Abstract
Objective:
To determine the risk, the benefit, and the main factors of prognosis of third liver resections for recurrent colorectal metastases
Summary Background Data:
Recurrence following liver resection is frequent after a first as after a second hepatectomy. Second liver resections yield a similar survival to that obtained with first liver resection, but little is known about third hepatectomy.
Methods:
This study reports a retrospective analysis of 60 patients who underwent a third liver resection for colorectal metastases in a 16-year experience (1984–2000). Patients were identified from a prospective database that collected 615 consecutive patients who cumulated 883 hepatectomies (615 first, 199 second, 60 thirds, and 9 fourths). Third hepatic resections were compared with first and second procedures, in terms of risk and benefit for the patient. Prognostic factors of survival after third hepatic resection were determined by univariate and multivariate analysis
Results:
A third hepatic resection was attempted in 68 of 115 of liver recurrences following a second hepatectomy (59%) and achieved in 88% of the cases (60 of 68). There was no intraoperative mortality or postoperative deaths within the 2 months. Fifteen patients developed postoperative complications (25%), a rate similar to that of first and second hepatectomies. Overall 5-year survival was 32% and disease-free survival was 17% after the third resection. Survival compared favorably to that of patients with recurrence following a second hepatectomy who could not be operated (5% at 3 years) or who failed to be resected (15% at 2 years, P = 0.0001). It also compared favorably to that of patients who underwent only two hepatectomies (5-year survival, 27%). When estimated from the time of first hepatectomy, survival was 65% at 5 years for the 60 patients who underwent three hepatic resections. Concomitant extrahepatic tumor was treated in 16 patients (27%) by 11 abdominal procedures and 5 pulmonary resections. By multivariate analysis, tumor size >30 mm for first liver metastases, presence of extrahepatic tumor at second hepatectomy, and noncurative pattern of third liver resection were independent prognostic factors of reduced survival.
Conclusions:
Third hepatectomy is safe and provides an additional benefit of survival similar to that of first and second liver resections. It is worthwhile when curative and integrated into an intended multimodal strategy of tumoral eradication.
This study analyses the risk, the benefit, and the prognostic factors of the outcome of 60 patients submitted to a third liver resection for colorectal metastases at a single institution. Third hepatectomy is safe and provides when potentially curative, a chance of survival similar to that of first and second liver resections.
Despite the curative intent of liver surgery for colorectal metastases, approximately 60% of the patients will present a tumor recurrence,1–4 30% of whom under the form of isolated liver metastases.1,3,5,6 Prevention of this recurrence relies on postoperative adjuvant chemotherapy with, however, controversial results7–10 and, if any, a limited incidence on the diminution of recurrences.8,9 Treatment mainly involves repeat hepatectomy, and it has been shown in recent years that second hepatectomy have similar risks and outcome as first liver resections.11–27 A new liver recurrence will occur in the same proportions of patients as following the first hepatectomy;3 64% in our experience18 and more than one half of these patients have solitary lesions.3,28 The problem arises in this situation as to whether a third liver resection should be reasonably envisaged in view of the risks and the results of such a procedure. Few series reported a third hepatectomy: the numbers totaled no more than 39 patients in a review of the literature published by Wanebo et al in 199629 and only 10 patients had available data in another review of Neeleman and Andersson.30 In this study, we conducted a retrospective analysis of a 16-year experience in hepatic resection of colorectal metastases to assess the perioperative mortality and morbidity of third liver resections as well as their outcome with the aim to define the risk to benefit ratio of these procedures and the main factors of prognosis.
PATIENTS AND METHODS
From January 1984 to December 2000, a total of 615 consecutive patients underwent hepatic resection for colorectal liver metastases at our institution. Of these, 416 patients had only a single procedure (68%), 139 had only two liver resections (22%), and 60 had three liver resections (10%). These 60 patients are the subject of the present study. They were identified from a prospective database collecting all the patients resected for colorectal metastases and their data were retrospectively reviewed. Patients with three hepatic resections (Group 3) were compared with those with only one or two hepatic resections (Group 1 and Group 2, respectively), in terms of tumor characteristics, of surgical treatment of primary and metastatic disease, and of outcome. To avoid any overlap of patients between groups and to allow adequate interpretation of the comparison between groups, each patient was affected to only one group in relation to the total number of hepatectomies he underwent. The objective was to define, if any, a different profile between patients submitted to a third or a second hepatectomy and those who underwent a single procedure. However, for the real assessment of the risk related to the procedure itself, all third hepatectomies were compared with all second and all first liver resections. A total of 883 hepatectomies (615 first, 199 second, 60 thirds, and 9 fourths were performed on the 615 patients).
The principle of our surgical treatment of metastases was to perform hepatic resection whenever possible and potentially curative, independently of the presence of negative factors of prognosis.31 All patients were followed in the same way after colectomy and after hepatic resection: serum carcinoembryonic antigen, carbohydrate antigen (CA 19–9) assay, and abdominopelvic ultrasound every 4 months, and abdominal and chest computed tomography (CT) scan every 8 months during the first 2 years. Thereafter, the periodicity of follow-up changed to a 6- and a 12-month basis, respectively.
Selection of Patients and Preoperative Management
Patients were selected for a third hepatectomy according to the same criteria yet reported for a second hepatectomy.18 The liver recurrence should be completely resected in the absence of any unresectable extrahepatic tumor at abdominal and chest CT scan. In recent years, hepatic magnetic resonance and positron emission tomography scan were done in cases where a diagnostic doubt persisted after the mentioned explorations.
