Abstract
These experiments examined the release of acetylcholine in the hippocampus and striatum when rats were trained, within single sessions, on place or response versions of food-rewarded mazes. Microdialysis samples of extra-cellular fluid were collected from the hippocampus and striatum at 5-min increments before, during, and after training. These samples were later analyzed for ACh content using HPLC methods. In Experiment 1, ACh release in both the hippocampus and striatum increased during training on both the place and response tasks. The magnitude of increase of training-related ACh release in the striatum was greater in rats trained on the response task than in rats trained on the place task, while the magnitude of ACh release in the hippocampus was comparable in the two tasks. Experiment 2 tested the possibility that the hippocampus was engaged and participated in learning the response task, as well as the place task, because of the availability of extra-maze cues. Rats were trained on a response version of a maze under either cue-rich or cue-poor conditions. The findings indicate that ACh release in the hippocampus increased similarly under both cue conditions, but declined during training on the cue-poor condition, when spatial processing by the hippocampus would not be suitable for solving the maze. In addition, high baseline levels of ACh release in the hippocampus predicted rapid learning in the cue-rich condition and slow learning in the cue-poor condition. These findings suggest that ACh release in the hippocampus augments response learning when extra-maze cues can be used to solve the maze but impairs response learning when extra-maze cues are not available for use in solving the maze.
Damage to different neural systems results in impairments of the acquisition and retention of different learning and memory tasks (Cohen and Squire 1980; Wagner et al. 1998; Gold et al. 2001; Packard and Cahill 2001; White and McDonald 2002; Poldrack and Packard 2003). The respective roles of the hippocampus and striatum in learning are particularly evident in the results of several experiments demonstrating double dissociations between damage to the hippocampus and striatum and impairments of learning and memory on different tasks (Packard et al. 1989; Packard and McGaugh 1992; Kesner et al. 1993; McDonald and White 1993). In general, lesions of the hippocampus most often impair learning in tasks with solutions based on the use of extramaze cues, while lesions of the striatum most often impair learning in tasks with solutions that depend on intra-maze cues or on a specific turn.
The findings of experiments using pharmacological manipulations of the hippocampus are generally consistent with the results evidenced after hippocampal damage. For example, injections of cholinergic antagonists directly into the hippocampus impair memory for tasks involving spatial learning (Carli et al. 1997; Farr et al. 1999, 2000; Degroot and Parent 2000), while similar injections into the striatum impair memory for tasks involving cued or response learning (Prado-Alcala et al. 1980, 1985; Diaz del Guante et al. 1993).
In some instances, tests of the effects of lesions or inactivation of the hippocampus and striatum show that down-regulation of one of the neural systems enhances learning and memory for tasks associated with the other brain area. For example, Chang and Gold (2004) found that lidocaine injections into the hippocampus impaired learning to find a food reward in one arm of a plus-shaped maze, that is, place learning, but enhanced learning to find the reward by turning to the right (or left), that is, response learning. One interpretation of such findings is that some neural systems compete with each other to gain control over learning; enhancement of learning in these instances provides some of the best evidence for multiple memory systems (White and McDonald 2002; Poldrack and Packard 2003; Gold 2004).
Support for the view that the hippocampus and striatum interact in a competitive manner on some tasks is also seen in experiments that examine ACh release during training on a dual-solution task, a T-maze that can be learned using either place or response solutions (Tolman 1948; Restle 1957; Packard and McGaugh 1996). McIntyre et al. (2003b) used in vivo microdialysis methods to measure ACh release in the hippocampus and striatum while rats were trained to a 9/10 criterion on the dual solution task. ACh release increased in both brain areas during training. Of particular interest, those rats with high ratios of ACh release in the hippocampus versus striatum either at baseline or during training exhibited place solutions on a probe test administered after the rats reached the criterion of 9/10 correct, while those rats with low ratios of ACh release in the hippocampus versus striatum exhibited response solutions. In another assessment of ACh release during training in the T-maze, Chang and Gold (2003) observed a similar relationship between baseline ACh release and selection of place versus response solutions and, in addition, showed that the use of place solutions early in training was accompanied by early increases of release of ACh in the hippocampus and the use of response solutions later in training by later increases in release of ACh in the striatum. Similar results were also seen as rats made a transition from place to turning responses in a rewarded spontaneous alternation task (Pych et al. 2005).
During training in the dual-solution T-maze, rats appear to learn both place and response solutions, but select one strategy or the other when able to make a choice on a probe trial. Thus, the results obtained examining ACh release and learning in the dual-solution T-maze do not necessarily predict relationships between release of ACh in the hippocampus and striatum on other tasks in which either a place or a response solution provides a better solution. The present experiments examined simultaneously ACh release in the hippocampus and striatum while rats learned food-rewarded mazes that were efficiently learned using either place or turning strategies. Beyond the general question of characterizing conditions in which ACh release responds to behavioral demands, one goal of the experiments was to determine whether ACh release regulated the relative contributions of the hippocampus and striatum to learning of tasks that require either place or turning solutions.
Results
Experiment 1
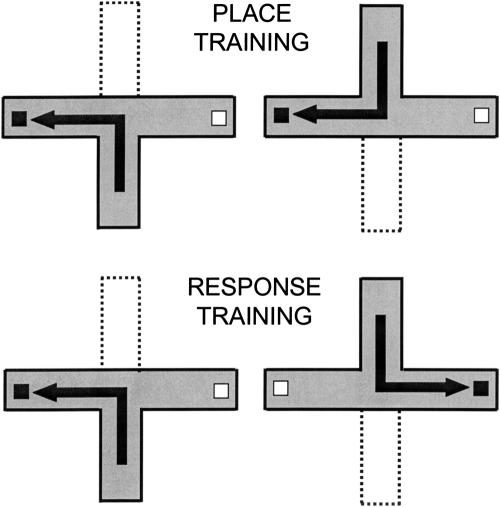
Using in vivo microdialysis with later HPLC assays, ACh release in the hippocampus and striatum were measured while rats were trained for 90 trials (one trial/min) on either place or response versions of a food-motivated T-maze (Fig. 1).
Figure 1.
Maze configuration for Experiment 1. In both tasks, the north and south arms were used exclusively as start arms and the east and west exclusively as goal arms. When a rat was started from the south, the north arm was blocked and vice versa. In the place task, rats were trained to find food in a particular spatial location (“to the west” in this example). In the response task, rats were trained to find food by repeating the same egocentric turn (“to the right” in this example).
Behavior
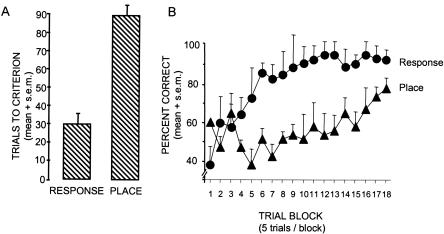
The behavioral results are shown in Figure 2. The groups of rats trained in each task showed significant learning during the 90 trials (F(17,272) = 5.35, P < 0.001). However, rats learned the response version of the maze significantly more quickly than they learned the place version (F(1,17) = 28.52, P < 0.001). The rats reached the criterion of 9/10 correct in means (+SEM) of 29.9 + 4.8 trials in the response version and 87.9 + 4.8 trials in the place version of the maze (t-test, P < 0.001).
