Abstract
The Polycomb group (PcG) of proteins represses homeotic gene expression through the assembly of multiprotein complexes on key regulatory elements. The mechanisms mediating complex assembly have remained enigmatic since most PcG proteins fail to bind DNA. We now demonstrate that the human PcG protein dinG interacts with CP2, a mammalian member of the grainyhead-like family of transcription factors, in vitro and in vivo. The functional consequence of this interaction is repression of CP2-dependent transcription. The CP2-dinG interaction is conserved in evolution with the Drosophila factor grainyhead binding to dring, the fly homologue of dinG. Electrophoretic mobility shift assays demonstrate that the grh-dring complex forms on regulatory elements of genes whose expression is repressed by grh but not on elements where grh plays an activator role. These observations reveal a novel mechanism by which PcG proteins may be anchored to specific regulatory elements in developmental genes.
Many of the seminal insights into developmental processes have come from studies of Drosophila, where stimulatory and inhibitory influences are finely balanced to determine body patterning. This is classically illustrated in studies of the homeotic pathways where the trithorax group of genes sustain active Hox gene expression and the Polycomb group (PcG) encodes stable repressors (for reviews, see references 5, 13, 46, and 59). Another key regulatory gene required for developmental balance encodes the transcription factor Grainyhead (GRH), also known as NTF-1 or ELF-1. GRH was initially identified through its ability to bind a cis element critical for transcriptional activation of the Dopa decarboxylase gene (7, 8, 18, 30). During development, GRH is initially involved in dorsal-ventral and terminal patterning of the early embryo through the formation of multiprotein complexes that repress transcription from the decapentaplegic, tailless, and zerknüllt genes (26, 39). Later, grainyhead is predominantly expressed in the cuticle-producing tissues and in the embryonic central nervous system, where its expression profile overlaps significantly with that of another developmental gene it regulates, Ultrabithorax (3, 7, 19, 71). The importance of grainyhead in Drosophila embryogenesis is emphasized by the dire developmental consequences associated with perturbed expression. Overexpression of wild-type or a dominant negative form of grh in early development is associated with embryonic death and, in the case of the wild-type factor, morphogenetic cuticular defects (4). Mutations abolishing the neuroblast-specific isoform of the protein cause laval-pupal death, and embryos which totally lack grh function do not hatch, exhibiting flimsy cuticles, grainy and discontinuous head skeletons, and patchy tracheal tubes (8, 71). Although no patterning defects are seen in these embryos due to the maternal provision of the factor in early embryogenesis, germ line clones generated from heterozygous grh/− females do show the predicted expansion of tll (26, 39).
In mammals, three highly related grainyhead-like genes have been previously identified. In humans they are known as LBP-1a, CP2 (LBP-1c/LSF/UBP-1), and LBP-9, whereas in mice they are referred to as Nf2d9, CP2, and CRTR-1, respectively (27, 32, 35, 40, 51, 68, 77, 78). Analogous to their Drosophila counterpart, the mammalian grainyhead-like genes also play important roles in development. CP2 is involved in fetal-erythroid expression of the globin genes through the formation of a heteromeric complex known as the stage selector protein (28, 29, 80). It is also involved in cellular processes including regulation of the interleukin-4, thymidylate synthase, and uroporphyrinogen III synthase promoters (11, 48, 63, 75). Nf2d9 is also a key developmental regulatory gene, fulfilling similar roles to CP2 (49). However, Nf2d9 also has important nonredundant functions, as evidenced by the embryo-lethal phenotype observed in mice nullizygous at this locus (S. M. Jane and J. M. Cunningham, unpublished data).
Structure-function and sequence analyses of CP2/LBP-1a/LBP-9 and GRH indicate that the DNA binding and protein dimerization domains are highly conserved. Up to 48% identity and 64% similarity are present in the DNA binding domain and 45% identity and 61% similarity are present in the dimerization domain, emphasizing the importance of homo- and heteromeric complex formation for their activity (58, 72). Based on this, we performed a protein interaction trap screen using human CP2 as the “bait” in an attempt to identify new genes that modulate the transcriptional activity of this family. We now report that the human RING finger protein dinG, a member of the PcG of proteins, specifically interacts with members of the grainyhead-like family. We also demonstrate that the Drosophila homologue of dinG interacts with GRH. These findings provide a novel mechanism by which a PcG complex can be directly targeted to specific sites on the DNA in key developmental gene-regulatory regions.
MATERIALS AND METHODS
Yeast two-hybrid library screen and analysis of protein interactions.
The yeast two-hybrid library screen was performed as described previously (20, 80). For analysis of mammalian protein interactions, the indicated fragments of the cDNAs encoding CP2, LBP-1a, dinG, and Ring1A were derived by PCR or restriction digestion and cloned into pGAD424 (a GAL4 transactivation domain vector, GAL4AD) (Clontech). The PCR fragments were sequenced in their entirety, and the restriction fragments were sequenced across the fusion junction with GAL4AD. The resulting plasmids were cotransformed into yeast as detailed above with or without 30 mM 3-amino-1,2,4-triazole. Positive interactions met the two criteria of growing on plates containing selection medium and testing β-galactosidase positive. Expression of the various plasmids was confirmed in yeast by Western analysis using antibodies that recognize the GAL4 DNA binding domain or GAL4AD (Clontech) (data not shown).
Expression of GST fusion proteins and affinity chromatography.
The CP2 or dRING cDNA was cloned in frame with the glutathione S-transferase (GST) coding sequence in the pGEX vector (Pharmacia). The GST fusion proteins were expressed in Escherichia coli strain BL21 and purified on glutathione-Sepharose (Pharmacia). Their integrity was confirmed by Coomassie staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For in vitro protein-protein interaction assays, 1 mg of GST or GST fusion protein was incubated for 1 h at 4°C with 10 ml of glutathione-Sepharose beads which had been preblocked with 5% milk. After extensive washing, the beads were resuspended in 200 ml of binding buffer (10 mM Tris-HCl [pH 7.9], 500 mM KCl, 0.1 mM EDTA, 150 mg of bovine serum albumin per ml, 0.1% Nonidet P-40, 10% glycerol) and incubated for 1 h at room temperature with [35S]methionine-labeled dinG or GRH. After extensive washing, retained proteins were eluted by boiling in SDS loading buffer and analyzed by SDS-PAGE and autoradiography.
Coimmunoprecipitation analyses.
The dinG coding region was cloned into the retroviral vector plasmid MSCV-HA at a unique XhoI site. This bicistronic vector contains (i) the murine stem cell virus (MSCV) 5′ long terminal repeat, (ii) a hemagglutinin (HA) epitope tag fused in frame with the amino-terminal end of the dinG coding sequence, (iii) the encephalomyocarditis virus internal ribosomal entry site, (iv) the green fluorescent protein cDNA, and (v) the MSCV 3′ long terminal repeat. This plasmid and a control plasmid lacking the dinG cDNA were transfected into 293T cells by calcium phosphate precipitation. After 48 h, cell lysates were prepared and initially precleared with normal rabbit serum (10 mg/ml) prior to incubation with preimmune serum or antisera to CP2 overnight at 4°C. A 50% slurry of protein G-Sepharose was added, and the mixture was incubated at 4°C for 1 h. The mixture was then centrifuged at 3,000 × g for 1 min, and the pellet was washed in 50 mM Tris-HCl (pH 7.9)—150 mM NaCl prior to being resuspended in SDS loading buffer. Samples were subjected to SDS-PAGE, transferred to polyvinylidene difluoride membranes, and blotted with antisera to HA. Signal detection was performed with the ECL system as specified by the manufacturer (Amersham Pharmacia).
Native coimmunoprecipitation was performed with nuclear extract from K562 cells generated by the method of Dignam (16). Antiserum against CP2 (or preimmune serum) was used for immunoprecipitation, and blotting was performed with anti-dinG, anti-HPC2, anti-Bmi-1, anti-M33, or anti-RYBP antisera. Signal detection was performed with the ECL system as specified by the manufacturer.
In vitro transcription assays.