All the recurrences were diagnosed during the routine follow-up of patients submitted to a liver resection. Preoperative chemotherapy was routinely used for patients with multiple lesions, with a short disease-free interval from the second hepatectomy, or patients with concomitant extrahepatic tumor. It consisted mainly in a combination of 5-fluorouracil, folinic acid, and oxaliplatin administered for 4 to 6 courses. Liver resection was performed in the absence of tumor progression likely to affect the curativity of surgery. A liver resection without preoperative chemotherapy was reserved to patients with unique liver recurrence occurring more than 1 year after the second hepatectomy, in the absence of any extrahepatic disease.
Operative Technique
The operative technique of repeat liver resections has been detailed previously.18 The overall policy of liver resection in the institution was based on the attempt of a radical resection by anatomic or wedge resection, sparing the highest amount of liver parenchyma as possible but provided as much as possible a margin of 1 cm from the tumor. Third hepatectomies were still more demanding than second liver resections stressing the need for all hepatic surgeons to integrate the further possibility of a repeat resection even at a second hepatectomy. Division of adhesions of the remnant liver to adjacent organs, especially the diaphragm, was time-consuming. The liver was also often more fragile and prone to bleed owing to the changes induced by chemotherapy. Because of the previous dissections, we often faced difficulties for the dissection of the hepatic pedicle, to allow a Pringle maneuver. Third hepatectomies obviously needed all the modern technological armamentarium of liver surgery, including intraoperative ultrasound, the ultrasonic dissector, the argon beam, and the bipolar coagulation forceps to reduce as much as possible intraoperative blood loss. Cryosurgery and/or radiofrequency devices were also very useful in combination to conventional surgery to treat nonresectable remnant lesions. By this, it was possible to extend the indications of liver resection to a significant proportion of patients who otherwise would have been contraindicated for surgery.
Postoperative Management
Systemic chemotherapy using mainly 5-fluorouracil and folinic acid and combined more recently to oxaliplatin or irinotecan, was delivered routinely after a third hepatic resection. The usual duration of treatment was 6 months. Discontinuation of the treatment was decided in case of confirmed tumoral remission on the biologic and on the imaging checkup. Patients were reviewed at 1 month and then every 4 months with evaluation of tumor markers, liver function tests, and hepatic ultrasonography. Abdominal and chest CT scan were performed every 8 months. In case of associated resectable extrahepatic recurrence, resection of the extrahepatic site was usually performed 2 to 3 months after hepatic surgery, with systemic chemotherapy in between, to prevent tumor progression.
Statistical Analysis
Chi square test and analysis of variance were respectively used to compare proportions and means between groups. Survival probabilities were estimated using the Kaplan-Meier method. The log-rank test was used to compare patient survivals for different prognostic factors. The stepwise Cox proportional hazard regression model was used to assess the independent factors of patient survival. A P value < 0.05 was considered statistically significant.
RESULTS
Evolution of the Frequency of Repeat Liver Resections for Colorectal Metastases
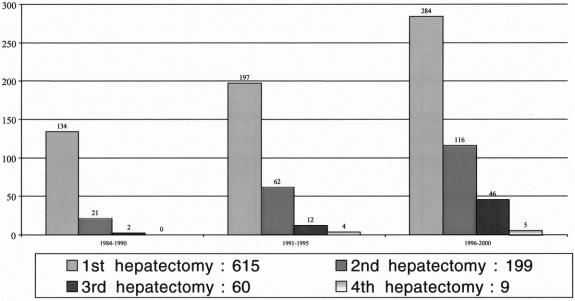
A significant increase in the number of repeat liver resections was observed in the study period. While repeat hepatectomies represented 23 of 157 overall resections (15%) in the period from 1984 to 1990, the proportion increased to 28% (78 of 275) in the period from 1991 to 1995 and 37% (167 of 451) in the period 1996 to 2000 (Fig. 1). Third hepatectomies represented 1% (2 of 157), 4% (12 of 275), and 10% (46 of 451) for the same periods, respectively.
FIGURE 1. Evolution of first and repeat liver resections of colorectal metastases in relation to three periods of time (1984–1990, 1991–1995, and 1996–2000) at Paul Brousse Hospital.
Feasibility of Third Hepatectomies
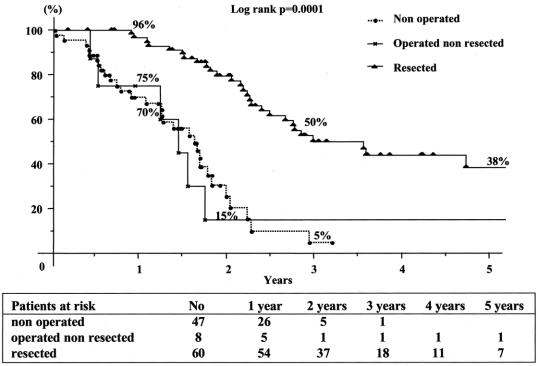
From the 199 patients who underwent a second hepatectomy, a tumor recurrence was identified in 156 cases (78%), 115 of which (58%) in the liver, either isolated or with associated extrahepatic recurrence. A third hepatectomy could not be envisaged in 47 of these patients (41%) because of tumoral diffusion. It was attempted in 68 patients (59%), 60 of whom underwent the procedure (52%). These resected patients represented 30% of the 199 patients who underwent a second hepatectomy. For the 8 patients who presented an intraoperative contraindication to a third resection, the cause was the impossibility to achieve a curative resection in 4 (3 upstaging of liver metastases, 1 carcinomatosis) and a technical impossibility of correct liver exposure related to fibrous adhesions of the remnant liver to adjacent organs, particularly to the diaphragm, in 4 patients. Two of these patients could be treated one by cryotherapy and the other by radiofrequency.
