Abstract
Objective:
To determine the long-term results of liver transplantation for well- or moderately differentiated hepatocellular carcinoma (HCC).
Summary Background Data:
HCC patient selection for liver transplantation remains controversial, and deciding exclusively on the strength of criteria such as number and size of nodules appears prognostically inaccurate.
Methods:
Since 1991, preoperative tumor grading has been used at our center to establish whether a patient with HCC is fit for transplantation. Poorly differentiated HCC cases were excluded, while size and number of nodules were not considered as absolute selection criteria. Thirty-three patients with moderately or well-differentiated HCC were prospectively studied after liver transplantation. A group of 15 patients with incidental HCC transplanted during the same period were also evaluated and compared with the 33 patients with preoperatively diagnosed HCC.
Results:
On histologic examination, 38% of the entire group of 48 patients did not meet the “Milan criteria” and 42% were pTNM stages III and IV. The median follow-up was 44 months. The 5-year actuarial survival rate was 75% and recurrence-free survival was 92%. HCC recurred in only 3 patients (6%). The only histomorphologic variable differing significantly between incidental and nonincidental HCC was nodule size. The timing of diagnosis (incidental vs. nonincidental HCC), the Milan criteria, and the TNM stage revealed no statistically significant impact on overall and recurrence-free survival rates.
Conclusions:
The routine pre-orthotopic liver transplantation tumor grading may represent a valid tool in the selection of unresectable HCC patients for transplantation.
In our series, the use of HCC grades (G1 and G2) to select candidates for OLT (based on preoperative FNAB) was associated with an extremely low rate of tumor recurrence, comparable with that of incidentally detected HCC, although nodule size and number were not used as selection criteria.
Over the last decade, the disappointing results reported in early publications on transplantation for hepatocellular carcinoma (HCC)1–3 and the scarce availability of liver donors4 have favored the introduction at many centers5–7 of stringent morphologic criteria (solitary nodule <5 cm, 3 nodules <3 cm) for listing HCC patients for orthotopic liver transplantation (OLT). Many papers8–13 have shown, however, that adopting these criteria carries the risk of a significant number of patients being refused a potentially curative solution. Conversely, a significant proportion of patients assumed to be good transplant candidates are actually at high risk of tumor recurrence.
Recent studies have shown that tumor grade and microscopic vascular invasion represent a much more direct indicator of the biologic progression of HCC and hence of posttransplant tumor recurrence risk.8,14–22 Only tumor grade can be routinely determined preoperatively, however, and may thus be a worthwhile criterion for selecting candidates for OLT.8,9,15,23–26 Several works published in the 1980s had already pointed out the relevance of the histologic features of HCC, and grade in particular, to the prognosis of patients undergoing resection or transplantation.27–31 Since 1991, our center has adopted a protocol for selecting HCC patients for OLT that considers preoperative grading as a means for excluding the biologically most aggressive cases, while size and number of nodules were not considered absolute selection criteria.
The aim of the present study was to evaluate the efficacy of OLT in a group of HCC patients selected on the basis of specific criteria, which excluded the biologically most aggressive cases according to grade. A group of patients with incidentally detected HCC transplanted during the same interval was also prospectively observed.
PATIENTS AND METHODS
Study Design
Preoperatively Known HCC (pkHCC)
Between July 1991 and May 2002, 133 HCC patients seen at our center were considered unresectable due to the morphologic features of the tumor, the severity of cirrhosis, or the patient's general conditions. HCC was histologically confirmed in all patients by ultrasound-guided percutaneous hepatic needle biopsy (FNAB). The grade of HCC differentiation was determined at the same time, according to the Edmonson-Steiner criteria.32
The following exclusion criteria were established for OLT short-listing: general contraindications to transplant (age, severe extrahepatic diseases, recent malignancies, compliance), extrahepatic spread or vascular invasion (preoperatively evident or suspected), and poorly differentiated HCC (G3) at pre-OLT FNAB.
