Abstract
Long-term memory is dependent on protein synthesis and inhibiting such synthesis following training results in amnesia for the task. Proteins synthesized during training must be transported to the synapse and disrupting microtubules with Colchicines, and hence, blocking transport, results in transient amnesia. Reactivating memory for a previously learned avoidance triggers a biochemical cascade analogous to that following the initial training and renders the memory labile once more to protein synthesis inhibitors. However, the reminder-induced cascade differs in certain key features from that following training. Here we show that in a one-trial passive avoidance task in chicks, in contrast with initial consolidation following training, memory following a reminder is not impaired by Colchicine. We conclude that recall after a reminder does not require synaptic access to somatically synthesized proteins in this task. Our results support the hypothesis that in the chick, a reminder may instead engage local protein synthesis at the synapse, rather than in the soma.
The translation of a short-term experience into longer term memory (consolidation) requires protein synthesis, presumed to be necessary for the resculpting of synapses (Hebb 1949). Inhibitors of protein synthesis, administered around the time of training or 4-6 h later, produce lasting amnesia for the task (Davis and Squire 1984; Rose 2000). Beyond this time, the memory is insensitive to the inhibitors and has been regarded as permanent (long-term memory). However, recently reconfirmed older observations show that reminding the animal of the previously learned experience renders the memory labile once more (Sara 2000a,b; Nader 2003; Dudai 2004). Administration of protein synthesis inhibitors in association with the reminder for an aversive experience produces amnesia for the task, in some cases apparently permanent (Nader et al. 2000; Nader 2003), in others more transient (Litvin and Anokhin 2000; Milekic and Alberini 2002; Eisenberg and Dudai 2004). This has prompted an ongoing debate, i.e., is the amnesia due to a blockade of the same biochemical cascade as is involved in the initial consolidation (hence, permanently preventing “reconsolidation”), or does it represent a temporary failure to access the memory (retrieval) (Nadel and Land 2000; Alberini 2005). Of course, in some senses this distinction is artificial, as any reminder inevitably constitutes a new experience and will involve some learning, which may be part of a process leading to extinction of the earlier memory (Vianna et al. 2001). A further complexity is added by the fact that even without reminder, putative memory traces are not entirely stable, migrating from one brain region to others over a period that may vary from hours to weeks (Myers and Davis 2002; Tronel and Sara 2002; Frankland and Bontempi 2005).
Our laboratory has been studying these phenomena using a one-trial passive avoidance task in young chicks and the protein synthesis inhibitor anisomycin (Ani) (Anokhin et al. 2002; Salinska et al. 2004). Ani administered around the time of reminder produces a transient amnesia for the passive avoidance response, but both the dose and the temporal dynamics of the effect are different from those producing amnesia in the hours following initial training. Furthermore, whereas the biochemical locus of change following training is in the intermediate medial mesopallium (IMMP, previously called IMHV) (Reiner et al. 2004), following a reminder it is in the region we had earlier (Rose 2000) identified as a putative “storage site” for the memory trace, the medial striatum (MS; previously called LPO).
One explanation for the differences in the amnestic effect of Ani could be that while the initial learning experience involves enhanced gene expression and somatic protein synthesis followed by the transport of the newly synthesized proteins to the synapse, re-evoking the experience by way of a reminder engages only local (dendritic/synaptic) protein synthesis. That such synthesis can occur in dendritic spines and presynaptic elements (synaptoneurosomes) is well established (Steward and Worley 2002; Tang and Schuman 2002). We reasoned that if this were the case, then while transiently blocking axonal and dendritic flow during consolidation should result in amnesia for the task, this would not be the case following recall of the experience. Such a transient blockade, lasting minutes to hours, occurs if microtubular structure is disrupted, which can be achieved by administration of Colchicine (Borisy and Taylor 1967a; Edson et al. 1993). In the experiments reported here we have examined the effects of Colchicine on recall for the passive avoidance task following both training and reminder.
