Abstract
Objective:
To evaluate the efficacy of a novel 2-stage treatment with reductive surgery plus percutaneous isolated hepatic perfusion (PIHP) for multiple hepatocellular carcinoma (HCC), which was previously unresectable.
Summary Background Data:
Surgical resection is the treatment of choice for HCC, but the majority of patients with advanced HCC are not suitable candidates. PIHP is a minimally invasive surgery that allows high-dose regional chemotherapy of the liver, and our phase II studies have shown its profound efficacy for the local control of advanced HCC.
Methods:
Twenty-five patients with multiple advanced HCC were enrolled in this prospective study. In the first stage, all patients underwent reductive hepatectomy: major hepatectomy in 13 patients and segmentectomy or less in 12. In 2 patients with subsegmentectomy, the retropancreatic and periportal metastatic lymph nodes were synchronously resected. Regardless of the type of hepatectomy, all patients routinely underwent cholecystectomy, and ligations of the right gastric artery and arterial collaterals of the remnant liver to increase the safety and efficacy of PIHP. In the second stage, PIHP with doxorubicin 60–120 mg/m2/treatment was planned for a period of 1 to 3 months after surgery.
Results:
Of 25 enrolled patients, 22 successfully underwent PIHP an average of 1.8 times for the local control of residual liver tumors. In the remaining 3 patients, PIHP was abandoned because 2 had rapid disease progression and 1 had liver failure after surgery. In 22 patients with the 2-stage treatment, 19 (86%) had objective local tumor control (10 complete remissions and 9 partial responses with a median response duration of 16 months). The actuarial survival rate of all 25 patients was 42% at 5 years.
Conclusions:
Reductive surgery plus PIHP produced a strong antitumoral effect on multiple advanced HCC, when liver function allows this concentrated treatment approach, and offers long-term survival in a subset of patients who were previously deemed to have unresectable disease.
This study evaluated the efficacy of a 2-stage treatment with reductive surgery plus percutaneous isolated hepatic perfusion in 25 patients with multiple advanced hepatocellular carcinoma. The results demonstrated that this dual treatment is highly effective in achieving local control of multiple advanced hepatocellular carcinoma and offers long-term survival in a subset of patients who were previously deemed to have unresectable disease.
Hepatocellular carcinoma (HCC) remains the third leading cause of cancer death in Japan. Although surgical resection represents the best hope of long-term survival, only 10%1,2 to 30%3 of HCC are amenable to operative resection because of either the extent of disease or cirrhosis-related liver dysfunction. Furthermore, even after resection with curative intent, patients often develop intra or extrahepatic recurrences.2,4–6
These circumstances have led many investigators to pursue less-invasive local therapies. In the past decade, percutaneous ethanol injection under ultrasonographic guidance has been used extensively to treat HCC around the world.7–9 However, it has recently been reported that percutaneous ethanol injection (PEI) was frequently associated with local recurrence.10,11 Other tumor ablation therapies, such as microwave coagulation therapy12 or radiofrequency ablation,13–15 are currently considered as alternative treatment options. These thermal ablative therapies seem to be adequate for the destruction of solitary HCCs associated with cirrhosis and are generally applied to those of less than 3 nodules each smaller than 3 cm. Hence, for HCC patients with larger tumors or multiple macro- and micrometastases, effective multimodality treatment approaches should be explored to improve the overall outcome and survival.
Percutaneous isolated hepatic perfusion (PIHP) is a form of high-dose regional chemotherapy that enables the administration of cytotoxic agents at a dose of up to 10 times the maximally tolerated dose while reducing exposure of the entire body to the major side effects of the agents.16–20 Our previous studies have shown that PIHP is a safe and effective treatment to achieve a high rate of tumor regression in multiple HCC and yields long-term survival in a subset of patients with otherwise intractable HCCs.17,19 In this study, we investigated the efficacy of a novel 2-stage treatment combining reductive surgery and PIHP for the local control of multiple advanced HCC. The treatment strategy lies in the attempt to achieve tumor mass reduction during the first stage operation and, at the same time, to increase the efficacy and safety of PIHP for the residual liver tumors in the second stage.
PATIENTS AND METHODS
Patients
This prospective study was performed between January 1992 and December 2001. In this 10-year period, we enrolled 25 patients with multiple advanced HCC to be treated with reductive hepatectomy and sequential PIHP. All patients were deemed to have adequate liver function reserve for the planned hepatectomy. In addition, all patients met the following eligibility criteria for PIHP: age 10–70 years; WHO performance status of 2 or less and life expectancy of more than 3 months; serum bilirubin 2.5 mg/dL or less; 15-minute indocyanine green retention rate (ICGR15) 35% or less; serum aspartate aminotransferase (AST) 300 IU/L or less; platelets 50,000/mm3 or more; no preexisting heart disease. We obtained written informed consent from each patient before enrollment. The study protocol was approved by the institutional review board of Kobe University Hospital for human investigation.