Ninety-three of the 133 patients (70%), having 1 or more of the above features, were excluded (Table 1). Among the 10 excluded G3 HCCs, 6 were initially TNM stage II, 5 met the Milan criteria, and 6 had the largest nodule < 5 cm. The median survival for this group of 10 patients was 6 months (range 1–19 months), despite several therapeutic strategies being attempted (transarterial chemo-embolization [TACE], percutaneous ethanol injection [PEI], chemotherapy [CT]). Moreover, 60% died with multiple bone and lung metastases.
TABLE 1. Exclusion Criteria in 93 Patients With Unresectable HCC

Forty of the 133 HCC patients (30%) joined the waiting list for OLT: 33 (82.5%) were transplanted and are the topic of the present study; 6 (15%) were still awaiting transplantation as of May 31st 2002; one (2.5%) was removed from the waiting list after developing neoplastic portal thrombosis 3 months after listing.
Incidental HCC (iHCC)
A total of 327 OLTs were performed at our center during the study period (1991–2002). In 15 cases (4.6%), HCC was diagnosed on the explanted liver. This group of patients was also followed up prospectively.
Patient Characteristics, Staging Procedures, Follow-up
The baseline features of the entire group are given in Table 2, including the characteristics of their iHCC and pkHCC. Forty-two patients (87%) had virus-related cirrhosis (HCV in 26, HBV in 8, both in 8) and liver function was severely compromised (Child B-C) in 41 cases (85%). Alpha-fetoprotein (AFP) levels were below 100 in 81% of patients at the time of listing. The proportion of patients with severe cirrhosis (Child C), but normal AFP levels was significantly higher among iHCC than among pkHCC cases (53% vs. 19%, P = 0.003; and 60% vs. 18%, P = 0.002).
TABLE 2. Baseline Characteristics of the 48 HCC Patients Undergoing OLT and Comparison Between iHCC and pkHCC Cases
The preoperative evaluation included: hepatic Doppler US, hepatic Lipiodol-arteriography, abdominal CAT, angio-CAT or angio-MRI (if vascular invasion was suspected), chest and brain CAT, and total-body bone scintigraphy to rule out extrahepatic metastases. These tests were repeated if patients remained on the transplant waiting list for more than 6 months. The median waiting time between final staging and transplant was 3 months (range 1–6 months). Preoperative staging (33 cases) revealed 14 patients (43%) in TNM stages III and IV, while 13 (39%) did not meet the “Milan criteria.”6 OLT was performed with a venovenous bypass in 13 (27%) of the 48 cases studied, while a “piggyback technique” was used in the other 35. In one case (2%), a split liver from a cadaveric donor was used, while there were no living donor liver transplantations in this series. During the transplant, complete hepatic artery lymphadenectomy was performed in all patients with pkHCC. The explanted liver underwent histologic assessment by an experienced pathologist who recorded HCC type, number and size of nodules, grade, microvascular and macrovascular invasion, any fibrous encapsulation, and lymph node involvement. In cases of death after transplantation, autopsy was performed to assess the cause of death and identify any disease recurrence. Posttransplant immunosuppressive therapy consisted of cyclosporine or tacrolimus in association with steroids, which were tapered off within 6 months of transplantation. Follow-up at 1, 3, and 6 months after transplantation, and every 6 months thereafter, always included liver US and AFP assay. Total-body CT was performed a year after transplantation or whenever tumor recurrence was suspected.
Associated Therapies
Most patients with pkHCC (94%) had TACE and/or other therapies (PEI, radiofrequency ablation [RF], CT) while on the waiting list (Table 2).
Up until 1998 (the first 24 study patients), all patients with HCC nodules exceeding 2 cm or multiple nodules of any size (T2, T3, T4 on histology of the explanted liver) were administered postoperative CT. From 1998 onwards (further 24 study patients), this approach was no longer applied to HCV-positive patients to reduce the risk of post-OLT viral recurrence. CT included 5-fluorouracil and carboplatin (1 cycle a week for 6 months). In all, 21 patients (44%) received CT after transplantation: 8 in the iHCC group (53% of the group) and 13 in the pkHCC group (39%).
Statistical Analysis
Post-OLT recurrence-free and overall survival rates were the end-point of the study. The χ2 test, Fisher exact test, and logistic regression, where appropriate, were used for comparisons (iHCC vs. pkHCC). The cumulative overall and recurrence-free survival rates were calculated by the Kaplan-Meier method. The survival curves were compared using the log-rank test. All statistical tests were two-tailed. Analyses were performed using the SAS Institute statistical package (JMP). Differences were considered significant at P < 0.05.