Characteristics of Primary Tumor, First and Recurrent Liver Metastases
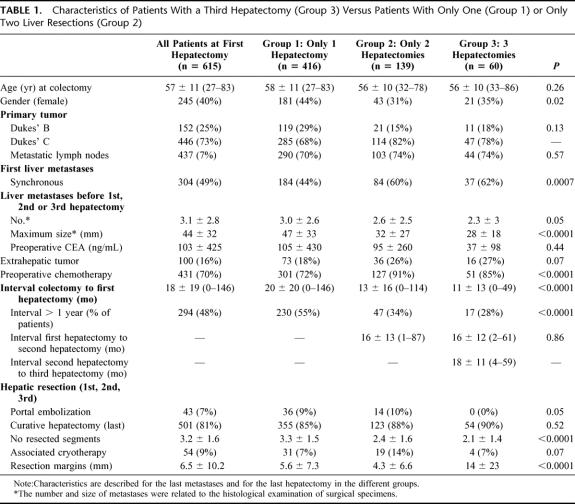
As far as patients submitted to a third hepatectomy were compared with those who underwent only one or only two hepatectomies (Table 1), no difference between the three groups was observed for age and for characteristics of the primary tumor. First liver metastases were more frequently synchronous in Group 3 than in Groups 1 and 2. Accordingly, the time interval between colectomy and first hepatectomy was lower in patients submitted to three liver resections, compared with patients who underwent one or two hepatectomies. This time interval was more than 1 year in 28% of patients with three liver resections as compared with 55% of those with a single procedure and 34% of those with two hepatectomies. No difference was observed for the time interval between first and second hepatectomy, similar for patients submitted to two liver resections and for patients submitted to three hepatectomies. The third hepatectomy was performed after a mean of 18 ± 11 months (range 4–59 months) after the second one.
TABLE 1. Characteristics of Patients With a Third Hepatectomy (Group 3) Versus Patients With Only One (Group 1) or Only Two Liver Resections (Group 2)
With regards to the characteristics of first or second liver metastases, no difference existed between groups. However, when the metastases of the last hepatectomy were considered, the maximum size and the number of liver metastases before the third hepatectomy were smaller than that of first and second hepatectomies. By contrast, there were more patients with extrahepatic tumor in Groups 3 and 2 than in Group 1, and preoperative chemotherapy was more frequently used before third and second hepatectomy than before first liver resection.
Surgical Treatment
Forty-six of the 60 third hepatectomies (76%) were limited liver resections (<3 segments) and 14 were major hepatectomies (24%), a proportion significantly lower than for first (62%) or for second hepatectomies (59%). Twelve (20%) were anatomic resections, a proportion also lower than first (56%) and second procedures (52%). Fifty-four of the 60 patients (90%) underwent a potentially curative resection, a curativity similar to that obtained with first (85%) and second hepatectomies (88%) Table 1). For the remaining 6 patients, the cause of noncurative treatment was the impossibility to achieve a complete resection of all the hepatic tumors in 4, unresectable celiac lymph nodes in 1, and diffuse carcinomatosis in 1. Portal embolization was not used before third hepatectomy as opposed to first and second liver resections (0% vs. 9% and 10%, respectively, P = 0.05). Concomitant extrahepatic tumor was found in 16 patients (27%), 10 of whom in the abdominal cavity, concerning the lymph nodes of the hepatic pedicle and/or of the celiac area (3 patients), the adrenal gland (1), the diaphragm (2), the colon (1), the stomach (1), or the peritoneum (1). One patient concomitantly has lymph nodes of the hepatic pedicle and a metastasis of the right adrenal gland. Most of these tumors were incidentally discovered during the procedure, and combined resections were only performed when potentially curative. Associated procedures involved 4 lymphadenectomies, 2 adrenalectomies, 2 partial resections of the diaphragm, 1 colectomy, 1 partial gastrectomy, and 1 resection of a limited carcinomatosis. Extra-abdominal metastases in 6 patients were exclusively localized in the lungs. Among these, 5 underwent a subsequent thoracotomy for pulmonary resection, 1 of which was bilateral.
Operative Mortality
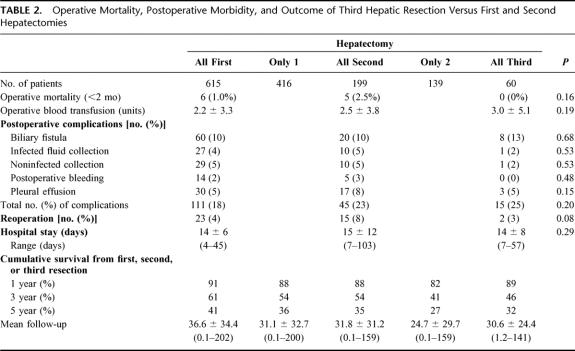
There was no intraoperative mortality or postoperative deaths within the 2 months in the 60 patients. The mean number of transfused blood units was 3.0 ± 5.1 similar to that of patients who underwent first or second hepatectomies (Table 2). Ten patients (17%) required 5 blood units or more, a proportion also similar to that of first and second hepatectomies (16% and 22%, respectively)
TABLE 2. Operative Mortality, Postoperative Morbidity, and Outcome of Third Hepatic Resection Versus First and Second Hepatectomies
Postoperative Complications
Fifteen patients developed postoperative complications (25%) (Table 2). There were 11 complications directly related to the hepatic resection (18%): 8 biliary leaks (ie, presence of bile through the abdominal drainage or within a collection), 1 of which required reoperation for bile peritonitis and 1 needed percutaneous drainage, 1 subphrenic abscess drained percutaneously, 1 transient liver insufficiency, and 1 caval thrombosis regressive after anticoagulation therapy. Four patients presented general complications (7%), 3 of whom had pleural effusions and one with traumatic intracranial hemorrhage needed urgent neurosurgery.
