Abstract
Introduction:
For patients with hepatocellular carcinoma (HCC) in particular, living donor liver transplant (LDLT) improves access to transplant. We report our results in 36 patients with HCC who underwent LDLT with a median follow-up >1 year.
Methods:
Underlying diagnoses included: hepatitis C (24), hepatitis B (9), cryptogenic cirrhosis (1), hemochromatosis (1), and primary biliary cirrhosis (1). Patients with tumors ≥ 5 cm received IV doxorubicin intraoperatively and 6 cycles of doxorubicin at 3-week intervals. Patients were followed with CT scan and alpha-fetoprotein levels every 3 months for 2 years posttransplant. Mean waiting time, pretransplant treatment, tumor variables, and survival were analyzed. Univariate and multivariate analysis were done to analyze tumor variables; Kaplan-Meier and log rank were used to compare survivals. P < 0.05 was considered significant.
Results:
Mean wait for LDLT was 62 days, compared with 459 days in 50 patients with HCC transplanted with cadaveric organs during the same time period (P = 0.0001). At median follow-up of 450 days, there have been 10 deaths due to non–tumor-related causes and 3 deaths from recurrence; recurrence has also been observed in 3 other patients. On univariate and multivariate analysis, bilobar distribution was the only significant tumor variable (P = 0.03, log rank = 0.02). Fifty-three percent of patients exceeded UNOS priority criteria. One- and two-year patient survivals were 75% and 60%, respectively. Freedom from recurrence at 365 and 730 days was 82% and 74%, respectively. Overall and in patients with HCC > 5 cm (n = 12), there were no statistically significant differences in survival or in freedom from recurrence between recipients of living donor and cadaveric grafts.
Conclusion:
Although one third of patients had tumors > 5 cm, the incidence of recurrence as well as patient survival and freedom from recurrence are comparable to results after cadaveric transplant. LDLT allows timely transplantation in patients with early or with large HCC.
In 36 patients with HCC who underwent living donor liver transplantation, the incidence of recurrence as well as patient survival and freedom from recurrence at a median follow-up >1 year are comparable to results after cadaveric transplant. Living donor liver transplantation allows timely transplantation in patients with early or with large HCC.
For patients with hepatocellular carcinoma (HCC), the only hope for cure lies in physical removal or destruction of the tumor prior to spread outside the liver.1,2 In most patients with HCC, however, cirrhosis and multicentricity preclude resection, leaving liver transplantation as the only approach with the potential to cure both the cirrhosis and the tumor.
Results of liver transplantation in patients with early unresectable HCC are comparable to results of liver transplantation for other indications.3 On the basis of these findings, UNOS prioritizes patients with a single tumor ≤ 5 cm or with 2–3 tumors all ≤ 3 cm. Patients with larger tumors, however, receive no priority and are thus in effect barred from receiving a cadaveric donor liver.
We recently reviewed our experience in 43 patients with HCC ≥ 5 cm who underwent cadaveric liver transplantation. At a mean follow-up of 55 months, 44% were alive and 48% were free of recurrence.4 By contrast, 3-year survival in patients with HCC treated only with chemotherapy is less than 25%.5,6
Over recent years, however, longer waiting times have severely limited cadaveric transplantation for HCC. At our center, the average waiting time among HCC patients is in excess of 1 year, and 16–23% of HCC patients who are listed for transplant eventually drop off the waiting list due to tumor progression.7,8 Living donor liver transplantation (LDLT) provides an alternative source of donor livers for transplantation, the allocation of which is independent of waiting time or UNOS criteria. For the patient who has a living donor, a donor organ is not a scarce resource.7,9
In patients who undergo transplantation for HCC, the median interval to recurrence is 12 months.3,4,6 Previously we reported our initial results in 27 patients with HCC who underwent LDLT, for whom the median follow-up was 236 days.7 In that series, 2 recurrences were noted. Here we report our extended results in these and other patients with HCC who underwent living donor liver transplantation, with a median follow-up > 1 year.
