Abstract
Corticotropin-releasing hormone (Crh) plays an important role in modulating physiological and behavioral responses to stress. Its actions are mediated through two receptors, Crhr1 and Crhr2. Urocortin (Ucn), a Crh-related neuropeptide and the postulated endogenous ligand for Crhr2, is a potential mediator of stress responses. We generated Ucn-deficient mice using embryonic stem cell technology to determine its role in stress-induced behavioral and autonomic responses. Unlike Crhr1- or Crhr2-deficient mice, Ucn-deficient mice exhibit normal anxiety-like behavior as well as autonomic regulation in response to stress. However, the mutant mice display an impaired acoustic startle response that is not due to an obvious hearing defect. Thus, our results suggest that Ucn does not play an essential role in stress-induced behavioral and autonomic responses. Ucn may modulate the acoustic startle response through the Ucn-expressing neuron projections from the region of the Edinger-Westphal nucleus.
The corticotropin-releasing hormone (Crh) system has long been reported to participate in coordinating autonomic, endocrine, and behavioral responses to stress. Urocortin (Ucn) is a mammalian member of the Crh family which was identified in screens of a rat brain cDNA expression library using a polyclonal antibody raised against fish urotensin I (31). Ucn exhibits 63% identity with fish urotensin I and 45% identity with Crh at the amino acid level. Ucn administration in vivo triggers many physiological and behavioral responses similar to those observed with Crh (26). Although there is some controversy concerning the details of the expression pattern of Ucn (2, 31, 34), Ucn and Crh are distinctively distributed in the rat brain. Consistent in all studies is the predominant expression of Ucn mRNA and protein in the Edinger-Westphal (EW) nucleus.
Ucn is capable of binding at high affinity to both Crhr1 and Crhr2 (7, 16, 22, 28) though it is believed to have a higher affinity for Crhr2 (31). On the basis of Ucn's affinity for Crhr2 and the colocalization of the Ucn fiber with Crhr2 (30), Ucn has been hypothesized to be the endogenous ligand for Crhr2.
Mice deficient for Crhr1 (27, 29) or Crhr2 (1, 4, 8) exhibit abnormal behavioral responses to stress; however, Crh-deficient mice have normal stress-induced behaviors (32). Additionally, the basal expression of Ucn is up-regulated in the EW nucleus in the absence of Crh (33). This led us to postulate that Ucn may play a role in mediating some aspects of the stress-induced responses previously ascribed to Crh.
To elucidate the functional role of Ucn, we generated Ucn-deficient mice using embryonic stem cell technology. Homozygous mutants were viable and fertile. No obvious differences between Ucn-deficient and wild-type mice were observed when their anxiety behavior and autonomic and endocrine responses to stress were assessed. However, Ucn-deficient mice exhibited a significantly reduced acoustic startle response (ASR), which is not due to an obvious hearing impairment. We suggest that Ucn may modulate the startle response through its projections to the pontine reticular nucleus.
MATERIALS AND METHODS
Generation of Ucn-deficient mice.
We isolated two phage genomic clones from the 3′ Hprt genomic library (38) with a Ucn cDNA probe. The clones were characterized by restriction enzyme digestion and partial sequencing (36). The 5′ homology arm is a 3.4-kb fragment (corresponding to nucleotide sequence 94 to 3508 of the mouse Ucn gene; GenBank accession no. AF038632) generated by long-range PCR with two primers (3629, 5′-CGA ACT GCG GCC GCG TAA GTC CTT TCC ATT GCT CTC, and 3630, 5′-TCT ATC TCG AGC CTC TGT ATC AAT GGT GCC GCC TG). An enhanced green fluorescent protein (EGFP)-LacZ fusion gene reporter and a floxed phosphoglycerate kinase neo (PGKneo) positive-selection marker were placed downstream of the 5′ homology arm and fused with Ucn exon 2. The 3′ homology arm is a 3-kb PCR fragment generated with two primers (3668, 5′-CAT TAG GCG CGC CGT ATG GGG TCA CGA AAG CCT TGA C, and 3673, 5′-CAT ATG GCC ATG ATG GCC TCT ACC TCT CTA ACC AAT GTC). Downstream of the 3′ arm is a polII promoter-driven thymidine kinase gene (TK) negative-selection marker. Out of 200 G418- and 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodouracil-resistant clones, we identified three targeting events by long-range PCR using primer pair 3625 (5′-TGT CTC CTG GTC TGA AGC TGC AGT CC; corresponding to nucleotides 32 to 57 in the gene sequence under accession no. AF038632) and 3744 (5′-GTT TAC GTC GCC GTC CAG CTC GAC; corresponding to the EGFP cDNA sequence) to detect recombination at the 5′ end and primer pair 3984 (5′-CGA GAT CAG CAG CCT CTG TTC CAC ATA CAC; in the 3′ untranslated region of the PGKneo cassette) and 3759 (5′-CGA GAT CAG CAG CCT CTG TTC CAC ATA CAC; 3 kb downstream of Ucn exon 2) to detect recombination at the 3′ end. Gene targeting was performed in AB2.2 129S7 embryonic stem cells. Blastocyst injection and chimeric mouse production have been described previously (24). A three-primer PCR strategy was developed to genotype animals. Primer pair 4043 (5′-GAC ACC AGG CAA CAG CTG GAG; annealing to a sequence in intron 1 of the Ucn gene) and 4044 (5′-GAA GGT GAG GTC GAT GGA CAG TG; in exon 2) give rise to a 406-bp fragment that is present only in the wild-type allele, and primers 4043 and 4045 (5′-TTT ACG TCG CCG TCC AGC TC; in the EGFP gene) give a 270-bp fragment that identifies the mutant allele. All the experiments except the ABR test were carried out on 129S7/C57BL/6-Tyrc-Brd F2-F4 hybrids at the age of 10 to 14 weeks. Both male and female mice were equally represented in all statistical analyses.