Results
Colchicine effect on recall following training
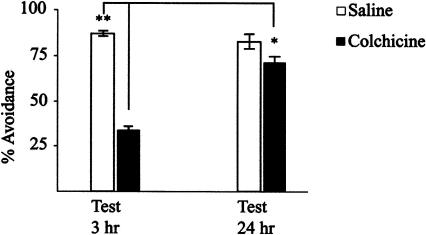
We began by replicating and extending an earlier study by Bell and Morgan (1981). These authors reported that bilateral injections of 5μg (15 nmol) Colchicine into the forebrain shortly after training resulted in a transient amnesia for the avoidance response. We began by confirming that Colchicine injected bilaterally into the IMMP at doses of up to 20 μg was without overt behavioral or toxic effects. Injections of Colchicine at 15 nmol at 30-10 min pre-training were without effect on acquisition of the task or recall in chicks tested at varying times up to 24 h subsequently (data not shown). However, in accordance with Bell and Morgan's observations, we found that bilateral injections of 15 nmol Colchicine/hemisphere into the IMMP, 15 min post-training, results in amnesia in chicks tested 3 h subsequently. The amnesia is, however, transient; chicks trained, injected with Colchicine as before, and tested 24 h later showed good recall (Fig. 1). If the Colchicine injections were delayed to 30 min post-training, however, no amnesia was apparent (data not shown).
Figure 1.
Effects of Colchicine on retention. Chicks were trained as described in the Materials and Methods. Colchicine (15 nmol/hemisphere) or saline was injected bilaterally into the IMMP 15 min post-training. Chicks were tested 3 and 24 h later. Results are presented as mean ± SEM, n = 45-60 for each group. (*) P ≤ 0.020; (**) P ≤ 0.0025. Statistical analysis of behavioral data was by analysis of variance (ANOVA), and where significant differences were found between groups, further analysis was made using a post hoc least-significant difference test.
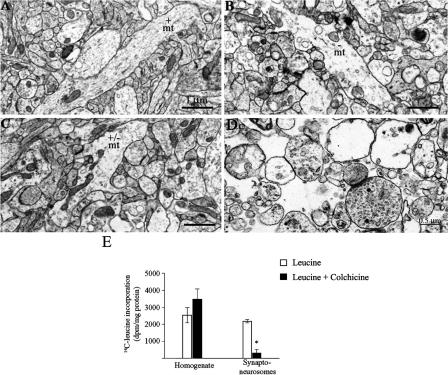
Electron micrographs from brain perfused 30 min after injection of Colchicine into the IMMP showed considerable disruption of microtubules in the region compared with controls (Fig. 2A,B). By 3 h post-injection, the microtubules in Colchicine-injected animals had begun to reform (Fig. 2C). To confirm that Colchicine does indeed also disrupt transport of newly synthesized proteins to the synapses, we injected 14-C leucine bilaterally into the IMMP, followed 15 min later by 15 nmol Colchicine. Three hours later, the chicks were killed, forebrain regions containing IMMP dissected, and crude synaptoneurosome fractions prepared by centrifugation. The purity of the synaptoneurosomal preparation was routinely checked by electron microscopy (Fig. 2D). Colchicine did not affect the specific radioactivity of incorporated leucine in the trichloroacetic acid-precipitated proteins in the homogenate, indicating that it was without effect on protein synthesis per se, but reduced the proportion of the de novo synthesized proteins recovered in synaptoneurosomes (Fig 2E). Thus, Colchicine does not disrupt protein synthesis, but merely blocks the delivery of the proteins to the synapse. This blockage is transient, as by 3 h, the microtubules in this region are partially reconstituted.
Figure 2.
Effect of Colchicine on microtubules and protein synthesis. Animals were injected bilaterally into the IMMP (as described in Fig. 1) either with saline or Colchicine (15 nmol/hemisphere) and perfused for electron microscopy 30 min or 3 h later. (A) Control perfused 3 h after saline injection. (B,C) Colchicne-injected animals perfused 30 min (B) and 3 h (C) later. (mt) Microtubules. (D) Electron micrograph of the synaptoneurosomal preparation. (E) 14C-leucine (L-[14C(U)]), 0.0018 MBq/hemisphere (ARC65) was injected bilaterally into the IMMP and followed 15 min later with injection of Colchicine (15 nmol/hemisphere). Controls received the same amount of 14C-leucine. Results are presented as mean ± SEM. n = 5 for each group. (*) P ≤ 0.0003. Statistical analysis of leucine incorporation data was by analysis of variance (ANOVA).