The CP2-dependent promoter plasmid for the in vitro transcription assays was prepared by subcloning three concatemerized copies of the murine α-globin promoter CP2 binding motif (5′-GAGCAAGCACAAACCAGCCAA-3′) upstream of a minimal γ-globin gene promoter (−35 to +46) linked to a luciferase reporter gene. Reactions were performed essentially as described previously (61). Crude HeLa cell extract (10 ml) was incubated with 2 nM supercoiled template DNA in the presence of 4 mM (each) rATP, rCTP, rUTP, and rGTP. As an internal control, all reaction mixtures contained a 0.2 nM concentration of a template containing the adenovirus major late (AdML) promoter (a kind gift of Robert Tjian). Recombinant CP2 (20 ng) or dinG (20 to 200 ng) was added to the reaction mixtures as indicated. Transcription assay mixtures were incubated for 30 min at 30°C. RNA synthesized in vitro was analyzed by primer extension. Fold increases in transcription were determined by densitometry from the results of three experiments.
DNA construction, cell lines, and transfection assays.
The human CP2 cDNA was cloned into the pEF-BOS mammalian expression vector as an XbaI fragment. The dinG cDNA was cloned as a BamHI-XhoI fragment into a modified version of the mammalian expression vector pCI-Neo containing three HA epitopes. The frame of the HA-dinG fusion was confirmed by sequencing. The thymidine kinase (TK)-dependent reporter construct contained the TK promoter linked to the Renilla luciferase gene. The CP2-dependent reporter construct is described above. The plasmids were transfected into the 293 cell line using calcium phosphate, and the cells were cultured for 48 h. Cells were lysed with Triton X-100, and luciferase activity was assayed using the Promega system on a Monolight 2001 luminometer (Analytical Luminescence Laboratories). The linearity of the assay was confirmed by using serial dilutions, and cell lysates had equivalent protein concentrations. Results were obtained from three independent experiments performed in triplicate.
EMSA.
Electrophoretic mobility shift assays (EMSA) were performed as described previously (28). The probes used were DRE-B (nucleotides 1914 to 1959 of the dpp VRR in the dpp promoter) (26), tor-RE (the footprint D sequence of the tll promoter) (39), and be2 (GRH binding site in the Ddc promoter) (71). Mammalian extracts were derived from the human placental cell line JEG-3. Drosophila embryo extract was a kind gift of David Tremethick (Australian National University). Antiserum against GRH was a kind gift of Sarah Bray (University of Cambridge) (7). Antiserum to dinG was raised in rabbit and shown to cross-react with Drosophila dring in Western blots (data not shown).
RESULTS
Isolation of dinG from a K562 erythroleukemia library.
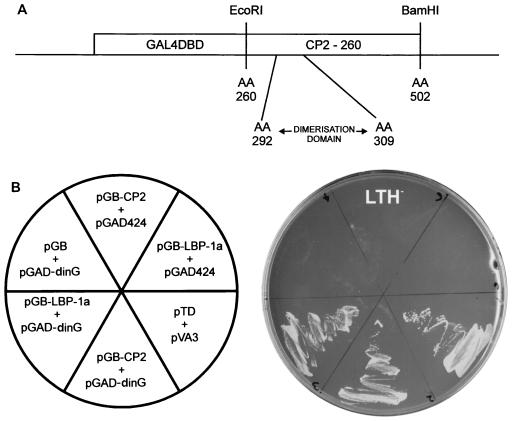
Biochemical studies in our laboratory and others have localized the protein dimerization domain of CP2 to a 750-bp fragment encompassing the carboxy terminus of the protein. Within this domain is a 17-amino-acid stretch (amino acids 292 to 309) that is absolutely required for protein dimerization. A cDNA sequence encoding the COOH-terminal 242 amino acids (amino acids 260 to 502) of CP2 was inserted into the yeast expression vector pGBT9. The resultant plasmid (GAL4-260-502) encodes a hybrid protein containing the DNA binding domain of GAL4 fused to CP2 residues 260 to 502 (Fig. 1A). The yeast reporter strain HF7C was transformed with this vector and an expression library from the K562 human erythroleukemia cell line fused to the sequences encoding the GAL4AD. This library was chosen because the tissue of origin expresses high levels of CP2 and is known to contain one specific CP2 partner, human NF-E4 (80). From approximately 5 × 106 clones screened, we isolated 100 clones that showed specific interaction with the CP2 bait. From this collection we identified about 40 clones that encoded CP2, an expected result in view of the ability of the protein to homodimerize. An additional 50 clones represented previously identified false-positive results from the two-hybrid screen. From the remaining clones, we identified and characterized one gene that encodes dinG, a human RING domain-containing protein, which is a member of the PcG of proteins (25). To ensure that the positive result in the two-hybrid assay was not influenced by the GAL4 component of each fusion protein, GAL4-CP2-260-502- and GAD-dinG-expressing plasmids were transfected separately into HF7C cells with the opposite yeast vector expressing no fusion protein. We also examined LBP-1a, another member of the grainyhead-like family of developmental transcription factors which is highly homologous to CP2 in the dimerization domain, in this assay. As shown in Fig. 1B, yeast growth on HIS (−) plates, which is indicative of a protein-protein interaction, was observed only in the context of CP2 or LBP-1a with the dinG-expressing plasmid. No interactions were observed with the empty vectors (Fig. 1B) or between dinG and a range of other factors including GATA-1, SCL, and EKLF (data not shown).
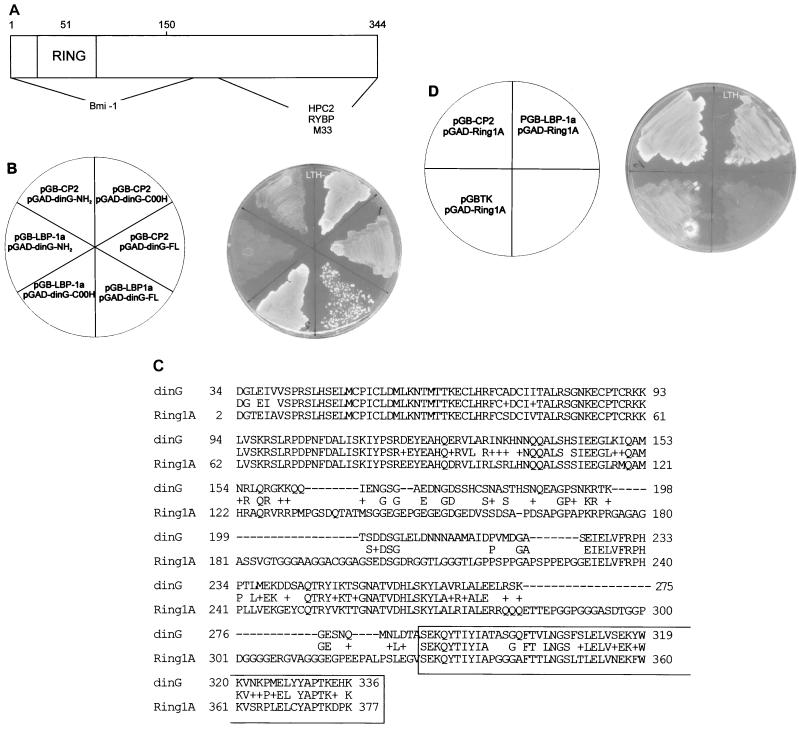
FIG. 1.
Isolation of the CP2-interacting protein, dinG. (A) Schematic of the bait construct used in the yeast two-hybrid screen of a K562 cDNA library. The coding sequence of CP2 from amino acids (AA) 260 to 502 were fused in frame with the GAL4 DNA binding domain (GAL4DBD). The minimal dimerization domain (amino acids 292 to 309) is shown. (B) Human dinG interacts specifically with CP2 and LBP-1a in yeast. (Left) The Saccharomyces cerevisiae reporter strain HF7C was transformed with the indicated plasmids. pGB-LBP-1a contains the carboxy-terminal half of LBP-1a analogous to pGB-CP2-260. pGAD-dinG was the original clone identified in the library screen and contains a dinG fragment encoding amino acids 257 to 336. pGB, pGAD424, and the positive controls pTD and pVA3 have been reported previously. (Right) Transformants were streaked onto synthetic medium plates lacking tryptophan, leucine, and histidine (LTH−) and incubated at 30°C for 3 days.
dinG interacts with CP2 in in vitro and cellular assays.