Ucn and Crh expression studies.
In situ hybridization using Ucn and Crh 35S-labeled riboprobes was performed as described previously (37). To detect Ucn gene expression using the targeted LacZ reporter, anesthetized mice were perfused with ice-cold fixative (4% paraformaldehyde in phosphate-buffered solution). After perfusion, the brains were dissected and fixed for 30 min in the same fixative on ice. Vibrotome-cut brain sections were collected and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overnight at room temperature to visualize the β-galactosidase activity.
Locomotor activity in the open field.
Locomotor activity was evaluated by placing mice into the center of a clear Plexiglas (40- by 40- by 30-cm) open-field arena and allowing them to explore for 30 min. Overhead incandescent light bulbs provided room lighting that measured approximately 800 lux inside the test chamber. In addition, white noise was present at approximately 55 dB inside the test arena. Activity in the open field was quantitated by a computer-operated Digiscan optical animal activity system (RXYZCM 16; Accuscan) containing 16 photoreceptor beams on each side of the arena, which divides the arena into 256 equally sized squares. Total distance (locomotor activity), vertical activity (rearing measured by the number of photobeam interruptions), and center distance (i.e., the distance traveled in the center of the arena) were recorded. The center distance was also divided by the total distance to obtain the center distance-to-total distance ratio. The center distance-to-total distance ratio can be used as an index of anxiety-related responses. Data were collected in 2-min intervals over the 30-min test session. Open-field activity data were analyzed by three-way (genotype, gender, and time) analysis of variance (ANOVA) with repeated measurements.
Light-dark exploration.
One week later mice were subjected to a light-dark exploration test; the apparatus for this test consists of a polypropylene chamber (44 by 21 by 21 cm) unequally divided into two chambers by a black partition containing a small opening. The large chamber is open and brightly illuminated (800 lux), while the small chamber is closed and dark. White noise is present in the room at approximately 55 dB in the test chamber. Mice were placed in the illuminated side and allowed to move freely between the two chambers for 10 min. The time to enter the dark chamber and the total number of transitions were recorded. Light-dark test data were analyzed by a two-way (genotype and gender) ANOVA.
Startle and prepulse inhibition of the startle.
Prepulse inhibition of ASRs was measured 1 week after the open-field tests by using the SR-Lab system (San Diego Instruments, San Diego, Calif.) (19, 20). A test session was started by placing a mouse in a Plexiglas cylinder, where it was left undisturbed for 5 min. A test session consisted of seven trial types. One trial type was a 40-ms, 120-dB sound burst used as the startle stimulus. There were five different acoustic prepulse-plus-acoustic startle stimulus trials. The prepulse sound was presented 100 ms before the startle stimulus. The 20-ms prepulse sounds were 74, 78, 82, 86, or 90 dB. Finally, there were trials where no stimulus was presented to measure baseline movement in the cylinders. Six blocks of the seven trial types were presented in pseudorandom order such that each trial type was presented once within a block of seven trials. The average intertrial interval was 15 s (ranged from 10 to 20 s). The startle response was recorded for 65 ms (measuring the response every 1 ms) starting with the onset of the startle stimulus. The background noise level in each chamber was 70 dB. The maximum startle amplitude recorded during the 65-ms sampling window was used as the dependent variable. The following formula was used to calculate the prepulse inhibition of a startle response: 100 − [(startle response on acoustic prepulse-plus-startle stimulus trials/startle response on startle alone trials) × 100]. Thus, a high percentage prepulse inhibition value indicates a good prepulse inhibition, i.e., the subject showed a reduced startle response when a prepulse stimulus was presented compared to the response when the startle stimulus was presented alone. Conversely, a low percentage prepulse inhibition value indicates poor prepulse inhibition, i.e., the startle responses with and without the prepulse were similar. Acoustic response amplitude data were analyzed by a two-way (genotype and gender) ANOVA. Prepulse inhibition data were analyzed by a three-way (genotype, gender, and prepulse sound level) ANOVA with repeated measures.