Colchicine effect on recall following a reminder
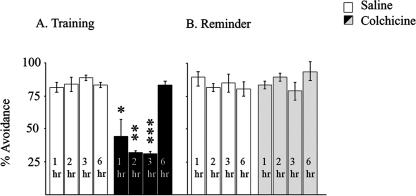
As Colchicine produces transient amnesia following training, we then asked whether it also does so following a reminder. In our earlier experiments (Anokhin et al. 2002; Salinska et al. 2004) we found that the post-reminder time window at which Ani causes transient amnesia in the chick does not co-incide exactly with that post-training; therefore, we divided chicks into two groups. The first was trained, as in Figure 1, injected with Colchicine 15 min later, and tested 1, 2, 3, or 6 h subsequently. Chicks in the second group were trained and remained in their pens for 24 h, after which they were given a reminder by presenting them with a dry chrome bead identical to the one that they had previously pecked and found to taste bitter. Birds that pecked the bead at the reminder (< 10%) were excluded at this point. Fifteen minutes later, birds that avoided the bead during the reminder were injected with Colchicine and tested 1, 2, 3, or 6 h subsequently. As Figure 3A shows, birds injected with Colchicine following training showed a transient amnesia, birds injected post-reminder showed normal retention (Fig. 3B).
Figure 3.
Effect of Colchicine on retention following training or reminder. Animals were trained as described in the Materials and Methods and reminded 24 h after training, as described in the text. (A) Chicks were either injected 15 min post-training and tested at 1, 2, 3, or 6 h subsequently, or (B) remained in their pens for 24 h, followed by a reminder and Colchicine injection and tested at 1, 2, 3, or 6 h post-reminder. Results are presented as mean ± SEM. n = 45-60 for each group. (*) P ≤ 0.005; (**) P ≤ 0.0007; (***) P ≤ 0.000025. Statistical analysis of behavioral data was by analysis of variance (ANOVA) and where significant differences were found between groups, further analysis was made using a post hoc least-significant difference test.
Anisomycin effect following a reminder
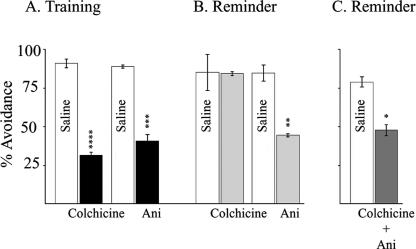
To confirm that, unlike the situation post-training, Ani and Colchicine differed in their effects post-reminder, we compared them directly in the same experiment. Chicks were trained and divided into two groups, and each group then subdivided into three. The first group was injected with saline or Ani (5 min post-training, the time-point used in our earlier experiments) (Salinska et al. 2004) or Colchicine (15 min post-training) and tested 3 h later as in Figure 1. The second group was given a reminder 24 h post-training and injected with Colchicine, Ani, or saline as before. Three hours later, all birds were tested as before. As Figure 4 shows, Ani-injected birds were amnesic following either training (Fig. 4A) or reminder (Fig. 4B), as predicted from our earlier results (Anokhin et al. 2002; Salinska et al. 2004), whereas in contrast to the situation post-training, Colchicine did not produce amnesia following the reminder. In a separate experiment also shown in Figure 4C, we examined the effect of combined injections of Ani 5 min pre-reminder and Colchicine 15 min post-reminder. The amnestic effect of Ani was unaffected by presence of Colchicine.
Figure 4.