Reoperation was required in 2 patients for the yet mentioned bile peritonitis and urgent neurosurgery (3%), a proportion similar to that of first and second resections (4% and 8%, respectively).
Outcome
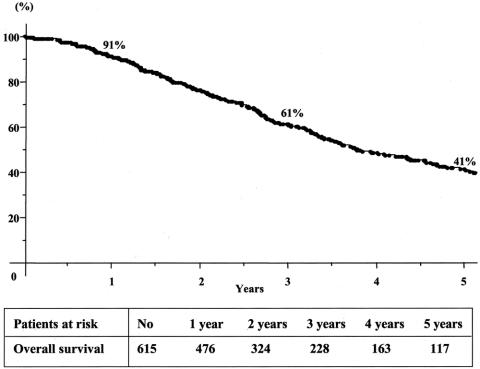
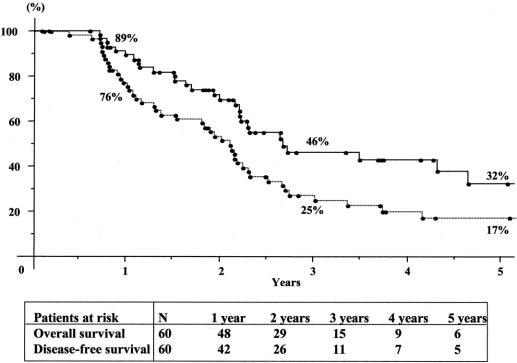
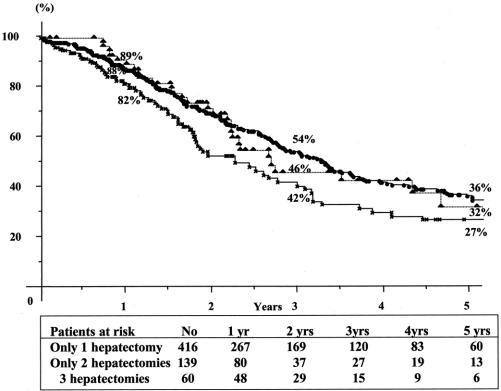
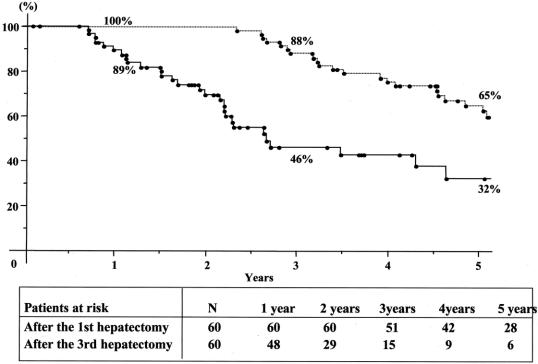
Overall 5-year survival of the total series was 41% from the time of first hepatectomy (Fig. 2) For third liver resections, overall 5-year survival was 32% and disease-free survival was 17% from the time of third hepatectomy (Fig. 3). Survival compared favorably to that of nonoperated patients (5% at 3 years) or to patients who failed to be resected (15% at 2 years, P = 0.0001) (Fig. 4). It also compared similarly to that of patients who underwent only one or two hepatectomies (36% and and 27%, respectively) (Fig. 5). When estimated from the time of first hepatectomy, survival was 65% at 5 years for the 60 patients who underwent three hepatic resections (Fig. 6). After a mean follow-up was 31 ± 24 months after the third hepatectomy (range 1.2–141), a new recurrence occurred in 44 of the 60 patients (73%), 22 (50%) only in the remnant liver, 10 (23%) both in the remnant liver and in extrahepatic sites (lungs 9 cases, lymph nodes 3 cases, diaphragm 1 case), and 12 (27%) only in extrahepatic sites (lungs 5, lungs and pelvis 1, lungs and vagina 1, bone 2, lymph nodes 1, adrenal 1, brain 1). Eighteen patients (41%) had a surgical treatment of their recurrence. Treatment of this recurrence included a fourth hepatectomy in 10 patients (23%), 2 of which followed by a pulmonary resection and a single pulmonary resection in 6 other patients (14%). Two other patients (4%) underwent an adrenalectomy and a partial resection of the vulva.
FIGURE 2. Cumulative survival after first hepatectomy for colorectal metastases.
FIGURE 3. Cumulate overall (—) and disease-free survival (—-) after third hepatic resection for colorectal metastases.
FIGURE 4. Cumulate survival from the time of recurrence after second hepatectomy in relation to the type of treatment of the recurrence.
FIGURE 5. Cumulative survival after the last hepatectomy in patients with only 1 (–•–), only 2 (–×–), or 3 (–▴–) hepatectomies.
FIGURE 6. Cumulate survival after the first (—-) and after the third (—) hepatectomy in the group of 60 patients with three hepatic resections.
Overall, the operations performed on these 60 patients included 190 hepatectomies, 4 combined cryotherapies, 13 intra-abdominal resections, and 13 pulmonary resections for a total of 220 procedures (3.7 operations per patient).
At present, 30 patients have died (50%) and 30 are currently alive (50%), 10 of whom without disease (33%). At 5 years, 6 patients are alive, 5 of whom without recurrence after the third hepatectomy.