No patients were candidates for surgical resection with curative intent or local ablative therapies because of the number, size, or bilobar distribution of the liver tumors. All patients underwent computed tomography (CT) scans of the chest and abdomen to investigate extrahepatic sites of metastases. If clinically indicated, patients underwent a bone scan. HCC staging was performed according to the International Union Against Cancer macroscopic staging criteria for primary malignant liver cancer. The diagnosis of HCC was confirmed histologically in all patients at the time of reductive surgery. Data from 5 of 25 patients were included in our previous studies,17,19 reporting the phase I and II aspects of PIHP for advanced HCC patients.
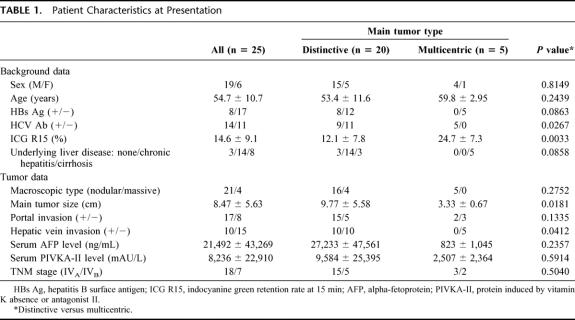
Table 1 lists the patients’ characteristics. The patients were categorized into 2 groups according to the pattern of tumor distribution in the liver: type 1 (n = 20), those with large and distinctive main tumors with satellite nodules; type 2 (n = 5), those with multicentric tumors. In type 1 patients, 3 had normal liver, and the remaining 17 had either chronic hepatitis or liver cirrhosis, whereas all 5 patients from the type 2 group had hepatitis C virus-related liver cirrhosis. The ICGR15 ranged from 4.7 to 32.1% with a mean of 14.6%, and type 1 patients had a significantly lower value compared with type 2 patients. The macroscopic features of HCC were mainly nodular, and the main tumor diameters were significantly greater in type 1 patients than in type 2. Regardless of the tumor type, all patients had multiple intrahepatic metastases of more than 5 nodules. Extrahepatic metastases were found in 7 of 25 patients; 4 had lung metastases, 1 had direct invasion to the transverse colon, and 2 had retropancreatic and periportal lymph node involvement, which constituted a solitary nodular mass, each measuring 5.2 cm and 6.5 cm in diameter, respectively. Seventeen patients (68%) had macroscopic invasion to the portal vein (VP), of which 3 had VP in the portal trunk (VP4), 6 in the first portal branch (VP3), and 8 in the second or more distal portal branch (VP1,2). All 9 patients with main portal vein involvement (VP3,4) had type 1 tumors. Vascular invasion to the major hepatic vein was found in 10 type 1 patients. In 2 patients with hepatic vein invasion, tumor involvement was demonstrated in the inferior vena cava.
TABLE 1. Patient Characteristics at Presentation
Reductive Surgery in the First Stage
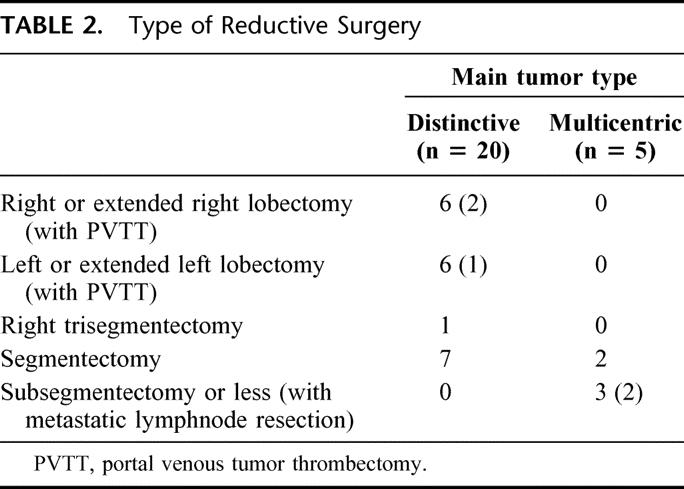
Table 2 shows the reductive surgery procedures according to the tumor type. In 20 patients with distinctive main tumors, 13 had major hepatectomies: 5 left lobectomies, 1 extended left lobectomy, 1 right lobectomy, 5 extended right lobectomies, and 1 right trisegmentectomy. In 3 patients with major hepatectomy, tumor thrombus in the portal trunk was concomitantly removed with venotomy of the portal vein. The remaining 7 patients with type 1 tumors underwent segmentectomy. By contrast, all 5 patients with type 2 tumors had minor hepatectomies: 2 segmentectomies and 3 subsegmentectomies. In 2 patients with subsegmentectomy, the solitary abdominal lymph node metastases were synchronously resected. Irrespective of the type of the first stage hepatectomy, all patients had residual liver tumors after operation.
TABLE 2. Type of Reductive Surgery
In all 25 patients, cholecystectomy and right gastric artery ligation were routinely performed to prevent extrahepatic perfusion of anticancer drugs during PIHP, and arterial collaterals to the remnant liver were disconnected to maximize the efficacy of PIHP in the second stage.