Effect of Colchicine or Ani on retention following reminder. Animals were trained as described in the Materials and Methods. (A) Chicks were either injected with Ani 5 min or Colchicine 15 min post-training and tested 3 h subsequently, or (B) remained in their pens for 24 h, reminded by a 30-sec presentation of the chrome bead, injected 5 min subsequently with Ani or 15 min later with Colchicine, and tested 3 h later with chrome and white beads as before. (C) Effect of combined injections of Ani and Colchicine on retention following a reminder. Controls were injected with saline. Results are presented as mean ± SEM. n = 45-60 for each group. (*) P ≤ 0.01; (**) P ≤ 0.007; (***) P ≤ 0.004; (****) P ≤ 0.002. Statistical analysis of behavioral data was by analysis of variance (ANOVA) and where significant differences were found between groups, further analysis was made using a post hoc least significant difference test.
In a separate control, we checked whether there was any interaction between the Colchicine injections and the reminder at 24 h. Birds were trained, and half given a reminder at 24 h. Fifteen minutes later, birds in each group were injected with Colchicine or saline, and recall at 27 h, were tested. There were no differences between groups (Table 1A). As previous experiments had shown, within 24 h, the putative memory trace for the passive avoidance task had migrated from the IMMP to the MS (Gilbert et al. 1991), and, furthermore, that a reminder 24 h post-training results in increased metabolic activity in the MS (Salinska et al. 2004); we checked the effect of Colchicine injections directly into the MS either post-training or post-reminder on recall 3 h subsequently. In neither case did Colchicine have any effect on either retention or discrimination (Table 1B).
Table 1.
Colchicine effect on memory
| IMMP
|
MS
|
||||
|---|---|---|---|---|---|
| A. | % Avoidance | B. | % Avoidance | ||
| Saline | Colchicine | Saline | Colchicine | ||
| Training | 91 | 100 | Training | 85 | 81 |
| Reminder | 89 | 77 | Reminder | 93 | 80 |
A. Colchicine (15 nmol/hemisphere) injected into IMMP.
B. Colchicine (15 nmol/hemisphere) injected into MS. n = 15–20 in each group.
Discussion
Colchicine has a variety of effects on intracellular structures (Borisy and Taylor 1967b; Wilson et al. 1999) and by virtue of disrupting microtubules, blocks axonal flow and, hence, the transport of de novo synthesized proteins to the synapse (Gardiol et al. 1999; Steward and Worley 2002). This blockade will include the proteins required for the synaptic restructuring necessary for memory consolidation, such as the cell-adhesion molecules (Mileusnic et al. 1995; Scholey et al. 1995), which are synthesized in the soma. Blocking axonal flow does not affect the synthesis of these proteins, but results in their accumulation in the soma until such time as the blockade is lifted. The data of Figure 2 confirms this for the chick. In contrast to the well-established finding that, as in other species and tasks, injection of Ani around the time of training in the passive avoidance task in the chick results in lasting amnesia (Davis and Squire 1984; Rose 2000), Colchicine produces transient amnesia. As shown in Figure 1, and as reported previously by Bell and Morgan (1981), following a Colchicine injection, memory, absent at 3 h, is restored by 24 h. Although this temporary blockade might have been a consequence of other effects of Colchicine on cell structure, such as tenascin filaments (Domnina et al. 1995), the most probable interpretation is that only when the microtubular block is lifted are the newly synthesized cell-adhesion molecules transported and, accordingly, enable memory consolidation to proceed. Implicit in this argument is that any putative synaptic “tags” (Frey and Morris 1998) must remain in place during the blockade of transport.
However, in contrast to the effect of Ani, disrupting axonal transport by injecting Colchicine into the IMMP did not affect retention following a reminder 24 h later (Fig. 3). One possible explanation for this would be that at 24 h, memory for the task is no longer dependent on the IMMP but on the MS, to which there is a projection via the archistriatum (Csillag 1999). Although pre-training lesions of the IMMP result in amnesia for the avoidance response, lesions made some 3 h post-training are without amnestic effect (Gilbert et al. 1991). In contrast, pre-training lesions of the MS are not amnestic, while post-training lesions are (see also, Salinska et al. 2004). However, post-reminder injections of Colchicine into the MS, like those into the IMMP, were without effect on subsequent recall. Further, Ani continued to produce amnesia even in the presence of Colchicine (Fig. 4), suggesting that in this case it is blocking the synthesis of synaptoneurosomal proteins on mRNA already present. There is good evidence both for the presence of such mRNA and for the functional significance of the proteins whose synthesis it makes possible (Job and Eberwine 2001; Tang and Schuman 2002) as well as for spine plasticity (Carlisle and Kennedy 2005). The failure of Colchicine to produce amnesia following a reminder is thus most straightforwardly explained on the assumption that, in contrast to the somatic protein synthesis required for consolidation of a new memory, a reminder of the experience that generates that memory involves only local synthesis at the synapse.