Prognostic Factors of Survival
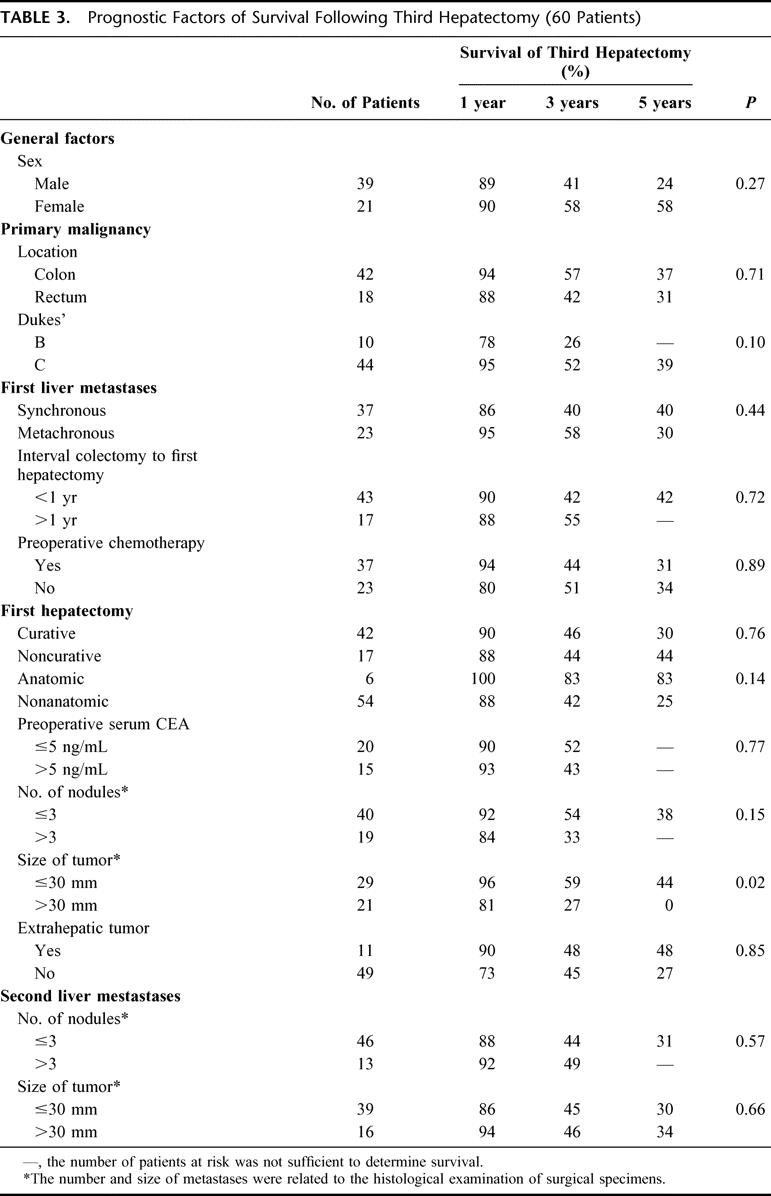
Within the multiple factors evaluated as potential determinants of prognosis after a third hepatectomy (Table 3), the characteristics of the primary tumor had no influence. A maximum size of first hepatic metastases exceeding 30 mm was associated with a decreased survival after third hepatectomy (27% vs. 59% at 3 years, P = 0.02). The characteristics of second metastases and of second resections were not predictive of outcome after third hepatectomy excepted for the presence of extrahepatic tumor associated with a poorer outcome (P = 0.09). While the number and the maximum size of third metastases had no influence on the outcome, the main factor of prognosis was the curative pattern of the third liver resection (5-year survival 35% vs. 0% for noncurative procedures, P = 0.002).
TABLE 3. Prognostic Factors of Survival Following Third Hepatectomy (60 Patients)
At multivariate analysis a tumor size >30 mm for first liver metastases (P = 0.01, risk ratio 3.0), the presence of extrahepatic tumor at second hepatectomy (P = 0.04, risk ratio 3.1), and the noncurative pattern of third liver resection (P = 0.009, risk ratio 5.9) were independent prognostic factors of reduced survival.
DISCUSSION
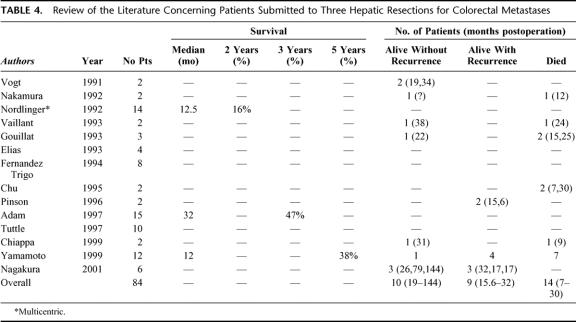
Repeat hepatectomy for colorectal recurrence is increasingly performed owing to recent advances in the early diagnosis of recurrence, in the efficacy of chemotherapy, and in the expertise of hepatic surgery. This approach has a reported survival benefit of 30% to 41% at 5 years,11–27 similar to that of primary liver resection.31 However, tumor recurrence is frequent after either a first1–4 or after a second resection.3,6,12,18 In our reported series of rehepatectomies, 41 of 64 patients (64%) had presented a recurrence after a median follow-up of 27 months.18 In the present study, the rate of recurrence after a second hepatectomy is 78% overall (ie, intrahepatic and/or extrahepatic) and 57% for the sole hepatic location. The problem arises in this situation as to whether a third liver resection could be indicated. Little has been reported to date to answer this question. In the most recent review of the literature (Table 4), 84 patients underwent a third liver resection for colorectal recurrence, but outcome was available in only 33 patients. No study was specifically dedicated to third hepatectomies, only 4 of 14 reports mentioned more than 10 patients, and finally only 2 patients survived longer than 5 years after the resection.
TABLE 4. Review of the Literature Concerning Patients Submitted to Three Hepatic Resections for Colorectal Metastases
The results of our study show that third hepatectomy is safe and provide an additional benefit of survival similar to that provided by first and second liver resections. There was no mortality within 60 days after the procedure and the 5-year survival was 32%.