Figure 2.
Learning curves during training in the place and response tasks. Rats trained in the response task learned more rapidly than did rats trained in the place task.
ACh release profiles during training
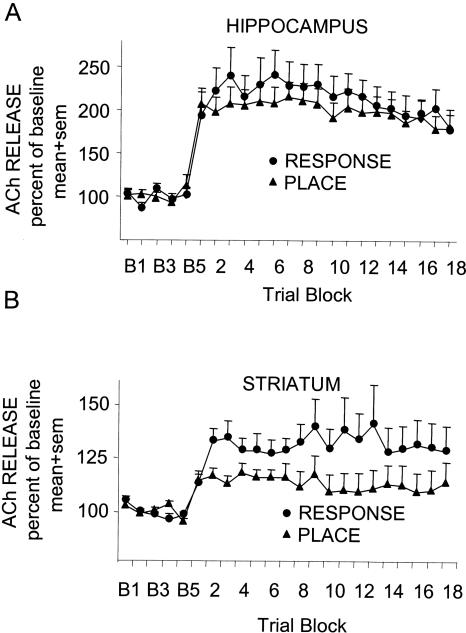
As shown in Figure 3A, ACh release in the hippocampus increased significantly during training on both the response and place tasks (F(22,352) = 27.57, P < 0.001). ACh release in the hippocampus increased to means of >200% of baseline values at the beginning of training on both tasks, remained at approximately those values throughout training, and did not differ by task throughout training (F(22,352) = 0.90, ns).
Figure 3.
Profiles of ACh released from the hippocampus (A) and striatum (B) of rats trained in either a place or response task. In both structures, training caused a significant increase in the amount of ACh released. Response training caused significantly more striatum ACh release than did place training.
As shown in Figure 3B, ACh release in the striatum also increased significantly during training on both tasks (F(22,352) = 6.884, P < 0.001). In addition, ACh release profiles differed according to task (F(22,352) = 1.89, P < 0.01). ACh release in the striatum of rats increased comparably on the first trial block on both tasks, but the rats trained on the response task showed a further increase in ACh release from Block 1 to Block 2 (matched t-test, P < 0.05), raising the ACh level to values that were then sustained throughout training. Thus, training in the response task resulted in higher overall levels of ACh release in the striatum than did training in the place task.
The ratio of hippocampus/striatum ACh release, a measure that has distinguished differences in learning strategies in some past experiments (Chang and Gold 2003; McIntyre et al. 2003b; Pych et al. 2005), did not differ significantly by task in the present experiment (data not shown).
Correlations of baseline release of ACh with rate of acquisition
McIntyre et al. (2003b) and Chang and Gold (2003) found that baseline release of ACh predicted the predominant use of place or response strategies after rats had been trained in a dual-solution T-maze. In the present experiment, neither the magnitude of ACh release in the striatum nor hippocampus at baseline, that is, prior to training, was significantly correlated with the number of trials to criterion in either task.
Histology
All rats had microdialysis probe placements in the ventral hippocampus and dorsolateral striatum.
Discussion
ACh release increased significantly in both the hippocampus and striatum while rats were trained in either the place or response versions of the four-arm maze. The magnitude of release of ACh in the striatum discriminated between the two versions of the task, with higher release of ACh in the striatum during training on the response task than during training on the place task.
In contrast to the result seen in the striatum, release of ACh in the hippocampus was comparable on the two tasks, and neither the response to training nor baseline levels of ACh release appeared to be related to the rates of acquisition of either the place or response versions of the task. The negative results were somewhat surprising because the magnitude of release of ACh in the ventral hippocampus has been positively associated with spatial working memory scores on spontaneous alternation tasks (Ragozzino et al. 1994, 1996; Pych et al. 2005). Release of ACh in the ventral hippocampus is also negatively associated with learning in a conditioned cue preference task (McIntyre et al. 2002) that is impaired by amygdala damage and enhanced by hippocampal damage or pharmacological down-regulation (McDonald and White 1993, 1995). Moreover, release of ACh in the ventral hippocampus predicts place versus response solutions on probe trials after training on a dual-solution T-maze (Chang and Gold 2003; McIntyre et al. 2003b), a maze similar to that used in Experiment 1. Although selection of place strategies seems to describe functions of the ventral hippocampus, the dorsal hippocampus appears to be more involved in spatial processing per se (Moser and Moser 1998), and would be a good target for measures of ACh release in future studies like the present one.
The absence of differences in release of ACh in the hippocampus across tasks might reflect the use of place information to solve the response task as well as the place task. For example, rats might learn a conditional solution to the response task using both place and response information, for example, if starting in the north arm, turn left. According to this view, any differences in the extent to which the hippocampus differentially participates in place versus response learning might be obscured by hippocampal involvement in both versions of the maze. Thus, ACh release in the hippocampus might be similar in the two tasks because it is engaged in both. In addition, the pattern of results suggests that, when both the hippocampus and striatum are engaged, the hippocampus controls the expression of learning even when ACh release in the striatum is different in the two tasks. This interpretation is consistent with past evidence that, after extensive training, rats can perform the dual-solution T-maze using either place or response solutions (Restle 1957; Packard and McGaugh 1996). The interpretation is also consistent with evidence that the relative roles of the hippocampus and striatum differ depending on the availability of extra-maze cues (Masuda and Iwasaki 1984; Mitchell and Hall 1988; Chang and Gold 2004). In addition, the substantial differences in the learning rates for the place and response versions of the task, differences that vary with such factors as cue availability (Chang and Gold 2004), might interfere with the ability to distinguish fully and equally the involvement of ACh release in the hippocampus and striatum during learning.
Results
Experiment 2
Experiment 1 found that ACh release in the striatum was higher during response learning than during place learning, suggesting that the higher level of striatum ACh release promoted response learning. In contrast, the measures of ACh in the hippocampus were unrelated to acquisition of either the place or response versions of the T-maze. These negative findings contrast with results obtained in other tasks in which release of ACh in the hippocampus appeared to be positively associated with place solutions and negatively associated with response solutions in a dual-solution maze as well as with a conditioned cue preference task (McIntyre et al. 2002, 2003b; Chang and Gold 2003).