Unequivocal support for this hypothesis would require a drug that specifically blocks synaptic/dendritic protein synthesis, but spares somatic protein synthesis, and preferably, the identification of the proteins involved; in the absence of such proof, our finding may be open to alternative interpretations—for instance, that the Ani-induced amnesia results not directly from Ani's effect as a protein synthesis inhibitor, but from some other or downstream consequence—such as a change in local levels of free amino acids as a result of inhibiting synthesis. However, what is now clear is that in both this avian aversive learning paradigm, as in some mammalian ones, the cellular events following a reminder do not simply recapitulate those following initial training. As long ago as 1969, Dawson and McGaugh found that electroshock, which is amnestic for conditioned fear training, if applied immediately post-training, is without effect post-reminder. More recent re-examination of the “reconsolidation” phenomenon has shown significant biochemical differences from the events following training. In mammalian systems, there are differences in the types of immediate early genes activated (Taubenfeld et al. 2001; Izquierdo and Cammarota 2004), while amygdalar circuits involved in consolidation are apparently not required for reconsolidation (Bahar et al. 2004). In the chick, the dose dependency of amnesia to agents such as Ani or 2-deoxygalactose, and the temporal dynamics of the amnestic response, differ between training and reminder (Anokhin et al. 2002). The circuitry involved also appears to differ, as a reminder enhances metabolic activity in the MS but not the IMMP, the region involved in the post-training cascade (Salinska et al. 2004).
The pharmacological lability of memory following a reminder is far from a universal phenomenon, and often seems to involve different molecular processes from those following initial training. Thus, in contextual fear conditioning in the rat, the transcription factor Zif268 is not required for initial consolidation, but is apparently engaged following a reminder as amnesia is induced by antisense oligos to it (Lee et al. 2004). Further, the effects of inhibitors appear to vary with species and task. For instance, in contrast to retrieval after a reminder for aversive learning tasks such as fear conditioning, passive or inhibitory avoidance or conditioned taste-aversion instrumental responses are not affected by protein synthesis inhibitors following a reminder (Hernandez and Kelley 2004). Yet, in a rewarding odor-discrimination task in rats (Torras-Garcia et al. 2005), as with passive avoidance in chicks (Litvin and Anokhin 2000), NMDA receptor blockade following reminder is amnestic. The effect of such inhibitors following aversive tasks is dependent on the interval between the initial experience and the reminder (Anokhin et al. 2002; Alberini 2005), and there is still a conflict of evidence over whether and under what conditions the amnesia is permanent rather than transient. The results presented in this study also require us to reconsider the nature of the biochemical processes initiated by the reminder, suggesting as they do that, at least in this form of avian learning, engage local synaptic and dendritic processes, not necessarily in the same cells or brain regions as those involved in initial consolidation. This distinction may account for the transient nature of the amnesia produced by protein synthesis inhibitors following a reminder in this experimental paradigm.
Materials and Methods
Animals and training
Ross Chunky chicks were hatched and reared in our own incubators. Day-old chicks were placed in pairs in pens, pre-trained, and trained as described previously (Lossner and Rose 1983). Briefly following a stabilization period of 1 h in the pens, the chicks were pre-trained by three presentations of a small (2.5 mm diameter) white bead over a 10-min period. Chicks that pecked at least twice during pre-training (>85%) were then presented with a 4-mm diameter chrome bead dipped in the bitter-tasting methylanthranilate for 20 sec. Chicks that pecked this bead and evinced a disgust response (>90%) were considered to be trained. At appropriate times, later chicks were tested by being offered the dry chrome bead for 30 sec and 5 min later, the pre-training white bead. Chicks that avoided the chrome bead but pecked the white on test were considered to have retained the memory for the aversive experience and to be able to discriminate. Retention was calculated as the percent in each group that showed avoidance and discrimination. Each chick was trained and tested only once. The differences between hatches were tested with ANOVA (Single factor analysis). Statistical analysis of behavioral data was by analysis of variance (ANOVA) and where significant differences were found between groups, further analysis was made using a post hoc least-significant difference test.