PIHP in the Second Stage
PIHP was planned at least twice until the patient developed unacceptable toxic effects or consent was withdrawn. The first PIHP was performed for a period of 1 to 3 months after reductive surgery, and the time intervals between the PIHP schedules ranged from 1 to 4 months. The dose rate of doxorubicin was determined according to the guidelines used in our previous studies.19 The first dose was set in the range of 100 mg/m2 to 120 mg/m2. For repeated PIHP, we lowered the dose to 90 mg/m2 if the pretreatment leukocyte counts were less than 4000/mm3 and to 60 mg/m2 if the values were less than 3000/mm3. In patients with values below 2500/mm3, PIHP was discontinued.
Before receiving PIHP, the patient underwent hepatic arteriography (HAG) to confirm the absence of reversals in the direction of blood flow through the portal vein, and a hepatic arterial infusion (HAI) catheter was placed by Seldinger’s technique in the angiography suite on the day of treatment. During HAI catheter placement, the gastroduodenal artery was always embolized with coils, and strict attention was directed to selective liver perfusion through the HAI catheter. PIHP was performed under general anesthesia in the operating room. Except for the first 2 patients with the double balloon technique, all patients underwent PIHP with the single catheter technique according to the method described elsewhere.18 In brief, a small cut-down incision was made on the groin and the femoral vein was exposed 2 to 3 cm above the saphenofemoral junction. After intravenous infusion of heparin (100 U/kg), the specially designed 4-lumen/2-balloon catheter was introduced into the retrohepatic inferior vena cava through the femoral vein. Hepatic effluent was isolated by balloon inflations, and pumped to charcoal filters via the fenestrations of one major catheter lumen. The filtered blood was returned straight to the right atrium through the other major lumen with an opening at the distal tip of the catheter. After confirming hemodynamic stability, the patient received a 15- to 30-minute continuous HAI of doxorubicin at a dose ranging from 60 mg/m2 to 120 mg/m2. After the end of HAI, extracorporeal drug filtration was maintained at least for 10 minutes, and the 4-lumen/2-balloon catheter was removed. After repairing the femoral vein, the skin incision was closed in a standard 2-layer fashion.
Evaluation of Toxicity and Local Tumor Control
Our primary endpoint was the rate of local tumor control. The secondary endpoints for efficacy were duration of response and overall survival. Survival lasted from the date of reductive hepatectomy to the date of death. All 25 patients had grossly measurable tumors left behind in the remnant livers. Thus, local tumor control was assessed by comparing the pre and post-PIHP CT findings and/or HAG findings according to WHO criteria:21 complete remission, defined as no evidence of neoplastic disease; partial response, defined as a reduction in total tumor load of more than 50%; stable disease, defined as no change, reduction of less than 50% or increase of less than 25%; and progressive disease, defined as an increase equal to or more than 25%. The duration of local tumor control was the time from first assessment to progression in responding patients. Postoperative examinations and follow-up studies were performed in accordance with the protocol described previously.19 Serum alphafetoprotein (AFP) and proteins induced by vitamin K absence or antagonist II (PIVKA II) concentrations were measured bimonthly during the first year and every 3 months thereafter.
Systemic and hepatic toxicities were graded according to the National Cancer Institute Common Toxicity Criteria.22 Hepatic toxicities were graded as elevations in liver function tests after the PIHP procedure. Nonhepatic toxicities were graded as those that did not reverse within 24 hours of the PIHP procedure.
Data Analysis
Results are given as mean ± standard deviation unless stated otherwise. Statistical analysis was performed as indicated with a statistical analysis program package (Stat View 5 SAS Institute Inc., Cary, NC). Statistical comparison between groups was performed using the χ2 test with Yates correction or the Fisher exact test where appropriate for nominal data, and the Mann-Whitney U test for numerical data. Cumulative survival rates and the median response duration were calculated using the Kaplan-Meier method, and compared between groups by the log-rank test. P < 0.05 was considered to be significant.
RESULTS
Morbidity and Mortality After the First-Stage Reductive Surgery
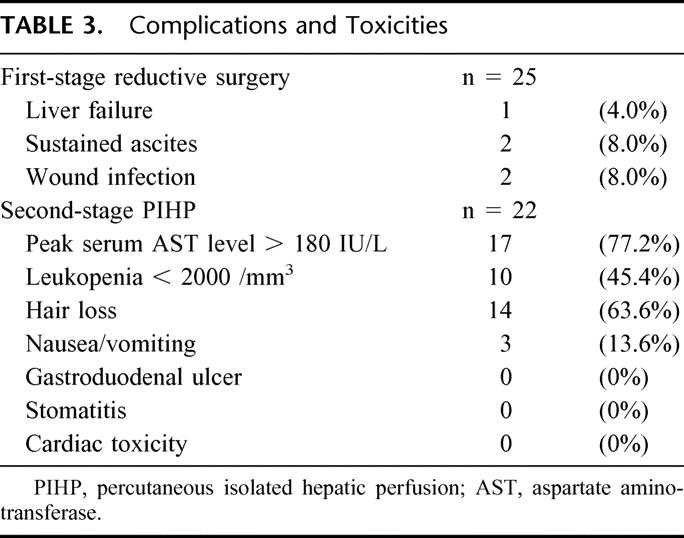
Table 3 lists the complications after reductive surgery. Postoperative complications occurred in 5 of 25 patients (20%); complications included 1 liver failure, 2 wound infections treated by percutaneous drainage, and 2 episodes of ascites. The postoperative course was uneventful in the remaining 20 patients.