Because it is possible that strategies associated with the hippocampus might provide a reasonable solution to the response version of the task used in Experiment 1, for example, a conditional place discrimination solution, the present experiment examined the possibility that associations of ACh release in the hippocampus, and perhaps also in the striatum, might be more readily apparent in tasks in which cue availability is manipulated. Therefore, Experiment 2 examined ACh release in the hippocampus and striatum in a response version of a four-arm plus-shaped maze under cue-rich and cue-poor conditions. The general maze and procedures were those used in Experiment 1 (Fig. 1), except all four arms were open and all arms were used as start arms on different trials. In the cue-rich condition, the room cues were similar to those in Experiment 1; in the cue-poor condition, the maze was surrounded by a curtain to obscure most extramaze cues. The four-arm maze was more difficult than that used in Experiment 1, in an attempt to offer a potentially augmented opportunity to observe the relationships between the neurochemical measures and learning. However, the increased difficulty resulted in only 50% of the rats reaching criterion within the single training session that included 120 trials (one trial/min). The results presented here are based on only those rats that completed training during the single session, in parallel with a single instance of microdialysis sample collection as in Experiment 1.
Behavior
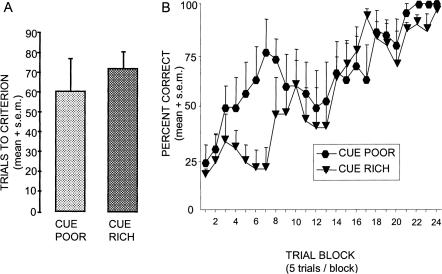
The learning measures for the two groups are shown in Figure 4. The mean number of trials to criterion for rats trained under cue-poor (61.2 + 15) versus cue-rich (mean = 72.5 + 7.7SEM) conditions did not differ significantly (P > 0.2). Significant acquisition was evident across trials (F(23,230) = 9.12, P < 0.001), but rate of acquisition did not differ according to cue condition (F(23,230) = 1.36, P = 0.13, ns), although the rats in the cue-poor condition appeared to show evidence of learning somewhat earlier than did the rats in the cue-rich condition.
Figure 4.
Trials to criterion (A) and learning curves (B) during response training in the plus-maze did not differ significantly across cue conditions.
ACh release under cue-rich versus cue-poor conditions
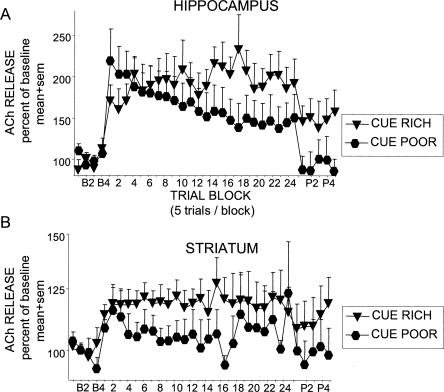
Figure 5 shows the percent change in ACh release in the hippocampus and striatum during training under the two cue conditions. In both the hippocampus and striatum, training resulted in significant increases in release of ACh above baseline evident throughout training (hippocampus: F(32,320) = 9.12, P < 0.001; striatum: F(32,320) = 2.32, P < 0.001). In the hippocampus (Fig. 5A), ACh release profiles differed according to cue condition (F(32,320) = 2.18, P < 0.001). ACh release in the hippocampus increased quickly and did so to a similar extent during early trials under each cue condition. However, of interest, release of ACh in the hippocampus was sustained and perhaps increased slightly across the 120 training trials in the cue-rich condition, but declined steadily and markedly during training in the cue-poor condition (T1 vs. T24, P < 0.01). After training in the cue-rich condition, ACh release declined somewhat, but was still well above baseline 25 min after training. In the cue-poor condition, ACh release declined back to baseline shortly after the last training trial.
Figure 5.
Profiles of ACh release from the hippocampus (A) and striatum (B) of rats trained in a response task in either a visually cue-rich or cue-poor environment. (A) A training-related increase in hippocampus ACh release occurred during response training in both the cue-rich and cue-poor conditions. Hippocampus ACh release increased to ∼200% of baseline values in both cue conditions during the first 20 min of training. Thereafter, the ACh release profiles diverge according to cue condition with levels of hippocampus ACh in rats trained in the cue-rich condition maintaining values >200% and hippocampus ACh in rats trained in the cue-poor condition steadily decreasing to eventually plateau at ∼150%. (B) A training-related increase in striatum ACh release occurred during response training in both the cue-rich and cue-poor conditions. Although ACh released in the striatum of rats trained in the cue-rich task appears to be slightly higher than those trained in the cue-poor task, no significant differences were found.
In the striatum (Fig. 5B), ACh release increased slightly, but not significantly, more under the cue-rich than under the cue-poor condition (area under the curve, t-test, P > 0.3). Training under both conditions resulted in generally sustained levels of ACh release in the striatum throughout training and, interestingly, quickly returned to baseline under the cue-poor condition but not the cue-rich condition, where the levels remained elevated throughout the 25 min of post-training sampling.
Baseline ACh release
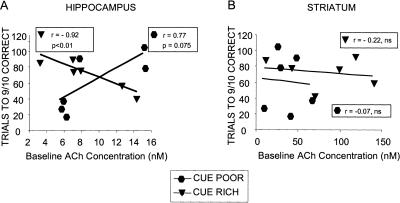
Baseline release of ACh in the hippocampus but not striatum predicted the rate of acquisition. As shown in Figure 6A, ACh release in the hippocampus of rats trained under the cue-rich condition was negatively associated (r = –0.92, P < 0.05) with trials to criterion on the response task. The rats with higher baseline release of ACh in the hippocampus were those with faster acquisition under the cued conditions. In contrast, ACh release in the hippocampus of rats trained under the cue-poor condition showed a trend (r = 0.77, P = 0.075) in the opposite direction, with those rats with higher levels of baseline ACh release in the hippocampus showing slower acquisition of the response task. These correlations differed significantly from each other (Fisher's z-transformation; z = 2.81, P < 0.01). In contrast, as shown in Figure 6B, parallel examinations of baseline release of ACh in the striatum before training on either cue condition revealed no significant correlations with learning rates.
Figure 6.
Relationship between baseline ACh release levels and learning. (A) Hippocampus ACh release levels at baseline (i.e., prior to training) correlated with the number of trials rats required to reach the learning criterion of 9/10 trials correct. However, in the cue-rich environment, higher levels of baseline hippocampus ACh release were correlated with faster learning, while in the cue-poor environment, higher levels of baseline ACh release were correlated with slower learning. (B) Baseline striatum ACh release levels were not correlated with number of trials to criterion in either cue condition.
Discussion
The findings of Experiment 2 show that the profile of ACh release in the hippocampus, but not striatum, during training on a response task varies with cue availability. ACh release in the hippocampus increased with the onset of response training under both cue conditions. However, the training-related increase in ACh release in the hippocampus was sustained in rats trained in the cue-rich condition but decreased across trials under the cue-poor condition. These findings suggest that the hippocampus remained activated throughout training when extra-maze cues were available but not when the cues were minimized.