METHODS
All patients who underwent LDLT for HCC at our center were included. Candidates for LDLT must meet United Network of Organ Sharing (UNOS) minimal listing criteria. Only patients with HCC recognized preoperatively were included in this study. Tumor evaluation included dual-phase helicoidal CT scan or MRI with gadolinium of the abdomen, chest CT scan, and bone scan. Patients with evidence of extrahepatic tumor spread, including invasion of the main portal vein, were excluded from transplant. Recipient selection unrelated to evaluation of HCC has been explained in previous publications.7,9,10 Follow-up of HCC patients after transplant included CT scans of the abdomen and chest and measurement of alpha-fetoprotein levels every 3 months during the first 2 years, every 6 months during the third year, and yearly thereafter. Steroids and tacrolimus-based immunosuppression were employed in all cases. Steroids were used at half-dose in the anhepatic phase in recipients with HCV. In HCC patients, tapering of immunosuppression was expedited during follow-up based on liver function tests.
Potential donors must be ABO compatible and >18-year-old. A multidisciplinary team (hepatologist, surgeon, coordinators, social worker, and psychiatrist) evaluates them. Biochemistry studies include coagulation profiles, complete blood count, SMA-18, serologic studies for hepatitis, human immunodeficiency virus, Epstein-Barr virus, cytomegalovirus, and VDRL, measurement of alpha1 antitrypsin, serum lipid profile, serum protein electrophoresis, and negative markers for autoimmune hepatitis. EKG and chest x-ray are done for cardiac evaluation. If BMI ≥ 28 or steatosis is suggested by radiologic examination, a liver biopsy is performed. After CT and MRI, an adequate assessment of the liver volume is mandatory, a graft recipient weight ratio ≥ 0.8% is preferred, and a residual 30% of liver volume with no steatosis is mandatory.
We recorded recipient age and gender, underlying liver disease, tumor size and location, Child-Pugh score, UNOS status at the time of transplant, and waiting time (measured as the number of days between completion of recipient evaluation and the date of transplant). Previous treatments for tumor were analyzed and correlated with the incidence of recurrence.
Tumor contraindications for LDLT were the presence of extrahepatic disease, as well as the presence of invasion of the main portal vein. Intrahepatic vascular invasion was not considered a contraindication to LDLT. Patients with tumors >5 cm underwent exploratory laparotomy to rule out extrahepatic disease on the planned day of transplantation, before the donor was anesthetized. These patients received a single dose of doxorubicin 10 mg/m2 IV intraoperatively, followed by 6 cycles of doxorubicin at 3 weekly intervals beginning 1 month postoperatively as previously described.4,7 The explanted liver was carefully sliced for pathologic study. We analyzed number of tumors, size, location, vascular invasion (none, microscopic, or macroscopic), as well as histologic grade. Patient and graft survival, and freedom from recurrence, were analyzed overall and with patients categorized by size of tumor (>5 cm or ≤ 5 cm). Results were compared with those in 165 patients with HCC who underwent cadaveric transplantation at our center between January 1992 and February 2002.
Statistical Analysis
Descriptive statistics were expressed as mean ± SD and median. Fisher exact test and χ2 were also used to compare groups. Logistic regression analysis was used to determine which of the tumor variables were independently associated with survival and recurrence after transplant. Actuarial survival was calculated by the Kaplan-Meier method; survival rates were compared using the log-rank test, with P < 0.05 considered significant.
RESULTS
Between August 1998 and April 2002, 927 patients with known or suspected HCC were evaluated at our center. Of these, 399 (43%) were initially considered to be potential liver transplant candidates, and 115 eventually were transplanted: 36 LDLT (31%), and 79 cadaveric. Overall during this period, 99 adults underwent LDLT at our hospital, for all indications.
The mean follow-up of the 36 patients who underwent LDLT for HCC is 450 ± 308 days; the median follow-up is 470 days. Four patients received left lobe grafts; 32 patients received right lobes. The mean waiting time for LDLT was 62 days.