Third hepatectomies are obviously addressed to a limited proportion of patients selected as shown by our study by the potentiality of a curative surgical treatment. Accordingly, the patients of the study represented 10% of the total group of patients resected for colorectal metastases (60 of 615), 32% of patients who underwent a second hepatectomy (60 of 199), and 52% of the patients who developed hepatic recurrence following a second liver resection (60 of 115). Noteworthy, extrahepatic metastases were not considered as a contraindication provided that they could be resected at the same time or further to the treatment of liver metastases. When indicated third hepatectomy was not always possible and feasibility was 88% (60 of 68). Refinements of liver imaging and improvements in the radiologic diagnosis of tumor recurrence as currently allowed by positron emission tomography) will probably decrease in the future the number of patients for whom a third hepatectomy is contraindicated at laparotomy.
A critical issue in performing a third hepatectomy is the risk of this iterative procedure. There has been concern that reresections may be associated to higher intraoperative bleeding32,33 and to higher rates of complications.33 Indeed, repeat hepatectomy are technically more demanding and more time-consuming, and third hepatectomy is still exposed at a higher risk of complications. However, owing to the currently available technology and expertise in liver surgery, it has been possible in our study to obtain perioperative courses similar to that of first and second liver resections. No more operative bleeding and no more complications than that observed after a first hepatectomy occurred after third hepatic resections. However, technical problems were faced in 4 of the 8 operated patients who failed to have a third resection indicating that these operations are obviously more difficult to achieve.
The benefit provided by third hepatic resection is currently demonstrated by the 5-year survival rate of 32% observed in our patients, a rate similar to that obtained after first or second hepatectomy. This compares favorably to the poor survival of nonoperated patients (5% at 3 years) or to that of patients who failed to be resected for recurrence after a second hepatectomy (15% at 2 years). A further demonstration of the benefit provided by third resection is a 5-year survival rate of 65% from the time of the first hepatectomy, a rate never described in the literature for patients affected of colorectal liver metastases. By comparison, survival of patients submitted to only one hepatectomy in our study was 36%, the difference in survival reflecting the positive impact of repeat hepatectomy on the final outcome of this selected group of patients. Similarly, the survival of patients submitted to only two liver resections was 27%, whereas it was 35% for the total group of patients submitted to at least two resections, the difference reflecting again the positive effect of a third or fourth hepatectomy, when feasible. One may argue the hypothesis that the benefit obtained in these 60 patients submitted to a third hepatectomy could have been related much more to a favorable biology of the tumor rather than to the surgical treatment per se. However, no spontaneous long-term survival has been reported,34,35 and even with more effective regimens of chemotherapy, the benefit in survival is limited with 0% to 2% of patients alive at 5 years.36,37
No major difference in terms of patient demographics and tumor staging was evidenced when comparing the different groups of patients having only one or only two liver resections to those with three liver resections. The only difference was the higher incidence of synchronous metastases in our study group, a factor more negative than positive in terms of outcome.2,38 No more difference was observed when comparing all first or all second procedures in the three groups of patients. Therefore, it was unlikely that patients submitted to three hepatic resections belong to a very favorable group of patients whose outcome could have been satisfactory without the need of surgical treatment. More likely, these patients have benefited from a combination of an aggressive surgical approach and a relatively favorable tumor biology.
This survival benefit was obtained through a close follow-up of resected patients and a constant attempt of tumor clearance by a variety of means, including repeat hepatic resections, preoperative and postoperative chemotherapy, adjuvant treatments such as cryotherapy radiofrequency or portal embolization, along with surgical treatment of extrahepatic localizations. For third hepatectomy, the curative patterns of resection appears as the main factor of a good outcome as it was previously reported for second hepatectomies.18 However, the prognostic value of the maximum size of first liver metastases and of the presence of extrahepatic disease at second hepatectomy clearly indicates that all the history of patient disease is concerned for the outcome after third liver resection.
An alternative treatment to repeat hepatectomy has emerged in the most recent years, based on the local treatment of metastases by radiofrequency.39–41 At present, ablative techniques are used in combination to conventional resection for nonresectable lesions, but no data are available on the long-term efficacy of radiofrequency in metastases. Recent results from our group indicate that the risk of local recurrence could be higher than expected at least for percutaneous procedures.42 Therefore, resection should be the preferred approach when possible and ablative techniques should be reserved to unresectable recurrences.
CONCLUSION
Third hepatic resections are safe although more technically demanding. They provide a survival benefit of 32% at 5 years to about one third of patients with colorectal recurrence following a second hepatectomy. This survival benefit cumulates that obtained with previous hepatic resections, leading to an expected survival of 65% at 5 years for this group of patients. Third hepatic resections appear worthwhile when curative and integrated to a strategy of tumor eradication when associated to resectable extrahepatic disease.
ACKNOWLEDGMENTS
The authors thank Valérie Delvart for performing all the statistical analysis of the study and for fruitful discussion on the methodology.
Discussion
Dr. P.A. Clavien: Professor Adam, thanks for sharing this impressive experience with us. I also had the privilege to read the manuscript prior to the presentation. You are providing important data regarding surgery at a time when we are seeing an increasing number of alternative ablation therapies, often marketed prior to conclusive demonstration of their efficacy. The 65% 5-yr survival after the first hepatectomy and the absence of operative or early postoperative death in your series should represent the standard to which other therapies should be compared.
I have 3 questions. First, your paper indicates actuarial survival estimated on the Kaplan-Meier method rather than actual survival. As the follow-up ranges from 1 month to many years, it would be worthwhile to know the actual survival rate, which might better represent the real survival benefit.
Second, your study covers a 16-year period, from 1984 until 2000, and many changes have occurred in several areas such as preoperative evaluation, surgical techniques and importantly the use of novel chemotherapeutic agents. Could you comment on how these changes have affected your indications for re-resection and results? On the same token, did some of these changes explain the increased number of 3rd hepatectomies in recent years? Could you also explain your strategy for “repeated” neoadjuvant chemotherapy?