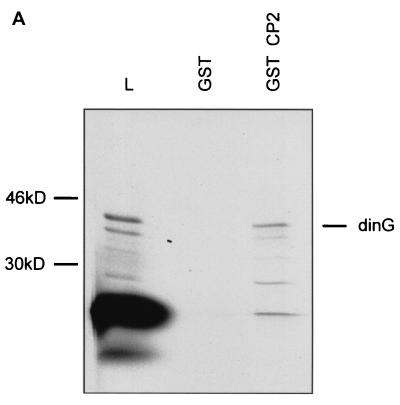
To confirm the interaction between dinG and CP2, we used in vitro GST chromatographic studies. For these experiments, radiolabeled in vitro-transcribed and translated full-length dinG was applied to a Sepharose matrix coupled to either GST alone or a fusion protein of GST and the carboxy terminus of CP2 containing the dimerization domain (GST-CP2). As seen in Fig. 2A, dinG was specifically retained on the GST-CP2 column.
FIG. 2.
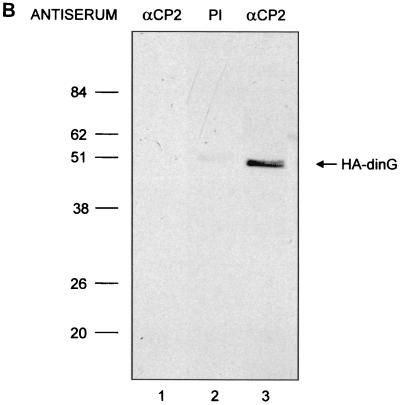
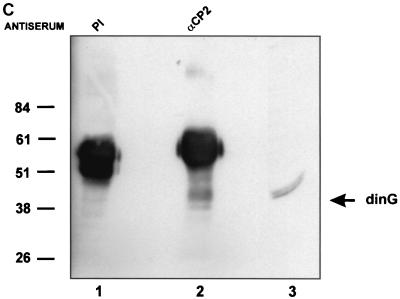
dinG interacts with CP2 in vitro and in a cellular context. (A) Interaction between CP2 and dinG. A fusion protein between GST and CP2 (GST-CP2) or GST alone was expressed in E. coli, and 1 mg of protein was bound to glutathione-Sepharose beads. The beads were then incubated with 2 ml of 35S-labeled in vitro-translated dinG in binding buffer for 1 h at room temperature (see Materials and Methods). After extensive washing, the beads were resuspended in SDS loading buffer and subjected to SDS-PAGE. The migration of the dinG load and the molecular mass standards are labeled. The identities of the slower-migrating species retained on the GST-CP2 matrix are unknown, but they could represent proteins that bridge the gap between CP2 and dinG. L, load. (B) 293 cells were transfected with an MSCV-based expression plasmid containing the dinG cDNA tagged at the amino terminus with the HA epitope or the MSCV vector with no insert. After 48 h, the cells were sorted by fluorescence-activated cell sorting and lysates were prepared from both transfections and immunoprecipitated with either anti-CP2 antiserum (lanes 1 and 3) or preimmune (PI) serum (lane 2). The immunoprecipitates from the MSCV lysate (lane 1) or MSCV-HA-dinG (lanes 2 and 3) were subjected to SDS-PAGE and blotted with monoclonal antiserum to HA. The migration of HA-dinG and the molecular mass standards (in kilodaltons) is indicated. (C) Coimmunoprecipitation of CP2 and dinG from native K562 cells. Nuclear extract from K562 cells was immunoprecipitated with polyclonal antiserum to CP2 (lane 2) or preimmune (PI) serum (lane 1). The immunoprecipitates were subjected to SDS-PAGE and blotted with antiserum to dinG. Crude K562 nuclear extract served as the positive control (lane 3). The intensely staining band in lanes 1 and 2 is the antibody heavy chain. The migration of the molecular mass standards is indicated.
To confirm the interaction between CP2 and dinG in mammalian cells, we performed coimmunoprecipitation experiments. The initial experiments used an MSCV-based expression vector containing an HA epitope fused in frame at the amino terminus with the dinG coding sequence (MSCV-HA-dinG). The human 293 cell line was transfected with this vector or the control empty vector that carries the green fluorescent protein gene (MSCV). Two days later, cells were sorted by fluorescence-activated cell sorting and whole-cell lysate was prepared. Lysates were immunoprecipitated with either antiserum to CP2 or preimmune serum, fractionated by SDS-PAGE, and immunoblotted with a monoclonal antibody which recognized the HA epitope. As shown in Fig. 2B, CP2 and HA-dinG were coimmunoprecipitated with the CP2-specific antiserum (lane 3) but not with preimmune serum (lane 2). Coimmunoprecipitation was clearly dependent on the presence of HA-dinG since it was not observed with lysates of 293 cells transfected with the MSCV vector alone (lane 1). The reverse experiments, in which immunoprecipitation was performed with the monoclonal antibody to HA and the precipitate was blotted with anti-CP2 antiserum, also showed an in vivo interaction between dinG and CP2 (data not shown).
To further confirm this interaction, a native coimmunoprecipitation experiment was performed using cell lysate from K562 cells. As shown in Fig. 2C, lysate immunoprecipitated with anti-CP2 antiserum and blotted with anti-dinG antiserum demonstrated a band of 40 kDa (lane 2) which comigrated with the immunoreactive species in K562 extract blotted with anti-dinG antiserum (lane 3). In contrast, no dinG was observed with lysate immunoprecipitated with preimmune serum (lane 1).
The RING domain of dinG is not required for its interaction with CP2 or LBP-1a.
Previous studies have defined the regions of dinG and its murine homologue Ring1B involved in the formation of a multiprotein complex with other PcG members. As shown in Fig. 3A, Bmi-1 requires an intact RING finger for binding to dinG. In contrast, HPC2 interacts with key amino acids in the COOH region of the dinG protein (25). The murine factors RYBP and M33 also interact with a carboxy-terminal domain of Ring1B (23, 55). To map the domain in dinG necessary for the interactions with CP2 and LBP-1a, we generated yeast expression vectors containing either full-length dinG or its amino terminus or carboxy terminus fused to the GAL4AD. These were transfected into yeast with the GAL4DB plasmids containing the dimerization domains of CP2 or LBP-1a and grown in the presence of selection medium lacking leucine, tryptophan, and histidine. As shown in Fig. 3B, the carboxy-terminal fragment of dinG was sufficient to mediate the protein interaction with both CP2 and LBP-1a, allowing yeast growth. Positive lacZ staining of these colonies confirmed the specificity of reporter gene activation (data not shown). Sequence comparison between dinG and the closely related PcG protein mRing1A (55) demonstrated 70% amino acid identity in the region mediating the interaction with CP2 and LBP-1a (Fig. 3C). We therefore examined whether Ring1A could also interact with the grainyhead-like factors. The sequence encoding the conserved carboxy-terminal region of the protein was fused in frame with the GAL4AD and screened against the dimerization domains of CP2 and LBP-1a in yeast. As shown in Fig. 3D, specific interactions were observed with both factors, as indicated by growth on histidine selection medium. Positive lacZ staining of these colonies again confirmed the specificity of reporter gene activation (data not shown).
FIG. 3.
The RING domain of dinG is not required for the interaction with CP2/LBP-1a. (A) Schematic representation of the dinG protein. The RING finger domain is marked, and the regions interacting with Bmi-1 and HPC2 are indicated. The region of the murine homologue of dinG, Ring1B, that interacts with RYBP and M33 is also shown. (B) Mapping the CP2/LBP-1a interaction domain of dinG. (Left) The S. cerevisiae reporter strain HF7C was transformed with the indicated plasmids. pGB-CP2-260 and pGB-LBP-1a are detailed in the legend to Fig. 1. pGAD-dinG-FL contains the entire dinG cDNA fused to the GAL4AD. pGAD-dinG-NH2 contains a dinG cDNA fragment encoding amino acids 1 to 165 fused to GAL4AD, and pGAD-dinG-COOH contains a dinG cDNA fragment encoding amino acids 286 to 336 fused to GAL4AD. (Right) Transformants were streaked onto synthetic medium plates lacking tryptophan, leucine, and histidine (LTH−) and incubated at 30°C for 3 days. (C) Sequence comparison of dinG and Ring1A. The deduced amino acid sequences encoded by dinG and Ring1A cDNAs are aligned using the BLAST algorithm (2). Amino acid identity is shown, and similarities are indicated by (+). Dashes represent gaps introduced to maximize the alignment. The region of dinG that interacts with CP2/LBP-1a is boxed. (D) Ring1A interacts with CP2/LBP-1a. (Left) The S. cerevisiae reporter strain HF7C was transformed with the indicated plasmids. pGB-CP2-260 and pGB-LBP-1a are detailed in the legend to Fig. 1. pGBTK has been described previously. pGAD-Ring1A contains a Ring1A cDNA fragment encoding amino acids 327 to 377 fused to GAL4AD. (Right) Transformants were streaked onto synthetic medium plates lacking tryptophan, leucine, and histidine (LTH−) and incubated at 30°C for 3 days.
