Abstract
Objective:
We evaluated the long-term impact of iliofemoral thrombosis (I-FDVT) on walking capacity, venous hemodynamic status, CEAP class, venous clinical severity, and quality of life, and determined the prevalence of venous claudication.
Materials and Methods:
All patients with prior I-FDVT, assessed at our institution since 1990, were called for follow-up. Those with walking impairment due to arterial disease (ABI < 1.0 postexercise) or unrelated causes and those thrombectomized or thrombolyzed were excluded; 39 patients (22–83 years, median 46 years) were included. Median follow-up was 5 years (range 1–23 years). Investigation included classification in CEAP and Venous Clinical Severity Scoring (VCSS) systems, air-plethysmography (outflow fraction [OF], venous filling index [VFI], residual volume fraction [RVF]) and venous duplex, treadmill (3.5 km/h, 10%) to determine initial (ICD) and absolute (ACD) claudication distances, and quality of life assessment (SF-36). Nonaffected limbs of patients with unilateral I-FDVT (37 of 39) comprised the control group. Data are presented as median and interquartile range.
Results:
A total of 81% of limbs with I-FDVT had superficial and deep reflux and 19% superficial reflux; reflux in control limbs was 29.7% (P < 0.001) and 27% (P > 0.2), respectively; 43.6% (17 of 39; 95% CI, 27–60%) of patients developed venous claudication ipsilateral to I-FDVT (ICD: 130 m, range 105–268 m), compelling 15.4% (6 of 39; 95% CI, 3.5–27%) to discontinue treadmill (ACD: 241 m, range 137–298 m). Limbs with prior I-FDVT had a lower OF (37%, range 32.2–43%; P < 0.001), abnormally higher VFI (3.8 mL/s, range 2.5–5.7 mL/s; P < 0.001), and RVF (45%, range 32.5–51.5%; P = 0.006), and clinical impairment in CEAP and VCSS systems (P < 0.0001). Patients with I-FDVT had impaired physical functioning (P = 0.02) and role (P = 0.033), general health (P = 0.001), social function (P = 0.047), and mental health (P = 0.043).
Conclusions:
A total of 43.6% of those with prior I-FDVT developed venous claudication compelling interruption of walking in 15.4%. Prior I-FDVT caused outflow impairment and a large residual venous volume and reflux, resulting in marked clinical and quality of life compromise. Standardized challenge enabled discrimination of those with clinically relevant impairment.
We evaluated the long-term impact of iliofemoral thrombosis (I-F DVT) on walking capacity, venous hemodynamic status, CEAP class, venous clinical severity score, and quality of life. Based on stringent exclusion criteria to eliminate walking impairment due to other causes, we found that 43.6% of those with prior I-F DVT develop venous claudication (ICD: 130 m, range 105–268 m), compelling interruption of walking in 15.4% (ACD: 241 m, range 137–298 m). Limbs with prior I-F DVT have a lower outflow fraction, higher reflux, and residual venous volumes (all P ≤ 0.006), resulting in marked clinical impairment in CEAP and VCSS systems (P < 0.0001), significantly compromising subjects’ perception of their physical functioning and role, general health, social function, and mental health.
The long-term sequelae of deep vein thrombosis (DVT) in the lower limb comprising the post-thrombotic syndrome, generate severe disability,1–6 and a marked compromise in the quality of life7,8 with serious socioeconomic implications.9,10 The associated impairment of venous hemodynamics in the lower extremity is initially the product of venous outflow obstruction11–13 and as this subsides with the natural lysis of thrombi,14–16 venous valvular incompetence develops.17–20 It is the combination of venous valvular incompetence, outflow obstruction, and calf muscle pump function derangement that generates the hemodynamic milieu most often associated with the occurrence of post-thrombotic complications.21,22 Intermittent venous claudication, defined as the presence of thigh/leg pain and tightness on vigorous exercise, which subsides with rest,23–26 mostly associated with iliofemoral venous outflow impairment,23,25–28 is probably the least investigated post-thrombotic symptom. In iliofemoral thrombosis, mainly due to clot propagation,29 trauma,26 pelvic surgery,30,31 cesarean section,26,32 pregnancy,33 insertion of femoral venous catheters34 and immobilization,31 claudication develops as the arterial leg inflow increases during strenuous exercise in the presence of a fixed outflow resistance, determined by the venous collaterals.23,26,28
Monitoring of venous outflow in limbs with venous claudication, using either direct pressure measurements or validated plethysmographic methods, has shown an increase in the peripheral venous pressure during exercise,24,25,27,28,35 the arm-foot pressure differential6,28,35 and attenuation in the proximal venous flow,21,26,35 reflecting a noncompensating outflow resistance.26,28,31,32,35
The CEAP stratification36 and the introduction of the venous clinical severity scoring system (VCSS) in chronic venous disease (CVD)37 make it easier to quantify the long-term clinical impact of iliofemoral thrombosis on the affected extremities, in the presence of well-accepted noninvasive methods for comprehensive venous hemodynamic assessment, such as air-plethysmography (APG).21,38,39
The purpose of this study was to evaluate the long-term impact of iliofemoral thrombosis (I-FDVT) on walking capacity, venous hemodynamic status, CEAP class, venous clinical severity and quality of life, and to determine the prevalence of venous claudication.
MATERIALS AND METHODS
All patients with prior I-FDVT managed conservatively with anticoagulation, who had been treated at our department over the previous 10 years, were invited to the vascular laboratory for noninvasive venous surveillance. Following informed consent, 39 patients, 21 women and 18 men, median age 46 years (range 22–83 years), meeting the inclusion and exclusion criteria listed in Table 1 were investigated. Thirty-seven (95%) of these patients had sustained unilateral I-FDVT and the remaining 2 bilateral. The median time elapsed since the acute I-FDVT episode was 5 (range 1–23) years. The contralateral nonaffected limbs of those with unilateral I-FDVT served as the control group. Seventy-eight limbs were studied overall, 41 with I-FDVT (17 on the right limb and 24 on the left) and 37 without previous thrombosis. The diagnosis of I-FDVT at the time of the acute episode was made using ascending venography (4 limbs; 9.8%), duplex (27 limbs; 65.8%), or both (10 limbs; 24.4%).