ASR profile.
Each test session began by placing a subject in the Plexiglas cylinder in the startle chamber, where it was left undisturbed for 5 min. After a 5-min acclimation, each subject was given 52 trials over a 13-min test period. There were 13 different sound levels presented: 70, 74, 78, 82, 86, 90, 94, 98, 102, 106, 110, 114, and 118 dB. Each stimulus was 40 ms and was presented four times in a pseudorandom order such that each sound level was presented with a block of 13 trials. The average intertrial interval was 15 s (ranged from 10 to 20 s). The startle response was recorded for 65 ms (measuring the response every 1 ms) starting with the onset of the startle stimulus. The maximum startle amplitude recorded during the 65-ms sampling window was used as the dependent variable.
Startle response profile data were analyzed by a three-way (genotype, gender, and sound level) ANOVA with repeated measurements with appropriate follow-up comparisons (simple-effect tests) to evaluate the overall differences between the two groups. To determine the startle threshold for each genotype, startle responses to the various stimuli for wild-type and mutant mice were analyzed individually by an ANOVA with repeated measurements followed by planned-contrast comparisons in which the intensity of the response to each stimulus was compared to that of the response following the presentation of the 70-dB (baseline, background sound level) stimulus. In the latter analysis, the threshold for response measurement was defined as the stimulus level which produces a response significantly higher (P < 0.05) than the measured baseline response to the 70-dB sound. To ensure that it was a stable response, the response to each of the remaining stimuli also had to be significantly different from that observed at baseline.
Elevated plus maze.
Mice were placed for 10 min on the elevated plus maze to test anxiety (14, 21). The maze consists of four arms, two with high, black walls and two without walls. Mice were placed in the intersection between the arms, and the number of entries and the time spent in open and closed arms were recorded. Elevated plus maze data were analyzed by a two-way (genotype and gender) ANOVA.
ABR.
The auditory brain stem response (ABR) was measured essentially as described previously (15). Briefly, mice (10 wild type, 9 Ucn−/−; age, 8 weeks) were anaesthetized with 45 mg of ketamine/kg of body weight and 5.4 mg of xylazine/kg, and electrodes were inserted at the vertex and behind the ear (reference electrode). Tucker-Davis Technology software and hardware were used to induce the stimuli and record the ABR data. Five-millisecond pulses were delivered through a closed-field earphone (Entymotic; ER-2) at a frequency of 20/s. The intensity levels of the stimuli ranged from 100 to 25 dB in 5-dB decrements re 0.001 V (RMS) to the earphone. ABR waveforms were based on an average of 500 responses at each intensity level. The results were analyzed with a two-tail Student t test.
Telemetric measurement of autonomic response.
Heart rates in conscious, free running mice (eight wild type, six Ucn−/−) were monitored with a radio transmitter (TA10ETA-F20 or TA10EA-F20; Data Sciences) implanted in the abdominal cavity and a radio receiver (RPC-1; Data Sciences) (17). The transmitter was turned on the night before restraining the mice. The baseline heart rates were monitored at 1-min intervals. A tail access rodent restrainer (Stoelting, Wood Dale, Ill.) was used to stress the mouse, and the heart rate was monitored every 10 s for 10 min. After the mouse was released from the restrainer, the heart rate was measured at 1-min intervals until it reached its baseline level. The heart rate during the entire experimental period was collected and stored using Dataquest A.R.T., version 2.0, software (Data Sciences). Each mouse was group caged with its littermate from weaning. Restraint experiments were performed between 2 and 3 p.m. and at least 7 days after the implantation surgery.
Plasma hormone measurements.
All the blood samples were collected without anesthesia. To determine the basal levels of hormones, mice (20 to 24 weeks old, n = 11/genotype) were undisturbed until the blood was collected between 11:00 a.m. and 12:00 p.m. by decapitating the mice within 1 min of initial cage disturbance. For the measurement of stress-induced hormone levels, mice (20 to 24 weeks old, n = 11/genotype) were placed in 50-ml Cornical tubes (plastic tubes with air holes) for 1 h and then decapitated. Trunk blood was collected in ice-cold EGTA (Amersham). Plasma was aliquoted after centrifugation at 4°C and frozen at −20°C until assayed. The corticosterone level was determined by a radioimmunoassay, and the catecholamines (epinephrine and norepinephrine) were measured by a radioenzymatic assay (23) (Endocrine Sciences, Calabasas Hills, Calif.).