HPC2 also contributes to the dinG-CP2 polycomb complex.
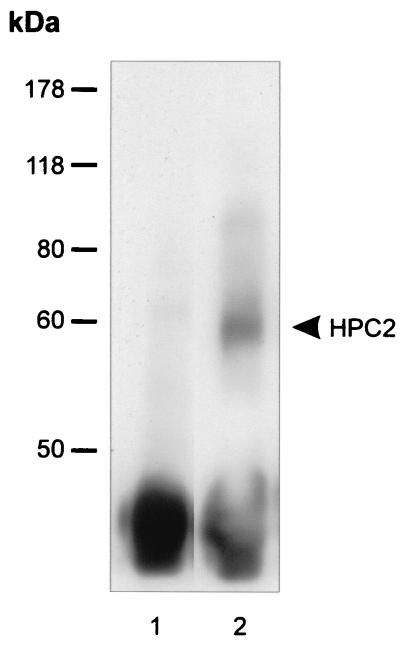
The dinG protein has previously been shown to form complexes in vivo with a variety of PcG family members including Bmi-1, HPC2, RYBP, and M33 (23, 25, 53). To determine whether any of these factors contribute to the dinG-CP2 complex, we performed further native coimmunoprecipitation experiments. K562 or murine erythroleukemia cell line extract was immunoprecipitated with anti-CP2 antiserum and blotted with anti-RYBP, anti-M33, anti-Bmi1, and anti-HPC2 antisera. As shown in Fig. 4, HPC2 was coimmunoprecipitated with anti-CP2 antiserum (lane 2) but not with preimmune sera (lane 1) under conditions identical to those used for the dinG immunoprecipitation. RYBP, M33, and Bmi-1 did not coimmunoprecipitate with CP2 and dinG (data not shown).
FIG. 4.
HPC2 with CP2 form a complex in a cellular context. Nuclear extract from K562 cells was immunoprecipitated with polyclonal antisera to CP2 (lane 2) or preimmune serum (lane 1). The immunoprecipitates were subjected to SDS-PAGE and blotted with antiserum to HPC2. The migrations of the molecular mass standards and of HPC2 (in kilodaltons) are indicated.
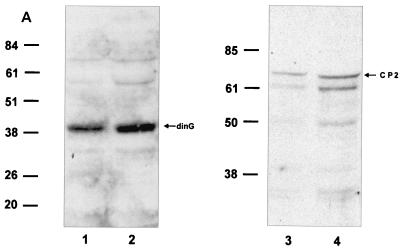
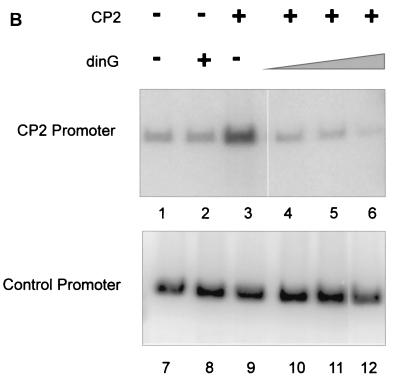
dinG functions as a repressor, blocking CP2-dependent transcriptional activation.
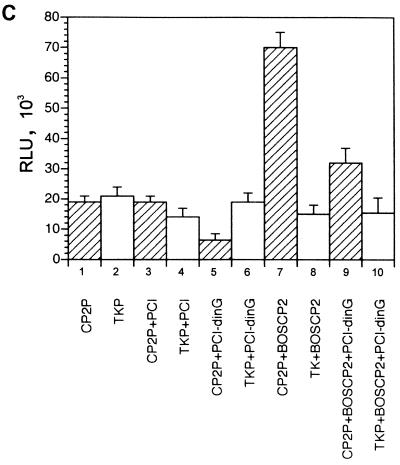
To examine the functional consequences of the PcG complex-CP2 interaction, we established a CP2-dependent in vitro transcription assay. A minimal promoter was linked to concatemers of the murine α-globin CP2 binding site, and transcription was initiated by the addition of HeLa cell nuclear extract. Western analysis of this extract using antibodies to CP2 and dinG, which give comparable signals on standard curves, revealed abundant dinG protein and more modest amounts of CP2 (Fig. 5A). In vitro transcription with extract alone produced a modest signal that was not affected by the addition of recombinant dinG (Fig. 5B, lanes 1 and 2). In contrast, the addition of recombinant CP2 augmented transcription threefold (lane 3). When recombinant dinG was also added to this reaction mixture, the effect of recombinant CP2 was abolished and transcriptional levels returned to those observed with the basic extract (lane 4). Addition of a fivefold excess of recombinant dinG had no additional effect (lane 5). However, the addition of a 10-fold excess of dinG reduced transcriptional levels to below those observed with the basic extract (lane 6). No effect was observed with CP2 or dinG with the control promoter (lanes 7 to 11), except for a slight reduction in transcription with the highest concentration of dinG (lane 12).
FIG. 5.
dinG specifically represses CP2-dependent transcription in vitro and in a cellular context. (A) Western analysis of HeLa cell nuclear extract. Nuclear extract prepared from 5 × 107 HeLa cells was subjected to SDS-PAGE and blotted with antiserum to dinG (10 μl [lane 1] or 20 μl [lane 2]) or CP2 (10 ml [lane 3] or 20 μl [lane 4]). These antibodies display comparable affinities to recombinant protein (data not shown). The blots were developed for equal times by ECL. The migrations of the dinG and CP2 proteins and the molecular mass standards (in kilodaltons) are indicated. (B) dinG-mediated repression of CP2-dependent transcription in vitro. (Top) Crude HeLa cell extract (10 μl) was incubated with 2 nM supercoiled template DNA containing the CP2-dependent promoter in the presence of 4 mM (each) rATP, rCTP, rUTP, and rGTP. (Bottom) As an internal control, all reaction mixtures contained a 0.2 nM concentration of a template containing the AdML promoter. Recombinant CP2 (20 ng) or dinG (20 to 200 ng) was added to the reaction mixtures as indicated. Transcription assay mixtures were incubated for 30 min at 30°C. RNA synthesized in vitro was analyzed by primer extension. (C) dinG-mediated repression of CP2-dependent transcription in a cellular context. 293 cells were transiently transfected with a CP2-dependent promoter linked to the luciferase reporter gene (hatched columns) and the TK promoter linked to the Renilla luciferase reporter gene (open columns) in the presence or absence of a dinG expression vector (PCI-dinG) and a CP2 expression vector (pEFBOS-CP2) as indicated. Transfection with the empty vector (pCI) served as the control. Luciferase levels were corrected for protein concentration, and values were derived from three independent experiments performed in triplicate.
To confirm these findings in a cellular context, we cotransfected the CP2-dependent promoter linked to a luciferase reporter gene and the TK promoter linked to a Renilla luciferase reporter gene into the 293 cell line in the presence and absence of a CP2 expression vector (pEFBOS-CP2) and a dinG expression vector (pCI-dinG). As shown in Fig. 5C, CP2-dependent transcription was repressed by dinG in both the absence (column 5) and presence (column 9) of pEFBOS-CP2. No effect was observed on the TK promoter in either setting (columns 6 and 10).
Identification of the Drosophila homologue of dinG that interacts with GRH.