TABLE 3. Complications and Toxicities
Three patients died early (within 4 months after surgery). In the first patient with left lobectomy and the second patient with extended right lobectomy, death occurred at 3 and 4 months, respectively, as a result of rapid progression of the residual tumors. The third patient died of liver failure 42 days after posterior segmentectomy, which caused repeated hemorrhage from the cut surface of the liver, beginning intermittently from the eighth postoperative day. This patient had an ICGR15 of 32%, and postoperatively developed massive ascitic leakage. These 3 patients could not receive PIHP for residual liver tumors.
Complications and Toxicity After Second-Stage PIHP
Of 25 patients, 22 successfully received PIHP for the local control of residual liver tumors. The number of courses of PIHP ranged from 1 to 3 times with an average of 1.8 times, and the cumulative dose of doxorubicin averaged 149±48 mg/m2/patient in this study. All 22 patients tolerated PIHP well with reversible doxorubicin-related side effects (Table 3). The majority of patients had transient chemical hepatitis, and 17 of 22 patients (77.2%) had grade 3 AST elevation (180–720 IU/L). The AST levels peaked on day 2, and returned to the baseline levels in all patients by day 7. When the maximal AST levels were assessed, the results from patients with cirrhosis (283±213 IU/L) and other patients (380±205 IU/L) did not significantly differ (P = 0.86). No patients had sclerosing cholangitis, as indicated by persistent hyperbilirubinemia. All patients had some degree of leukopenia, and grades 3 and 4 leukopenia were encountered in 9 patients (40.9%) and 1 (4.5%), respectively. Gastrointestinal toxicities including stomatitis, gastritis, and ulcers were not observed in any patients.
Local Tumor Control by Reductive Surgery Plus PIHP
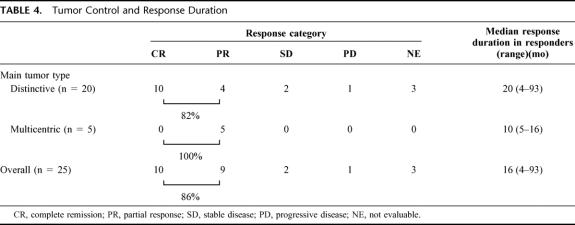
All but 3 patients with early death after reductive surgery were assessable for local tumor control (Table 4). Of 22 patients with the 2-stage treatment, 10 had complete remission (CR), and 9 had a partial response (PR) with an objective response rate of 86%. Of note, all 10 patients with CR had type 1 tumors, and, except for one, had chronic hepatitis as an underlying liver disease. The radiologic responses (CT and HAG findings) of 2 responders are shown in Figures 1 and 2, respectively. The median duration of response in 19 responding patients was 16 months (range, 4–93 months). There was a trend toward longer duration of response in responders with type 1 tumors compared with those with type 2 tumors (P = 0.05). All patients but one had serum tumor marker elevation of either AFP and/or PIVKA-II at entry, and all 19 patients with objective tumor response (CR plus PR) had a greater than 90% reduction in serum AFP or PIVKA-II levels; 10 had a normalized tumor marker level at 1 year.
TABLE 4. Tumor Control and Response Duration
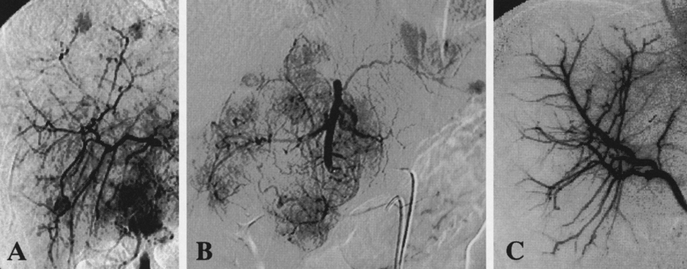
FIGURE 1. HAG findings of a 34-year-old man with hepatitis B virus-related chronic hepatitis (ICGR15 = 5.1%). The patient underwent extended left lobectomy of the liver at the first stage. The second stage PIHP with 100 mg/m2 doxorubicin was performed twice 1 and 2 months after hepatectomy. The serum AFP decreased from the pretreatment level of 64,520 ng/mL to 9 ng/mL 4 months after hepatectomy. However, he died of recurrent disease 16 months after reductive hepatectomy. A, Pretreatment HAG performed via the right hepatic artery, which originated from the superior mesenteric artery. Multiple intrahepatic metastases were noted in the right lobe. B, Pretreatment HAG via the left hepatic artery branching from the celiac axis showing massive type tumors measuring 8.5 cm in the left lobe. C, HAG 5 months after hepatectomy showed a complete clearance of the residual tumors in the remnant liver.