RESULTS AND DISCUSSION
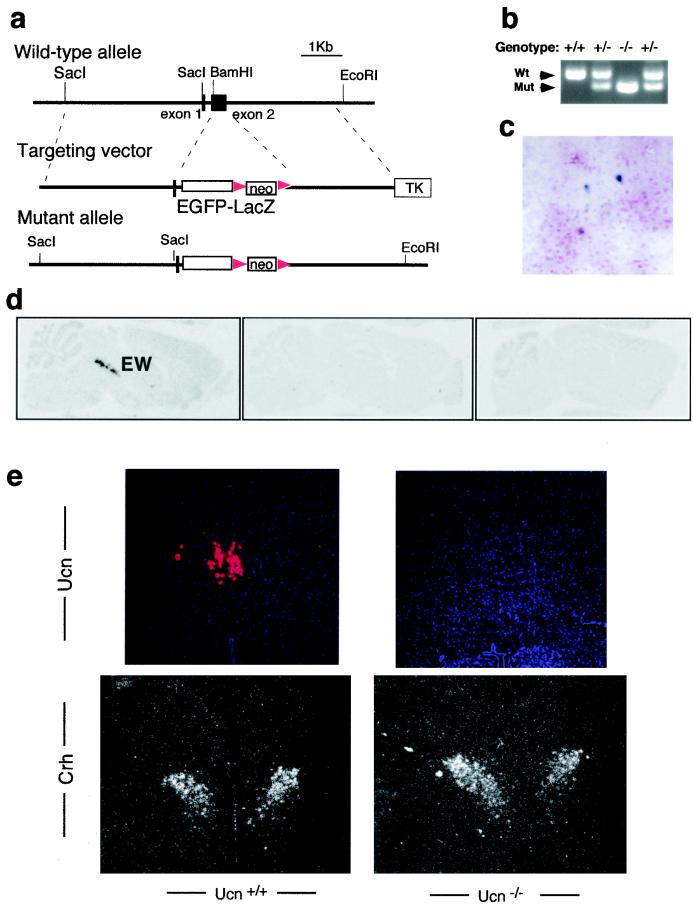
To define the in vivo function of Ucn, embryonic stem cell technology was used to generate mice with a targeted mutation in the Ucn gene (Fig. 1a). The targeting vector replaced the entire coding exon of Ucn with an EGFP-LacZ fusion reporter and a PGKneo selection cassette. The targeted allele is null and was designed to serve as a reporter of Ucn gene expression. Intercrosses of Ucn+/− mice generated homozygous mutants at the expected Mendelian ratio. Ucn−/− mice were fully viable and fertile and had normal body weights. In situ hybridization analyses on brain sections confirmed that there was no Ucn mRNA expression in the region of the EW nucleus of homozygous mutant mice (Fig. 1d and e), while similar levels of Crh mRNA expression were observed in the paraventricular nucleus (Fig. 1e). In the Ucn+/− and Ucn−/− mice (Fig. 1c), we detected β-galactosidase activity of the reporter allele in the region of the EW nucleus.
FIG. 1.
Targeted disruption of the Ucn gene. (a) Mouse Ucn gene and targeting vector. The entire coding sequence of exon 2 was deleted by homologous recombination and replaced with an EGFP-LacZ reporter and floxed PGKneo cassette. Triangles, loxP sites. (b) PCR analysis of genomic DNA from wild-type (Wt), heterozygous, and homozygous mutant (Mut) mice. (c) Expression of the targeted reporter allele. X-Gal staining of Vibrotome brain sections containing the EW nuclei from Ucn−/− mice are shown. (d) 35S-labeled Ucn riboprobe in situ hybridization of serial coronal brain sections showing that Ucn is primarily in the EW nuclei. (e) In situ hybridization for Ucn in EW nuclei (top) and for Crh in paraventricular nuclei (bottom). Silver grain autoradiograph images merged with Hoechst 33258 DNA staining are shown. Note that the Ucn signal is absent in the Ucn−/− mice and that Crh was similarly expressed in mice with both genotypes.
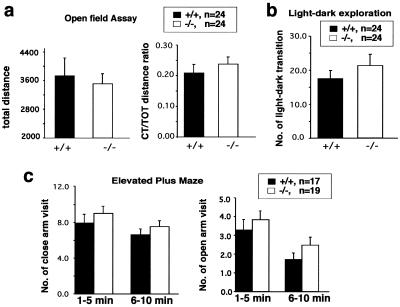
Administration of Ucn has been shown to trigger anxiety-like behaviors (18, 26). To determine the role of Ucn in mediating the stress response, we examined Ucn−/− mice for anxiety responses using the open-field, light-dark exploration, and elevated plus maze tests. In the open-field assay, Ucn−/− and wild-type mice showed similar levels of locomotor activity (P > 0.05) as measured by total distance traveled and similar anxiety-related behavior (P > 0.05) as measured by center/total distance ratio (Fig. 2a). There was no significant difference between Ucn−/− and Ucn+/+ mice in the light-dark exploration assay or the elevated plus maze assay (P > 0.05) (Fig. 2b and c). We found no significant interactions between genotype and gender in the open-field, light-dark exploration and elevated plus maze assays (P > 0.05 in each case). Thus no significant difference between genotypes was detected in the three anxiety-related behavioral tests (Fig. 2).