A second main result was that baseline release of ACh predicted the rate of acquisition of the response task under both cue conditions. However, the direction of the correlation differed under cue-poor versus cue-rich conditions. When visual cues were readily available, those rats that learned quickly were those with high levels of ACh release in the hippocampus. When cues were minimized, those rats that learned quickly were those with low levels of ACh release in the hippocampus. This pattern of results, together with the finding that ACh release declined during training in the cue-poor condition, is consistent with the following interpretation: In the cue-rich condition, response learning can be accomplished both by using a turning strategy, for example, turn right, or by using a conditional place strategy, for example, turn right when facing south. Because high levels of ACh release in the hippocampus are associated with rapid learning under cue-rich conditions, the results suggest that the hippocampus can participate effectively in integrating place information while learning to turn in a specific direction. The processing of this information by the hippocampus may function in concert with the striatum, where release of ACh also increases and is sustained during training. In the cue-poor condition, place information is minimally available and therefore not a source of information effective in solving the response task. Therefore, in the cue-poor condition, hippocampal processing may compete with the striatum for control over learning, slowing acquisition by engaging ineffective strategies for learning.
An additional and unexpected finding was that ACh release in the hippocampus and striatum remained well above baseline during the 25 min after training in the cue-rich condition, but not the cue-poor condition, suggesting that ACh may continue to participate in the processing of information in the post-training period in the cue-rich condition.
Conclusions
The present findings, that release of ACh increased in both striatum and hippocampus on all task versions examined, are consistent with those observed previously on a dual-solution T-maze and a rewarded spontaneous alternation task (Chang and Gold 2003; McIntyre et al. 2003a,b; Pych et al. 2005). An important new finding observed here is that the magnitude of the training-related increase in ACh release can vary with task. In Experiment 1, the magnitude of the increase in release of ACh in the striatum during training on a response task was significantly greater than that observed during training on a place task. A second new finding, seen in Experiment 2, is that there appears to be active titration of ACh release during training such that training-related increases in ACh release in the hippocampus diminish under cue-poor training conditions when hippocampal involvement is not useful, or may even be detrimental, to learning. The correlations between absolute levels of baseline ACh release in the hippocampus when rats are trained on a cue-poor and cue-rich conditions support this view. High levels of release of baseline ACh in the hippocampus are associated with rapid learning under cue-rich training conditions but with slow learning under cue-poor conditions. These findings imply that the level of activation in a neural system that is not generally related to a particular task may have important consequences for learning in that task. In this regard, the findings offer another example of possible competition between memory systems.
These findings suggest an important role for ACh in regulating memory systems and memory formation processes within those systems (Gold 2003, 2004). In addition to roles for ACh in learning and memory, as discussed here, many reports identify a role for neocortical ACh in attentional processes (Sarter et al. 2003, 2005; Hasselmo and McGaughy 2004). When applied to the present results examining ACh release in the striatum and hippocampus, generalized attention does not explain the differences across tasks, although directed attention—for example, via hippocampus and striatum for the use of allocentric versus egocentric cues—may be another way to explain the differential activation of these memory systems.
At a systems level, the findings reported here reveal information about the relative contributions of the hippocampus and striatum to learning of tasks with different cognitive demands, supporting views of multiple memory systems and showing that release of ACh is one marker of the relative activation of these memory systems. In addition to viewing ACh as a marker of activation, there is considerable reason to view release of ACh as an important direct contributor to memory processing in these systems (Prado-Alcala 1985; Gold 2003, 2004; Power et al. 2003; Ragozzino 2003). Particularly germane to the present experiments, several reports demonstrate that manipulations of cholinergic functions in the hippocampus and striatum can influence learning and memory processes. Injections of muscarinic agonists and antagonists into the striatum (e.g., Prado-Alcala et al. 1984; Diaz del Guante et al. 1993; Lazaris et al. 2003; Tzavos et al. 2004) or hippocampus (e.g., Izquierdo et al. 1992; Ohno et al. 1994; Kim and Levin 1996; Riekkinen Jr et al. 1997; Kobayashi and Iwasaki 2000; Ferreira et al. 2003; Rogers and Kesner 2004) enhance and impair learning and memory, respectively, in many tasks. Such findings suggest that ACh plays a central role in learning and memory processing in the hippocampus and striatum. However, experiments have not yet been performed, to our knowledge, to assess differential roles of manipulations of cholinergic mechanisms within the context of different neural systems, that is, using tasks designed to tease apart the respective roles of the hippocampus and striatum, as well as other systems important to learning and memory. In addition, while ACh release in the striatum comes from interneurons contained within the striatum (Graybiel 1995; Pollack 2001) and in the hippocampus from projection neurons originating in medial septum/diagonal band regions (Mesulam et al. 1983; Frotscher and Leranth 1985; Dutar et al. 1995), the bases for differential control of ACh release in the striatum and hippocampus during tasks as seen in the present experiments is unknown. In addition, the ways in which the differential processing of the hippocampus and striatum are melded into coherent learned behaviors is also unknown, although speculations have suggested that the information is either maintained truly independently across neural systems or is collected into a common neural system that integrates the outputs of multiple memory systems (for reviews, see White and McDonald 2002; Gold 2004; Mizumori et al. 2004).
While these system-level questions remain, the possible importance of ACh for modulating learning and memory is supported by many examinations of mechanisms by which ACh might regulate memory and neural plasticity. Particularly in neocortex and the hippocampus, there is considerable evidence that release of ACh regulates several forms of neurophysiologically assessed plasticity (Segal and Auerbach 1997; Weinberger 2003, 2004; Adams et al. 2004; Hasselmo and McGaughy 2004). ACh enhancement of signal-to-noise ratios (Hasselmo 1995; Gu 2002) and increased neural excitability (Weiss et al. 2000; Disterhoft and Oh 2003) are likely to contribute importantly to ACh regulation of neural and behavioral plasticity.
In addition to neurophysiological mechanisms underlying cholinergic regulation of neural plasticity, the cellular responses may include activation of several signal transduction mechanisms including, for example, protein kinase C γ (Rossi et al. 2005), extra-cellular signal-regulated kinase (Rosenblum et al. 2000; Berkeley et al. 2001), cGMP (Gillette and Mitchell 2002), and activation of transcription factors (Greenberg et al. 1986), such as cyclic AMP response element binding protein (CREB) (Dineley et al. 2001; Greenwood and Dragunow 2002; Hu et al. 2002). Of specific relevance here, differences in activation of cholinergic receptors in the hippocampus and striatum under different task demands might be associated with similar training-related differences in hippocampal versus striatal levels of phosphorylated CREB and induction of c-Jun and c-Fos after training (Colombo et al. 2003; Colombo 2004; Teather et al. 2005).
Thus, at several levels of analysis, ACh is likely to have a key role in regulating neural plasticity and memory. Further characterization of the training conditions under which ACh is released in different brain regions, together with manipulations of cholinergic mechanisms, will be important as will parallel assessments of the cellular responses through which ACh acts on learning and memory.