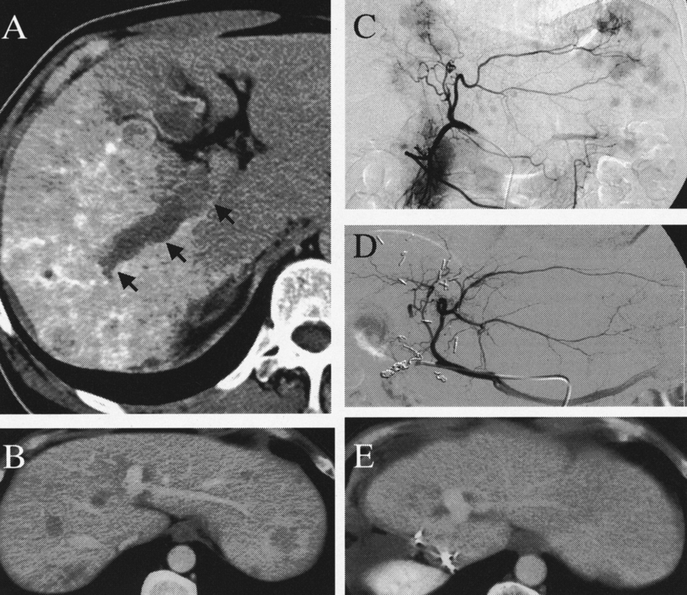
FIGURE 2. HAG and CT scan of a 53-year-old man with hepatitis C virus-related liver cirrhosis (ICGR15 = 16.9%). He underwent extended right lobectomy with portal vein tumor thrombectomy at the first stage. The second-stage PIHP was repeated twice in a period of 1 to 3 months after hepatectomy, using 100 mg/m2, and 80 mg/m2 doxorubicin, respectively. The serum AFP decreased from the pretreatment level of 20,104 ng/mL to 6 ng/mL 4 months after hepatectomy. He is currently well with no evidence of disease recurrence 14 months after reductive hepatectomy. A, Computed tomography (CT) scan before hepatectomy demonstrated massive type tumors measuring 7.5 cm in the right lobe. Arrows indicate tumor thrombus in the portal vein. B, Contrast-enhancement CT scan before treatment showed disseminated tumors in the left lobe of the liver. C, Pretreatment HAG via the left hepatic artery also demonstrated disseminated tumors in the future remnant liver. D, HAG of the left hepatic artery 8 months after hepatectomy showed the complete clearance of the residual tumors in the remnant liver. E, Contrast enhancement CT scan 9 months after hepatectomy also confirmed the complete clearance of residual hepatic tumors.
Survival and Cause of Death
At the time of the final analysis (October 31, 2002), the median follow-up of all 25 patients was 25 months after hepatectomy (range, 3–97 months). In 22 patients with the 2 stage treatment, 10 patients died at 5*, 9*, 11*, 13, 16, 18, 20, 25, 25, and 29 months after reductive surgery (* indicates survivals of patients with VP3,4); 7 died of tumor progression in the liver, 2 died of respiratory failure caused by multiple lung metastases, and 1 died of massive cerebral infarction caused by tumor embolism. The remaining 12 patients are alive and well, as of this writing, 10, 13, 14*, 20, 29*, 36, 55, 57, 59, 64, 93*, and 97* months after reductive surgery.
The 1-year and 5-year actuarial survival rates of all 25 patients were 76% and 42%, respectively (Fig. 3). In 22 patients with the 2-stage treatment, the rates were 86% and 47% at 1 and 5 years, respectively. Figures 4, 5, and 6 show survival curves of all 25 patients according to tumor type (distinctive versus multicentric), TNM stage (IVA vs. IVB), and macroscopic portal involvement (VP positive vs. VP negative), respectively. Although statistical significance was not reached, patients with type 1 tumors had a better survival compared with those with type 2 tumors (P = 0.18), and all 7 patients surviving more than 3 years had type 1 tumors. Patients with stage IVB disease had an apparently worse prognosis compared with those with IVA disease (P = 0.04). VP-positive and -negative patients showed a similar survival curve (P = 0.76). Of additional note, survivals of cirrhotic and noncirrhotic patients did not significantly differ (P = 0.28; data not shown).