FIG. 2.
Normal anxiety-like behavior of Ucn−/− mice. (a). Ucn−/− and wild-type mice show no difference in their basal locomotor activities (total distance; F1,44 = 0.285, P = 0.596) and anxiety response (center/total [CT/TOT] distance ratio; F1,44 = 0.674, P = 0.416) in an open-field test. (b) In light-dark exploration, Ucn−/− and wild-type mice show similar numbers of transitions between the light and dark chambers (F1,43 = 0.772, P = 0.384). (c) Ucn−/− mice show no significant difference from control mice in the elevated plus maze assay (P > 0.132 in each case).
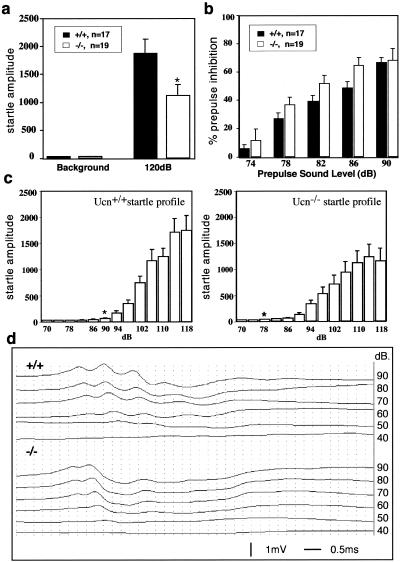
Startle is a fast reflexive response to a sudden and intense acoustic, tactile, or visual stimulus. Administration of Crh but not Ucn increases the ASR in rats (6). This effect is believed to be mediated through Crhr1 because it can be blocked by an antagonist of Crhr1 (12). However, Crh-deficient mice exhibit a normal ASR (32). To address the role of Ucn in the ASR, we examined the startle response in Ucn−/− mice. Surprisingly, we found that Ucn-deficient mice displayed an impaired ASR. The amplitude of the overall startle response to a 120-dB sound stimulus was significantly lower in Ucn−/− mice than in Ucn+/+ mice (Fig. 3a). In addition, there was a significant genotype-gender interaction (P < 0.05). Simple-effect analysis of the genotype-gender interaction indicated that female Ucn−/− mice displayed a reduced startle response that was not significantly different from that of wild-type females (P > 0.05) but that the startle response in male mice was significantly different (P < 0.05) from the startle response in male wild-type mice.
FIG. 3.
Impaired ASR in Ucn−/− mice. (a) Ucn−/− mice display an impaired ASR. The startle amplitude in the Ucn−/− mice is significantly decreased compared to that in wild-type mice (P < 0.05). (b) Ucn−/− and wild-type mice show similar levels of prepulse inhibition of the startle response (P > 0.5 in each case). The level of preinhibition of the ASR obtained with each of the five prepulse stimuli is presented for both Ucn+/+ and Ucn−/− mice. (c) Different sensitivities of the ASR in Ucn−/− and Ucn+/+ mice. Amplitudes of response to various sound levels by mice with both genotypes are shown. Note that although Ucn−/− mice had a significantly dampened response, they were actually more sensitive to low sound levels than wild-type mice. ∗, threshold value of the startle response for mice with each genotype. (d) Hearing assessment based on ABR in Ucn+/+ and Ucn−/− mice. There were no statistically significant differences in thresholds and interpeak latencies in response to click stimuli ranging from 40 to 90 dB.
Since the ASR measures the movement response to a sudden and intense sound, it is possible that the impaired response may be caused by a hearing defect. However, Ucn−/− and Ucn+/+ mice displayed similar levels of prepulse inhibition (PPI) of the ASR, even at low levels of sound stimuli (Fig. 3b). There was no genotype-gender interaction for the PPI data.
Finally, a startle characterization curve was generated to determine the threshold sound level necessary to elicit a significant startle response. The startle threshold, defined as the stimulus level which produces a significantly higher response (P < 0.05) than the baseline response measured at 70 dB, for Ucn−/− mice was 78 dB; for wild-type mice it was 90 dB. Consistent with the threshold data, results from the ANOVA revealed a significant genotype-sound level interaction (P = 0.004). Post hoc analyses revealed that the startle response of Ucn −/− mice was actually higher than that of wild-type mice at several of the low sound levels (i.e., 78, 82, 86, and 90 dB; P < 0.05 for each). In contrast to what was shown by the startle data obtained during the PPI test, there was no genotype-gender interaction in the startle threshold experiment. The data from the startle curve confirmed that, although Ucn−/− mice have a lower maximum startle response, they are more sensitive at low sound levels (Fig. 3c).