Drugs
Chicks were injected at different time points pre- or post-training into the left and right IMMP or MS. Bilateral intracranial injections were made using a custom-built head holder (Davis et al. 1982) and a 5-μL Hamilton syringe fitted with a plastic sleeve to allow appropriate penetration. Correct placement was ensured by and was routinely visually monitored post-mortem. Anisomycin (ANI; Sigma) was dissolved in sterile 3 M HCl made in saline solution and the pH adjusted to 7.4 with 3 M NaOH. Colchicine (Sigma) was dissolved directly in sterile saline. The total dose of Ani was 125 nmol/hemisphere, and for Colchicine, 15 nmol/hemisphere. The volume of injected drugs was 2 μL.
Protein synthesis and axonal flow
Chicks were injected as above with 14C-leucine (L-[14C(U)]), 0.0018 MBq/hemisphere) (ARC65), followed 15 min later by injection of Colchicine (15 nmol/hemisphere) (group LC). Controls received the same amount of 14C-leucine (group L). n = 5 for each group. Three hours later, birds were decapitated and IMMP samples were dissected out and homogenized in an ice-cold buffered sucrose solution (1:10 w/v, 5mM HEPES at pH 7.4, 0.32 M sucrose) supplemented with protease inhibitor cocktail (Roche, Diagnostic) for synaptoneurosomal preparation (Oestreicher and van Leeuwen 1975). The total homogenate was centrifuged for 10 min at 1000g. The supernatant was centrifuged for 45 min at 17,000g to give a crude synaptoneurosomal and mitochondrial pellet. The obtained pellet was resuspended in ice-cold buffered sucrose and spun over a 1.2 M sucrose cushion to remove remains of myelin and larger mitochondria. The final pellet was enriched in synaptoneurosomes (see Fig. 2D). Aliquots were taken at each step throughout the procedure for protein (Bradford 1976) and for total and incorporated 14C-leucine measurement (see Fig. 2E). Specific radioactivity (dpm/mg protein) was determined by trichloroacetic acid precipitation.
Acknowledgments
We thank Ms. M. Nikolakopoulou and Ms. H. Davies for the perfusion of animals and electron micrographs of chick brains and synaptoneurosomes. All procedures were carried out under Home Office licence to S.P.R.R.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.38005.
References
- Alberini, C.M. 2005. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes. Trends Neurosci. 28: 51-56. [DOI] [PubMed] [Google Scholar]
- Anokhin, K.V., Tiunova, A.A., and Rose, S.P.R. 2002. Reminder effects—reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive avoidance task in young chicks. Eur. J. Neurosci. 15: 1759-1765. [DOI] [PubMed] [Google Scholar]
- Bahar, A., Dorfman, N., and Dudai, Y. 2004. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur. J. Neurosci. 19: 1115-1118. [DOI] [PubMed] [Google Scholar]
- Bell, G.A. and Morgan, I.G. 1981. The effects of Colchicine and vinblastine on memory in chicks. Behav. Brain Res. 2: 301-322. [DOI] [PubMed] [Google Scholar]
- Borisy, G.G. and Taylor, E.W. 1967a. The mechanism of action of Colchicine: Binding of Colchicine-3H to cellular protein. J. Biol. Chem. 34: 525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— 1967b. The mechanism of action of Colchicine: Colchicine binding to sea urchin eggs and the mitotic apparatus. J. Cell. Biol. 34: 535-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M.M. 1976. A rapid and sensitive method for the quantification of micrograms of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254. [DOI] [PubMed] [Google Scholar]
- Carlisle, H.J. and Kennedy, M.B. 2005. Spine architecture and synaptic plasticity. Trends Neurosci. 28: 182-187. [DOI] [PubMed] [Google Scholar]