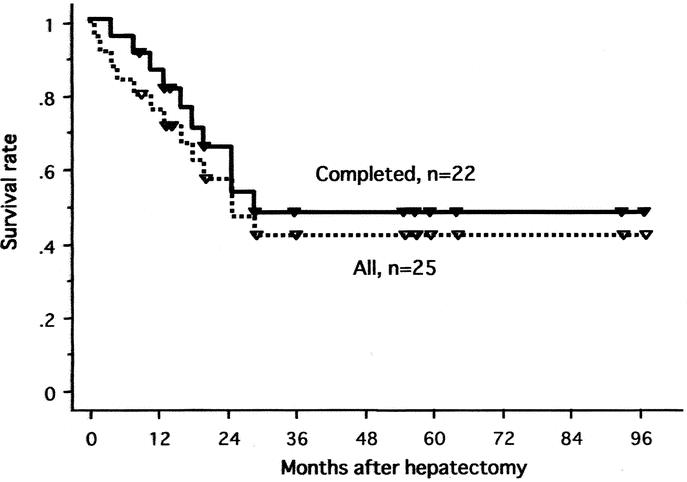
FIGURE 3. Survival curves of all 25 patients and 22 patients with hepatectomy and PIHP.
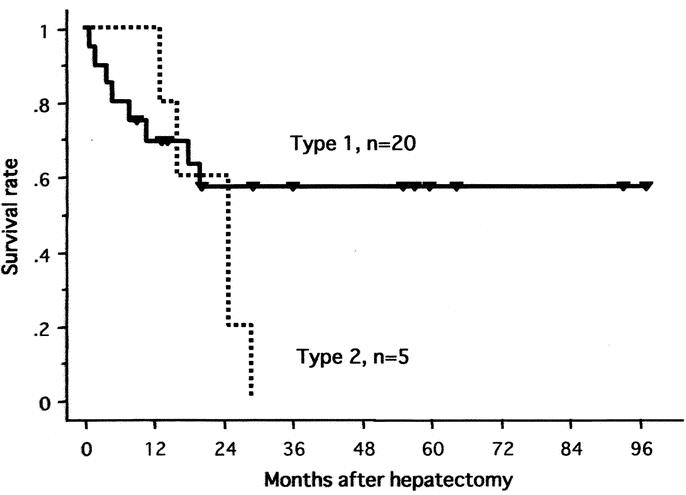
FIGURE 4. Survival curves of all 25 patients according to the tumor type (type 1, distinctive versus type2, multicentric).
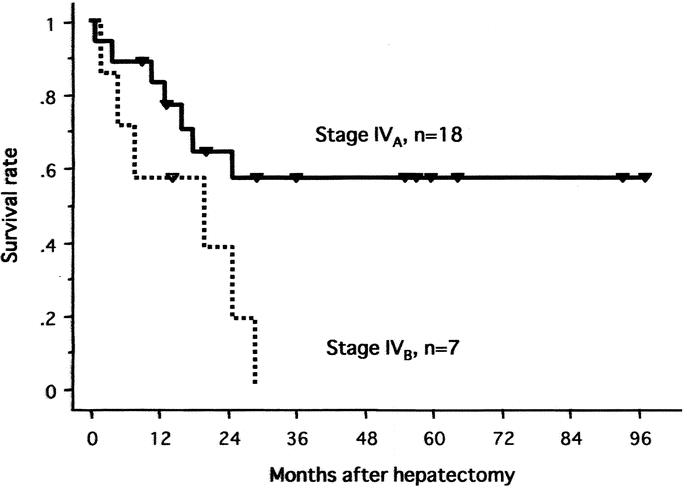
FIGURE 5. Survival curves of all 25 patients according to TNM stage (IVA vs. IVB).
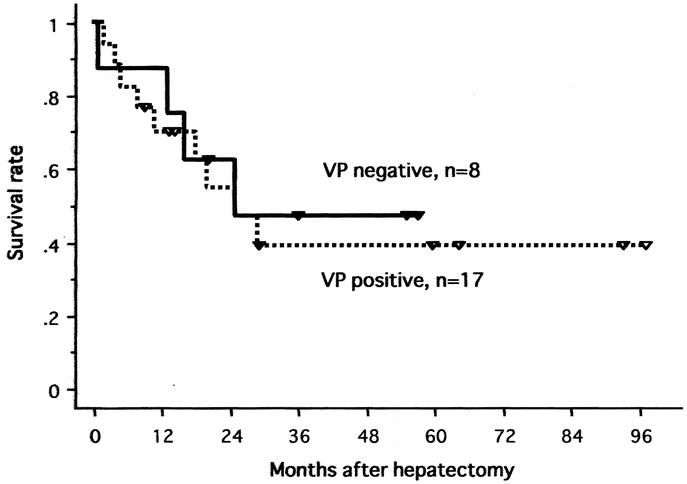
FIGURE 6. Survival curves of all 25 patients according to macroscopic portal involvement (VP positive vs. VP negative).
DISCUSSION
Since 1989, our center has been using PIHP in the treatment of multiple advanced HCC. In 1998, we reported that the overall survival rates in 28 consecutive patients who underwent PIHP were 67.5% at 1 year and 39.5% at 5 years with a response rate of 63%.19 This profound efficacy of PIHP in local tumor control has been reproducibly proven in our recent updating series with the accrual of additional patients,23 now totaling 67 patients. Based on these results, PIHP is currently considered to be a standard treatment in our institution and has also been extended to an aggressive combination with reductive hepatectomy for previously unresectable HCC. The present study documents the safety, efficacy, and survival outcome of the 2-stage treatment with reductive hepatectomy plus PIHP in 25 patients.