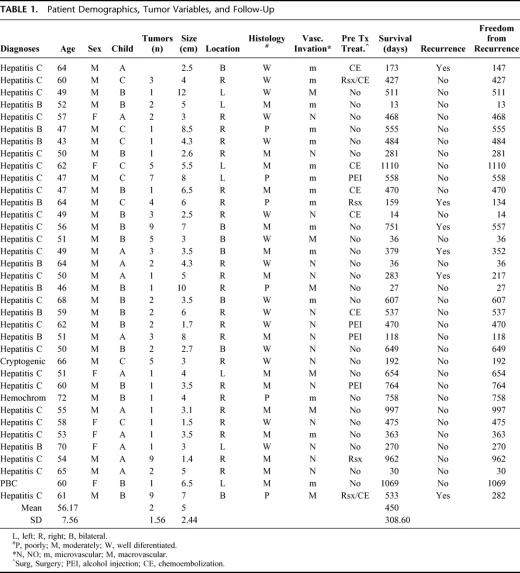
Demographics, pathology, pretransplant treatment, follow-up, and recurrence are presented in Table 1. Fifty-three percent of the patients exceeded UNOS priority criteria.
TABLE 1. Patient Demographics, Tumor Variables, and Follow-Up
There were no major complications or deaths among the donors. Complications included wound infection,4 bile leak from the cut surface of the liver (managed by percutaneous drainage),2 prolonged ileus,1 and small bowel obstruction (treated laparoscopically).1
Recipient complications included bile leak,1 biliary stricture,2 mycotic aneurysm,2 hepatic artery thrombosis,1 portal vein thrombosis,1 veno-occlusive disease requiring retransplant,1 colonic perforation,1 distal Roux-Y anastomotic leak,1 retrocaval bleeding,2 and small-for-size syndrome.2 Fifteen patients required reoperations, including 2 who were retransplanted.
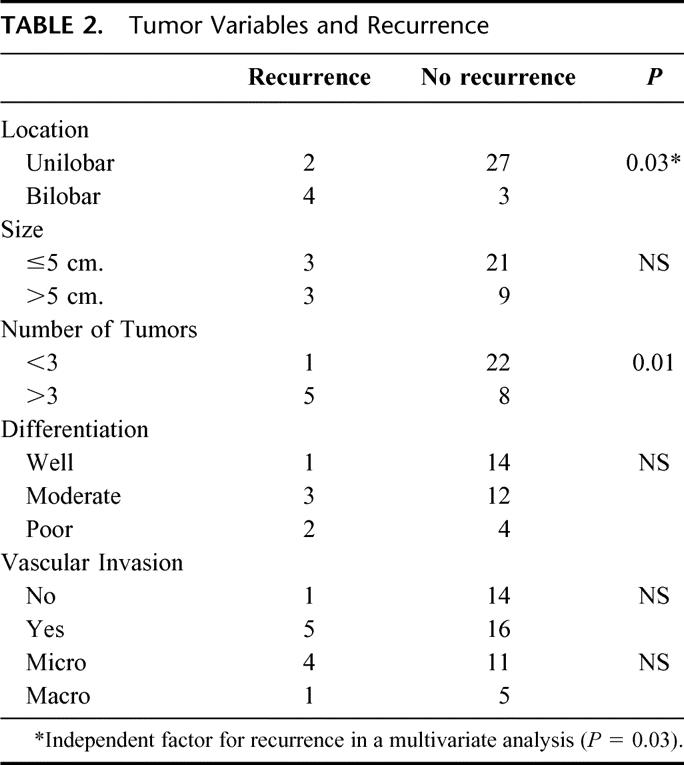
Six patients developed tumor recurrence. The mean time to recurrence was 282 ± 158 days. On univariate analysis, bilobar distribution (P = 0.03) and number of tumors >3 (P = 0.01) were risk factors for recurrence. On multivariate analysis, only bilobar distribution significantly affected freedom from recurrence (log rank = 0.02) (Fig. 3). None of the remaining variables had a statistically significant effect on recurrence (Table 2) or patient survival. Pretransplant treatment had no significant effect on the incidence of recurrence (3 of 23 patients with no pretransplant treatment had recurrence, versus 3 of 13 who received treatment; P = 0.3).