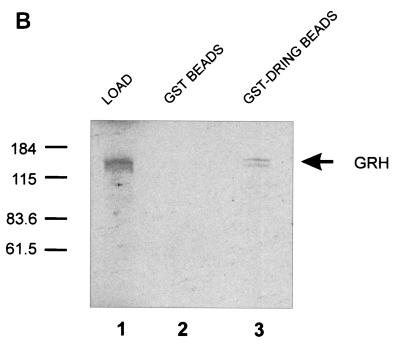
Strong evolutionary conservation of amino acid sequence exists between the mammalian and Drosophila members of the grainyhead-like family. The likelihood of a similar conservation of function led us to postulate the existence of a Drosophila homologue of dinG. Database searches identified a sequence that we have termed dring, which has 44% identity and 61% similarity to the dinG amino acid sequence and 50% identity and 68% similarity in the domain of the dinG protein which interacts with the GRH-like family (Fig. 6A). To determine whether the Drosophila factor DRING could interact with GRH, we generated radiolabeled in vitro-transcribed and translated GRH for GST chromatography assays. As shown in Fig. 6B, GRH was specifically retained on a GST-DRING matrix but not on GST alone, confirming the evolutionary conservation of this interaction.
FIG. 6.
DRING, the Drosophila homologue of dinG, interacts with GRH. (A) Sequence comparison of dinG and DRING. The deduced amino acid sequences encoded by dinG and dring cDNAs were aligned by using the BLAST algorithm (2). Amino acid identity is shown, and similarities are indicated by plus signs. Dashes represent gaps introduced to maximize the alignment. The region of dinG that interacts with CP2/LBP-1a is boxed. (B) GST chromatography of DRING and GRH. A fusion protein between GST and DRING (GST-DRING) or GST alone was expressed in E. coli, and 1 mg of protein was bound to glutathione-Sepharose beads. The beads were then incubated with 2 ml of 35S-labeled in vitro-translated GRH in binding buffer for 1 h at room temperature (see Materials and Methods). After extensive washing, the beads were resuspended in SDS loading buffer and subjected to SDS-PAGE. The migrations of the GRH load and the molecular mass standards (in kilodaltons) are indicated.
GRH-like factors and dinG-like PcG proteins form a complex on GRH DNA binding sites that mediate transcriptional repression.
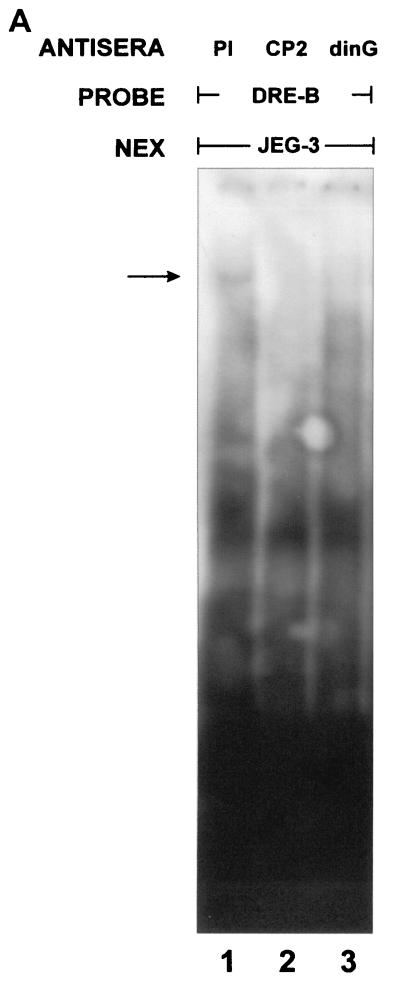
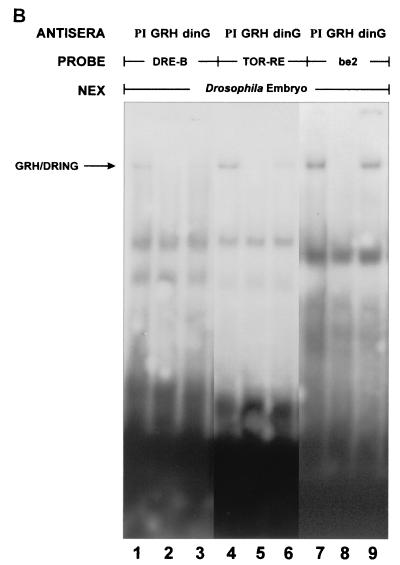
Our experiments to this point indicated that dinG could interact with CP2 and repress transcription from a CP2-dependent promoter. These data were generated in the context of a concatemerized consensus CP2 binding site. No physiological target genes of CP2-mediated repression have been identified in mammalian systems. In contrast, the regulatory regions in the dpp and tll genes involved in GRH-mediated repression have been clearly defined in vivo (26, 39). In view of the significant homology between GRH and CP2 in the DNA binding domain, we initially examined whether the CP2-dinG complex could form on the GRH-responsive element in the dpp promoter. A probe containing the DRE-B region of the dpp promoter was studied in an EMSA in the presence of nuclear extract from the mammalian cell line JEG-3. As shown in Fig. 7A, addition of this extract to the DRE-B probe resulted in the formation of a DNA-protein complex. This complex was ablated by the addition of either anti-CP2 (lane 2) or anti-dinG (lane 3) antiserum. To extend this observation, we examined whether the GRH-DRING complex could assemble on the regulatory regions in the dpp and tll genes that are critical for GRH-mediated repression (26, 39). Probes containing the DRE-B region of the dpp promoter (26) and the tor-RE element in the tll promoter (39) were studied in an EMSA with Drosophila embryo extract in the presence and absence of anti-GRH antiserum or anti-dinG antiserum (which cross-reacts with the Drosophila DRING protein) (data not shown). The be2 element of the Ddc promoter (where GRH functions as a transcriptional activator) (71) was also studied. As shown in Fig. 7B, a complex consisting of at least GRH and DRING formed on both the dpp and tll elements (lanes 1 and 4). In both settings, the complex was ablated (or shifted out of the gel) by anti-GRH (lanes 2 and 5) and anti-dinG (lanes 3 and 6) antisera. In contrast, the complex formed on the Ddc promoter (lane 7) was ablated by the addition of anti-GRH antiserum (lane 8) but remained unchanged in the presence of anti-dinG antiserum (lane 9).
FIG. 7.
GRH-like factors and RING PcG form a complex on GRH DNA binding sites that mediate transcriptional repression. (A) Binding of the CP2-dinG complex to DNA. Nuclear extract from the human JEG-3 cell line was studied in an EMSA with a dpp promoter probe (DRE-B) in the presence of preimmune (PI) serum (lane 1), anti-CP2-specific antiserum (lane 2), or anti-dinG antiserum (lane 3). The migration of the CP2-dinG-DNA complex is indicated by an arrow. (B) Binding of the GRH-DRING complex to GRH DNA binding sites that mediate transcriptional repression. Nuclear extract from early (120-min) Drosophila embryos was studied in an EMSA with two probes known to contain GRH-dependent repressor elements (DRE-B from the dpp promoter [26] and TOR-RE from the tll promoter [39]) and one probe containing a GRH-dependent activator sequence (be2 from the Ddc promoter [71]). Reaction mixtures contained preimmune (PI) serum (lanes 1, 4, and 7), anti-GRH antiserum (lanes 2, 5, and 8), or anti-dinG antiserum (which cross-reacts with DRING) (lanes 3, 6, and 9).
DISCUSSION
In Drosophila, the PcG maintains repression of homeotic genes in patterns established by the gap, pair rule, and segment polarity genes (33, 38, 64). In PcG mutant embryos, patterns of homeotic gene expression are established correctly, but later in development, expression occurs outside their normal boundaries, and as a consequence, homeotic transformations are seen (17, 33, 65, 76). The products of the PcG of genes form functional multiprotein complexes as evidenced by the enhancement of the homeotic phenotypes observed with combinations of PcG mutations, from the colocalization of PcG proteins to salivary gland polytene chromosome bands and from coimmunoprecipitation experiments (22, 31, 37, 41, 57, 67, 69). These complexes are conserved in evolution, with both murine and human factors forming heteromeric links with other members of the family (25, 52, 56, 74).
The PcG has been suggested to act through a variety of mechanisms. These include the compaction of chromatin, rendering it insensitive to transcription factors (45, 81), stabilization of nucleosome arrays preventing remodeling by complexes such as SWI-SNF (21, 57), inhibition of general transcription factor-mediated transcriptional activation through interactions with TAFII proteins (9, 54), alteration of histone acetylation (73), interference with promoter-enhancer interactions (47), and formation of silencer-promoter complexes (5, 46). This regulation is mediated in part by PcG response elements (PREs), cis-acting DNA elements which maintain the expression boundaries of homeotic genes (12, 14, 24, 43, 60). More recently, PcG binding has also been demonstrated outside PREs, in core promoters (44). These sites are functionally important and in some circumstances may act cooperatively with upstream PREs (14, 42).