RESULTS
Histopathological Data and Tumor Staging
The histopathological features of the HCCs are summarized in Table 3. In the pkHCC group, liver hilum lymph nodes were always negative for infiltration at histology. None of the patients involved in the study had macroscopic vascular invasion. In all pkHCC patients (33 cases), histopathology on the explanted liver was fully concordant with preoperative findings in terms of grading. The 15 iHCCs were also found well- to moderately differentiated on pathologic assessment in all cases.
TABLE 3. Histopathological Characteristics (of Explanted Liver) of 48 HCC Patients and Comparison Between iHCC and pkHCC Cases
When the pathologic features of the iHCCs and pkHCCs were compared, only nodule size differed significantly (1.8 vs. 2.5; P = 0.03). At post-OLT staging, 42% of the patients were classified as pTNM stages III-IV and 38% did not meet the Milan criteria. No significant differences emerged in the distribution of iHCC and pkHCC patients according to TNM stage or Milan criteria. The findings in pre-OLT imaging studies were different from the pathologic findings in a large number of patients (Table 4).
TABLE 4. Accuracy of Imaging Techniques in pkHCC Staging (33 patients)

Overall and Recurrence-Free Survival Analysis
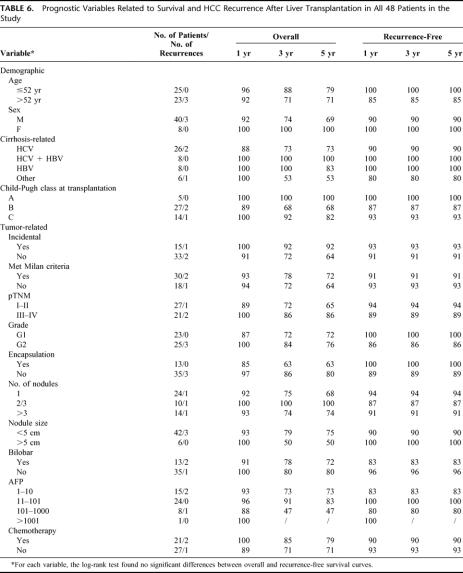
As of May 31st 2002, the median follow-up for the whole study group (48 patients) was 44 months (range 3–120 months). Four patients (8.3%) were retransplanted, 2 owing to acute liver failure (within a month of the first transplant) and 2 because of recurrent viral-related cirrhosis (12 and 16 months after the first transplant). At histopathology, the explanted livers showed no signs of tumor recurrence. Overall mortality was 19% (9 cases), and only in 2 patients (4%) was death related to tumor recurrence (Table 5). Altogether, there were only 3 cases of HCC recurrence (6%), all histologically confirmed, 3, 10, and 11 months after OLT (median time to recurrence 7 months). The patient with the earliest recurrence was treated with 12 cycles of PEI on 2 recurrent liver lesions and is still alive and disease free 88 months after transplantation. One-, 3-, and 5-year actuarial survival rates were, respectively, 94%, 79%, and 75%. Recurrence-free survival remained constant at 92% since all relapses occurred within the first year (Fig. 1). The survival curve for HCC-related transplants at our center coincides with the curve for OLT for nonmalignant diseases (Fig. 2). The prognostic significance of the main demographic, clinical, and pathologic variables in our series is shown in Table 6. As was to be expected, given the low number of tumor recurrences, none of these variables had a statistically significant impact on overall and disease-free survival, and none revealed any statistically significant differences when analyzed separately in relation to the iHCCs and pkHCCs. Two recurrences occurred among the pkHCC patients and 1 in the iHCC group: all 3 met the Milan criteria before transplantation and only 1 did not after histologic assessment on the explanted liver (Fig. 3).
TABLE 5. Cause of Death and Features of the 9 Patients Who Died After OLT

FIGURE 1. Overall (A) and recurrence-free (B) survival curves after OLT in the 48 patients enrolled in the study. Standard errors at 3 years are 7% and 4.6%, respectively; 95% confidence intervals are represented by dashed lines.

