Abstract
Objective:
To examine the effects of perioperative rhG-CSF administration on immune function in patients subjected to major surgery.
Summary Background Data:
Severe trauma, such as major surgery, initiates acute immunodysfunction which predisposes the patient towards infectious complications.
Methods:
Sixty patients undergoing elective surgery received either recombinant human granulocyte colony-stimulating factor/rh G-CSF (Filgrastim) or a placebo perioperatively. At several time points before and after the surgical intervention immunofunctional parameters were assessed.
Results:
Leukocyte counts and serum levels of anti-inflammatory mediators (IL-1ra and TNF-R) were increased in Filgrastim-treated patients, while the post-operative acute phase response was attenuated. Monocyte deactivation (reduced TNF-α release and HLA-DR expression) and lymphocyte anergy (impaired mitogenic proliferation and reduced TH1 lymphokine release) were blunted and the incidence and severity of infectious complications were reduced.
Conclusions:
These results suggest that Filgrastim treatment reinforces innate immunity, enabling better prevention of infection. Thus, this unique combination of hematopoietic, anti-inflammatory and anti-infectious effects on the innate immune system warrants further study of clinical efficacy and sepsis prophylaxis.
Severe trauma, such as major surgery, initiates acute immunodysfunction, which predisposes the patient toward infectious complications. Sixty patients undergoing elective surgery received either recombinant human granulocyte colony-stimulating factor/rh G-CSF (Filgrastim) or a placebo perioperatively. RhG-CSF treatment resulted in maintained lymphocyte and monocyte responsiveness as well as itreduced incidence and severity of infectious complications compared to the placebo population. These results suggest that rhG-CSF treatment reinforces innate immunity, thus preventing posttraumatic multiple organ dysfunction.
Major surgery carries an increased risk of infectious complications. The body reacts systemically to trauma by the acute phase reaction. Local trauma and inflammation are accompanied by anti-inflammatory counterregulation, which leaves immune cells in a state of anergy, impairing host defense against infection.1 Post-traumatic immunoparalysis involves both monocytes and lymphocytes and is reviewed in detail elsewhere.2 The capacity of monocytes to react to an inflammatory stimulus by releasing tumor necrosis factor (TNF)-α is diminished.3 This correlates with decreased expression of HLA-DR, a major histocompatibility complex subtype, on their surface.4 Lymphocytes have a reduced capacity to produce TH1 type cytokines, interleukin-2 (IL-2) and interferon-γ (IFN-γ), and are inhibited in their ability to proliferate5,6 while production of TH2 type cytokines such as IL-4 is up-regulated.7 Prevention of post-traumatic immunoparalysis and reduction of the risk of trauma-induced infection could be brought about by strengthening and priming host defense before trauma, improving the immune system’s capacity to cope with invading pathogens locally and preventing into a systemic anti-inflammatory response.
The granulocyte colony-stimulating factor (G-CSF) plays a central role in the endogenous response to infection and inflammation.8 G-CSF stimulates hematopoiesis and primes granulocytes for enhanced immune defense. On the other hand, the release of proinflammatory mediators such as TNF-α, IL-1β, and IL-12 by monocytes in response to an infectious stimulus is reduced by G-CSF, while these cells are primed toward an increased release of anti-inflammatory mediators such as IL-1ra, sTNF-receptors p55 and p75.9,10 Therefore, G-CSF seems to be a promising candidate to signal the immune system to prepare for defense, while not activating immune cells directly, and to limit the inflammatory response by attenuating monocytes, allowing more moderate reactions but not paralyzing these regulators.
Data from preclinical studies support this hypothesis: Faster recruitment of neutrophils to the site of infection,11 reduced spreading of bacteria12,13 and better survival of endotoxin-induced hepatotoxicity and shock14,15 as well as multimicrobial sepsis16–18 have been reported in rodent models in which G-CSF was administered before or with trauma. Improved cardiovascular function, endotoxin clearance, and survival with G-CSF treatment were also reported in canine models of septic shock.19,20 Only a few human trials of G-CSF as sepsis prophylaxis have been carried out. G-CSF treatment significantly reduced infectious complications in 19 cancer patients undergoing esophagectomy compared with 77 control patients.21 G-CSF treatment reduced the incidence of multiple organ failure in 756 pneumonia patients22 as well as in 37 liver allograft recipients.23
The evidence from the above-described animal and limited clinical studies suggested to us that prophylactic treatment with G-CSF at the time a risk can be anticipated, such as before an operation, may offer protection from infections and lower the incidence of sepsis. Thus, with this original study, we wanted to investigate the impact of continuous perioperative rhG-CSF administration on postoperative immunoinflammatory function as well as the incidence and outcome of infections in patients having high-risk surgery.