TABLE 1. Inclusion and Exclusion Criteria
Examination Protocol
The examination protocol entailed: a detailed history with emphasis on the risk factors for deep vein thrombosis; physical examination including assessment of clinical severity using the CEAP and VCSS classifications; evaluation of the ankle brachial pressure indices (ABPIs); lower limb venous duplex ultrasound and comprehensive air-plethysmography; and a treadmill exercise challenge to determine the walking distances attainable following a 15-minute rest. The short-form health survey questionnaire (SF-36)41–43 was answered on completion of the noninvasive investigations.
Risk Factors
Patients were assessed for the presence of risk factors at the time of the acute episode. These factors, mainly associated with deep vein thrombosis, were as follows: age >60 years (n = 12); past thrombosis (n = 4); surgery performed just prior to the occurrence of I-FDVT (n = 11); thrombophilia (n = 4); malignancy (n = 2); varicose veins (n = 8); hormone replacement therapy or oral contraception (n = 5); and obesity (n = 5). Twelve patients were smokers. Four (10.2%) patients had a history of clinically overt pulmonary embolism requiring an inferior vena cava filter. Thirty-one (80.5%) patients had one or more predisposing factors for DVT in their history at the time of I-FDVT. At the time of our long-term follow-up assessment, 17 (43.6%) patients were on oral anticoagulation treatment with warfarin.
Clinical Examination
On clinical examination, 7 (17.9%) patients had developed overt collateral veins in the pubic area rerouting venous return from the affected iliofemoral side to the contralateral unaffected one. All patients had a full complement of infra-aortic pulses, and there was no clinical evidence of peripheral vascular disease, based on resting and postexercise ABPIs (>1.0). Examination included evaluation of peripheral motor and sensory nerve function, and comprehensive orthopaedic assessment of the spine and lower limb joints. The limbs were stratified according to the CEAP system,36 and quantification of clinical severity was performed using the venous clinical severity score (VCSS) introduced by Rutherford et al.37
Venous Hemodynamics
Duplex scanning was performed using the HP Sonos 2500, fitted with a linear array scan-head (7.5/5.0 MHz), as previously described.44,45 Quantification of the venous hemodynamics was performed using APG (ACI-Medical, Inc., Sun Valley, CA) bilaterally. The hemodynamic parameters to follow were measured as previously described:46,47 venous volume (VV) in mL, venous filling index (VFI) in mL/s, ejection fraction (EF) in %, residual volume fraction (RVF) in %, and outflow fraction (OF) in %.
Treadmill Exercise Challenge
Treadmill exercise was carried out on a Powerjog M10, (Sport Engineering Ltd, Birmingham, UK). Following preliminary familiarization with the treadmill equipment, patients were asked to walk at a set speed of 3.5 km/h and 10% inclination for 1 minute. Timed-evaluation of the postexercise ABPIs was then conducted using a pair of calibrated sphygmomanometers and a sensitive biphasic Doppler (Dyna D 800, AHS, Paris; 1 MHz). After 15 minutes rest, patients were asked to walk for a maximum time period of 10 minutes, approximately 600 m, at a speed of 3.5 km/h and 10% inclination. The distance at which patients first experienced persistent symptoms consistent with venous claudication was noted as the initial claudication distance (ICD) of the affected limb. The distance at which the severity of claudication compelled discontinuation of the treadmill challenge was designated the absolute claudication distance (ACD). Venous claudication was defined as a persistent and uncomfortable tightness, ache, or pain in the thigh or calf which presented during the treadmill challenge, and worsened with exercise time.23–26 Patients who had to give up the treadmill challenge, due to shortness of breath or unrelated causes were excluded from the claudication distance analysis.
Quality of Life
The Short-Form health survey questionnaire (SF-36)41,42 was used in the assessment of the impact of I-FDVT on the quality of life. Analysis and interpretation were conducted according to the SF-36 manual.42,43 In all eight parameters evaluated, the performance of each study patient with I-FDVT was matched and compared with the mean performance (norms) of healthy subjects adjusted for age and sex.42
Statistical Analysis
Analysis of unrelated quantitative data of three or more groups of patients was performed using the nonparametric Kruskal-Wallis test, following a Kolmogorov-Smirnov distribution test; comparison of two sets of unrelated data was performed using the Mann-Whitney U test. Two sets of paired data were compared with the Wilcoxon test. The Bonferroni correction was applied when required. Point estimates and 95% confidence intervals of the differences (95% CI) are provided. Univariate linear regression analysis (Spearman’s rank test) was performed to establish associations. Differences in proportions were evaluated using the χ2 test. Data are presented as median and interquartile ranges. A P value of <5%, was considered as statistically significant, unless otherwise stated.
RESULTS
Venous Claudication (Treadmill Exercise)
Seventeen patients (17 of 39; 43.6%; 95% CI, 27–60%) developed venous claudication in the limb with prior I-FDVT during treadmill exercise at an ICD of 130 m (range 105–268 m). The severity of claudication compelled 6 patients (6 of 39; 15.4%; 95% CI, 3.5–27%) to discontinue the treadmill at an ACD of 241 m (range 137–298 m). None of the control limbs developed claudication within the investigated maximum walking distance (600 m; P < 0.001) (Fig. 1).
