft of a size smaller than GW/ESLW ratio of <0.40 provided that the middle hepatic vein is patent. However, such conclusion is derived from weighing of the liver graft at the backtable. Although statistically a strong correlation of the graft weight measured at the backtable with the CT volumetry was seen, CT volumetry could be sometimes erroneous. Moreover, even though the patient could survive with a graft smaller than the GW/ESLW ratio of 0.40, ultrastructural evidence of damage in the sinusoidal cells was seen in the liver grafts.32 To provide a safety margin and ensure a uniformly good result, the preoperative selection of donors should still be based on the right lobe volume measured by CT volumetry/ESLW ratio of 0.40.
CONCLUSION
Occlusion of the middle hepatic vein, small graft size ≤0.35, and hypothermia during operation are determinants of hospital mortality of right lobe LDLT. With a completely patent middle hepatic vein, a graft size of >35% of the estimated standard graft weight of the recipient may be sufficient for recipient survival. A perfect design of right lobe LDLT should include a graft size of at least ≥35% of the estimated standard graft weight of the recipient, construction of large and unimpeded hepatic venous drainage, inclusion of the middle hepatic vein in the graft, and procedures to avoid hypothermia and massive blood transfusion during the operation. Venovenous bypass, which predisposes to hypothermia and coagulopathy, appears to be unnecessary in right lobe LDLT.
Discussion
Dr. J.B. Belghiti: I want to congratulate your group for these excellent results, especially with no mortality among your last 53 recipients. These results confirm the importance of a complete venous drainage of the right graft through the middle hepatic vein (MHV). To evaluate in the donor the impact of the harvesting of the MHV, we retrospectively studied two groups of right graft donors with and without the MHV. Although we observed a slight increase of the duration of the parenchymal section (132 vs. 151 minutes) and of blood loss (639 vs. 784 mL), there was no significant difference concerning the morbidity rate between the two groups (55 vs. 33%). The analysis of the postoperative liver function showed no difference between the two groups concerning the peak of transaminases and bilirubin levels nor in the drop of prothrombin time. Moreover, the analysis of the rate of regeneration at day 5 was similar in the two groups.
You demonstrated that a complete venous drainage can improve the early function of a small graft (<35%).
My question is: what is the impact of the recipient status receiving such small grafts?
Dr. S.T. Fan: Thank you very much for your question and your kind comments. I have not shown the data of the recipients completely. The MELD score exceeds 30 in most of the patients. They are relatively sick patients, and actually a lot of the patients, about 45% of them, had acute or acute and chronic liver failure. In the March 2002 issue of the British Journal of Surgery, we reported the result of right lobe live donor liver transplantation for patients with acute liver failure and that group of patients is part of the patients reported in this paper. Patients with acute or chronic liver failure had a similar outcome with this technique compared with patients with cirrhosis. I am glad that you concur with us that the inclusion of the middle hepatic vein is necessary and safe in right lobe live donor liver transplantation.
Thank you.
Dr. Th. E. Starzl: I have been quite interested in the phenomena that you described before, and again today. The results are slightly counterintuitive, as already has been implied. The most surprising thing is the 60-year line of demarcation between the “good” young from the less satisfactory old donor. Would it be possible to give us some idea of the number of donors below and above 60? Because the mean age of your two groups is almost the same (57 years in the first group and 59 years in the second), it could be inferred that the number of young patients must be very small indeed. It would be very helpful to see the age distribution in detail.
Dr. M. Makuuchi: I am duly impressed by Professor S.T. Fan’s presentation. We also used the same outflow reconstruction as you did, but I do not think the shape of reconstruction site was satisfactory. So, we use a rectangular-shaped cryopreserved vein graft between the right and middle hepatic veins. The vein graft was then sutured to the common orifice composed by recipient IVC and the three hepatic veins. The venous patch was bulging to the ventral side. Another method we use is the double IVC method, which is useful when a few to several short hepatic veins are found in the right liver graft. The short hepatic veins are anastomosed to the cryopreserved IVC on the back table, and the graft IVC was sutured side-to-side to the recipient IVC.
Footnotes
The study was supported by the Distinguished Research Achievement Award and the Committee on Research and Conferences Grant of the University of Hong Kong.
Reprints: Professor Sheung-Tat Fan, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong. E-mail: hrmsfst@hkucc.hku.hk.