One enigma of PcG function is the inability of most of these factors to bind DNA. Of the many Drosophila and mammalian genes defined in this group, few are known to encode a protein with DNA binding properties. One of these is Drosophila pleiohomeotic (PHO), which encodes a zinc finger protein related to the mammalian transcription factor YY1 (10). More recently, PSC has also been shown to have direct DNA binding activity (21). The mammalian factor mel-18 also binds regulatory regions in the Hox genes in vitro (34). The inability of the other PcG proteins to directly bind DNA has led to the proposal of a number of models to explain their targeting to specific genes. These include the generation of DNA binding activity that is dependent on multiple proteins in the complex, binding of PcG proteins to a distinct chromatin structure rather than a specific sequence motif (42), and interactions with noncoding RNA at specific chromosomal sites (1). An additional model suggests that PcG complex formation at specific sites is dependent on recruitment by DNA binding proteins, analogous to the assembly of enhanceosomes. Candidates for this function have included ESC and E(Z) (50, 66), Zeste (54), Hunchback (HB) (79), and the HB interacting protein dMi-2 (36). Recently, the E2F6 transcription factor has been shown to participate in a Bmi-1-containing PcG complex, although it is unclear whether this results in targeting to specific DNA sequences (70). We have now demonstrated that recruitment of a PcG complex to a specific DNA target can be achieved by a family of non-Pc DNA binding proteins known to regulate developmental gene expression. Underlying this DNA-protein complex assembly is the interaction between the key developmental GRH-like proteins and the PcG member dinG. This interaction is highly conserved in evolution, with the Drosophila homologues of both proteins (GRH and DRING, respectively) also shown to interact.
The murine RING finger proteins Ring1A and Ring1B and their human homologues RING1 and dinG have previously been shown to form multiprotein complexes with a variety of PcG proteins (23, 25, 52, 55). The Ring1B/dinG proteins complex with the mammalian PcG homologues Bmi-1, HPC2 (53), and HPH1 (52) and the mouse homologue of HPH2, MPh2 (25). Similarly, the Ring1A/RING proteins interact with Bmi-1 and HPC2. Both factors also interact with the repressor protein RYBP and M33 (23). Despite this, the Ring proteins have only recently been established as members of the PcG with the demonstration of homeotic transformations in mice carrying Ring1A loss- and gain-of-function mutations (15). Our data identifying the Drosophila homologue of dinG further strengthens this conclusion.
In addition to their role in assembly of multiprotein PcG complexes, the mammalian Ring proteins, analogous to many other PcG members, have repressor activity when tethered to DNA through a GAL4 DNA binding domain (52, 55). Our studies now provide a mechanism whereby this activity can potentially be targeted to specific gene regulatory sequences in a wide variety of developmental genes. Our in vitro and cellular transcription studies demonstrate that dinG represses transcription when recruited to a CP2-dependent promoter but not when recruited to a control promoter, emphasizing the functional specificity of the CP2-dinG interaction (Fig. 5B and 6B). The potential physiological relevance of this is emphasized by the demonstration of a GRH-DRING complex on DNA regulatory elements involved in GRH-mediated gene repression. This transcriptional repression is in keeping with the models of PcG function that invoke their participation in silencer-promoter complexes anchored to the regulatory sequences by specific DNA binding proteins (5, 46). The domain responsible for the repressor activity of the Ring proteins resides in the amino terminus and is therefore distinct from the region that interacts with the grh-like proteins (55). Our in vivo immunoprecipitation studies indicate that only HPC2 contributes to the dinG-CP2 PcG complex. The other known partners of dinG were not observed in the complex. The lack of M33/RYBP may be explained by competition with CP2 and HPC2 for dinG binding. The lack of Bmi-1 suggests that PcG complexes may vary depending on the promoter context. The grh-like interaction domain of dinG, which is critical for CP2-dependent transcriptional repression, is functionally conserved in both Ring1A/RING1 and Ring1B/dinG, suggesting that the different PcG complexes assembling on the Ring proteins are both available to the grh-like factors (Fig. 3B and D).
The PcG-mediated repression in reporter constructs has previously been shown to completely reverse with increased levels of transcription factors (81). Our in vitro transcription analysis and cellular transfection experiments support this, since increased amounts of CP2 overcame the dinG-mediated repression in both systems. Repression is restored by the addition of recombinant dinG in vitro or the dinG expression vector in vivo, indicating that the balance between the factors is a major determinant of the transcriptional activity of the promoter. This phenomenon is specific, since no repression of two non-CP2-dependent promoters is seen and reduction in basal transcription is observed in vitro only in the setting of a 10-fold excess of dinG with both the CP2-dependent and control promoters (Fig. 5B).
Although the components of the Ring PcG are well established, the developmental genes that these complexes regulate have been more elusive. Overexpression of RING1 has been shown to strongly repress En-2, the mammalian homologue of the Drosophila PcG target gene engrailed (52). Interestingly, the engrailed promoter is known to bind GRH (62). Other potential Ring (DRING) protein target genes can be gleaned from the known functions of grainyhead. During Drosophila embryogenesis, grainyhead influences dorsal/ventral and terminal patterning and other developmental events through the regulation of key genes, including decapentaplegic, tailless, Ultrabithorax, and engrailed (6, 26, 39, 62). Although grainyhead is known to be a transcriptional activator in some contexts, its effects on patterning are achieved through repression of dpp and tll by an unknown mechanism. Site-specific recruitment of a PcG complex to the core promoters of these genes seems an attractive model to explain this gene repression and is supported by our EMSA results Additional experiments linking DRING to GRH repressor elements in vivo and studies examining the effects of perturbed dring expression during Drosophila development are in progress.
ADDENDUM
During the preparation of this paper, the Drosophila homologue of dinG (dRING1) was identified and shown to exist in association with the sequence-specific DNA binding factor Zeste and with numerous TATA binding protein-associated factors (dTAFIIs) (54).
Acknowledgments
We thank Amy McEwen for technical support, Rob Saint for advice, and Robert Tjian and Michael Kyba, Hugh Brock and Richard Jones for plasmids.
This work was supported by the NHMRC of Australia, The Wellcome Trust (S.M.J.), the Anti-Cancer Council of Victoria (D.R.C.), NIH PO1 HL53749-06, Cancer Center Support CORE grant P30 CA 21765, the American Lebanese Syrian Associated Charities (ALSAC), and the Assisi Foundation of Memphis.