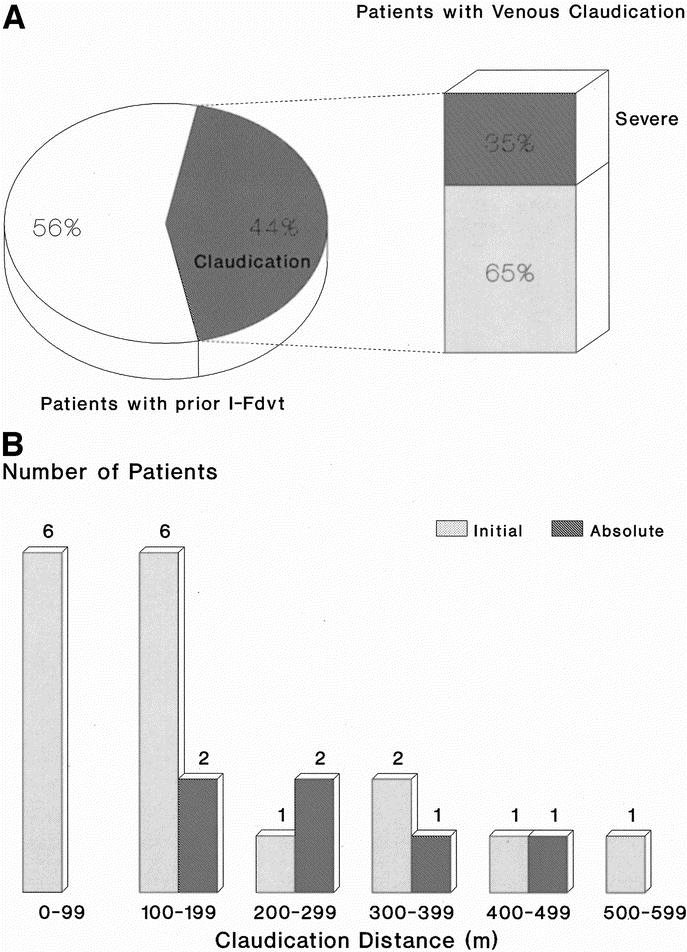
FIGURE 1. A: The prevalence of intermittent venous claudication among 39 patients with prior I-FDVT, after a median 5-year (range 1–23 years) follow-up period; 43.6% (17 of 39) developed intermittent claudication at a median initial claudication distance of 130 m (interquartile range 105–268 m), the severity of which compelled 6 of them [35% (6 of 17)] or 15.4% (6 of 39) of the total to discontinue the treadmill at a median absolute claudication distance of 241 m (interquartile range 137–298 m). B: Distribution of initial and absolute claudication distances among the 17 patients of our study population with prior iliofemoral thrombosis and intermittent venous claudication.
Hemodynamic Evaluation
Of the limbs with prior I-FDVT, 81% had venous reflux in both superficial and deep systems; reflux was confined to superficial system alone in 19%. In control limbs, reflux in both superficial and deep systems was present in 29.7% (P < 0.001), and in superficial system only in 27% (P > 0.2). The substandard OF (37%, range 32.2–43%) in limbs with prior I-FDVT was significantly lower (P < 0.001) than control limbs (49%, range 44–52%). VFI of limbs with prior I-FDVT was abnormal (3.8 mL/s, range 2.5–5.7 ml/s) and significantly higher (P < 0.001) than control limbs (1.6 mL/s, range 1.4–1.9 ml/s); similarly, RVF (45%, range 32.5–51.5%) was abnormal and higher (P = 0.006) than control limbs (35%, range 24–40%). EF and VV were similar in the two limb groups (P = 0.13 and P = 0.27, respectively) (Figs. 2,3, and 4).
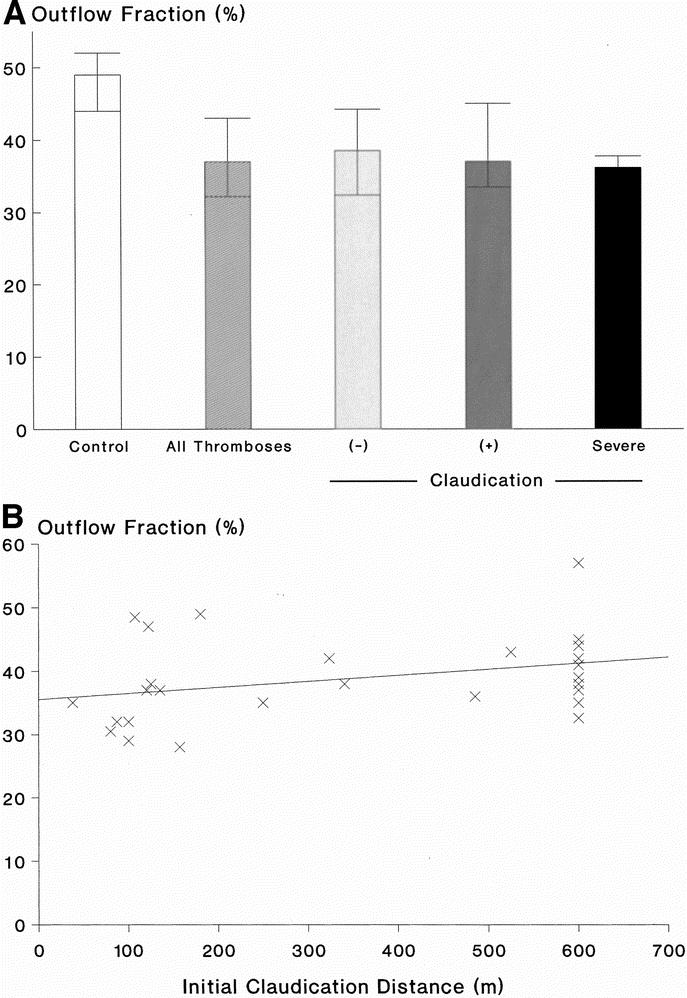
FIGURE 2. A: Outflow fraction [OF] (%) in 37 control limbs and 41 limbs with prior I-FDVT (P < 0.001), on the left. On the right, the limbs with prior I-FDVT are subdivided into those with (+) and without (–) claudication, and those with severe claudication compelling cessation of walking; differences among the three subgroups were not significant (P = 0.58). Data are expressed as median and interquartile ranges. B: Outflow fraction (%) in limbs with prior I-FDVT plotted against the initial claudication distance (m) (r = 0.43, P = 0.018, Spearman’s two-tailed). Patients with shortness of breath were excluded.