MATERIALS AND METHODS
Sixty patients were included in a randomized, placebo-controlled, double-blind study that was performed according to the requirements of the Helsinki declaration. The patients either received placebo or 5 μg recombinant human granulocyte colony-stimulating factor/rh G-CSF (Filgrastim)/kg s.c. on days −2, 1, and 3 relative to surgery (Group A) or 5 μg/kg on day −2 and 2 μg/kg on each of the 5 subsequent days (Group B). The total dose received was 15 μg Filgrastim/kg body weight in both groups (Fig. 1).
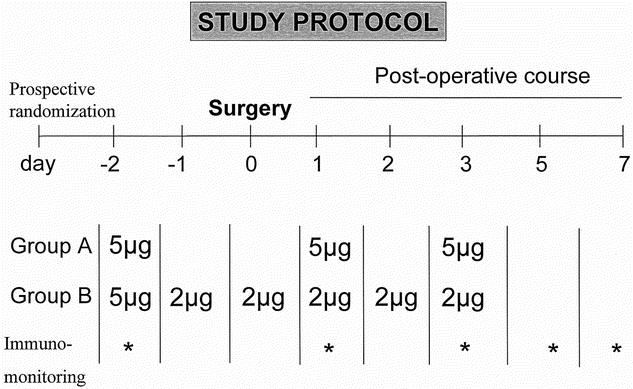
FIGURE 1. The experimental protocol of the study is depicted. Patients in the treatment group received rh G-CSF (15 μg/kg body weight) over 6 perioperative days either as 3 bolus administrations of 5 μg/kg body weight or as a continuous administration of 2 μg/kg body weight following an initial bolus of 5 μg/kg body weight.
The study design was approved by the ethical review board of the Ludwig-Maximilians-University Munich. Exclusion criteria were pregnancy, immunosuppression (chemotherapy, steroid medication, AIDS, etc.), acute inflammatory diseases (indicated by increased C-reactive protein serum levels), and preexisting renal failure requiring hemodialysis. The underlying diseases for all included patients were associated with malignancy. All patients gave informed consent. No side effects of subcutaneous Filgrastim administration were observed.
The types of surgery performed in the placebo versus Filgrastim group and other demographic data are depicted in Table 1. As it has been documented, the groups did not differ significantly in terms of age, gender distribution, and magnitude of surgical trauma as expressed through the length of operation time (4.3 ± 0.4 hours in the placebo vs. 4.6 ± 0.2 hours in the Filgrastim group).
TABLE 1. Patient Demographics

Whole Blood Incubation
Blood samples for immunomonitoring were withdrawn on days -2, 1, 3, 5, and 7. Differential white blood cell counts were assessed at every time of blood collection. Whole blood incubations were performed in sterile Vacutainers (Sarstedt, Nümbrecht, Germany)24,25; 5 mL 20% whole blood in RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 100 IU penicillin, 100 μg/mL streptomycin and 2.5 IU heparin (Hoffmann LaRoche, Grenzach-Whylen, Germany) was stimulated with 10 μg/mL LPS from Salmonella abortus equi (Sigma-Aldrich, Deisenhofen, Germany). After 24 hours at 37°C vials were shaken and centrifuged (300 g, 5 minutes). Cytokines were measured in serum or supernatants by ELISA based on antibody pairs against IL-1ra, IL-2, G-CSF (R&D, Wiesbaden, Germany), IL-4 (Pharmingen, Hamburg, Germany), TNF-α, IFN-γ (Endogen, Biozol, Eching, Germany), and sTNF-R1 and 2 (Bender MedSystems, Vienna, Austria). C-reactive protein was measured with the immunoturbidimetric kit Tina-quant CRP (Roche Diagnostics, Germany).