FIGURE 3. Patient survivals by tumor distribution (unilobar versus bilobar).
TABLE 2. Tumor Variables and Recurrence
Three patients with recurrent HCC are alive. Two of the 3 developed intrahepatic recurrence 147 and 332 days posttransplant. Initially, they received intrahepatic chemotherapy (to reduce local progression of the tumor and because arterial embolization was felt to be dangerous after transplant), but the second patient was recently discovered to have multiple intrathoracic metastases. The third patient was diagnosed with intrathoracic recurrence 217 days after transplant.
Thirteen patients (38%) died. Eight died perioperatively of transplant-related complications (sepsis related to bile leak, n = 4; sepsis after mycotic aneurysm rupture, n=1; sepsis after portal vein thrombosis, n=1; sepsis after retransplantation for massive graft necrosis due to veno-occlusive disease, n=1; sepsis due to aspiration pneumonia, n=1). One patient died 192 days after transplant of chronic rejection; another patient died 470 days after retransplant for HCV recurrence; these 2 patients were both free of tumor recurrence. One patient died 555 days after transplant with lung cancer, without HCC recurrence.
The other 3 patients with recurrent disease died of tumor recurrence, at 751 days (intra- and extrahepatic tumors diagnosed at 557 days posttransplant); 134 days (45 days after diagnosis of intrahepatic and extrahepatic recurrence); and 533 days (tumor recurrence diagnosed at 282 days after transplant and treated with radiofrequency ablation).
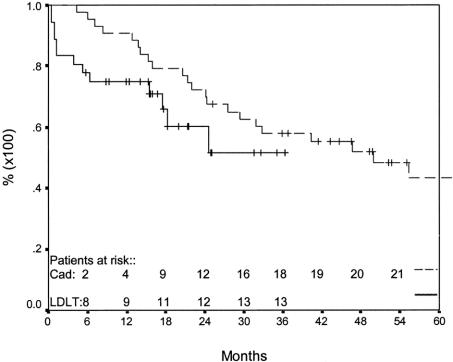
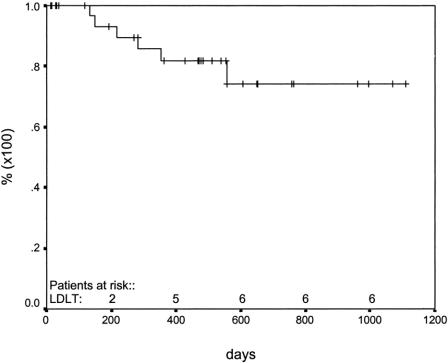
One- and two-year patient survivals were 75% [95% CI = 67.1–82.9%] and 60% [95% CI = 48.5–71.5%], respectively (Fig. 1), while 1- and 2-year graft survivals were 68% [95% CI = 59.9–76.1%] and 59% [95% CI = 49.5–68.5%], respectively. Figure 1 also shows the comparison between our series patient survival and Roayiae's patients with >5 cm tumors transplanted with cadaveric organs. Freedom from recurrence at 365 and 730 days was 82% [95% CI = 74.6–89.4%] and 74% [95% CI = 64.2–83.8%], respectively (Fig. 2).
FIGURE 1. Patient survival after living donor liver transplant for HCC compared with cadaveric liver transplant in patients with HCC ≥ 5 cm HCC.
FIGURE 2. Freedom from recurrence after living donor liver transplantation for HCC.
The mean time between evaluation and transplant for LDLT was 62 days, versus 459 days for HCC patients who received cadaveric livers (P = 0.0001).