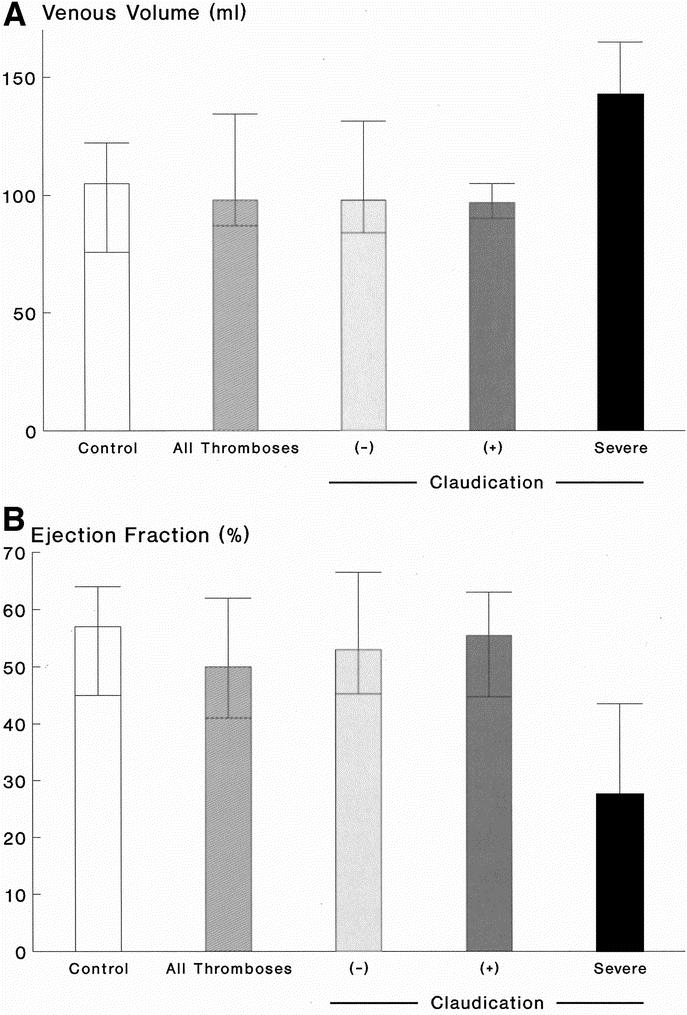
FIGURE 3. A: Venous volume [VV] (mL) in 37 control limbs and 41 limbs with prior I-FDVT (P = 0.27), on the left. On the right, the limbs with prior I-FDVT are subdivided into those with (+) and without (–) claudication, and those with severe claudication compelling cessation of walking; differences among the three subgroups were not significant (P = 0.58). Data are expressed as median and interquartile ranges. B: Ejection fraction [EF] (%) in 37 control limbs and 41 limbs with prior I-FDVT (P = 0.13), on the left. On the right, the limbs with I-FDVT are subdivided into those with (+) and without (–) claudication, and those with severe claudication compelling cessation of walking; differences among the three subgroups were significant overall (P = 0.014) [(–) vs. severe, P = 0.003; (+) vs. severe, P = 0.016]. Data are expressed as median and interquartile ranges.
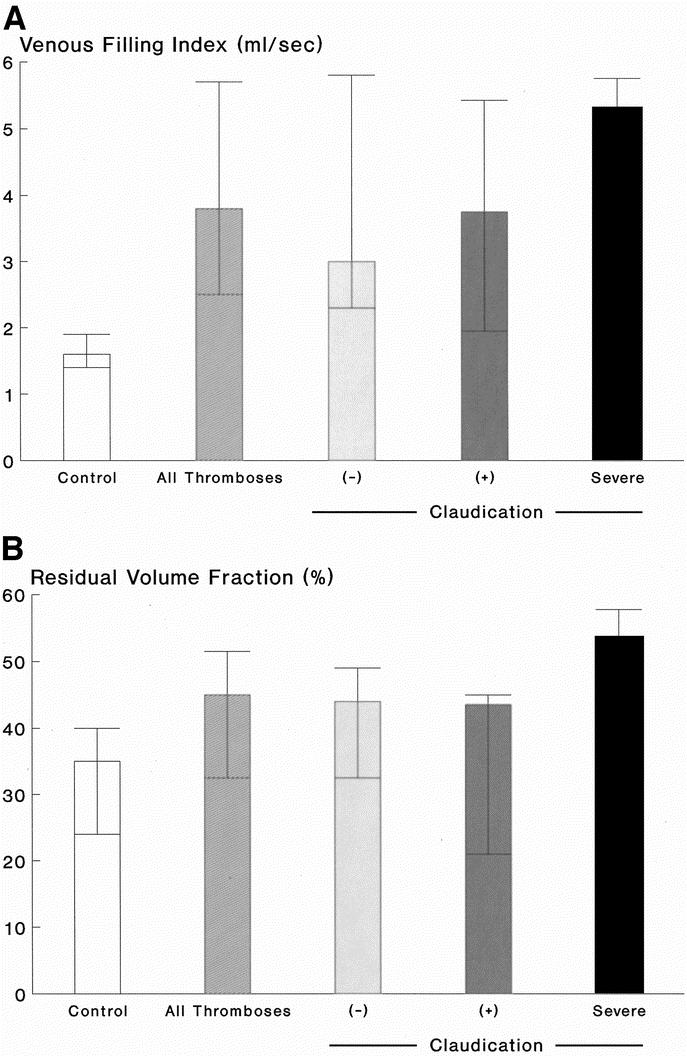
FIGURE 4. A: Venous filling index [VFI] (mL/s) in 37 control limbs and 41 limbs with prior I-FDVT (P < 0.001), on the left. On the right, the limbs with prior I-FDVT are subdivided into those with (+) and without (–) claudication, and those with severe claudication compelling cessation of walking; differences among the three subgroups were not significant overall (P = 0.22) [(–) vs. severe, P = 0.09; (+) vs. severe, P = 0.18]. Data are expressed as median and interquartile ranges. B: Residual volume fraction [RVF] (%) in 37 control limbs and 41 limbs with prior I-FDVT (P = 0.006), on the left. On the right, the limbs with prior I-FDVT are subdivided into those with (+) and without (–) claudication, and those with severe claudication compelling cessation of walking; differences among the three subgroups were significant overall (P = 0.05) [(–) vs. severe, P = 0.03; (+) vs. severe, P = 0.03]. Data are expressed as median and interquartile ranges.
Clinical Assessment
Compared with the control limbs, those with prior I-FDVT had a significant clinical impairment confirmed using both the CEAP and VCSS systems. The median CEAP stratification of the limbs with prior I-FDVT was 3 classes (95% CI, 2–3 classes) higher (P < 0.0001) than the control limbs. Similarly, limbs with prior I-FDVT had a higher severity score (P < 0.0001) on the VCSS scale compared with the control limbs with a point estimate of 5 points (95% CI, 3–6 points) (Fig. 5).