REFERENCES
- 1.Trotter JF, Wachs M, Everson GT, et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074-1082. [DOI] [PubMed] [Google Scholar]
- 2.Boillot O, Dawahra M, Mechet I, et al. Liver transplantation using a right liver lobe from a living donor. Transplant Proc. 2002;34:773-776. [DOI] [PubMed] [Google Scholar]
- 3.Cuomo O, Troisi R, Militerno G, et al. Living orthotopic liver transplant using right lobe: our experience in the first 19 donors. Transplant Proc. 2001;33:3801-3802. [DOI] [PubMed] [Google Scholar]
- 4.Steinmuller T, Pascher A, Sauer IM, et al. Living donor liver transplantation of the right liver lobe between adults [in German]. Dtsch Med Wochenschr. 2002;127:1067-1071. [DOI] [PubMed] [Google Scholar]
- 5.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SG, Park KM, Hwang S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation. 2002;74:54-59. [DOI] [PubMed] [Google Scholar]
- 7.Marcos A, Orloff M, Mieles L, et al. Functional venous anatomy for right-lobe grafting and techniques to optimize outflow. Liver Transpl. 2001;7:845-852. [DOI] [PubMed] [Google Scholar]
- 8.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7:680-686. [DOI] [PubMed] [Google Scholar]
- 9.Inomata Y, Uemoto S, Asonuma K, et al. Right lobe graft in living donor liver transplantation. Transplantation. 2000;69:258-264. [DOI] [PubMed] [Google Scholar]
- 10.Higashiyama H, Yamaguchi T, Mori K, et al. Graft size assessment by preoperative computed tomography in living related partial liver transplantation. Br J Surg. 1993;80:489-492. [DOI] [PubMed] [Google Scholar]
- 11.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [PubMed] [Google Scholar]
- 12.Fan ST, Lo CM, Liu CL, et al. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336-340. [DOI] [PubMed] [Google Scholar]
- 13.Fan ST. Adult-to-adult living liver transplantation. Adv Surg. 2001;35:187-202. [PubMed] [Google Scholar]
- 14.Fan ST, Yong BH, Lo CM, et al. Right lobe live donor liver transplantation with or without venovenous bypass. Br J Surg. 2003;90:48-56. [DOI] [PubMed] [Google Scholar]
- 15.Lo CM, Fan ST, Liu CL, et al. Hepatic venoplasty in living donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation. 2003;75:358-360. [DOI] [PubMed] [Google Scholar]
- 16.Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan ST, Lo CM, Liu CL, et al. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [DOI] [PubMed] [Google Scholar]
- 19.Fan ST, Lo CM, Liu CL, et al. The safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. in press. [DOI] [PMC free article] [PubMed]
- 20.Lee S, Park K, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812-814. [DOI] [PubMed] [Google Scholar]
- 21.Kubota K, Makuuchi M, Takayama T, et al. Successful hepatic vein reconstruction in 42 consecutive living related liver transplantations. Surgery. 2000;128:48-53. [DOI] [PubMed] [Google Scholar]
- 22.Shaw BW Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcos A, Ham JM, Fisher RA, et al. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg. 2000;231:824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Hulst VP, Henny CP, Moulijn AC, et al. Veno-venous bypass without systemic heparinization using a centrifugal pump: a blind comparison of a heparin bonded circuit versus a non heparin bonded circuit. J Cardiovasc Surg (Torino). 1989;30:118-123. [PubMed] [Google Scholar]
- 25.Neelakanta G, Colquhoun S, Csete M, et al. Efficacy and safety of heat exchanger added to venovenous bypass circuit during orthotopic liver transplantation. Liver Transpl Surg. 1998;4:506-509. [DOI] [PubMed] [Google Scholar]
- 26.Mor E, Jennings L, Gonwa TA, et al. The impact of operative bleeding on outcome in transplantation of the liver. Surg Gynecol Obstet. 1993;176:219-227. [PubMed] [Google Scholar]
- 27.Fan ST, Lo CM, Liu CL, et al. Causes of hospital death in patients undergoing liver transplantation. HPB. 1999;1:85-89. [Google Scholar]
- 28.Testa G, Malagó M, Nadalin S, et al. Right-liver living donor transplantation for decompensated end-stage liver disease. Liver Transpl. 2002;8:340-346. [DOI] [PubMed] [Google Scholar]
- 29.Makuuchi M, Kawasaki S, Noguchi T, et al. Donor hepatectomy for living related partial liver transplantation. Surgery. 1993;113:395-402. [PubMed] [Google Scholar]
- 30.Kawasaki S, Makuuchi M, Matsunami H, et al. Preoperative measurement of segmental liver volume of donors for living related liver transplantation. Hepatology. 1993;18:1115-1120. [PubMed] [Google Scholar]
- 31.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112-1116. [DOI] [PubMed] [Google Scholar]
- 32.Man K, Fan ST, Lo CM, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256-264. [DOI] [PMC free article] [PubMed] [Google Scholar]