Peripheral Blood Mononuclear Cell (PBMC) Preparation
PBMCs were prepared from 25 mL heparinized (10 IU/mL) whole blood drawn from the cubital vein or central venous catheters at 8 AM (± 1 hour) into a syringe (B. Braun Medical AG, Emmenbruecke, Switzerland) using a standard Ficoll-Hypaque (Biochrome, Berlin, Germany) density gradient. The blood was layered on top of the Ficoll Hypaque at a 2:1 ratio in 50-mL tubes and centrifuged for 20 minutes at 500 g at room temperature. PBMCs were collected from the interface, washed twice in HBSS (Gibco, Eggenstein, Germany), and resuspended in either RPMI 1640 (Gibco) containing 10% fetal calf serum (Vitromex, Vilshofen, Germany) and 0.1 mg/mL gentamycin (Merk, Darmstadt, Germany) or phosphate-buffered saline (B. Braun Medical AG) containing 0.1% sodium azide. Viability of PBMC was consistently >95% as determined by trypan blue exclusion.
PBMC Culture
Cells (2 X 106 cells/mL) were stimulated with either LPS (10 μg/mL) or phytohemagglutinin (PHA HA16/17; 2,5 μg/mL; Wellcome, Burgwedel, Germany) and incubated for 24 hours or 48 hours (37°C, 6% CO2, and 90% humidity). Cell-free supernatants were collected and stored at −80°C until assayed for cytokine production. Lymphocyte proliferation over 48 hours in response to PHA was determined by 3H-thymidine incorporation as described.26
Surface Receptor Expression
PBMCs resuspended in phosphate-buffered saline/0.1% sodium azide were double labeled with 1 μg/106 cells of anti-CD14-fluorescein-isothiocyanate (FITC) (RM052; mouse IgG2a) and anti-HLA-DR-phycoerythrin (PE) (Immu-357; mouse IgG1) or with corresponding control antibodies, all from Immunotech (Marseille, France). Samples were incubated for 30 minutes on ice in the dark, and then washed twice with phosphate-buffered saline/0.1% sodium azide. Samples were kept on ice in the dark and all measurements were analyzed within 30 minutes after completing the staining procedure with a Coulter EpicsXL (Beckman Coulter GmbH, Krefeld, Germany) fitted with a 488-nm argon laser and filter settings for FITC (530 nm wide bandpass filter) and PE (575 nm dichronic filter). After appropriate instrument settings and spectral compensations were performed, the instrument settings were not changed and stability was regularly checked. A minimum of 10,000 events was assessed using log-amplified fluorescence signals and linearly amplified side- and forward-scatter signals. XL2 software (Beckman Coulter) was used to analyze the data.
Statistics
Statistical analysis was performed by one-way analysis of variance followed by Tukey test or Dunn’s test with SigmaStat program. P values less than 0.05 were considered significant (*P ≤ 0.05. **P ≤ 0.001).
RESULTS
Filgrastim treatment was well tolerated in all patients. No significant differences were observed between the two Filgrastim treatment regimens; therefore, the groups were combined in the analyses presented.
As expected, Filgrastim initiated a rapid neutrophilia, which elevated the total leukocyte counts to 2 to 3 times placebo values throughout the treatment period (Fig. 2a). Monocyte counts were slightly increased in the treatment group over preoperative values, while these counts remained stable in placebo blood. Interestingly, the slump in lymphocyte counts observed after the operation in placebo-treated patients (50% and 57% of preoperative values on days +1 and +3, respectively) was less dramatic and more short-lived in Filgrastim-treated patients (67% and 115% of preoperative counts on days +1 and +3). Furthermore, Filgrastim treatment induced increased serum levels of IL-1ra and soluble TNF-receptors p55 and p75, while placebo levels were not affected by the surgical interventions (Table 2).