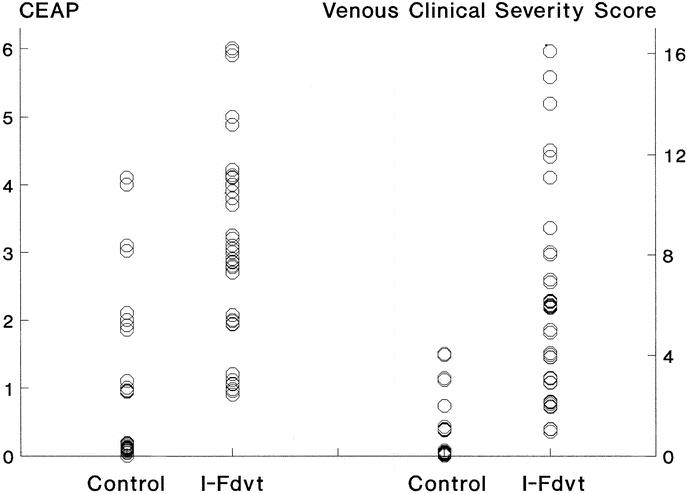
FIGURE 5. Clinical stratification of 41 limbs with prior I-FDVT and 37 control limbs according to the CEAP36 and the VCSS (venous clinical severity scoring)37 systems. Limbs with prior I-FDVT were significantly worse than the control limbs in both systems (both P < 0.0001).
Risk Factors
Correlational analysis of the risk factors for DVT, including age, obesity, malignancy, varicose veins, thrombophilia, surgery preceding the onset of I-FDVT, contraceptive pills or hormone replacement therapy, and history of congestive cardiac failure, expressed as a cumulative number per subject evaluated, failed to yield statistical significance in relation to the CEAP class (r = 0.049), VCSS grade (r = −0.029), ACD (r = −0.09), VFI (r = −0.034), RVF (r = 0.088), and OF (r = −0.16) (Spearman’s rank test).
Quality of Life (SF-36)
Quality of life in patients with prior I-FDVT was significantly impaired in 5 of the 8 parameters assessed using the SF-36. Physical functioning (P = 0.02), physical role (P = 0.033), general health (P = 0.001), social function (P = 0.047), and mental health (P = 0.043) were all quantified as worse than those of healthy subjects adjusted for age and sex. Role emotional, vitality, and bodily pain were not significantly affected (all, P ≥ 0.085) in our sample volume (Fig. 6).
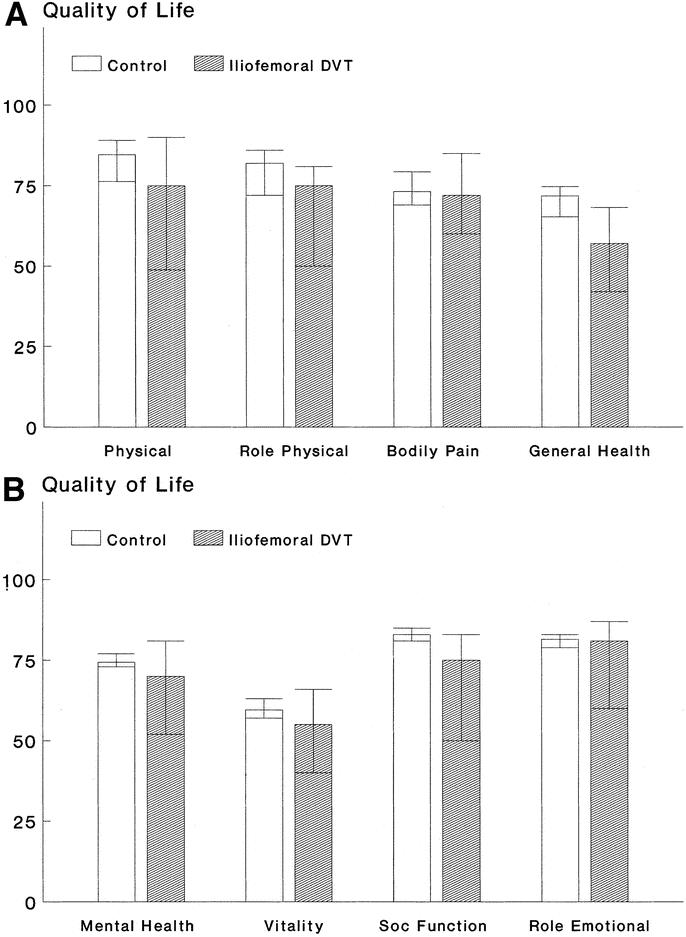
FIGURE 6. Quality of life assessment of 39 patients with prior I-FDVT according to the Short-Form 36 health survey questionnaire, in comparison with an equal number of healthy individuals matched for age and sex. Patients with prior I-FDVT had a significantly worse perception of their physical function (P = 0.02), physical role (P = 0.033), general health (P = 0.001) [a], social function (P = 0.047), and mental health (P = 0.043) [b]; there was no difference in the bodily pain (P = 0.57) [a], vitality (P = 0.085), and role emotional (P = 0.4).
DISCUSSION
Over 3 decades since the entity of venous claudication was first acknowledged, its prevalence and clinical significance among patients with iliofemoral thrombosis remain undetermined. Among 20 patients with I-FDVT treated with conventional anticoagulation and followed-up for 5 years, Akesson et al48 identified three patients with venous claudication (15%). In the largest series on chronic venous obstruction consisting of 385 limbs, of which 137 (28%) were further investigated, Raju and Fredericks28 found 44 (11.4%) with severe hemodynamic compromise (Stages III-IV); however, claudication rates were not provided. Venous claudication was reported by Labropoulos et al12 to occur in 8% of patients (n = 70) with chronic venous dysfunction due to past DVT; claudication affected only those (n = 30) with prior I-FDVT; however, the method of its elicitation was not clarified. Recently, Neglen et al6 reported the presence of pain in 94% of the post-thrombotic limbs (n = 78) on which they performed endovascular iliac vein reconstruction to relieve outflow obstruction; in 27 of these limbs (35%), the pain was assessed as >4 on a 0–10 analogue scale (maximum 10). However, whether this was true venous claudication or constant pain due to post-thrombotic complicated CVD was not elucidated. Killewich et al26 identified only seven patients with venous claudication in their extensive practice over a 3-year span, without disclosing information on its prevalence; claudication was evaluated using a treadmill exercise challenge, which varied both in speed and inclination from subject to subject. Isolated cases have been presented by others.24,25,32,49
Venous claudication and its severity among patients with a confirmed prior I-FDVT were evaluated in the current study using a standardized treadmill exercise challenge, similar to that used for the investigation of peripheral vascular disease. Seventeen of these patients (43.6%, 17 of 39) were found to suffer from venous claudication. Claudication set in after a median distance of 130 m (range 105–268 m) on the treadmill at a speed of 3.5 km/h with a moderate inclination (10%). Of these patients, 9 (23%, 9 of 39) could complete a 10-minute walking challenge (600 m) without a noticeable deterioration of their symptoms; a further 2 (5.1%, 2 of 39) had to stop before the scheduled completion of the treadmill challenge due to either physical exhaustion or worsening shortness of breath, their claudication being of nonlimiting severity; in 6 claudicants (15.4%, 6 of 39), symptoms would gradually escalate forcing them to call for the termination of the treadmill at a median distance of 241 m (range 137–298 m). The remainder of the investigated patients with prior I-FDVT (n = 22 of 39) were either entirely free of venous claudication during the treadmill challenge (36%, 14 of 39) or had to quit the challenge earlier due to either physical exhaustion or worsening breathlessness (20.5%, 8 of 39).