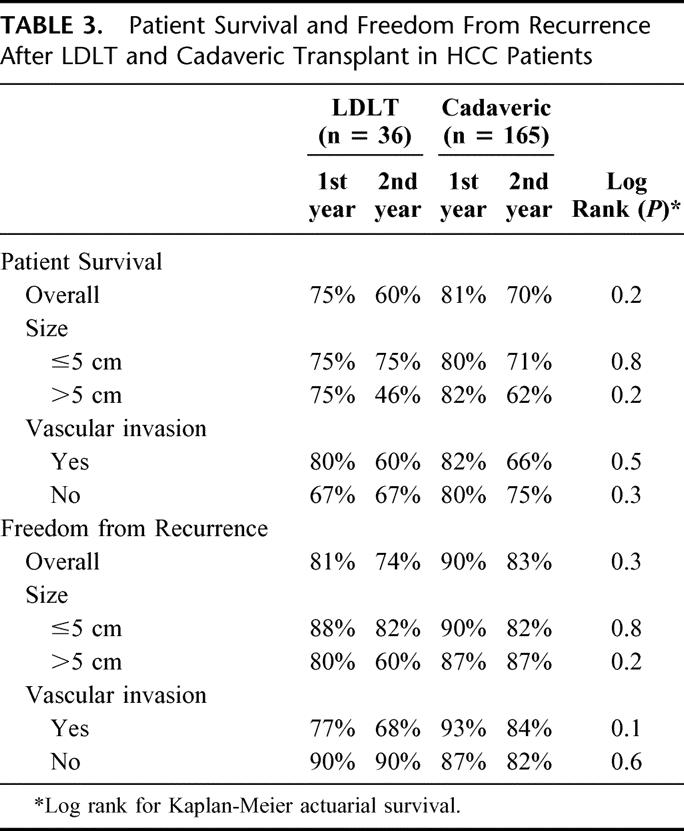
In both the overall group and in the subset of patients with HCC > 5 cm, there were no statistically significant differences in survival or in freedom from recurrence between recipients of living donor and cadaveric grafts (Table 3).
TABLE 3. Patient Survival and Freedom From Recurrence After LDLT and Cadaveric Transplant in HCC Patients
DISCUSSION
The incidence of HCC and the associated mortality continue to rise.5,11–13 In more than 90% of patients, HCC develops in the setting of underlying liver disease. Three-year survival in patients with cirrhosis and untreated HCC is close to 0%.1,5
Transplantation is currently the only life-saving therapy for patients with unresectable HCC and cirrhosis. Several authors have shown that tumors have an increased recurrence rate and disappointing results after transplant.3,6,14–16 The waiting time for cadaveric transplants is one of the factors that affect all candidates, but those with HCC are particularly likely to suffer because of the risk that the tumor will grow and metastasize before a donor organ is available.
Various authors who examined risk factors for HCC recurrence after transplantation have reported that the recurrence rate begins to increase significantly as tumor diameter approaches 5 cm.3,8,14–19 The fact that vascular invasion commonly begins as tumor diameter approaches 5 cm likely explains the correlation of diameter with recurrence; indeed, when larger tumors are found not to have vascular invasion (as is commonly observed in the setting of hepatitis B), posttransplant recurrence is relatively unlikely.20 In 1996, in a classic paper, Mazzaferro et al confirmed the impact of tumor size on cadaveric liver transplantation for patients with small HCC, reporting 83% overall patient survival and 75% overall recurrence-free survival at 4 years.3 Based on those data, UNOS establish the current listing criteria for HCC patients.
But in this new century, researchers have begun to reassess the importance of tumor size, extending the upper limit for acceptable survival after transplant to > 7 cm or total tumor diameter >8 cm.4,20–23 For example, we have shown that 48% of patients with tumors >5 cm transplanted using a multimodality adjuvant protocol were free of disease 4.5 years after transplant.4 Of the 43 patients in that study, 15 had stage 3 or 4 tumors (TNM classification). In these patients, 2-year survival was 69%, and 2-year recurrence-free survival was 80%.