Third, an obvious question, is how selection of patients may have influenced your results. Possibly, the single most important factor, which has influenced patient survival over the past few years, is related to our improved ability to identify extra-hepatic diseases. PET scan has become a standard preoperative test in our practice, and as reported by others, has enable us to change our indication for surgery in about 20% of cases due to the identification of extra-hepatic diseases, otherwise not diagnosed by other modalities. Could you comment whether you achieved better selection of patients over the recent years, which may have influenced your results, and whether you use PET scan routinely in your patients? I enjoyed this paper very much; it will pave the way for new therapeutic approaches.
Dr. R.A. Adam: Thank you, Professor Clavien, for your questions.
In response to your first question concerning the real survival of patients, I can say at present, that after a mean follow-up of 31 months, half of the patients have died and half are alive, 10 of whom without disease. After 5 years, 6 patients are alive, 5 of whom without recurrence after the third hepatectomy. Of course, not all of the patients have reached at least 5 years follow-up to get a definitive opinion on the proportion of patients who could really obtain this long-term survival.
Concerning chemotherapy, we have experienced as other centers, many changes in the study period, owing to the emergence of new drugs. Five-Fluorouracile and Folinic Acid have been used in the early experience of this series. Oxaliplatine has been added from the nineties and more recently Irinotecan has been combined either to 5-FU alone or to 5-FU and Oxaliplatine. The results of our series probably reflects the better efficacy of chemotherapy in recent years and it is interesting to note that chronologically the increasing performance of repeat hepatectomy (more than 30% of all the resections performed in our unit presently) has corresponded to the better efficacy of chemotherapy. This is particularly important in the subset of patients with third hepatectomy since these patients have demonstrated a real propensity to develop recurrence and chemotherapy is assumed to be very useful in the pre or the post operative setting to prevent a new recurrence.
With regard to your third question concerning the PET scan, we have had really a limited experience of the investigation in this long lasting series. From the few cases that we have managed combining the PET scan with ultrasound and CT scan, my feeling has been that we obtained more additional information for extra-hepatic disease than for intra-hepatic tumors.
Dr. D. Jaeck: Congratulations for this interesting study. In your series you reported 27 patients out of 68 who presented with extrahepatic disease and 4 with lymph nodes metastases. Did you use the PET scan to select your patients for a third hepatic resection? In the case of extrahepatic disease detected during the operation, what was your policy and particularly in case of positive lymph node found in the hepatic pedicle or in the celiac area? In our experience, there was no survivors one year after resection of liver metastase in case of celiac lymph node involvement. Did you limit the indication for third hepatic resection in elderly patients? In this population how did you choose between percutaneous radiofrequency ablation and third hepatic resection?
Finally, did you study the biology of the tumor and if yes, did you use these data, such as proliferation index or microsatellite instability, before deciding to perform a third hepatic resection?
Dr. R.A. Adam: Thank you, Professor Jaeck, for your congratulations and questions. Concomitant extra-hepatic tumor was found indeed in a quarter of the patients that underwent a third hepatic resection, 10 of whom in the abdominal cavity. For the 4 patients with positive lymph nodes, all had lymph nodes of the hepatic pedicule. Our policy is different as to whether the discovery of extra hepatic disease is made before or during operation. If unexpected extra-hepatic disease is diagnosed during operation, we proceed on hepatectomy combined to resection of extra hepatic disease when the overall approach is curative. When the diagnosis of extra hepatic disease and particularly of lymph node metastases is made before the operation, our policy is always to initiate chemotherapy and to proceed after 3 to 6 courses of chemotherapy to resection only when the metastases are downstaged or controlled by the treatment.
Your second question concerning the age of the patient is very important. By reviewing recently our series on resection for colorectal metastases in elderly patients, we have observed as you, similar results as for younger patients. This is of course an argument to propose liver resection and even repeat liver resection in well-selected elderly patients. As far as tumor biology is concerned, we have not had the opportunity to assess any difference in the biology of the tumor between elderly and younger population.
Dr. H. Bismuth: What about the fifth hepatectomy?
Dr. R.A. Adam: I hope to speak on this in one of the next ESA meetings... .
Footnotes
Reprints: Prof René Adam, Centre Hépato-Biliaire, Hopital Paul Brousse, 14 Av PV Couturier, 94800 Villejuif, France. E-mail: rene.adam@pbr.ap-hop-paris.fr.