Materials and Methods
Experiment 1
Subjects
Male Sprague-Dawley rats (Harlan, Oregon Barrier 236B) weighing ∼300 g at the beginning of this experiment were used. Rats arrived at our facility at least 1 wk prior to undergoing surgery to implant guide cannulae. Rats were housed individually in clear plastic cages, had food and water ad libitum, and were maintained on a 12-h light/12-h dark cycle. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Microdialysis guide cannulae were implanted in rats under sodium pentobarbital anesthesia (50 mg/kg, i.p.). Rats were placed in a stereotaxic device with horizontal skull (Paxinos and Watson 1986). One guide cannula (CMA/11; Carnegie Medicin) was positioned stereotaxically to terminate in the dorsolateral striatum (coordinates given relative to bregma for A/P and M/L and dura for D/V; A/P = 0.0, M/L = 4.1, D/V = 2.0), and a second cannula was positioned to terminate in the contralateral ventral hippocampus (A/P = 5.8, M/L = 5.0; D/V = 2.5). Four stainless steel anchor screws were implanted into the dorsal surface of the skull. The guide cannulae were implanted and, using dental cement, secured to the anchor screws and skull.
Maze training
Rats were trained in a four-arm plus-shaped maze with floor and walls made of black Plexiglas (Fig. 1). The arms of the maze (12.5 cm wide by 46 cm long by 7 cm high) extended radially from a central square platform (sides = 13 cm); the floor of the maze was positioned 0.7 m above the floor. Food cups were located at the ends of each arm. On each trial, one cup was baited with one-half Frosted Cheerio (General Mills). The arm directly opposite the start arm was blocked with a black Plexiglas inset (13.5 cm wide) so that the maze formed a “T” shape. The training room (3 m × 2.4 m) contained a moderate density of cues including high-contrast posters and dark-colored three-dimensional objects set against a light-colored wall.
Prior to training, rats were given a minimum of 1 wk to recover their body weights to pre-surgery levels. At that time, a food restriction regimen began and continued throughout the next 7–9 d until the day of training, at which time rats weighed 80%–85% of their baseline weights. During the 7–9 d prior to training, the rats were handled for 3 min each day before receiving their daily aliquot of food. The aliquot included a measured amount of rat chow and three Frosted Cheerios, the latter to reduce possible neophobia to the Frosted Cheerios reward used during training. Behavioral training began once rats reached the target body weight.
Rats were trained in either a place or a response version of the maze (90 trials, 1 trial/min). In the place version, rats were trained to go to the arm located in a particular spatial location of the testing room (e.g., the arm pointing west) for food reward. In the response task, rats were trained to consistently make the same body turn (e.g., turn to the right) at the choice-point for food reward (Fig. 1). Two of the four arms of the maze were used as start arms (north and south), and the other two arms were used as goal arms (east and west); start arm location was varied pseudorandomly.
At the beginning of each trial, one-half piece of cereal was placed at the end of the goal arm, and the rat was removed from a conical holding cage (36 cm high, 24 cm wide at the base, and 36 cm wide at the top) and placed in a start arm facing the center of the maze. After either eating the reward or reaching the end of an incorrect arm, the rat was taken out of the maze and placed back in the holding cage. The maze was rotated 90° clockwise after each trial so that olfactory cues could not provide systematic cues for learning. Exactly 60 sec elapsed between the beginning of one trial and the beginning of the next trial. Training was completed within a single session. Nine rats each were trained on place and response versions of the maze; three additional rats were not included because of technical difficulties with collections of microdialysis samples.
Microdialysis/HPLC
Approximately 2.5 h prior to the beginning of training, each rat was removed from its home cage and placed in a holding cage located in the testing room. After being transported to the testing room, a 2-mm microdialysis probe was inserted into and then removed from the striatum and a 3 mm probe was inserted into and removed from the contralateral hippocampus in order to minimize changes in neurotransmitter levels at the time of training due to tissue damage caused by probe insertion (CMA/11; Carnegie Medicin). The rat remained in a holding cage until the beginning of training. Then, 1 h after the initial probe insertion, probes were again inserted into the hippocampus and striatum, where they remained for the duration of training. Hippocampus microdialysis probe efficiencies were 12% and 10% in the place and response tasks, respectively; striatum microdialysis probe efficiencies were 8% and 7% in the place and response tasks, respectively.
Artificial cerebral spinal fluid (aCSF), containing 200 nM of the acetylcholinesterase inhibitor neostigmine, was perfused through the microdialysis probes continuously at a rate of 1.5 μL/min (contents of aCSF in mM: 128 NaCl, 2.5 KCl, 1.3 CaCl2, 2.1 MgCl2, 0.9 NaH2PO4; at pH 7.4). The aCSF also contained 1.0 mM glucose in hippocampal perfusate and 0.7 mM glucose in the striatal perfusate. These glucose concentrations in the microdialysis perfusates match baseline extra-cellular glucose levels in awake rats of 1.0 and 0.7 mM in the hippocampus and striatum, respectively (McNay and Gold 1999; McNay et al. 2001).
Samples collected during the first hour of microdialysis were discarded to provide time for baseline stabilization (Westerink and Timmerman 1999). Immediately prior to the beginning of training, four 5-min samples were collected from each brain structure to establish baseline extra-cellular ACh levels. During training, 5-min samples (7.5 μL) were collected from each brain structure. This protocol yielded a total of 46 samples from each animal ([5 baseline + 18 training] × [2 probes] = 46). Samples were frozen at –70°C for up to 4 wk before being assayed for ACh content.
High performance liquid chromatography (HPLC) with electrochemical detection (BAS; Bioanalytical Systems) was used to determine ACh concentrations in the microdialysis samples, and 5 μL of each microdialysis sample was injected into the system via an injection valve with a 10-μL loop (Rheodyne model 9725i). The assay system included an ion-exchange microbore analytical column (BAS P/N MF 8904, 530 × 1 mm), a microbore ACh/choline immobilized enzyme reactor containing acetylcholinesterase and choline oxidase (BAS P/N MF-8903, 50 × 1 mm), a 6-mm glassy fiber electrode (BAS P/N MF 1095) that was coated with a redox polymer film containing horseradish peroxidase, an auxiliary electrode with radical flow electrochemical thin-layer cell, and a 13-mm thin-layer gasket and an Ag/AgCl reference electrode; the working electrode held a 100 mV potential relative to the reference electrode. Flow rate was maintained at 140 μL/min by a Shimadzu LC-10ADvp pump with microstep plunger. The mobile phase contained 50 mM Na2HPO4 and 0.005% Pro-Clin (BAS P/N CF-2150) and was adjusted to a pH of 8.5. The sensitivity of this system was below 5 fmol, and assays were completed within 12.5 min. In the place task, baseline ACh concentrations in samples collected from the hippocampus were 9.4 ± 1.2 nM and in the striatum 37.2 ± 3.1 nM; in the response task, baseline ACh concentrations were 7.6 ± 1.9 nM in the hippocampus and 26.2 ± 4.8 nM in the striatum.