Patients who face a long wait for a cadaveric transplant need treatment with chemoembolization, ethanol injection, or radiofrequency ablation to prevent progression while waiting.17,18 LDLT brings the opportunity to offer timely transplantation to patients with HCC; when LDLT is feasible, the need for pretransplant treatment is eliminated, reducing overall morbidity.19,20
Since August 1998, HCC has been the leading indication for adult LDLT in our program, accounting for 36 of 99 cases (36%). Recently, we published an initial analysis of our results in 27 patients.7 At a median follow-up of 236 days, 2 patients had developed recurrence. The freedom from recurrence rate was 83%, and 1-year survival was 81%. Encouraged by these results, we continued to include patients in our protocol. Currently, at a median follow-up of 470 days, 6 of 36 have recurrence, at a mean recurrence time of 282 days.
In our present series, 3 patients with tumors <5 cm and 3 with tumors >5 cm developed recurrence. This finding shows once again the unpredictability of the posttransplant course in individual HCC patients. Iwatsuki et al14 wrote that bilobar tumors, size, and vascular invasion were independently significant factors for tumor-free survival. In our series, on univariate analysis, the presence of more than 3 tumors and bilobar distribution were significant risks factors. On multivariate analysis, bilobar distribution was the only independent risk factor for poor prognosis.
Although vascular invasion was not significantly associated with recurrence in our series, 5 of the 6 patients with recurrence did have vascular invasion.
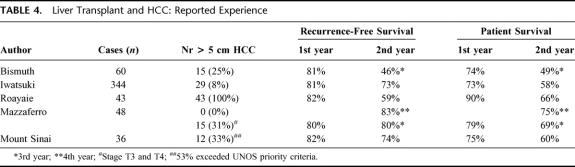
When we compare our results with classic studies (Table 4), our results with LDLT in our cohort, in which 33% of patients had larger tumors, are no different than results in other studies with similar follow-up but fewer patients with large tumors.
TABLE 4. Liver Transplant and HCC: Reported Experience
In the study by Roayaie, which was the first to focus only on patients with larger tumors, only cadaver organs were used.4 In our series of patients who underwent LDLT, 33% had tumors >5 cm, and 53% exceeded UNOS criteria; no other study has had such a large proportion of LDLT recipients with large tumors. Survival in our patients with large tumors did not differ in the living donor and cadaver groups, and tumor size did not affect tumor recurrence.
Patients who face a long wait for a cadaveric transplant need treatment with chemoembolization, ethanol injection, or radiofrequency ablation to prevent progression while waiting.24,25 The mean waiting time for LDLT was 62 days in our series, compared with 459 days in HCC patients who received cadaveric grafts. LDLT gives the opportunity to offer timely transplantation to patients with HCC and when LDLT is feasible, the need for pretransplantation treatment is eliminated, reducing overall morbidity.19,20,26
This shorter waiting time was not associated with higher patient or recurrence-free survival. We were, however, performing transplants on patients who would not have been eligible for cadaver livers or who would likely have been taken off the waiting list due to tumor progression. Indeed, disease progression while on the waiting list was the leading cause for excluding patients from transplant. We have increased the proportion of our patients with HCC who received liver transplants from 20% with cadaver transplant only to 29% with the addition of LDLT.
For reasons that are unclear, patients who underwent LDLT for HCC, despite being in better physiologic condition than the typical patient undergoing transplant for cirrhosis, had a higher perioperative mortality rate than patients undergoing LDLT for other indications. We have not discovered any common factors among HCC patients who died perioperatively, and we do not believe there is a causal association between HCC and perioperative mortality.
Offering LDLT to patients with HCC raises complex issues for the donor, the recipient, and the medical team.27–29 Still, it permits us to offer timely transplantation to patients with HCC without using scarce cadaveric organs. For patients with tumors exceeding the current criteria for prioritization, LDLT offers the only realistic hope for survival. The results of the current study show that overall and disease-free survival of patients undergoing LDLT for HCC is comparable to those of patients undergoing cadaveric transplants for similar tumors. Clearly, longer follow-up is required to determine which patients with HCC will have the highest likelihood of survival after LDLT. Nonetheless, given the results of this study, we feel justified in continuing to offer LDLT to patients with HCC.