The ASR is mediated by a neuronal circuit that consists of the auditory nerve, the ventral cochlear nucleus, the dorsal nucleus of the lateral lemniscus, the caudal pontine nucleus, and spinal interneurons and motor neurons (5). The amplitude and sensitivity of the ASR can be modulated by a number of physiological and pathological conditions (see reference 10 for a review). The impaired ASR observed in Ucn−/− mice cannot be explained by a hearing defect since Ucn−/− mice were more sensitive than wild-type mice to low levels of sound stimuli. It has been reported in rats that Ucn is expressed in the lateral superior olivary nucleus (2), a region involved in auditory sensory function. We were unable to confirm this either by in situ hybridization or by β-galactosidase staining of the reporter allele. Furthermore, we compared the hearing ability of wild-type mice to that of mutant mice by the ABR test. We observed no significant difference between wild-type and mutant mice in their multiwave response and interpeak latency at the range of 70- to 90-dB sound stimuli (Fig. 3d). Although we cannot rule out the possibility that there are other hearing defects in the Ucn-deficient mice, we favor the interpretation that the impaired ASR is not due to a hearing defect. The simplest explanation of the ASR data may come from the observation that in rats Ucn fibers descending from EW nucleus are found in the brain stem pontine reticular nucleus (2), a key element of the primary ASR (3, 5, 11, 35). We propose that Ucn may modulate the startle response through its projections to the pontine reticular nucleus. Keep in mind that the projections observed by retrograde labeling of Ucn fibers have been reported only in rats. The neuronal basis for the ASR defect in Ucn−/− mice remains to be determined experimentally.
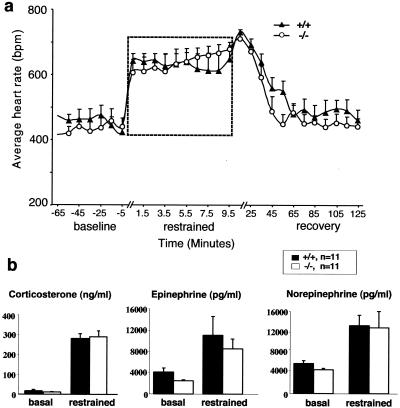
Ucn is predominantly expressed around the EW nucleus in the midbrain (31) (Fig. 1c to e). The projections from this nucleus, including Ucn-expressing neuron fibers, have been found in the various Crhr1-expressing nuclei, some of which are located in the regions of the brain stem and spinal cord (2, 9, 30). Stress (induced by physical restraint) has been shown to increase Ucn expression in the EW nucleus, suggesting that Ucn might play a role in stress-induced autonomic control (33). We assessed the autonomic response by monitoring the heart rate in free-running animals using telemetric radio transmitters. The heart rates, measured in both Ucn+/+ and Ucn−/− mice, showed no statistically significant differences at baseline, during physical restraint and during recovery (Fig. 4a). Consistent with the telemetry data, stress-induced endocrine responses, as indicated by the plasma corticosterone, epinephrine, and norepinephrine levels (Fig. 4b), in the Ucn−/− and Ucn+/+ mice did not differ. Finally, we detected similar increases (8 to 10%) in heart rates in both Ucn+/+ and Ucn−/− mice in response to single housing (data not shown). These results suggest that Ucn does not play a significant role in stress-induced autonomic control.
FIG. 4.
Normal stress-induced autonomic and endocrine responses in Ucn−/− mice. (a) Average heart rate is plotted as a function of time during 1 h before physical restraint, during 10 min of restraint, and during 2 h of recovery. The period of physical restraint (dashed box) is enlarged (note the change of scale on the x axis). Ucn−/− and control mice show no significant difference in the restraint test (P > 0.05 in each case). Significance was assessed by two-way ANOVA (genotype and time). bpm, beats per minute. (b) Ucn−/− and Ucn+/+ mice show similar plasma corticosterone, norepinephrine, and epinephrine levels under baseline and stressed conditions (P > 0.05 in each case).
In summary, Ucn-deficient mice exhibit normal stress-induced responses, including anxiety-like behavior, autonomic control, and endocrine secretion. Ucn-deficient mice have a reduced startle response to loud sounds, though they appear to be more sensitive to low-level sounds. The startle threshold for wild-type mice was 90 dB, consistent with previous observations (19), but the threshold for Ucn−/− mice was 78 dB. In addition, the Ucn-deficient mice exhibit a more pronounced response to low sound levels than wild-type mice. Future studies will be necessary to identify and understand the mechanism for this apparent paradox in startle response.