REFERENCES
- 1.Akhtar, A., D. Zink, and P. B. Becker. 2000. Chromodomains are protein-RNA interaction modules. Nature 407:405-409. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Attardi, L. D., and R. Tjian. 1993. Drosophila tissue-specific transcription factor NTF-1 contains a novel isoleucine-rich activation motif. Genes Dev. 7:1341-1353. [DOI] [PubMed] [Google Scholar]
- 4.Attardi, L. D., D. Von Seggern, and R. Tjian. 1993. Ectopic expression of wild-type or a dominant-negative mutant of transcription factor NTF-1 disrupts normal Drosophila development. Proc. Natl. Acad. Sci. USA 90:10563-10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz, M., and J. Muller. 1995. Transcriptional silencing of homeotic genes in Drosophila. Bioessays 17:775-784. [DOI] [PubMed] [Google Scholar]
- 6.Biggin, M. D., and R. Tjian. 1988. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell 53:699-711. [DOI] [PubMed] [Google Scholar]
- 7.Bray, S. J., B. Burke, N. H. Brown, and J. Hirsh. 1989. Embryonic expression of a family of Drosophila proteins that interact with a central nervous system regulatory element. Genes Dev. 3:1130-1145. [DOI] [PubMed] [Google Scholar]
- 8.Bray, S. J., and F. C. Kafatos. 1991. Developmental function of Elf-1: an essential transcription factor during embryogenesis in Drosophila. Genes Dev. 5:1672-1683. [DOI] [PubMed] [Google Scholar]
- 9.Brelling, A., B. M. Turner, M. E. Bianchi and V. Orlando. 2001. General transcription factors bind promoters repressed by Polycomb group proteins. Nature 412:651-655. [DOI] [PubMed] [Google Scholar]
- 10.Brown, J. L., D. Mucci, M. Whiteley, M.-L. Dirksen, and J. A. Kassis. 1998. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol. Cell 1:1057-1064. [DOI] [PubMed] [Google Scholar]
- 11.Casolaro, V., A. M. Keane-Myers, S. L. Swendeman, C. Steindler, F. Zhong, M. Sheffery, S. N. Georas, and S. J. Ono. 2000. Identification and characterisation of a critical CP2-binding element in the human interleukin-4 promoter. J. Biol. Chem. 275:36605-36611. [DOI] [PubMed] [Google Scholar]
- 12.Castelli-Gair, J., and A. Garcia-Bellido. 1990. Interactions of Polycomb and trithorax with cis regulatory regions of Ultrabithorax during the development of Drosophila melanogaster. EMBO J. 9:4267-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalli, G., and V. Paro. 1998. Chromo-domain proteins: linking chromatin structure to epigentic regulation. Curr. Opin. Cell Biol. 10:354-360. [DOI] [PubMed] [Google Scholar]
- 14.Chan, C. S., L. Rastelli, and V. Pirrotta. 1994. A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13:2553-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Mar Lorente, M., C. Marcos-Gutierrez, C. Perez, J. Schoorlemmer, A. Ramirez, T. Magin, and M. Vidal. 2000. Loss- and gain-of-function mutations show a Polycomb group function for Ring1A in mice. Development 127:5093-5100. [DOI] [PubMed] [Google Scholar]
- 16.Dignam, J. D. 1990. Preparation of extracts from higher eukaryotes. Methods Enzymol. 182:194-203. [DOI] [PubMed] [Google Scholar]
- 17.Duncan, I. M. 1982. Polycomblike: a gene that appears to be required for the normal expression of the bithorax and antennapedia gene complexes of Drosophila melanogaster. Genetics 102:49-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dynlacht, B. D., L. D. Attardi, A. Admon, M. Freeman, and R. Tjian. 1989. Functional analysis of NTF-1, a developmentally regulated Drosophila transcription factor that binds neuronal cis elements. Genes Dev. 3:1677-1688. [DOI] [PubMed] [Google Scholar]
- 19.Dynlacht, B. D., L. D. Attardi, A. Admon, M. Freeman, and R. Tjian. 1989. Functional analysis of NTF-1, a developmentally regulated Drosophila transcription factor that binds neuronal cis elements. Genes Dev. 3:1677-1688. [DOI] [PubMed] [Google Scholar]
- 20.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 21.Francis, N. J., A. J. Saurin, Z. Shao, R. E. Kingston. 2001. Reconstitution of a functional core polycomb repressive complex. Mol. Cell 8:545-556. [DOI] [PubMed] [Google Scholar]
- 22.Franke, A., M. DeCamillis, D. Zink, N. Cheng, H. W. Brock, and R. Paro. 1992. Polycomb and polyhomeotic are constituents of a multimeric protein complex in chromatin of Drosophila melanogaster. EMBO J. 11:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia, E., C. Marcos-Gutierrez, M. del Mar Lorente, J. C. Moreno, and M. Vidal. 1999. RYBP, a new repressor protein that interacts with components of the mammalian Polycomb complex and with transcription factor YY1. EMBO J. 18:3404-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyurkovics, H., J. Gausz, J. Kummer, and F. Karch. 1990. A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J. 9:257-258.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemenway, C. S., B. W. Halligan, and L. S. Levy. 1998. The Bmi-1 oncoprotein interacts with dinG and MPh2: the role of RING finger domains. Oncogene 16:2541-2547. [DOI] [PubMed] [Google Scholar]
- 26.Huang, J.-D., T. Dubnicoff, G.-J. Liaw, Y. Bai, S. A. Valentine, J. M. Shirokawa, J. A. Lengyel, and A. J. Courey. 1995. Binding sites for transcription factor NTF-1/Elf-1 contribute to the ventral repression of decapentaplegic. Genes Dev. 9:3177-3189. [DOI] [PubMed] [Google Scholar]
- 27.Huang, N., and W. L. Miller. 2000. Cloning of factors related to HIV-inducible LBP proteins that regulate steroidogenic factor-1-independent human placental transcription of the cholesterol side-chain cleavage enzyme, p450scc. J. Biol. Chem. 275:2852-2858. [DOI] [PubMed] [Google Scholar]
- 28.Jane, S. M., P. A. Ney, E. F. Vanin, D. L. Gumucio, and A. W. Nienhuis. 1992. Identification of a stage selector element in the human gamma-globin gene promoter that fosters preferential interaction with the 5′ HS2 enhancer when in competition with the beta-promoter. EMBO J. 11:2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jane, S. M., A. W. Nienhuis, and J. M. Cunningham. 1995. Hemoglobin switching in man and chicken is mediated by a heteromeric complex between the ubiquitous transcription factor CP2 and a developmentally specific protein. EMBO J. 14:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, W. A., C. A. McCormick, S. J. Bray, and J. Hirsh. 1989. A neuron-specific enhancer of the Drosophila dopa decarboxylase gene. Genes Dev. 3:676-686. [DOI] [PubMed] [Google Scholar]
- 31.Jones, C. A., J. Ng, A. J. Peterson, K. Morgan, J. Simon, and R. S. Jones. 1998. The Drosophila esc and E(z) proteins are direct partners in polycomb group-mediated repression. Mol. Cell. Biol. 18:2825-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, K. A., P. A. Luciw, and N. Duchange. 1988. Structural arrangements of transcription control domains within the 5′-untranslated leader regions of the HIV-1 and HIV-2 promoters. Genes Dev. 2:1101-1114. [DOI] [PubMed] [Google Scholar]
- 33.Jurgens, G. 1985. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature 316:153-155. [Google Scholar]
- 34.Kanno, M., M. Hasegawa, A. Ishida, K. Isono, and M. Taniguchi. 1995. mel-18, a Polycomb group-related mammalian gene encodes a transcriptional negative regulator with tumor suppressive activity. EMBO J. 14:5672-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato, H., M. Horikoshi, and R. G. Roeder. 1991. Repression of HIV transcription by a cellular protein. Science 251:1476-1478. [DOI] [PubMed] [Google Scholar]
- 36.Kehle, J., D. Beuchle, S. Treuheit, B. Christen, J. A. Kennison, M. Bienz, and J. Muller. 1998. dMi-2, a hunchback-interacting protein that functions in Polycomb repression. Science 282:1897-1900. [DOI] [PubMed] [Google Scholar]
- 37.Kyba, M., and H. W. Brock. 1998. The Drosophila polycomb group protein Psc contacts Ph and Pc through specific conserved domains. Mol. Cell. Biol. 18:2712-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis, E. B. 1978. A gene complex controlling segmentation in Drosophila. Nature 276:565-570. [DOI] [PubMed] [Google Scholar]
- 39.Liaw, G.-J., K. M. Rudolph, J.-D. Huang, T. Dubnicoff, A. J. Courey, and J. A. Lengyel. 1995. The torso response element binds GAGA and NTF-1/Elf-1, and regulates tailless by relief of repression. Genes Dev. 9:3163-3176. [DOI] [PubMed] [Google Scholar]
- 40.Lim, L. C., S. L. Swendeman, and M. Sheffery. 1992. Molecular cloning of the alpha-globin transcription factor CP2. Mol. Cell. Biol. 12:828-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonie, A., R. D'Andrea, R. Paro, and R. Saint. 1994. Molecular characterisation of the Polycomblike gene of Drosophila melanogaster, a trans acting regulator of homeotic gene expression. Development 120:2629-2636. [DOI] [PubMed] [Google Scholar]