The (43.6%) incidence of venous claudication among patients with prior I-FDVT in our study indicates that its clinical significance has been unequivocally underestimated and almost certainly undermanaged. The inevitable bias introduced by the consideration of only those who had survived the physical challenge of time, and the exclusion of the less fit of patients who were unable to withstand the stress of treadmill exercise, suggests that the incidence of claudication may in effect be of even greater proportions than that yielded in our study population. In addition, as both recanalization and collateralization are promoted with time-distance from the onset of I-FDVT,13,14,50 our findings emanating from a median 5-year follow-up probably represent a better venous outflow impairment than that of patients in closer time-proximity to their thrombotic event.
Determination of venous outflow using occlusive-APG showed that limbs with prior I-FDVT, despite the years elapsed since its occurrence allowing for a natural flow compensation, continued to show a significant impairment in comparison with the control group, comprised of the “contralateral” nonaffected limbs. Except for three subjects, venous claudication developed in limbs with substandard (12 of 17, 70.6%) or borderline (2 of 17, 11.6%) outflow capacity, and the latter measured as OF (%) correlated significantly (P = 0.018) with the initial claudication distances. Occlusive APG was chosen for venous outflow quantification due to its noninvasiveness, reproducibility, and validation in correlation with direct venous pressure measurements and the arm-foot vein pressure differential.21,51 There is no gold standard currently to detect and determine the degree of outflow obstruction.6 Even the invasive procedures, including arm/foot pressure differential, reactive hyperaemia pressure increase, and indirect resistance calculations, are insensitive and do not define the level of obstruction.6,12,35 Our findings on the impairment of venous outflow in limbs with prior iliofemoral thrombosis confirm previous data by others.4,12,21,26,35,40,48 Higher outflow or minimal impairment has been reported by Tripolitis et al32 and Janssen et al,50 respectively.
The current study confirms previous reports demonstrating an abnormally large amount of reflux in follow-up assessment of limbs with prior I-FDVT;4,6,12,26,35,48 the median VFI in these limbs was 90% higher than the upper normal limit. In the presence of outflow obstruction and valvular incompetence, the abnormally elevated RVF in the limbs with prior I-FDVT was not an unexpected finding, reflecting the development of venous hypertension. The elevation of RVF, which is well correlated with the ambulatory venous pressure in chronic venous disease,21,46 is also in agreement with previous reports4,6,12,24–26,28,35, although the ambulatory venous pressure may well not increase as in limbs with severe (grade IV) obstruction.28
In the long-term, venous hypertension has been reported to cause morphologic damage to the skeletal muscle manifested by atrophy, denervation, and myopathy52 and mediated probably by an inflammatory response.53,54 A significant impairment in the calf muscle pump among limbs with prior I-FDVT48 and complicated CVD (CEAP4–6)50 has been previously reported; foot volumetry and a supine venous pump function test were used, the latter being reported55 to correlate well with direct venous pressure measurements. The calf muscle pump function in our series was not found to be significantly impaired overall (P = 0.13) in limbs with prior I-FDVT, although the median EF, reflecting the ability of the exercising muscle to eject the calf volume up in the thigh, was attenuated by 12% in comparison with the control group. However, in the presence of severe venous claudication compelling cessation of walking, EF was attenuated by >50% compared with the control limbs, or the remaining limbs with prior I-FDVT (P = 0.014).
The hostile venous hemodynamic environment in limbs afflicted by I-FDVT, generated mainly by a combination of venous outflow obstruction, the large amount of venous reflux, and small attenuation in calf muscle pump function, was reflected in the considerable likelihood (34.2%, 14 of 41) of skin changes or ulceration, which was over 6 times higher (P < 0.01) than that (5.4%, 2 of 37) among the control group. The clinical consequences of the venous hemodynamic derangements associated with I-FDVT were demonstrable using two clinical classification systems. In the CEAP system,36 the limbs with prior I-FDVT were on average three clinical classes worse (95% CI, 2–3 classes) than the control ones; the relative distribution of limbs in the CEAP0–2 and CEAP3–6, classes was 29.3% and 70.7% in the I-FDVT group, and 86.5% and 13.5% in the control group, respectively (P < 0.001). The clinical discrepancy between the two groups was also confirmed using the VCSS system, introduced in 2000 to complement the CEAP and to enable quantification and grading of elements that could change in response to therapy or disease.37 VCSS is quantified depending on the presence or absence of pain; varicose veins; venous edema; skin pigmentation, inflammation, or induration; the use of compression therapy; and the number, duration and size of active ulcers; all stratified on four different levels (0–3; maximum 30). Limbs with prior I-FDVT had a median VCSS score of 6 points (range 1–16), significantly worse than the control ones, most of which scored nil. The significant correlation noted between the grades of the two systems (r = 0.84, P < 0.001) is in support of their complementary application.37
Analysis of the risk factors for DVT, present among the subjects with prior I-FDVT at the time they sustained thrombosis, and expressed as a cumulative number, failed to identify a significant correlation with the clinical status of CVD according to CEAP, the clinical severity in the VCSS system, walking ability (ACD), and global venous hemodynamics (VFI, RVF, OF). A higher propensity to developing DVT, attached to these factors, probably constitutes only a part of the different variables determining the extent and sites of thrombosis or the severity of outflow obstruction and valvular incompetence, which define the hemodynamic impairment leading to CVD.