During the 10-year study period, 514 patients with HCC have been admitted to our institution, of which 182 patients (35.4%) have undergone hepatectomy, including 25 patients in the present series. This implies that reductive hepatectomy with PIHP constituted 13.7% of all hepatectomies, and has contributed to approximately a 4.8% increase in the overall resection rate at our institution.
In this study, all patients underwent reductive surgery prior to PIHP. There are several oncological advantages of this treatment order. First, patients with a large HCC are frequently accompanied by arterial collaterals to the tumor originating from adjacent organs such as the diaphragm and the major omentum.24 In fact, this phenomenon was commonly observed in the current series. Surgical intervention at the first stage can eliminate these tumor feeding arteries, which otherwise affect drug perfusion to the tumor during PIHP. An impressively increased response rate of 86% in this study supports our rationale of performing hepatectomy at the first stage. Moreover, 6 of 25 patients had arterial variations such as the right hepatic artery originating from the superior mesenteric artery or the left hepatic artery from the left gastric artery, as shown in Figures 1 and 2. In these patients, simplification of the arterial inflow vessels to the remnant liver could be achieved during the first stage operation.
Another concern with the 2-stage treatment is portal involvement. It is well known that HCC patients with major portal invasion tend to develop splanchnic venous congestion. Once the portal trunk is completely obstructed with tumor thrombus, a life-threatening sequelae of massive ascites, variceal hemorrhage, and hepatic failure may ensue.25 The present series included 9 patients with tumor thrombus in the portal trunk or the first portal branch, of which 3 patients had a tumor thrombus reaching the portal trunk. In these patients, portal vein tumor thrombectomy at the first stage operation could prevent impending portal circulatory failure, and expand the therapeutic window for sequential PIHP for residual liver tumors.
Apart from the potential risk of liver failure, VP is a well-recognized causative factor for intrahepatic tumor dissemination, which mainly accounts for the dismal prognosis in this group of patients.25,26 Of interest, our data showed that the survival outcome of VP-positive patients was equivalent to that of VP negative patients and that the majority of patients with an objective response had portal invasion. Thus, we believe the improved survival of VP-positive patients can be ascribed to treatment-related antitumor effects. In this study, the majority of patients underwent major hepatectomy, which profoundly reduced the hepatic vascular bed. This is beneficial in terms of the local drug delivery of anticancer agents during PIHP, since the relative dose rate per gram liver tissue administered via the hepatic artery is increasing.
In the current series of multiple advanced HCC, the 5-year survival rates were 42% for all 25 patients, and 57% for 18 stage IVA patients treated with reductive hepatectomy and PIHP. To date, a few centers have concentrated on the role of hepatectomy for multiple advanced HCC.24–26 Shimada et al26 reported that 27 stage IVA patients with noncurative hepatectomy followed by postoperative lipiodolization had a 4-year survival rate of 20%. More recently, Ikai et al25 reported the survival outcomes of stage IVA HCC without lymph node metastasis after surgical resection. In their series of 150 patients, 90 patients underwent curative resection, while the remaining 60 patients had reductive hepatectomy plus multidisciplinary treatments consisting of intraoperative ethanol injection, and postoperative chemoembolization. They reported that the 5-year survival rates were 15% for all patients, and 7.7% for 71 patients with vascular invasion. Our series included 7 patients with stage IVB disease, and 17 patients with vascular invasion. In addition, all patients had noncurative resection because of extensive intrahepatic metastases. Thus, it appears that our survival data exceed those reported previously by others.
With a favorable survival profile, the current treatment strategy of reductive hepatectomy plus PIHP is encouraging for patients with previously unresectable HCC. However, the selected target population in our study precludes the generalization of our results to all unresectable HCC and highlights the need for the selective recruitment of candidates for the aggressive treatment approach. In this regard, our data shows that patients with type 1 tumors had a better liver function reserve, and were likely to survive longer than those with type 2 tumors. Accordingly, we believe that reductive hepatectomy plus PIHP should become a major treatment option for those patients with technically resectable type 1 tumors, and preserved liver function adequate for planned hepatectomy.
Our findings showed that patients with stage IVB HCC had an apparently worse prognosis compared with those with stage IVA disease. However, it is noteworthy that there were some patients with stage IVB disease, who survived more than 20 months after treatment in this study. Such midterm survivors in stage IVB patients have rarely been reported by others.25,26 Although the role of the current treatment strategy in this patient population remains to be determined by further studies, we feel that it could be justified as the best palliative treatment, if the extrahepatic disease were resectable, or deemed unlikely to be a major life-limiting factor.