- 42.Muller, J. 1995. Transcriptional silencing by the Polycomb protein in Drosophila embryos. EMBO J. 14:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller, J., and M. Bienz. 1991. Long range repression conferring boundaries of Ultrabithorax expression in the Drosophila embryo. EMBO J. 10:3147-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlando, V., E. P. Jane, V. Chinwalla, P. J. Harte, and R. Paro. 1998. Binding of trithorax and Polycomb proteins to the bithorax complex: dynamic changes during early Drosophila embryogenesis. EMBO J. 17:5141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paro, R. 1990. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 6:416-421. [DOI] [PubMed] [Google Scholar]
- 46.Pirrotta, V. 1998. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 93:333-336. [DOI] [PubMed] [Google Scholar]
- 47.Pirrotta, V., and L. Rastelli. 1994. White gene expression, repressive chromatin domains and homeotic gene regulation in Drosophila. Bioessays 16:549-556. [DOI] [PubMed] [Google Scholar]
- 48.Powell, C. M. H., T. L. Rudge, Q. Zhu, L. F. Johnson, and U. Hansen. 2000. Inhibition of the mammalian transcription factor LSF induces S-phase-dependent apoptosis by downregulating thymidylate synthase expression. EMBO J. 19:4665-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramamurthy, L., V. Barbour, A. Tuckfield, D. R. Clouston, D. Topham, J. M. Cunningham, and S. M. Jane. 2001. Targeted disruption of the CP2 gene, a member of the NTF family of transcription factors. J. Biol. Chem. 276:7836-7842. [DOI] [PubMed] [Google Scholar]
- 50.Rastelli, L., C. S. Chan, and V. Pirrotta. 1993. Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on enhancer of zeste function. EMBO J. 12:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodda, S., S. Sharma, M. Scherer, G. Chapman, and P. Rathjen. 2001. CRTR-1, a developmentally regulated transcriptional repressor related to the CP2 family of transcription factors. J. Biol. Chem. 276:3324-3332. [DOI] [PubMed] [Google Scholar]
- 52.Satijn, D. P., M. J. Gunster, J. van der Vlag, K. M. Hamer, W. Schul, M. J. Alkema, A. J. Saurin, P. S. Freemont, R. van Driel, and A. P. Otte. 1997. RING1 is associated with the polycomb group protein complex and acts as a transcriptional repressor. Mol. Cell. Biol. 17:4105-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satijn, D. P. E., and A. P. Otte. 1999. RING1 interacts with multiple Polycomb group proteins and displays tumorigenic activity. Mol. Cell. Biol. 19:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saurin, A. J., Z. Shao, H. Erdjument-Bromage, P. Tempst, and R. E. Kingston. 2001. A Drosophila Polycomb group complex includes zeste and dTAFII proteins. Nature 412:655-660. [DOI] [PubMed] [Google Scholar]
- 55.Schoorlemmer, J., C. Marcos-Gutierrez, F. Were, R. Martinez, E. Garcia, D. P. E. Satijn, A. P. Otte, and M. Vidal. 1997. Ring1A is a transcriptional repressor that interacts with the polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 16:5930-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sewalt, R. G. A. B., J. van der Vlag, M. J. Gunster, K. M. Hamer, J. L. den Blaauwen, D. P. E. Satijn, T. Hendrix, R. van Driel, and A. P. Otte. 1998. Characterization of interactions between the mammalian Polycomb group proteins Enx1/EZH2 and EED suggests the existence of different mammalian Polycomb group complexes. Mol. Cell. Biol. 18:3586-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao, Z., F. Raible, R. Mollaaghababa, J. R. Guyon, C.-T. Wu, W. Bender, and R. E. Kingston. 1999. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98:37-46. [DOI] [PubMed] [Google Scholar]
- 58.Shirra, M. K., Q. Zhu, H.-C. Huang, D. Pallas, and U. Hansen. 1994. One exon of the human LSF gene includes conserved regions involved in novel DNA-binding and dimerization motifs. Mol. Cell. Biol. 14:5076-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon, J. 1995. Locking in stable states of gene expression: transcriptional control during Drosophila development. Curr. Opin. Cell Biol. 7:376-385. [DOI] [PubMed] [Google Scholar]
- 60.Simon, J., A. Chaing, W. Bender, M. Shimell, and M. O'Connor. 1993. Elements of the Drosophila bithorax complex that control repression by Polycomb-group products. Dev. Biol. 158:131-144. [DOI] [PubMed] [Google Scholar]
- 61.Smale, S. T., M. C. Schmidt, A. J. Berk, and D. Baltimore. 1990. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc. Natl. Acad. Sci. USA 87:4509-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soeller, W. C., S. J. Poole, and T. Kornberg. 1988. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 2:68-81. [DOI] [PubMed] [Google Scholar]
- 63.Solis, C., G. I. Aizencang, K. H. Astrin, D. F. Bishop, and R. J. Desnick. 2001. Uroporhyrinogen III synthase erythroid promoter mutations in adjacent GATA1 and CP2 elements cause congenital erythropoietic porphyria. J. Clin. Investig. 107:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Struhl, G. 1981. A gene product required for correct initiation of segmental determination in Drosophila. Nature 293:36-41. [DOI] [PubMed] [Google Scholar]
- 65.Struhl, G., and M. Akam. 1985. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 4:3259-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Struhl, G., and D. Brower. 1982. Early role of the esc+ gene product in the determination of segments in Drosophila. Cell 31:285-292. [DOI] [PubMed] [Google Scholar]
- 67.Strutt, H., and R. Paro. 1997. The Polycomb group protein complex of Drosophila melanogaster has different compositions at different target genes. Mol. Cell. Biol. 17:6773-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sueyoshi, T., R. Kobayasi, K. Nishio, K. Aida, R. Moore, T. Wada, H. Handa, and M. Negishi. 1995. A nuclear factor (NF2d9) that binds to the male-specific p450 (Cyp 2d-9) gene in mouse liver. Mol. Cell. Biol. 15:4158-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tie, F., T. Furuyama, and P. J. Harte. 1998. The Drosophila Polycomb group proteins ESC and E(Z) bind directly to each other and co-localise at multiple chromosomal sites. Development 125:3483-3496. [DOI] [PubMed] [Google Scholar]
- 70.Trimarchi, J. M., B. Fairchild, J. Wen, and J. A. Lees. 2001. The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc. Natl. Acad. Sci. USA 98:1519-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uv, A. E., E. J. Harrison, and S. J. Bray. 1997. Tissue-specific splicing and functions of the Drosophila transcription factor Grainyhead. Mol Cell. Biol. 17:6727-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uv, A. E., C. R. L. Thompson, and S. J. Bray. 1994. The Drosophila tissue-specific factor grainyhead contains novel DNA-binding and dimerization domains that are conserved in the human protein CP2. Mol. Cell. Biol. 14:4020-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Vlag, J., and A. P. Otte. 1999. Transcriptional repression mediated by the human polycomb group protein EED involves histone deacetylation. Nat. Genet. 23:474-478. [DOI] [PubMed] [Google Scholar]
- 74.van Lohuizen, M., M. Tijms, J. W. Voncken, A. Schumacher, T. Magnuson, and E. Wientjens. 1998. Interaction of mouse Polycomb-group proteins Enx1 and Enx2 with Eed: indication for separate Pc-G complexes. Mol. Cell. Biol. 18:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volker, J. L., L. E. Rameh, Q. Zhu, J. DeCaprio, and U. Hansen. 1997. Mitogenic stimulation of resting T cells causes rapid phosphorylation of the transcription factor LSF and increased DNA-binding activity. Genes Dev. 11:1435-1446. [DOI] [PubMed] [Google Scholar]
- 76.Weeden, C., K. Harding, and M. Levine. 1986. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell 44:739-748. [DOI] [PubMed] [Google Scholar]
- 77.Wu, F. K., J. A. Garcia, D. Harrich, and R. B. Gaynor. 1988. Purification of the human immunodeficiency virus type 1 enhancer and TAR binding proteins EBP-1 and UBP-1. EMBO J. 7:2117-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon, J.-B., G. Li, and R. G. Roeder. 1994. Characterization of a family of related cellular transcription factors which can modulate human immunodeficiency virus type I transcription in vitro. Mol. Cell. Biol. 14:1776-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, C. C., and M. Bienz. 1992. Segmental determination in Drosophila conferred by hunchback (hb), a repressor of the homeotic gene Ultrabithorax (Ubx). Proc. Natl. Acad. Sci. USA 89:7511-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou, W.-L., D. R. Clouston, X. Wang, L. Cerruti, J. M. Cunningham, and S. M. Jane. 2000. Induction of human fetal globin gene expression by a novel erythroid factor, NF-E4. Mol. Cell. Biol. 20:7662-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zink, D., and R. Paro. 1995. Drosophila Polycomb group regulated chromatin inhibits the accessibility of a trans-activator to its target DNA. EMBO J. 14:5660-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]