The presence of venous claudication and the clinical severity of CVD in the affected limbs were evidently reflected in the quality of life of these patients. Prior I-FDVT resulted in a significant impairment of 5 of the 8 parameters assessed using the short form health survey questionnaire (SF-36).41–43 In comparison with the norms of healthy subjects, adjusted for age and sex, patients with prior I-FDVT believed that their physical functioning and role were compromised, perceived their general health as being substandard, and felt that both their social function and mental health were worse than previously. In all these respects, the performance of patients with prior I-FDVT parallels that of arteriopaths suffering from intermittent claudication.56,57 The questionnaire was completed by the entire group; yet more than half did not complain of claudication, leading to the assumption that those with venous claudication may have a quality of life impairment exceeding that of arterial claudicants, probably due to the cumulative effects of outflow impairment and venous insufficiency in post-thrombotic limbs.
CONCLUSION
The current study has shown that 43.6% (17 of 39; 95% CI, 27–60%) of limbs with prior I-FDVT suffered from venous claudication, compelling early cessation of walking in 15.4% (6 of 39; 95% CI, 3.5–27%). Five years on average after the acute event was sustained, limbs with prior I-FDVT had overall impaired venous outflow, abnormally high amount of venous reflux, and significantly increased residual venous volumes reflecting the presence of venous hypertension. These hemodynamic changes were associated with an overt clinical compromise resulting in a gross deterioration in most quantifiable aspects of quality of life. Assessment of patients with prior I-FDVT in conditions of standardized exercise challenge enabled identification of the subgroup of patients meriting further intervention.
Footnotes
Reprints: K. Delis, MD, PhD, FRCSI, Waller Cardiac Ward, Vascular Secretaries, St. Mary’s Hospital, Praed Street, Paddington, London W2 INY, United Kingdom. E-mail: k.delis@ic.ac.uk.
REFERENCES
- 1.Gjores JE. The incidence of venous thrombosis and its sequelae in certain districts of Sweden. Acta Chir Scand. 1956;(suppl 206). [PubMed]
- 2.Mavor GE, Galloway JM. Iliofemoral venous thrombosis: pathological considerations and surgical management. Br J Surg. 1969;56:45–59. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 19961;125:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Plate G, Eklof B, Norgren L, et al. Venous thrombectomy for iliofemoral vein thrombosis: 10-year results of a prospective randomised study. Eur J Vasc Endovasc Surg. 1997;14:367–374. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SR, Solymoss S, Lamping DL, et al. Long-term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000;15:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neglen P, Berry MA, Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20:560–571. [DOI] [PubMed] [Google Scholar]
- 7.Beyth RJ, Cohen AM, Landefeld CS. Long-term outcomes of deep-vein thrombosis. Arch Intern Med. 1995;155:1031–1037. [PubMed] [Google Scholar]
- 8.Comerota AJ, Throm RC, Mathias SD, et al. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000;32:130–137. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell TF, Browse NL, Burnand KG, et al. The socio-economic effects of an iliofemoral venous thrombosis. J Surg Res. 1977;22:483–488. [DOI] [PubMed] [Google Scholar]
- 10.Hume M. Venous ulcers, the vascular surgeon, and the Medicare budget. J Vasc Surg. 1992;16:671–673. [PubMed] [Google Scholar]
- 11.Franzeck UK, Schalch I, Jager KA, et al. Prospective 12-year follow-up study of clinical and hemodynamic sequelae after deep vein thrombosis in low-risk patients (Zurich study). Circulation. 1996;93:74–79. [DOI] [PubMed] [Google Scholar]
- 12.Labropoulos N, Volteas N, Leon M, et al. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg. 1997;132:46–51. [DOI] [PubMed] [Google Scholar]
- 13.Meissner MH, Caps MT, Zierler BK, et al. Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–833. [DOI] [PubMed] [Google Scholar]
- 14.Killewich LA, Bedford GR, Beach KW, et al. Spontaneous lysis of deep venous thrombi: rate and outcome. J Vasc Surg. 1989;9:89–97. [PubMed] [Google Scholar]
- 15.van Ramshorst B, van Bemmelen PS, Hoeneveld H, et al. Thrombus regression in deep venous thrombosis: quantification of spontaneous thrombolysis with duplex scanning. Circulation. 1992;86:414–419. [DOI] [PubMed] [Google Scholar]
- 16.Masuda EM, Kessler DM, Kistner RL, et al. The natural history of calf vein thrombosis: lysis of thrombi and development of reflux. J Vasc Surg. 1998;28:67–73; discussion 73––74. [DOI] [PubMed] [Google Scholar]
- 17.Moore DJ, Himmel PD, Sumner DS. Distribution of venous valvular incompetence in patients with the postphlebitic syndrome. J Vasc Surg. 1986;3:49–57. [DOI] [PubMed] [Google Scholar]
- 18.Markel A, Manzo RA, Bergelin RO, et al. Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg. 1992;15:377–382; discussion 383––384. [PubMed] [Google Scholar]
- 19.van Ramshorst B, van Bemmelen PS, Hoeneveld H, et al. The development of valvular incompetence after deep vein thrombosis: a follow-up study with duplex scanning. J Vasc Surg. 1994;19:1059–1066. [DOI] [PubMed] [Google Scholar]
- 20.Haenen JH, Janssen MC, van Langen H, et al. Duplex ultrasound in the hemodynamic evaluation of the late sequelae of deep venous thrombosis. J Vasc Surg. 1998;27:472–478. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaides AN, Sumner DS. Outflow fraction. In: Nicoalides AN, Sumner DS, eds. Investigation of Patients With Deep Vein Thrombosis and Chronic Venous Insufficiency. London: Med-Orion, 1991:61. [Google Scholar]
- 22.Araki CT, Back TL, Padberg FT, et al. The significance of calf muscle pump function in venous ulceration. J Vasc Surg. 1994;20:872–877; discussion 878––879. [DOI] [PubMed] [Google Scholar]