REFERENCES
- 1.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 2.Scheele J, Stangl R, Altendorf-Hofmann A, et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 3.Nordlinger B, Jaeck D, Guiguet M, et al. Surgical resection of hepatic metastases: multicentric retrospective study by the French Association of Surgery. In: Nordlinger B, Jaeck D, eds. Treatment of Hepatic Metastases of Colorectal Cancer. New York: Springer-Verlag, 1992:129–146. [Google Scholar]
- 4.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzetti F, Bignami P, Morabito A, et al. Patterns of failure following surgical resection of colorectal cancer liver metastases. Ann Surg. 1987;205:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone MD, Cady B, Jenkins RL, et al. Surgical therapy for recurrent liver metastases from colorectal cancer. Arch Surg. 1990;125:718–722. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz M, Muller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–254. [DOI] [PubMed] [Google Scholar]
- 8.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 9.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. J Clin Oncol. 2002;20:1499–1505. [DOI] [PubMed] [Google Scholar]
- 10.Portier G, Rougier P, Milan C, et al. Adjuvant systemic chemotherapy (CT) using 5-fluorouracil (FU) and folinic acid (FA) after resection of liver metastases (LM) from colorectal (CRC) origin. Results of an intergroup phase III study (trial FFCD-ACHBTH-AURC 9002). J Clin Oncol. (Proc ASCO.) [DOI] [PubMed]
- 11.Bozzetti F, Bignami P, Montalto F, et al. Repeated liver resection for recurrent metastases from colorectal cancer. Br J Surg. 1992;79:146–148. [DOI] [PubMed] [Google Scholar]
- 12.Vaillant JC, Balladur P, Nordlinger B, et al. Repeat liver resection for recurrent colorectal metastases. Br J Surg. 1993;80:340–344. [DOI] [PubMed] [Google Scholar]
- 13.Nordlinger B, Vaillant JC, Guiguet M, et al. Survival benefit of repeat liver resection for recurrent colorectal metastases. J Clin Oncol. 1994;12:1491–1496. [DOI] [PubMed] [Google Scholar]
- 14.Fong Y, Blumgart LH, Cohen A, et al. Repeat hepatic resection for metastatic colorectal cancer. Ann Surg. 1994;220:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Trigo V, Sharmsa F, Sugarbaker PH, et al. Repeat liver resection for colorectal metastasis. Surgery. 1995;117:296–304. [DOI] [PubMed] [Google Scholar]
- 16.Pinson CW, Wright JK, Chapman WC, et al. Repeat hepatic surgery for colorectal cancer metastasis to the liver. Ann Surg. 1996;223:765–773; discussion 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuttle TM, Curley SA, Roh MS. Repeat hepatic resection as effective treatment of recurrent colorectal liver metastases. Ann Surg Oncol. 1997;4:125–130. [DOI] [PubMed] [Google Scholar]
- 18.Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51–60; discussion 60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu QD, Vezeridis MP, Avradopoulos KA, et al. Repeat hepatic resection for recurrent colorectal cancer. World J Surg. 1997;21:292–296. [DOI] [PubMed] [Google Scholar]
- 20.Kin T, Nakajima Y, Kanehiro H, et al. Repeat hepatectomy for recurrent colorectal metastases. World J Surg. 1998;22:1087–1091. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto J, Kosuge T, Shimada K, et al. Repeat liver resection for recurrent colorectal liver metastases. Am J Surg. 1999;178:275–281. [DOI] [PubMed] [Google Scholar]
- 22.Chiappa A, Zbar AP, Biella F, et al. Survival after repeat hepatic resection for recurrent colorectal metastases. Hepatogastroenterology. 1999;46:1065–1070. [PubMed] [Google Scholar]
- 23.Muratore A, Polastri R, Bouzari H, et al. Repeat hepatectomy for colorectal liver metastases: a worthwhile operation? J Surg Oncol. 2001;76:127–132. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Sakaguchi T, Yokoi Y, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129:421–428. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H, Katoh H, Kondo S, et al. Repeat hepatectomy for recurrent hepatic metastases from colorectal cancer. Hepatogastroenterology. 2001;48:828–830. [PubMed] [Google Scholar]
- 26.Nagakura S, Shirai Y, Suda T, et al. Multiple repeat resections of intra- and extrahepatic recurrences in patients undergoing initial hepatectomy for colorectal carcinoma metastases. World J Surg. 2002;26:141–147. [DOI] [PubMed] [Google Scholar]
- 27.Petrowsky H, Gonen M, Jarnagin W, et al. Second liver resection are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Vega T, Donahue E, Doolas A, et al. A ten year experience with hepatic resection. Surg Gynecol Obstet. 1984;159:223–228. [PubMed] [Google Scholar]
- 29.Wanebo HJ, Chu QD, Avradopoulos KA, et al. Current perspectives on repeat hepatic resection for colorectal carcinoma: a review. Surgery. 1996;119:361–371. [DOI] [PubMed] [Google Scholar]
- 30.Neeleman N, Andersson R. Repeated liver resection for recurrent liver cancer. Br J Surg. 1996;83:893–901. [DOI] [PubMed] [Google Scholar]
- 31.Adam R. The importance of visceral metastasectomy in colorectal cancer. Ann Oncol. 2000;11(suppl 3):29–36. [DOI] [PubMed] [Google Scholar]
- 32.Elias D, Lasser PH, Hoang JM, et al. Repeat hepatectomy for cancer. Br J Surg. 1993;80:1557–1562. [DOI] [PubMed] [Google Scholar]
- 33.Aramaki M, Kawano K, Kai T, et al. Postoperative complications of repeat hepatectomy for liver metastases from colorectal carcinoma. Hepatogastroenterology. 2000;47:478–480. [PubMed] [Google Scholar]
- 34.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. [DOI] [PubMed] [Google Scholar]
- 35.Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. [DOI] [PubMed] [Google Scholar]
- 36.Thirion P, Wolmark N, Haddad E, et al. Survival impact of chemotherapy in patients with colorectal metastases confined to the liver: a re-analysis of 1458 non-operable patients randomized in 22 trials and 4 meta-analyses. Meta-Analysis Group in Cancer. Ann Oncol. 1999;10:1317–1320. [DOI] [PubMed] [Google Scholar]
- 37.Hobday TJ, Kugler JW, Mahoney MR, et al. Long term survivors of metastatic colorectal cancer treated with chemotherapy only: a North Central Cancer Treatment Group review. J Clin Oncol. 2002;20:4574–4580.12454115 [Google Scholar]
- 38.Sugihara K, Hojo Moriya Y, et al. Pattern of recurrence after hepatic resection for colorectal metastases. Br J Surg. 1993;80:1032–1035. [DOI] [PubMed] [Google Scholar]
- 39.Elias D, De Baere T, Smayra T, et al. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89:752–756. [DOI] [PubMed] [Google Scholar]
- 40.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. [DOI] [PubMed] [Google Scholar]
- 42.Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339; discussion 1340. [DOI] [PubMed] [Google Scholar]