Histology
Within 1 wk of behavioral testing, the rats were deeply anesthetized with sodium pentobarbital (75 mg/kg) and perfused intracardially with physiological saline followed by a 10% formalin solution. After perfusion, the brains were removed and post-fixed in a 10% formalin/30% sucrose solution for 4–7 d. Brains were then cut into 50-μm sections using a Leica 1800 cryostat. Every fourth section was mounted on slides beginning with the first section through which the dialysis probe had extended; slides were stained with cresyl violet and examined under light microscopy for verification of cannulae placements.
Statistical analyses
Two-way ANOVAs were used to analyze learning curves, striatum ACh release, and hippocampal ACh release using correct choices in blocks of five trials or percent of baseline ACh release in samples temporally coincident with the blocks of five trials as a within-subjects variable and task (place or response) as a between-subjects variable. Two-tailed t-tests were used to compare differences in the number of trials to criterion. Pearson correlations were used to analyze correlations between baseline ACh release in both hippocampus and striatum and number of trials to the criterion of 9/10 correct.
Experiment 2
Subjects
Subjects were 24 male Sprague-Dawley rats (Harlan, Oregon Barrier 236B). Three additional rats were not included in the data analysis—one rat because it did not perform the task and two because, at the time of histological assessment of probe placement, infection was evident surrounding the cannula tract.
The rats were ∼70 d old and weighed ∼300 g at the beginning of this experiment. All general housing and surgery procedures were as in Experiment 1. Rats arrived at our facility 1 wk prior to undergoing guide cannula surgery. After at least 1 wk of recovery from surgery, rats were placed on a food restriction regimen to reduce their body weights to 80%–85% of baseline weights.
Maze training
Rats were trained in a response version of a four-arm maze, similar to that used in Experiment 1, with a floor made of plywood painted flat black and walls made of clear Plexiglas. The arms of the maze (13 cm wide by 46 cm long by 17 cm high) extended radially from a central square platform (sides = 13 cm); the floor of the maze was situated 0.7 m above the floor. Food cups were affixed to the end of each arm and baited with one-half Frosted Cheerio (General Mills). In the cue-rich condition, the testing room environment was identical to that in Experiment 1. In the cue-poor condition, the maze was placed in a circular arena (2 m wide × 2.5 m high) that was surrounded by beige opaque shower curtains, approximately the same color as the walls of the testing room. The maze was illuminated by four 25 W incandescent light bulbs directed at the four corners of the room.
Once a rat reached its target body weight, behavioral testing began. Each rat was trained in a single session of 120 trials to make either a right or left turn at the maze choice-point to enter the goal arm. All four arms of the maze were used as both start and goal arms during training of each rat. The maze was rotated 90° clockwise after each trial to dissociate intra-maze and extramaze cues. On each trial, the rat was removed from a holding cage and placed in a start arm facing the center of the maze. After either eating the reward or reaching an incorrect arm, the rat was taken out of the maze and placed back in the holding cage until the start of the next trial. To match the timing of collection of microdialysis samples to the training trials, each trial began exactly 60 sec after the beginning of the prior trial, resulting in the 5-min microdialysis samples corresponding to blocks of five trials.
Of the 24 rats used in this experiment, 12 were trained under cue-rich conditions and 12 under the cue-poor conditions. This task proved considerably more difficult than the tasks used in Experiment 1. Under each cue condition in the present experiment, exactly half of the rats reached the learning criterion of 9/10 correct choices and half did not. Because of this, the final groups included Ns = 6 for each cue condition.
Microdialysis/HPLC
The microdialysis and analytical procedures were as described in Experiment 1. During training, 24 samples, 5 min each, were collected from each probe; each training sample corresponds to five training trials, yielding 66 samples from each animal [(4 baseline + 24 training + 5 post-training) × (2 microdialysis probes) = 66].
Statistical analyses
Trials to criterion were compared across cue conditions using two-tailed t-tests. ANOVAs were used to compare the learning curves and ACh release across conditions. Relationships between ACh release at baseline with trials to criterion were assessed with Pearson correlations.
Histology
As in Experiment 1, placements of microdialysis probes were identified after the behavioral portion of the experiment in brains stained with cresyl violet.
Acknowledgments
This work was supported by research grants from NIA (AG 07648) and NIDA (DA 016951), and by the University of Illinois Initiative on Aging and the Office of the Vice-Chancellor for Research.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.33105.
References
- Adams, S.V., Winterer, J., and Muller, W. 2004. Muscarinic signaling is required for spike-pairing induction of long-term potentiation at rat Schaffer collateral-CA1 synapses. Hippocampus 14: 413-416. [DOI] [PubMed] [Google Scholar]
- Berkeley, J.L., Gomeza, J., Wess, J., Hamilton, S.E., Nathanson, N.M., and Levey, A.I. 2001. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol. Cell. Neurosci. 18: 512-524. [DOI] [PubMed] [Google Scholar]
- Carli, M., Luschi, R., and Samanin, R. 1997. Dose-related impairment of spatial learning by intrahippocampal scopolamine: Antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav. Brain Res. 82: 185-194. [DOI] [PubMed] [Google Scholar]
- Chang, Q. and Gold, P.E. 2003. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J. Neurosci. 23: 3001-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2004. Inactivation of dorsolateral striatum impairs acquisition of response learning in cue-deficient, but not cue-available, conditions. Behav. Neurosci. 118: 383-388. [DOI] [PubMed] [Google Scholar]
- Cohen, N.J. and Squire, L.R. 1980. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science 210: 207-210. [DOI] [PubMed] [Google Scholar]
- Colombo, P.J. 2004. Learning-induced activation of transcription factors among multiple memory systems. Neurobiol. Learn. Mem. 82: 268-277. [DOI] [PubMed] [Google Scholar]
- Colombo, P.J., Brightwell, J.J., and Countryman, R.A. 2003. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J. Neurosci. 23: 3547-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot, A. and Parent, M.B. 2000. Increasing acetylcholine levels in the hippocampus or entorhinal cortex reverses the impairing effects of septal GABA receptor activation on spontaneous alternation. Learn. Mem. 7: 293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz del Guante, M.A., Carbonell-Hernandez, C., Quirarte, G., Cruz-Morales, S.E., Rivas-Arancibia, S., and Prado-Alcala, R.A. 1993. Intrastriatal injection of choline accelerates the acquisition of positively rewarded behaviors. Brain Res. Bull. 30: 671-675. [DOI] [PubMed] [Google Scholar]