In conclusion, the 2-stage treatment with reductive hepatectomy plus PIHP offers long-term survival in a subset of multiple HCC patients who were previously deemed to have unresectable disease. We believe the improved survival demonstrated in this study can be directly ascribed to the strong antitumoral effect of PIHP on local control in combination with hepatectomy. Thus reductive hepatectomy plus PIHP should become a major treatment option for a selected group of multiple HCC patients with distinctive main tumors, and preserved liver function adequate for planned hepatectomy.
Footnotes
Supported by Grants-in-Aid for Scientific Research (06454386 and 09470267) and the new Therapeutic Technology Development Program 2000 from the Japanese Ministry of Education.
Reprints: Y. Ku, Department of Gastroenterological Surgery, Graduate School of Medical Sciences, Kobe University, 7-5-2 Kusunoki-cho, Chuo-ku, Kobe 650–0017, Japan. E-mail: yonson@med.kobe-u.ac.jp.
REFERENCES
- 1.Mor E, Tur Kaspa R, Sheiner P, et al. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med. 1998;129:643–653. [DOI] [PubMed] [Google Scholar]
- 2.Bismuth H, Houssin D, Ornowski J, et al. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986;10:311–317. [DOI] [PubMed] [Google Scholar]
- 3.Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–284. [PMC free article] [PubMed] [Google Scholar]
- 4.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki Y, Imaoka S, Masutani S, et al. Influence of coexisting cirrhosis on long-term prognosis after surgery in patients with hepatocellular carcinoma. Surgery. 1992;112:515–512. [PubMed] [Google Scholar]
- 6.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925–929. [DOI] [PubMed] [Google Scholar]
- 8.Vilana R, Bruix J, Bru C, et al. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992;16:353–357. [DOI] [PubMed] [Google Scholar]
- 9.Livraghi T, Giorgio A, Mariu G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa S, Yamasaki N, Hiwaki T, et al. Factors that predict intrahepatic recurrence of hepatocellular carcinoma in 81 patients initially treated by percutaneous ethanol injection. Cancer. 1999;86:1682–1690. [DOI] [PubMed] [Google Scholar]
- 11.Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319–S328. [DOI] [PubMed] [Google Scholar]
- 12.Hamazoe R, Hiraoka Y, Ohtani S, et al. Intraoperative microwave tissue coagulation as treatment for patients with nonresectable hepatocellular carcinoma. Cancer. 1994;75:794–800. [DOI] [PubMed] [Google Scholar]
- 13.Jiao LR, Hansen DD, Havilik R, et al. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999;177:33–306. [DOI] [PubMed] [Google Scholar]
- 14.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies. Ann Surg. 1999;230:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku Y, Saitoh M, Nishiyama H, et al. Extracorporeal removal of anticancer drugs in hepatic arterial infusion: the effect of direct hemoperfusion combined with venovenous bypass. Surgery. 1990;107:273–281. [PubMed] [Google Scholar]
- 17.Ku Y, Fukumoto T, Iwasaki T, et al. Clinical pilot study on high-dose intraarterial chemotherapy with direct hemoperfusion under hepatic venous isolation in patients with advanced hepatocellular carcinoma. Surgery. 1995;117:510–519. [DOI] [PubMed] [Google Scholar]
- 18.Ku Y, Fukumoto T, Maeda I, et al. Single catheter technique of hepatic venous isolation and extracorporeal charcoal hemoperfusion for malignant liver tumors. Am J Surg. 1997;173:103–109. [DOI] [PubMed] [Google Scholar]
- 19.Ku Y, Iwasaki T, Fukumoto T, et al. Induction of long-term remission in advanced hepatocellular carcinoma with percutaneous isolated liver chemoperfusion. Ann Surg. 1998;227:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravikumar TS, Pizzorno G, Bodden W, et al. Percutaneous hepatic vein isolation and high-dose hepatic arterial infusion chemotherapy for unresectable liver tumors. J Clin Oncol. 1994;12:2723–2736. [DOI] [PubMed] [Google Scholar]
- 21.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. [DOI] [PubMed] [Google Scholar]
- 22.Division of Cancer Treatment. Guidelines for the Reporting of Adverse Drug Reactions. Bethesda, MD: National Care Institute; 1988. [Google Scholar]
- 23.Ku Y, Tominaga M, Iwasaki T, et al. Long-term results of percutaneous isolated hepatic perfusion for advanced hepatocellular carcinoma. Kan-Tan-Sui. 1999;39:869–876. [PubMed] [Google Scholar]
- 24.Yamamoto M, Iizuka H, Matsuda M, et al. The indications for tumor mass reduction surgery and subsequent multidisciplinary treatments in stage IV hepatocellular carcinoma. Surgery Today Jpn J Surg. 1993;23:675–681. [DOI] [PubMed] [Google Scholar]
- 25.Ikai I, Yamaoka Y, Yamamoto Y, et al. Surgical intervention for patients with stage IVA hepatocellular carcinoma without lymph node metastasis. Ann Surg. 1998;227:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada M, Takenaka K, Kawahara N, et al. Surgical treatment strategy for patients with stage IV hepatocellular carcinoma. Surgery. 1996;119:517–522. [DOI] [PubMed] [Google Scholar]