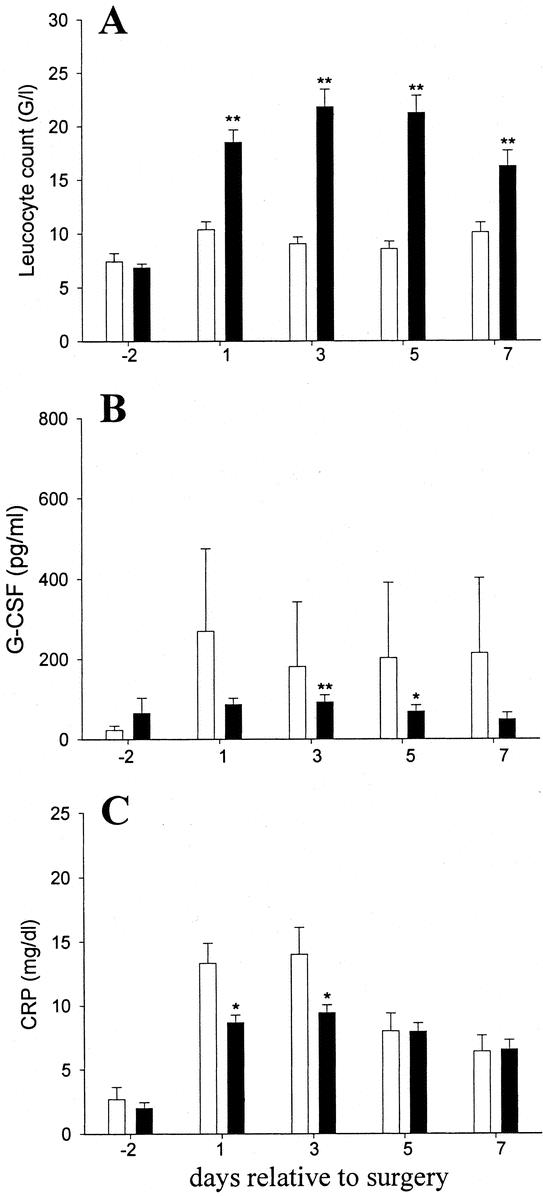
FIGURE 2. Perioperative Filgrastim treatment blunts trauma-induced acute phase reaction. Patients received placebo (empty bars) or Filgrastim (black bars) perioperatively. Total leukocyte counts (a), C-reactive protein (b) and G-CSF (c) serum concentrations are shown as means ± SEM *P ≤ 0.05; **P ≤ 0.001, relative to placebo values.
TABLE 2. Filgrastim-Treated Patients Have Higher Circulating Anti-inflammatory Mediators After Surgery

The postoperative acute phase reaction indicated by C-reactive protein and G-CSF levels in the serum was blunted in Filgrastim-treated patients (Fig. 2b,c). Filgrastim treatment further decreased the severity and duration of monocyte inactivation: the attenuated decrease in HLA-DR expression on monocytes from Filgrastim-treated patients (Fig. 3a) correlated with a better maintained capacity for release of TNF-α, IL-6, and IL-1ra in response to LPS stimulation ex vivo in the Filgrastim treatment group (Fig. 3b-d).
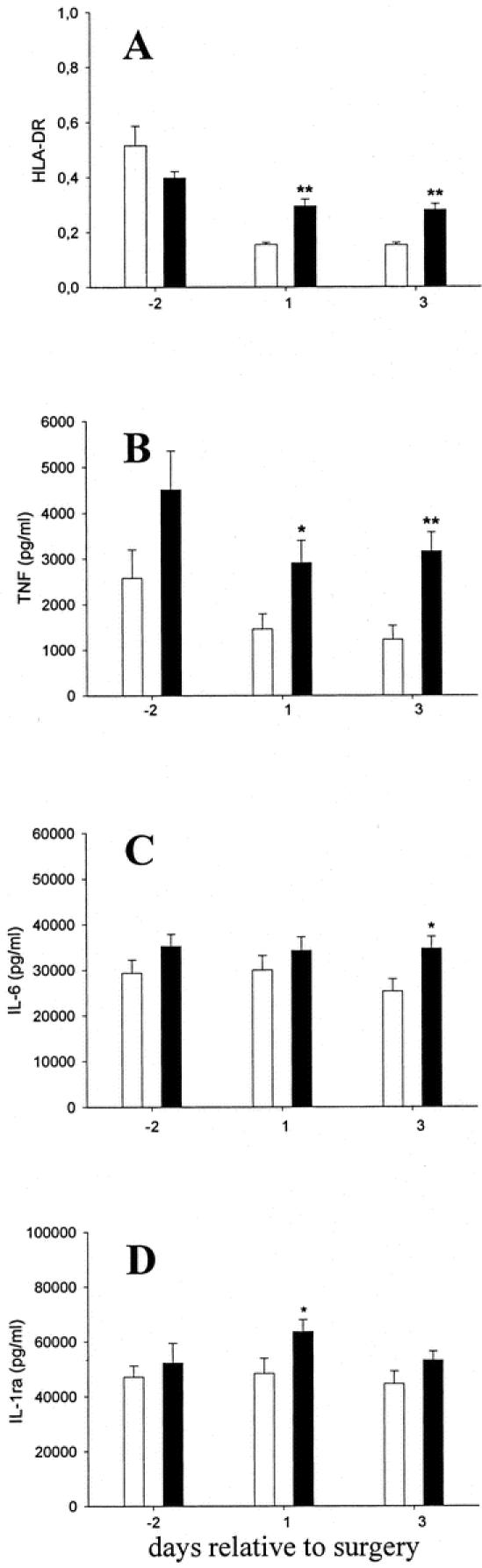
FIGURE 3. Perioperative Filgrastim treatment blunts trauma-induced monocyte deactivation. Patients received placebo (empty bars) or Filgrastim (black bars) perioperatively. a: HLA-DR expression on monocytes. LPS-induced TNF-α (b), IL-6 (c), and IL-1ra (d) release in diluted whole blood. Data represent means ± SEM. *P ≤ 0.05; **P ≤ 0.001, relative to placebo values.
PHA-induced lymphocyte proliferation dropped to half of preoperative values 1 day after surgery in the placebo group but was increased in the Filgrastim-treated group over preoperative values (Table 3). Also, the release of the TH1 lymphokine IL-2 and IFN-γ, which also dropped in the placebo group after surgery, was increased in the Filgrastim-treated group over placebo values. Furthermore, the IL-2/IL-4 ratio increased in treated patients compared with the placebo group after surgery (Table 3).
TABLE 3. Perioperative Filgrastim Treatment Prevents Lymphocyte Anergy and Maintains TH1-TH2 Balance

A list of adverse events in both treatment arms is documented (Table 4). As shown, 6 of 20 patients in the control group had infectious complications (30%), including 2 lethal outcomes, compared with 5 (13%) infectious complications that were observed in 40 Filgrastim-treated patients. Although the blood loss of 1650 ± 266 mL during surgery was more severe in the Filgrastim-treated group (1078 ± 164 mL in the placebo group, not significant), the average time Filgrastim-treated patients spent in the intensive care unit tended to be less (64 ± 26 hours) than placebo treated patients (156 ± 103, not significant). The average hospitalization time did not differ significantly between the two groups