In contrast to what was found for mice deficient for receptors Crhr1 and Crhr2, neither Crh nor Ucn alone plays an essential role in the stress-induced behavioral responses. It is formally possible that Crh and Ucn are redundant with regard to their functions in stress-mediated responses. More interestingly, our studies suggest that, besides Crh and Ucn, one or more Crh-like peptides that act on Crh receptors to mediate stress-induced behavioral and autonomic control exist. For such actions, Ucn-related candidates Ucn II and III have recently been identified (13, 25). Continued analysis of these Ucn-related proteins may further clarify the key processes by which anxiety is regulated.
Acknowledgments
We thank S. Rivera and J. Wesley for technical assistance and J. A. Bouwknecht for advice on statistical analysis.
This work was supported by the Baylor Mental Retardation Research Center and grants from the NIH to A.B., M.D.B., and W.E.B.
X. Wang and H. Su contributed equally to this work.
REFERENCES
- 1.Bale, T. L., A. Contarino, G. W. Smith, R. Chan, L. H. Gold, P. E. Sawchenko, G. F. Koob, W. W. Vale, and K. F. Lee. 2000. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypersensitive to stress. Nat. Genet. 24:410-414. [DOI] [PubMed] [Google Scholar]
- 2.Bittencourt, J. C., J. Vaughan, C. Arias, R. A. Rissman, W. W. Vale, and P. E. Sawchenko. 1999. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J. Comp. Neurol. 415:285-312. [PubMed] [Google Scholar]
- 3.Carlson, S., and J. F. Willott. 1998. Caudal pontine reticular formation of C57BL/6J mice: responses to startle stimuli, inhibition by tones, and plasticity. J. Neurophysiol. 79:2603-2614. [DOI] [PubMed] [Google Scholar]
- 4.Coste, S. C., R. A. Kesterson, K. A. Heldwein, S. L. Stevens, A. D. Heard, J. H. Hollis, S. E. Murray, J. K. Hill, G. A. Pantely, A. R. Hohimer, D. C. Hatton, T. J. Phillips, D. A. Finn, M. J. Low, M. B. Rittenberg, P. Stenzel, and M. P. Stenzel-Poore. 2000. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 24:403-409. [DOI] [PubMed] [Google Scholar]
- 5.Davis, M., D. S. Gendelman, M. D. Tischler, and P. M. Gendelman. 1982. A primary acoustic startle circuit: lesion and stimulation studies. J. Neurosci. 2:791-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones, D. N., R. Kortekaas, P. D. Slade, D. N. Middlemiss, and J. J. Hagan. 1998. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology 138:124-132. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto, T., R. V. Pearse, C. R. Lin, and M. G. Rosenfeld. 1995. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc. Natl. Acad. Sci. USA 92:1108-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishimoto, T., J. Radulovic, M. Radulovic, C. R. Lin, C. Schrick, F. Hooshmand, O. Hermanson, M. G. Rosenfeld, and J. Spiess. 2000. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 24:415-419. [DOI] [PubMed] [Google Scholar]
- 9.Klooster, J., H. J. Beckers, G. F. Vrensen, and J. J. van der Want. 1993. The peripheral and central projections of the Edinger-Westphal nucleus in the rat. A light and electron microscopic tracing study. Brain Res. 632:260-273. [DOI] [PubMed] [Google Scholar]
- 10.Koch, M. 1999. The neurobiology of startle. Prog. Neurobiol. 59:107-128. [DOI] [PubMed] [Google Scholar]
- 11.Koch, M., K. Lingenhohl, and P. K. Pilz. 1992. Loss of the acoustic startle response following neurotoxic lesions of the caudal pontine reticular formation: possible role of giant neurons. Neuroscience 49:617-625. [DOI] [PubMed] [Google Scholar]
- 12.Koob, G. F., and S. C. Heinrichs. 1999. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 848:141-152. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, K., C. Li, M. H. Perrin, A. Blount, K. Kunitake, C. Donaldson, J. Vaughan, T. M. Reyes, J. Gulyas, W. Fischer, L. Bilezikjian, J. Rivier, P. E. Sawchenko, and W. W. Vale. 2001. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 98:7570-7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lister, R. G. 1987. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92:180-185. [DOI] [PubMed] [Google Scholar]
- 15.Liu, M., F. A. Pereira, S. D. Price, M. Chu, C. Shope, D. Himes, R. A. Eatock, W. E. Brownell, A. Lysakowski, and M. J. Tsai. 2000. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 14:2839-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovenberg, T. W., C. W. Liaw, D. E. Grigoriadis, W. Clevenger, D. T. Chalmers, E. B. De Souza, and T. Oltersdorf. 1995. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA 92:836-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills, P. A., D. A. Huetteman, B. P. Brockway, L. M. Zwiers, A. J. Gelsema, R. S. Schwartz, and K. Kramer. 2000. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J. Appl. Physiol. 88:1537-1544. [DOI] [PubMed] [Google Scholar]