- Csillag, A. 1999. Striato-telencephalic and striato-tegmental circuits: Relevance to learning in domestic chicks. Behav. Brain Res. 98: 227-236. [DOI] [PubMed] [Google Scholar]
- Davis, H.P. and Squire, L.R. 1984. Protein synthesis and memory: A review. Psychol. Bull. 96: 518-559. [PubMed] [Google Scholar]
- Davis, J.L., Pico, R.M., and Cherkin, A. 1982. Arginine vasopressin enhances memory retroactively in chicks. Behav. Neural. Biol. 35: 242-250. [DOI] [PubMed] [Google Scholar]
- Dawson, R.G. and McGaugh, J.L. 1969. Electroshock effects on a reactivated memory trace: Further examination. Science 166: 525-527. [DOI] [PubMed] [Google Scholar]
- Domnina, L.V., Ivanona, O.Y., and Vasilev, J.M. 1995. Effect of microtubule-specific drugs upon spatial organization of extracellular matrix in fibroblastic cultures Cell Biol. Int. 19: 743-748. [DOI] [PubMed] [Google Scholar]
- Dudai, Y. 2004. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55: 51-86. [DOI] [PubMed] [Google Scholar]
- Edson, K.J., Lim, S.S., Borisy, G.G., and Letourneau, P.C. 1993. FRAP analysis of the stability of the microtubule population along the neuritis of chick sensory neurons. Cell Motil. Cytoskeleton 25: 59-72. [DOI] [PubMed] [Google Scholar]
- Eisenberg, M. and Dudai, A. 2004. Reconsolidation of fresh, remote, and extinguished fear memory in medaka: Old fears don't die. J. Neurosci. 20: 3397-3403. [DOI] [PubMed] [Google Scholar]
- Frankland, P.W. and Bontempi, B. 2005. The organization of recent and remote memories. Nat. Rev. Neurosci. 6: 119-130. [DOI] [PubMed] [Google Scholar]
- Frey, U. and Morris, R.E.M. 1998. Synaptic tagging for late maintenance of hippocampal long term potentiation. Trends Neurosci. 21: 181-188. [DOI] [PubMed] [Google Scholar]
- Gardiol, A., Racca, C., and Triller, A. 1999. Dendritic and post-synaptic protein synthetic machinery. J. Neurosci. 19: 168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, D.B., Patterson, T.A., and Rose, S.P.R. 1991. Dissociation of brain sites necessary for registration and storage of memory for a one-trial passive avoidance task in the chick. Behav. Neurosci. 105: 553-561. [DOI] [PubMed] [Google Scholar]
- Hebb, D.O. 1949. The organization of behavior. Wiley, New York. [DOI] [PubMed]
- Hernandez, P.J. and Kelley, A.E. 2004. Long-term memory for instrumental responses does not undergo protein synthesis-dependent reconsolidation upon retrieval. Learn. Mem. 11: 748-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, I. and Cammarota, M. 2004. Zif and the survival of memory. Science 304: 829-830. [DOI] [PubMed] [Google Scholar]
- Job, C. and Eberwine, J. 2001. Localization and translation of mRNA in dendrites and axons. Nat. Rev. Neurosci. 2: 889-897. [DOI] [PubMed] [Google Scholar]
- Lee, J.L.C., Everitt, B.J., and Thomas, K.L. 2004. Independent cellular processes for hippocampal memory consolidation and reconsolidation Science 304: 839-843. [DOI] [PubMed] [Google Scholar]
- Litvin, O.O. and Anokhin, K.V. 2000. Mechanisms of memory reorganization during retrieval of acquired behavioural experience in chicks: The effects of protein synthesis inhibition in the brain. Neurosci. Behav. Physiol. 30: 1357-1363. [DOI] [PubMed] [Google Scholar]
- Lossner, B. and Rose, S.P.R. 1983. Passive avoidance training increases fucokinase activity in right forebrain base of day-old chicks. J. Neurochem. 41: 1357-1363. [DOI] [PubMed] [Google Scholar]
- Milekic, M.H. and Alberini, C.M. 2002. Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36: 521-525. [DOI] [PubMed] [Google Scholar]