TABLE 4. Perioperative rhG-CSF Administration: Eventful Clinical Course

DISCUSSION
Trauma induced by extensive surgery may initiate an exaggerated acute phase reaction coupled with monocyte deactivation and lymphocyte anergy.2,27 This combination severely compromises the immune system, predisposing the patient to infection and sepsis. When the time of surgery is known, sepsis prophylaxis could be administered. However, such prophylactic strategies should neither block the normal, protective inflammatory reaction to bacterial invasion nor preactivate the immune cells.
In this trial, rhG-CSF was administered prophylactically before and after surgery and thus represents the first investigation, to our knowledge, with a study design like this. We found that rhG-CSF resulted in increased levels of natural circulating antagonists of TNF-α and IL-1, ie, TNF-R p55/p75 and IL-1ra, in rhG-CSF treated patients, thus increasing the threshold for an inflammatory reaction to occur. G-CSF administration to healthy volunteers9 also resulted in a massive increase of counterregulatory and anti-inflammatory cytokines such as soluble TNF-receptors and IL-1ra. While G-CSF administration to healthy volunteers also caused a reproducible increase of proinflammatory mediators such as TNF-α after ex vivo and in vitro stimulation with LPS, the increase of their antagonistic molecules was significantly higher, thus indicating that in general the impact of G-CSF on anti-inflammtory cytokine patterns appears to be more pronounced than its effect on proinflammatory cytokine patterns. While this observation was corroborated in our study for TNF-α versus TNF receptors, conversely we could not confirm down-regulating effects of G-CSF in general on proinflammatory lymphokines like IL-2 and IFN-γ, respectively an up-regulatory effect on IL-4 or IL-10 as described by other investigators.9,27,28 These contrasting (respectively) inconsistent findings are to be explained through the different timing (continuous vs. pretraumatic or post-traumatic) of G-CSF administration. A very striking example for the most differential effects that are exerted through G-CSF on cytokine release has been found by Pajkrt et al in human endotoxemia when G-CSF was administered either intravenously 2 hours or subcutaneously 24 hours before endotoxin.29 When administered 2 hours before endotoxin, G-CSF actually augmented an LPS-induced inflammatory cytokine response with high IL-6 levels, whereas when given 24 hours before LPS challenge, G-CSF attenuated the LPS-induced proinflammatory state and caused down-regulation of inflammation.