Footnotes
Reprints: Gabriel E. Gondolesi, MD, Mount Sinai Hospital, Box 1104, 1 Gustave Levy Place, New York, NY 10029. E-mail: gabriel.gondolesi@mountsinai.org.
REFERENCES
- 1.Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992;15:948–963. [DOI] [PubMed] [Google Scholar]
- 2.Calvet X, Bruix J, Bru C, et al. Natural history of hepatocellular carcinoma in Spain. Five year's experience in 249 cases. J Hepatol. 1990;16:6–72. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;14:728–729. [DOI] [PubMed] [Google Scholar]
- 4.Roayaie S, Frischer J, Emre S, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 cm. Ann Surg. 2002;235:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–137. [DOI] [PubMed] [Google Scholar]
- 6.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. [DOI] [PubMed] [Google Scholar]
- 7.Gondolesi G, Muñoz L, Matsumoto C, et al. Hepatocellular carcinoma: a prime indication for living donor liver transplantation. J Gastrointestinal Surg. 2002;6:102–107. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Bustamante J, Castells A, et al. Liver transplantation for hepatocellular carcinoma. Results of a restrictive policy. Hepatology. 1996;24:350A. [Google Scholar]
- 9.Miller C, Gondolesi G, Florman S, et al. One hundred and nine living donor liver transplants in adults and children. A single center experience. Ann Surg. 2001;234:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiano T, Kim-Schluger L, Gondolesi G, et al. Adult living donor liver transplantation: the hepatologist's perspective. Hepatology. 2000;33:3–10. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda K, Saitoh S, Koida I, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53. [PubMed] [Google Scholar]
- 13.Ebara M, Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis: a study in 22 patients. Gastroenterology. 1986;90:289–298. [DOI] [PubMed] [Google Scholar]
- 14.Iwatsuki S, Dvorchik I, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klintmalm G. Liver transplantation for hepatocellular carcinoma. A registry report of impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bismuth H, Adam R, Raccuia J, et al. Liver transplantation for hepatocellular carcinoma in cirrhosis. Transplant Rev. 1996;10:13–23. [Google Scholar]
- 18.McPeake J, O'Grady J, Zaman S, et al. Liver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcome. J Hepatol. 1993;18:226–234. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto J, Iwatsuki S, Kosuge T, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roayaie S, Ben Haim M, Emre S, et al. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B vs hepatitis C: a western experience. Ann Surg Oncol. 2000;7:764–770. [DOI] [PubMed] [Google Scholar]
- 21.Marsh J, Dvorchik I, Bonham C, et al. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;8:538–543. [DOI] [PubMed] [Google Scholar]
- 22.Tan K, Rela M, Ryder S, et al. Experience of orthotopic liver transplantation and hepatic resection for hepatocellular carcinoma less than 8 cm in patients with cirrhosis. Br J Surg. 1995;82:253–256. [DOI] [PubMed] [Google Scholar]
- 23.Yao F, Ferrel L, Bass N, et al. Liver transplantation for hepatocellular carcinoma: expansion of tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 24.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion, 701–703. [DOI] [PMC free article] [PubMed]
- 25.Spreafico C, Marchiano A, Regalia E, et al. Chemoembolization of hepatocellular carcinoma in patients who undergo liver transplantation. Radiology. 1994;192:682–690. [DOI] [PubMed] [Google Scholar]
- 26.Sarasin F, Majno P, Llovet J, et al. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost effectiveness perspective. Hepatology. 2001;33:1073–1079. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto T, Yamoaka Y, Tanaka K, et al. Quality of life among donors of liver transplants to relatives. N Engl J Med. 1993;329:363–364. [DOI] [PubMed] [Google Scholar]
- 28.Trotter JF, Talamentes M, McClure M, et al. Right hepatic lobe donation for living donor liver transplantation: impact on donor quality of life. Liver Transpl. 2001;7:485–493. [DOI] [PubMed] [Google Scholar]
- 29.Kim-Schluger L, Florman SS, Schiano T, et al. Donor quality of life after adult liver transplant donation. Transplantation 2004 (in press).