- Mileusnic, R., Rose, S.P.R., Lancashire, C., and Bullock, S. 1995. Characterization of antibodies specific for chick brain N-CAM which cause amnesia for a passive avoidance task. J. Neurochem. 64: 2598-2606. [DOI] [PubMed] [Google Scholar]
- Myers, K.M. and Davis, M. 2002. System-level reconsolidation: Reengagement of the hippocampus with memory reactivation. Neuron 36: 340-343. [DOI] [PubMed] [Google Scholar]
- Nadel, L. and Land, C. 2000. Memory traces revisited. Nat. Rev. Neurosci. 1: 209-212. [DOI] [PubMed] [Google Scholar]
- Nader, K. 2003. Memory traces unbound. Trends Neurosci. 26: 65-72. [DOI] [PubMed] [Google Scholar]
- Nader, K., Schafe, G.E., and LeDoux, J.E. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722-726. [DOI] [PubMed] [Google Scholar]
- Oestreicher, A.B. and van Leeuwen, C. 1975. Isolation and partial characterization of fractions enriched in synaptosomes from chick brain. J. Neurochem. 24: 251-259. [DOI] [PubMed] [Google Scholar]
- Reiner, A., Perkel, D.J., Bruce, L.L., Butler, A.B., Csillag, A., Kuenzel, W., Medina, L., Paxinos, G., Shimizu, T., Striedter, G., et al. 2004. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 473: 377-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, S.P.R. 2000. God's organism: The chick as a model for the study of learning and memory. Learn. & Mem. 7: 1-17. [DOI] [PubMed] [Google Scholar]
- Salinska, A., Bourne, R.C., and Rose, S.P.R. 2004. Reminder effects: The molecular cascade following a reminder in young chicks does not recapitulate that following training on a passive avoidance task. Eur. J. Neurosci. 19: 3042-3047. [DOI] [PubMed] [Google Scholar]
- Sara, S.J. 2000a. Retrieval and reconsolidation: Towards a neurobiology of remembering. Learn. Mem. 7: 73-84. [DOI] [PubMed] [Google Scholar]
- ——— 2000b. Strengthening the shaky trace through retrieval. Nat. Rev. Neurosci. 1: 212-213. [DOI] [PubMed] [Google Scholar]
- Scholey, A., Mileusnic, R., Schachner, M., and Rose, S.P.R. 1995. A role of the neural cell adhesion molecule L1 in consolidation of memory for a passive avoidance task in the chick. Learn. Mem. 2: 17-25. [DOI] [PubMed] [Google Scholar]
- Steward, O. and Worley, P. 2002. Local synthesis of proteins at synaptic sites on dendrites: Role in synaptic plasticity and memory consolidation. Neurobiol. Learn. Mem. 78: 508-527. [DOI] [PubMed] [Google Scholar]
- Tang, S.J. and Schuman, E.M. 2002. Protein synthesis in the dendrite. Phil. Trans. Roy. Soc. B 357: 521-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld, S.M., Milekic, M.H., Monti, B., and Alberini, C.M. 2001. The consolidation of new but not reactivated memory requires hippocampal C/EBPb. Nat. Neurosci. 4: 813-818. [DOI] [PubMed] [Google Scholar]
- Torras-Garcia, M., Lelong, J., Tronel, S., and Sara, S.J. 2005. Reconsolidation after remembering an odor-reward association requires NMDA receptors. Learn. Mem. 12: 18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel, S. and Sara, S.J. 2002. Mapping of olfactory memory circuits: Region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn. Mem. 9: 105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna, M.R.M., Szapiro, G., McGaugh, J.L., Median, J.H., and Izquierdo, I. 2001. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus Proc. Nat. Acad. Sci. 98: 12251-12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, L., Panda, D., and Jordan, M.A. 1999. Modulation of microtubule dynamics by drugs: A paradigm for the actions of cellular regulators. Cell Struct. Funct. 24: 329-335. [DOI] [PubMed] [Google Scholar]