In our study, the trauma-induced acute phase response, as represented by C-reactive protein measurements, was less in rhG-CSF-treated patients. Therefore, the inflammatory response to trauma was alleviated but not completely abolished by rhG-CSF treatment. A similar observation occurred for the anti-inflammatory counterreaction: Serum levels of anti-inflammatory IL-1ra were significantly increased in rh-GCSF-treated patients in comparison to controls, and postoperative HLA-DR expression on monocytes from rhG-CSF-treated patients was not suppressed compared with controls. Thus, the capacity of monocytes to release TNF-α when stimulated with endotoxin ex vivo was increased in rhG-CSF-treated patients over controls. Taken together, all markers for monocyte deactivation were improved by rhG-CSF treatment. The same was observed for markers of lymphocyte anergy: lymphocyte proliferative capacity and TH1-TH2 balance, which were altered in placebo patients, but maintained in rhG-CSF-treated patients after surgery.
G-CSF, a hematopoietic growth factor, has to be considered as a multipotent molecule that exerts its effects not only directly on granulocytes but also on other leukocyte lineages like monocytes and T lymphocytes, as shown in this study. The glycoprotein G-CSF is present at low concentrations (∼25 μg/mL) in the serum of healthy volunteers.30 Several studies have shown that G-CSF plasma levels are increased following trauma and the acute phase of infection.31,32 Therapeutic administration of low-dose G-CSF (10 mg/kg) into healthy volunteers, compared with the dose of 15 mg/kg given in our study, elevated the serum G-CSF to levels in the upper range of those reached by endogenous production during infection.33 Interestingly, clinical examinations showed that elevated G-CSF levels at the onset of sepsis decreased significantly within a few days in survivors but remained persistently increased in nonsurviving sepsis patients.33 In contrast, patients who do not respond to infection with increased G-CSF production have a worse prognosis than patients who respond with G-CSF production.34 Thus, it can be believed that therapeutic administration of G-CSF to trauma patients may be beneficial to the host by increasing or accelerating the response to inflammation or infection.
At present, exogenous G-CSF is widely used clinically and has been proven to be effective and safe in reducing the incidence of infection and sepsis in neutropenic and non-neutropenic immunocompromised patients.34–36 The safety and tolerance of G-CSF administration in these patients prone to sepsis are well established.
In non-neutropenic post-traumatic/postoperative patients with a high risk of sepsis, as reported by Weiss et al,37 none of the patients treated with rhG-CSF developed sepsis; however, three patients in the control group did. As well in our study, although the number of patients was small, there was a difference in outcome between placebo and rhG-CSF-treated patients. Of 20 placebo patients, 4 developed sepsis with multiorgan failure and 2 of them died, but in 40 rhG-CSF treated patients, only 3 developed sepsis with organ failure and all recovered (Table 4).
Over recent years, we have understood through a multitude of studies that trauma not only elementarily alters the specific performance of each cell type, but probably more importantly, it affects the loss of control capacity and modulatory surveillance that physiologically monocyte and T cells possess for each other within a number of regulatory loops. This loss of regulatory function occurs instantaneously at the moment of injury and probably, most importantly, leads to irreversible monocytic deactivation. We had to learn that reprogramming cellular dysfunction after traumatic stress is not possible. Rather, we have to supply the biologic system with a sufficient quantity of functionally mature leukocytes that allow a timely normalization of the immunoinflammatory performance. As this study shows, the administration of human recombinant growth factor, namely, G-CSF, can centrally accomplish the conservation of an innate immune system also under most stressful conditions such as major surgery, thus preventing life-threatening clinical scenarios such as second-hit multiple organ failure. We do believe that further trials of rhG-CSF in sepsis prophylaxis, perhaps in even higher risk populations, are warranted.