- 18.Moreau, J. L., G. Kilpatrick, and F. Jenck. 1997. Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport 8:1697-1701. [DOI] [PubMed] [Google Scholar]
- 19.Paylor, R., and J. N. Crawley. 1997. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 132:169-180. [DOI] [PubMed] [Google Scholar]
- 20.Paylor, R., M. Nguyen, J. N. Crawley, J. Patrick, A. Beaudet, and A. Orr-Urtreger. 1998. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn. Mem. 5:302-316. [PMC free article] [PubMed] [Google Scholar]
- 21.Pellow, S., P. Chopin, S. E. File, and M. Briley. 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14:149-167. [DOI] [PubMed] [Google Scholar]
- 22.Perrin, M., C. Donaldson, R. Chen, A. Blount, T. Berggren, L. Bilezikjian, P. Sawchenko, and W. Vale. 1995. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. USA 92:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peuler, J. D., and G. A. Johnson. 1977. Simultaneous single isotope radioenzymatic assay of plasma epinephrine, norepinephrine, and dopamine. Life Sci. 21:625-636. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez-Solis, R., A. C. Davis, and A. Bradley. 1993. Gene targeting in embryonic stem cells. Methods Enzymol. 225:855-878. [DOI] [PubMed] [Google Scholar]
- 25.Reyes, T. M., K. Lewis, M. H. Perrin, K. S. Kunitake, J. Vaughan, C. A. Arias, J. B. Hogenesch, J. Gulyas, J. Rivier, W. W. Vale, and P. E. Sawchenko. 2001. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 98:2843-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skelton, K. H., M. J. Owens, and C. B. Nemeroff. 2000. The neurobiology of urocortin. Regul. Pept. 93:85-92. [DOI] [PubMed] [Google Scholar]
- 27.Smith, G. W., J. M. Aubry, F. Dellu, A. Contarino, L. M. Bilezikjian, L. H. Gold, R. Chen, Y. Marchuk, C. Hauser, C. A. Bentley, P. E. Sawchenko, G. F. Koob, W. Vale, and K. F. Lee. 1998. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20:1093-1102. [DOI] [PubMed] [Google Scholar]
- 28.Stenzel, P., R. Kesterson, W. Yeung, R. D. Cone, M. B. Rittenberg, and M. P. Stenzel-Poore. 1995. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol. Endocrinol. 9:637-645. [DOI] [PubMed] [Google Scholar]
- 29.Timpl, P., R. Spanagel, I. Sillaber, A. Kresse, J. M. Reul, G. K. Stalla, V. Blanquet, T. Steckler, F. Holsboer, and W. Wurst. 1998. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor I. Nat. Genet. 19:162-166. [DOI] [PubMed] [Google Scholar]
- 30.Van Pett, K., V. Viau, J. C. Bittencourt, R. K. Chan, H. Y. Li, C. Arias, G. S. Prins, M. Perrin, W. Vale, and P. E. Sawchenko. 2000. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 428:191-212. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan, J., C. Donaldson, J. Bittencourt, M. H. Perrin, K. Lewis, S. Sutton, R. Chan, A. V. Turnbull, D. Lovejoy, C. Rivier, et al. 1995. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378:287-292. [DOI] [PubMed] [Google Scholar]
- 32.Weninger, S. C., A. J. Dunn, L. J. Muglia, P. Dikkes, K. A. Miczek, A. H. Swiergiel, C. W. Berridge, and J. A. Majzoub. 1999. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc. Natl. Acad. Sci. USA 96:8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weninger, S. C., L. L. Peters, and J. A. Majzoub. 2000. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology 141:256-263. [DOI] [PubMed] [Google Scholar]
- 34.Wong, M. L., A. al-Shekhlee, P. B. Bongiorno, A. Esposito, P. Khatri, E. M. Sternberg, P. W. Gold, and J. Licinio. 1996. Localization of urocortin messenger RNA in rat brain and pituitary. Mol. Psychiatry 1:307-312. [PubMed] [Google Scholar]
- 35.Yeomans, J. S., C. M. Hempel, and C. A. Chapman. 1993. Axons and synapses mediating startle-like responses evoked by electrical stimulation of the reticular formation in rats: symmetric and asymmetric collision effects. Brain Res. 617:309-319. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, L., C. J. Donaldson, G. W. Smith, and W. W. Vale. 1998. The structures of the mouse and human urocortin genes (Ucn and UCN). Genomics 50:23-33. [DOI] [PubMed] [Google Scholar]
- 37.Zheng, B., D. W. Larkin, U. Albrecht, Z. S. Sun, M. Sage, G. Eichele, C. C. Lee, and A. Bradley. 1999. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400:169-173. [DOI] [PubMed] [Google Scholar]
- 38.Zheng, B., A. A. Mills, and A. Bradley. 1999. A system for rapid generation of coat color-tagged knockouts and defined chromosomal rearrangements in mice. Nucleic Acids Res. 27:2354-2360. [DOI] [PMC free article] [PubMed]