- Dineley, K.T., Westerman, M., Bui, D., Bell, K., Ashe, K.H., and Sweatt, J.D. 2001. β-Amyloid activates the mitogen-activated protein kinase cascade via hippocampal α7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. J. Neurosci. 21: 4125-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft, J.F. and Matthew, Oh M. 2003. Modulation of cholinergic transmission enhances excitability of hippocampal pyramidal neurons and ameliorates learning impairments in aging animals. Neurobiol. Learn. Mem. 80: 223-233. [DOI] [PubMed] [Google Scholar]
- Dutar, P., Bassant, M.H., Senut, M.C., and Lamour, Y. 1995. The septohippocampal pathway: Structure and function of a central cholinergic system. Physiol. Rev. 75: 393-427. [DOI] [PubMed] [Google Scholar]
- Farr, S.A., Uezu, K., Flood, J.F., and Morley, J.E. 1999. Septo-hippocampal drug interactions in post-trial memory processing. Brain Res. 847: 221-230. [DOI] [PubMed] [Google Scholar]
- Farr, S.A., Flood, J.F., and Morley, J.E. 2000. The effect of cholinergic, GABAergic, serotonergic, and glutamatergic receptor modulation on posttrial memory processing in the hippocampus. Neurobiol. Learn. Mem. 73: 150-167. [DOI] [PubMed] [Google Scholar]
- Ferreira, A.R., Furstenau, L., Blanco, C., Kornisiuk, E., Sanchez, G., Daroit, D., Castro e Silva, M., Cervenansky, C., Jerusalinsky, D., and Quillfeldt, J.A. 2003. Role of hippocampal M1 and M4 muscarinic receptor subtypes in memory consolidation in the rat. Pharmacol. Biochem. Behav. 74: 411-415. [DOI] [PubMed] [Google Scholar]
- Frotscher, M. and Leranth, C. 1985. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: A combined light and electron microscopic study. J. Comp. Neurol. 239: 237-246. [DOI] [PubMed] [Google Scholar]
- Gillette, M.U. and Mitchell, J.W. 2002. Signaling in the suprachiasmatic nucleus: Selectively responsive and integrative. Cell Tissue Res. 309: 99-107. [DOI] [PubMed] [Google Scholar]
- Gold, P.E. 2003. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol. Learn. Mem. 80: 194-210. [DOI] [PubMed] [Google Scholar]
- ———. 2004. Coordination of multiple memory systems. Neurobiol. Learn. Mem. 82: 230-242. [DOI] [PubMed] [Google Scholar]
- Gold, P.E., McIntyre, C.K., McNay, E.C., Stefani, M.R., and Korol, D.L. 2001. Neurochemical referees of dueling memory systems. In Essays in honor of James L. McGaugh (eds. P.E. Gold and W.T. Greenough), pp. 219-248. American Psychological Press, Washington, DC.
- Graybiel, A.M. 1995. The basel ganglia. Tr. Neurosci. 18: 60-62. [PubMed] [Google Scholar]
- Greenberg, M.E., Ziff, E.B., and Greene, L.A. 1986. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science 234: 80-83. [DOI] [PubMed] [Google Scholar]
- Greenwood, J.M. and Dragunow, M. 2002. Muscarinic receptor-mediated phosphorylation of cyclic AMP response element binding protein in human neuroblastoma cells. J. Neurochem. 82: 389-397. [DOI] [PubMed] [Google Scholar]
- Gu, Q. 2002. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111: 815-835. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M.E. 1995. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav. Brain Res. 67: 1-27. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M.E. and McGaughy, J. 2004. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog. Brain Res. 145: 207-231. [DOI] [PubMed] [Google Scholar]
- Hu, M., Liu, Q.S., Chang, K.T., and Berg, D.K. 2002. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol. Cell. Neurosci. 21: 616-625. [DOI] [PubMed] [Google Scholar]
- Izquierdo, I., da Cunha, C., Rosat, R., Jerusalinsky, D., Ferreira, M.B., and Medina, J.H. 1992. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav. Neural Biol. 58: 16-26. [DOI] [PubMed] [Google Scholar]
- Kesner, R.P., Bolland, B.L., and Dakis, M. 1993. Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp. Brain Res. 93: 462-470. [DOI] [PubMed] [Google Scholar]
- Kim, J.S. and Levin, E.D. 1996. Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: Effects on spatial working memory in rats. Brain Res. 725: 231-240. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T. and Iwasaki, T. 2000. Functional dissociation of striatal and hippocampal cholinergic systems in egocentric and allocentric localization: Effect of overtraining. Nihon Shinkei Seishin Yakurigaku Zasshi 20: 113-121. [PubMed] [Google Scholar]
- Lazaris, A., Cassel, S., Stemmelin, J., Cassel, J.C., and Kelche, C. 2003. Intrastriatal infusions of methoctramine improve memory in cognitively impaired aged rats. Neurobiol. Aging 24: 379-383. [DOI] [PubMed] [Google Scholar]
- Masuda, Y. and Iwasaki, T. 1984. Effects of caudate lesions on radial maze behavior in rats. Jpn. Psychol. Res. 26: 42-49. [Google Scholar]
- McDonald, R.J. and White, N.M. 1993. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 107: 3-22. [DOI] [PubMed] [Google Scholar]
- ———. 1995. Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned-cue preference in the rat. Hippocampus 5: 189-197. [DOI] [PubMed] [Google Scholar]
- McIntyre, C.K., Pal, S.N., Marriott, L.K., and Gold, P.E. 2002. Competition between memory systems: Acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J. Neurosci. 22: 1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, C.K., Marriott, L.K., and Gold, P.E. 2003a. Cooperation between memory systems: Acetylcholine release in the amygdala correlates positively with performance on a hippocampus-dependent task. Behav. Neurosci. 117: 320-326. [DOI] [PubMed] [Google Scholar]
- ———. 2003b. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol. Learn. Mem. 79: 177-183. [DOI] [PubMed] [Google Scholar]
- McNay, E.C. and Gold, P.E. 1999. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: Effects of microdialysis flow rate, strain, and age. J. Neurochem. 72: 785-790. [DOI] [PubMed] [Google Scholar]
- McNay, E.C., McCarty, R.C., and Gold, P.E. 2001. Fluctuations in brain glucose concentration during behavioral testing: Dissociations between brain areas and between brain and blood. Neurobiol. Learn. Mem. 75: 325-337. [DOI] [PubMed] [Google Scholar]
- Mesulam, M.M., Mufson, E.J., Wainer, B.H., and Levey, A.I. 1983. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1–Ch6). Neurosci. 10: 1185-1201. [DOI] [PubMed] [Google Scholar]
- Mitchell, J.A. and Hall, G. 1988. Caudate-putamen lesions in the rat may impair or potentiate maze learning depending upon availability of stimulus cues and relevance of response cues. Q.J. Exp. Psychol. B 40: 243-258. [PubMed] [Google Scholar]
- Mizumori, S.J., Yeshenko, O., Gill, K.M., and Davis, D.M. 2004. Parallel processing across neural systems: Implications for a multiple memory system hypothesis. Neurobiol. Learn. Mem. 82: 278-298. [DOI] [PubMed] [Google Scholar]
- Moser, M.B. and Moser, E.I. 1998. Functional differentiation in the hippocampus. Hippocampus 8: 608-619. [DOI] [PubMed] [Google Scholar]
- Ohno, M., Yamamoto, T., and Watanabe, S. 1994. Blockade of hippocampal M1 muscarinic receptors impairs working memory performance of rats. Brain Res. 650: 260-266. [DOI] [PubMed] [Google Scholar]
- Packard, M.G. and Cahill, L. 2001. Affective modulation of multiple memory systems. Curr. Opin. Neurobiol. 11: 752-756. [DOI] [PubMed] [Google Scholar]
- Packard, M.G. and McGaugh, J.L. 1992. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav. Neurosci. 106: 439-446. [DOI] [PubMed] [Google Scholar]
- ———. 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 65: 65-72. [DOI] [PubMed] [Google Scholar]
- Packard, M.G., Hirsh, R., and White, N.M. 1989. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. J. Neurosci. 9: 1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G. and Watson, C. 1986. The rat brain in stereotaxic coordinates. Academic Press, New York.