- 23.Cockett FB, Thomas ML, Negus D. Iliac vein compression: its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J. 1967;2:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjordal RI. Intermittent venous claudication: a report of two cases. Acta Chir Scand. 1970;136:641–645. [PubMed] [Google Scholar]
- 25.Hobbs JT. The post-thrombotic syndrome. In: Hobbs JT, ed. The Treatment of Venous Disorders. Philadelphia: Lippincott, 1977:253–271. [Google Scholar]
- 26.Killewich LA, Martin R, Cramer M, et al. Pathophysiology of venous claudication. J Vasc Surg. 1984;1:507–511. [PubMed] [Google Scholar]
- 27.Negus D, Cockett FB. Femoral vein pressures in post-phlebitic iliac vein obstruction. Br J Surg. 1967;54:522–525. [DOI] [PubMed] [Google Scholar]
- 28.Raju S, Fredericks R. Venous obstruction: an analysis of one hundred thirty-seven cases with hemodynamic, venographic, and clinical correlations. J Vasc Surg. 1991;14:305–313. [PubMed] [Google Scholar]
- 29.Krupski WC, Bass A, Dilley RB, et al. Propagation of deep venous thrombosis identified by duplex ultrasonography. J Vasc Surg. 1990;12:467–474; discussion 474––475. [PubMed] [Google Scholar]
- 30.Reilly MK, McCabe CJ, Abbott WM, et al. Deep venous thrombophlebitis following aortoiliac reconstructive surgery. Arch Surg. 1982;117:1210–1211. [DOI] [PubMed] [Google Scholar]
- 31.Plate G, Einarsson E, Eklof B. Etiologic spectrum in acute iliofemoral venous thrombosis. Int Angiol. 1986;5:59–64. [PubMed] [Google Scholar]
- 32.Tripolitis AJ, Milligan EB, Bodily KC, et al. The physiology of venous claudication. Am J Surg. 1980;139:447–448. [DOI] [PubMed] [Google Scholar]
- 33.Mogensen K, Skibsted L, Wadt J, et al. Thrombectomy of acute iliofemoral venous thrombosis during pregnancy. Surg Gynecol Obstet. 1989;169:50–54. [PubMed] [Google Scholar]
- 34.Joynt GM, Kew J, Gomersall CD, et al. Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000;117:178–183. [DOI] [PubMed] [Google Scholar]
- 35.Neglen P, Raju S. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583–589. [DOI] [PubMed] [Google Scholar]
- 36.Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–645. [DOI] [PubMed] [Google Scholar]
- 37.Rutherford RB, Padberg FT, Comerota AJ, et al. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–1312. [DOI] [PubMed] [Google Scholar]
- 38.Comerota AJ, Harada RN, Eze AR, et al. Air plethysmography: a clinical review. Int Angiol. 1995;14:45–52. [PubMed] [Google Scholar]
- 39.Goren G. Regarding “Variability and reliability of air plethysmographic measurements for the evaluation of chronic venous disease”. J Vasc Surg. 1998;28:753–755. [DOI] [PubMed] [Google Scholar]
- 40.Kalodiki E, Calahoras LS, Delis KT, et al. Air plethysmography: the answer in detecting deep venous thrombosis. J Vasc Surg. 2001;33 (in press). [DOI] [PubMed]
- 41.Trust MO. SF-36 Health Survey. Scoring Manual for English Language Adaptations. Australia/New Zealand, Canada, United Kingdom, 1994.
- 42.Ware JE Jr. SF-36 Health Survey: Manual and Interpretation Guide. Boston: 1997.
- 43.McHorney CA, Ware JE, Lu JF, et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- 44.Delis KT, Ibegbuna V, Nicolaides AN, et al. Prevalence and distribution of incompetent perforating veins in chronic venous insufficiency. J Vasc Surg. 1998;28:815–825. [DOI] [PubMed] [Google Scholar]
- 45.Delis KT, Husmann M, Kalodiki E, et al. In situ haemodynamics of perforating veins in chronic venous insufficiency. J Vasc Surg. 2001;33 (in press). [DOI] [PubMed]
- 46.Christopoulos D, Nicolaides AN, Cook A, et al. Pathogenesis of venous ulceration in relation to the calf muscle pump function. Surgery. 1989;106:829–835. [PubMed] [Google Scholar]
- 47.Ibegbuna V, Delis K, Nicolaides AN. Effect of lightweight compression stockings on venous haemodynamics. Int Angiol. 1997;16:185–188. [PubMed] [Google Scholar]
- 48.Akesson H, Brudin L, Dahlstrom JA, et al. Venous function assessed during a 5 year period after acute iliofemoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990;4:43–48. [DOI] [PubMed] [Google Scholar]
- 49.Bautista E, Martinez J, Gagne P. Symptomatic venous hypertension because of occult iliofemoral deep vein thrombosis: a report of two cases. Cardiovasc Surg. 1998;6:598–603. [DOI] [PubMed] [Google Scholar]
- 50.Janssen MCH, Haenen JH, van Asten WJC, et al. Clinical and haemodynamic sequelae of deep venous thrombosis: retrospective evaluation after 7–13 years. Clin Sci. 1997;93:7–12. [DOI] [PubMed] [Google Scholar]
- 51.Nicolaides AN, Christopoulos D. Quantification of venous reflux and outflow obstruction with air plethysmography. In: Bernstein EF, ed. Vascular Diagnosis, 4th ed. St. Louis: Mosby-Year Book, 1993:915–921. [Google Scholar]
- 52.Taheri SA, Heffner R, Williams J, et al. Muscle changes in venous insufficiency. Arch Surg. 1984;119:929–931. [DOI] [PubMed] [Google Scholar]
- 53.Pappas PJ, You R, Rameshwar P, et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor-beta1 gene expression and protein production. J Vasc Surg. 1999;30:1129–1145. [DOI] [PubMed] [Google Scholar]
- 54.Ascer E, Mohan C, Gennaro M, et al. Interleukin-1 and thromboxane release after skeletal muscle ischemia and reperfusion. Ann Vasc Surg. 1992;6:69–73. [DOI] [PubMed] [Google Scholar]
- 55.Janssen MCH, Claassen JAHR, van Asten WJC, et al. Validation of the supine venous pump function test: a new non-invasive tool in the assessment of deep venous insufficiency. Clin Sci. 1996;483–488. [DOI] [PubMed]
- 56.Patterson RB, Pinto B, Marcus B, et al. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg. 1997;25:312–318; discussion 318––319. [DOI] [PubMed] [Google Scholar]
- 57.Hicken GJ, Lossing AG, Ameli FM. Assessment of generic health-related quality of life in patients with intermittent claudication. Eur J Vasc Endovasc Surg. 2000;20:336–341. [DOI] [PubMed] [Google Scholar]