Footnotes
Reprints: Eugen Faist, MD, FACS, Department of Surgery, Ludwig-Maximilians-University, Klinikum Grosshadern, Marchioninistrasse 15, 81377 Munich, Germany. E-mail: Faist@gch.med.uni-muenchen.de.
REFERENCES
- 1.Green DR, Faist E. Trauma and the immune response. Immunol Today. 1988;9:253–255. [DOI] [PubMed] [Google Scholar]
- 2.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–459. [DOI] [PubMed] [Google Scholar]
- 3.Schinkel C, Licht K, Zedler S, et al. Interferon-gamma modifies cytokine release in vitro by monocytes from surgical patients. J Trauma. 2001;50:321–327. [DOI] [PubMed] [Google Scholar]
- 4.Volk HD, Reinke P, Falck P, et al. Diagnostic value of an immune monitoring program for the clinical management of immunosuppressed patients with septic complications. Clin Transplant. 1989;3:246–252. [Google Scholar]
- 5.Faist E, Schinkel C, Zimmer S, et al. Inadequate interleukin-2 synthesis and interleukin-2 messenger expression following thermal and mechanical trauma in humans is caused by defective transmembrane signalling. J Trauma. 1993;34:846–853. [DOI] [PubMed] [Google Scholar]
- 6.Hensler T, Hecker H, Heeg K, et al. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zedler S, Faist E, Ostermeier B, et al. Postburn constitutional changes in T-cell reactivity occur in CD8+ rather than in CD4+ cells. J Trauma. 1997;42:872–880. [DOI] [PubMed] [Google Scholar]
- 8.Hartung T, von Aulock S, Wendel A. Role of granulocyte colony-stimulating factor in infection and inflammation. Med Microbiol Immunol. 1998;187:61–69. [DOI] [PubMed] [Google Scholar]
- 9.Hartung T, Docke WD, Gantner F, et al. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–2489. [PubMed] [Google Scholar]
- 10.Hartung T, Doecke WD, Bundschuh D, et al. Effect of Filgrastim treatment on inflammatory cytokines and lymphocyte functions. Clin Pharmacol Ther. 1999;66:415–424. [DOI] [PubMed] [Google Scholar]
- 11.Lister PD, Gentry MJ, Preheim LC. Granulocyte colony-stimulating factor protects control rats but not ethanol-fed rats from fatal pneumococcal pneumonia. J Infect Dis. 1993;168:922–926. [DOI] [PubMed] [Google Scholar]
- 12.Patton JHJ, Lyden SP, Ragsdale DN, et al. Granulocyte colony-stimulating factor improves host defense to resuscitated shock and polymicrobial sepsis without provoking generalized neutrophil-mediated damage. J Trauma. 1998;44:750–758. [DOI] [PubMed] [Google Scholar]
- 13.Lang CH, Bagby GJ, Dobrescu C, et al. Effect of granulocyte colony-stimulating factor on sepsis-induced changes in neutrophil accumulation and organ glucose uptake. J Infect Dis. 1992;166:336–343. [DOI] [PubMed] [Google Scholar]
- 14.Görgen I, Hartung T, Leist M, et al. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992;149:918–924. [PubMed] [Google Scholar]
- 15.Vollmar B, Messner S, Wanner G, et al. Immunomodulatory action of G-CSF in a rat model of endotoxin-induced liver injury: an intravital microscopic analysis of Kupffer cell and leukocyte response. J Leukoc Biol. 1997;62:710–718. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly M, Silver GM, Greenhalgh DG, et al. Treatment of intra-abdominal infection with granulocyte colony-stimulating factor. J Trauma. 1992;33:679–682. [DOI] [PubMed] [Google Scholar]
- 17.Barsig J, Bundschuh DS, Hartung T, et al. Control of fecal peritoneal infection in mice by colony-stimulating factors. J Infect Dis. 1996;174:790–799. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz W, Reimund KP, Weitzel F, et al. Granulocyte colony-stimulating factor prophylaxis before operation protects against lethal consequences of postoperative peritonitis. Surgery. 1994;116:925–934. [PubMed] [Google Scholar]