- Poldrack, R.A. and Packard, M.G. 2003. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia 41: 245-251. [DOI] [PubMed] [Google Scholar]
- Pollack, A.E. 2001. Anatomy, physiology, and pharmacology of the basal ganglia. Neurologic Clin. 19: 523-534. [DOI] [PubMed] [Google Scholar]
- Power, A.E., Vazdarjanova, A., and McGaugh, J.L. 2003. Muscarinic cholinergic influences in memory consolidation. Neurobiol. Learn. Mem. 80: 178-193. [DOI] [PubMed] [Google Scholar]
- Prado-Alcala, R.A. 1985. Is cholinergic activity of the caudate nucleus involved in memory? Life Sci. 37: 2135-2142. [DOI] [PubMed] [Google Scholar]
- Prado-Alcala, R.A., Cruz-Morales, S.E., and Lopez-Miro, F.A. 1980. Differential effects of cholinergic blockade of anterior and posterior caudate nucleus on avoidance behaviors. Neurosci. Lett. 18: 339-345. [DOI] [PubMed] [Google Scholar]
- Prado-Alcala, R.A., Cepeda, G., Verduzco, L., Jimenez, A., and Vargas-Ortega, E. 1984. Effects of cholinergic stimulation of the caudate nucleus on active avoidance. Neurosci. Lett. 51: 31-36. [DOI] [PubMed] [Google Scholar]
- Prado-Alcala, R.A., Fernandez-Samblancat, M., and Solodkin-Herrera, M. 1985. Injections of atropine into the caudate nucleus impair the acquisition and the maintenance of passive avoidance. Pharmacol. Biochem. Behav. 22: 243-247. [DOI] [PubMed] [Google Scholar]
- Pych, J.C., Chang, Q., Colon-Rivera, C., and Gold, P.E. 2005. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol. Learn. Mem. 84: 93-101. [DOI] [PubMed] [Google Scholar]
- Ragozzino, M.E. 2003. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol. Learn. Mem. 80: 257-267. [DOI] [PubMed] [Google Scholar]
- Ragozzino, M.E., Wenk, G.L., and Gold, P.E. 1994. Glucose attenuates a morphine-induced decrease in hippocampal acetylcholine output: An in vivo microdialysis study in rats. Brain Res. 655: 77-82. [DOI] [PubMed] [Google Scholar]
- Ragozzino, M.E., Unick, K.E., and Gold, P.E. 1996. Hippocampal acetylcholine release during memory testing in rats: Augmentation by glucose. Proc. Natl. Acad. Sci. 93: 4693-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino, M.E., Pal, S.N., Unick, K., Stefani, M.R., and Gold, P.E. 1998. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J. Neurosci. 18: 1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restle, F. 1957. Discrimination of cues in mazes: A resolution of the place-vs.-response question. Psychol. Rev. 64: 217-228. [DOI] [PubMed] [Google Scholar]
- Riekkinen Jr., P., Schmidt, B., and Riekkinen, M. 1997. Behavioral characterization of metrifonate-improved acquisition of spatial information in medial septum-lesioned rats. Eur. J. Pharmacol. 323: 11-19. [DOI] [PubMed] [Google Scholar]
- Rogers, J.L. and Kesner, R.P. 2004. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learn. Mem. 11: 102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum, K., Futter, M., Jones, M., Hulme, E.C., and Bliss, T.V. 2000. ERKI/II regulation by the muscarinic acetylcholine receptors in neurons. J. Neurosci. 20: 977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, M.A., Mash, D.C., and deToledo-Morrell, L. 2005. Spatial memory in aged rats is related to PKCγ-dependent G-protein coupling of the M1 receptor. Neurobiol. Aging 26: 53-68. [DOI] [PubMed] [Google Scholar]
- Sarter, M., Bruno, J.P., and Givens, B. 2003. Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory? Neurobiol. Learn. Mem. 80: 245-256. [DOI] [PubMed] [Google Scholar]
- Sarter, M., Hasselmo, M.E., Bruno, J.P., and Givens, B. 2005. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 48: 98-111. [DOI] [PubMed] [Google Scholar]
- Segal, M. and Auerbach, J.M. 1997. Muscarinic receptors involved in hippocampal plasticity. Life Sci. 60: 1085-1091. [DOI] [PubMed] [Google Scholar]
- Teather, L.A., Packard, M.G., Smith, D.E., Ellis-Behnke, R.G., and Bazan, N.G. 2005. Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiol. Learn. Mem. 84: 75-84. [DOI] [PubMed] [Google Scholar]
- Tolman, E.C. 1948. Cognitive maps in rats and men. Psych. Rev. 55: 189-208. [DOI] [PubMed] [Google Scholar]
- Tzavos, A., Jih, J., and Ragozzino, M.E. 2004. Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsomedial striatum on response reversal learning. Behav. Brain Res. 154: 245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, A.D., Schacter, D.L., Rotte, M., Koutstaal, W., Maril, A., Dale, A.M., Rosen, B.R., and Buckner, R.L. 1998. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281: 1188-1191. [DOI] [PubMed] [Google Scholar]
- Weinberger, N.M. 2003. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol. Learn. Mem. 80: 268-284. [DOI] [PubMed] [Google Scholar]
- ———. 2004. Specific long-term memory traces in primary auditory cortex. Nat. Rev. Neurosci. 5: 279-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C., Preston, A.R., Oh, M.M., Schwarz, R.D., Welty, D., and Disterhoft, J.F. 2000. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J. Neurosci. 20: 783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink, B.H. and Timmerman, W. 1999. Do neurotransmitters sampled by brain microdialysis reflect functional release? Anal. Chim. Acta 373: 263-274. [Google Scholar]
- White, N.M. and McDonald, R.J. 2002. Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem. 77: 125-184. [DOI] [PubMed] [Google Scholar]