- 19.Waring PM, Presneill J, Maher DW, et al. Differential alterations in plasma colony-stimulating factor concentrations in meningococcaemia. Clin Exp Immunol. 1995;102:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman BD, Quezado Z, Zeni F, et al. rG-CSF reduces endotoxemia and improves survival during E. coli pneumonia. J Appl Physiol. 1997;83:1467–1475. [DOI] [PubMed] [Google Scholar]
- 21.Mansmann G, Engert A, Hübel K. Application of G-CSF in the nonneutropenic host. Onkologie. 1998;21:124–127. [Google Scholar]
- 22.Andresen J, Movahhed H, Nelson S. Filgrastim (r-metHuG-CSF) in pneumonia. In: Morstyn G, Dexter TM, Foote M, eds. Filgrastim (r-metG-CSF) in Clinical Practice, 2nd ed. New York: Marcel Dekker, 1998:429–446. [Google Scholar]
- 23.Foster PF, Mital D, Sankary HN, et al. The use of granulocyte colony-stimulating factor after liver transplantation. Transplantation. 1995;59:1557–1563. [PubMed] [Google Scholar]
- 24.Hartung T, Pitrak DL, Foote M, et al. Filgrastim restores interleukin-2 production in blood from patients with advanced human immunodeficiency virus infection. J Infect Dis. 1998;178:686–692. [DOI] [PubMed] [Google Scholar]
- 25.Hartung T, von Aulock S, Freitag M, et al. Blood cytokine response of low-dose molgramostim (rhGM-CSF)-treated patients. Cytokine. 2000;12:1570–1574. [DOI] [PubMed] [Google Scholar]
- 26.Faist E, Ertel W, Cohnert T, et al. Immunoprotective effects of cyclooxygenase inhibition in patients with major surgical trauma. J Trauma. 1990;30:8–17. [DOI] [PubMed] [Google Scholar]
- 27.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood. 1999;93:425–439. [PubMed] [Google Scholar]
- 28.Pan L, Delmonte JJr, Jalonen CK, et al. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422. [PubMed] [Google Scholar]
- 29.Pajkrt D, Manten A, van der Poll T, et al. Modulation of cytokine release and neutrophil function by granulocyte colony-stimulating factor during endotoxemia in humans. Blood. 1997;90:1415. [PubMed] [Google Scholar]
- 30.Ibolgaufts H. Lexikon der Zytokine. München: Medikon Verlag, 1992.
- 31.Kawakami M, Tsutsumi H, Kamakawa T, et al. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood. 1990;76:1962–1964. [PubMed] [Google Scholar]
- 32.Tanaka H, Ishikawa K, Nishino M, et al. Changes in granulocyte colony-stimulating factor concentration in patients with trauma and sepsis. J Trauma. 1996;40:718–725. [DOI] [PubMed] [Google Scholar]
- 33.Gross-Weege W, Weiss M, Schneider M, et al. Safety of a low-dosage Filgrastim rhG-CSF treatment in non-neutropenia surgical intensive care patients with an inflammatory process. Intensive Care Med. 1997;23:16–22. [DOI] [PubMed] [Google Scholar]
- 34.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992;327:28–35. [DOI] [PubMed] [Google Scholar]
- 35.Welte K, Grabilove J, Bronchud MH, et al. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907. [PubMed] [Google Scholar]
- 36.Ishikawa K, Tanaka H, Matsuoka T, et al. Recombinant human granulocyte colony-stimulating factor attenuates inflammatory responses in septic patients with neutropenia. J Trauma. 1998;44:1046–1047. [DOI] [PubMed] [Google Scholar]
- 37.Weiss M, Gross-Weege W, Schneider M, et al. Enhancement of neutrophil function by in vivo filgrastim treatment for prophylaxis of sepsis in surgical intensive care patients. J Crit Care. 1995;10:21. [DOI] [PubMed] [Google Scholar]
- 38.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864. [PubMed] [Google Scholar]