FIGURE 2. Comparison between overall survival curves of HCC-related OLT (iHCC and pkHCC) versus OLT for nonmalignant disease. The log-rank test found no statistically significant differences.
TABLE 6. Prognostic Variables Related to Survival and HCC Recurrence After Liver Transplantation in All 48 Patients in the Study

FIGURE 3. A: Comparison between recurrence-free survival curves for iHCC versus pkHCC cases. The log-rank test found no statistically significant differences. B: Comparisons between recurrence-free survival curves for HCC that did versus did not meet the Milan criteria after histologic assessment. The log-rank test found no statistically significant differences.
The 2 variables (number and size of nodules) were unrelated with the overall and recurrence-free survival of the 48 patients. As for the pTNM classification, the 3 recurrences occurred in 1 pTNM stage I patient and 2 pTNM IV patients.
DISCUSSION
Hepatocellular carcinoma now represents the fifth most frequent malignant tumor in the world (564,000 cases a year) and the third cause of death due to cancer.33,34 Liver transplantation is the only option capable of simultaneously curing both HCC and the underlying liver disease. The chances of benefiting from OLT are hindered, however, by the limited number of donors, resulting in a high risk of exclusion due to disease progression while awaiting transplantation.4 The core problem is thus the careful selection of patients who can benefit from liver transplantation, which also means identifying patients whose risk of recurrence is as low as possible. The adoption of strict macromorphological selection criteria (size and number of nodules) has led to a stunning improvement in the survival rate for transplanted HCC patients in the last 10 years, but several recent studies have shown the prognostic limitations of such criteria.8,9,12,18,26
First of all, relying exclusively on the tumor's macromorphological characteristics may result in misdiagnosis due mainly to the limits of imaging techniques12,35. As pointed out in a report from Milan,6 27% of patients exceeded the original study entry criteria at histologic examination of the explanted liver.6 In the present study, the pre-OLT stage was inconsistent with the post-OLT stage in 16 pkHCC patients (48%) according to TNM stages, and in 5 (14%) according to the Milan criteria (Table 4).
Furthermore, the macromorphological characteristics of HCC give an imprecise estimate of the tumor's aggressiveness. In a study by Kirimlioglu et al,13 almost 15% of small HCCs (<5 cm) developed aggressive features, rather like flat carcinomas of the bladder and colon. In a series of 120 patients transplanted for HCC, Jonas et al8 reported HCC recurrence in 17% of cases (it was the main cause of death after OLT), a G3 HCC in 17% of patients and histologically confirmed vascular invasion in 40%, despite using the Milan criteria to select patients for OLT.
Conversely, tumor differentiation at the time of transplantation represents a direct index of the disease's biologic aggressiveness and is probably a more accurate indicator of the risk of recurrence.9,18,26 Klintmalm9 analyzed the impact of tumor features on survival and recurrence in 422 transplanted patients (International Register of Hepatic Tumors) and showed that, in well-differentiated HCC, tumor size and vascular invasion did not affect survival or tumor recurrence, while grading was the only independent prognostic factor emerging from multivariate analysis. Tamura et al26 claimed even patients with HCCs >5 cm may have excellent survival prospects if the tumor is well-differentiated. This being so, there may be a considerable proportion of patients who would benefit from transplantation but would be refused such a solution if the Milan criteria were followed. This could become a major issue if new strategies for increasing the donor pool (living related, split, marginal livers) make OLT available to a larger number of HCC patients. In the complex setting of living donor liver transplantation, in particular, where the donor has a strong will for dedication and there is no waiting list problem, less selective enrollment criteria for HCC patients are claimed.34,35 In our experience, 13 patients (39% of the pkHCC group) underwent OLT despite not meeting the Milan criteria preoperatively (Table 2), but none of them had HCC recurrence.
Recent studies have shown the importance of microscopic vascular invasion as an independent prognostic factor for HCC patients undergoing resection or transplantation,8,14–21 but this histopathological parameter cannot be used for preoperative selection because it is only assessable by histopathology on the explanted liver. Some studies have shown that the main predictor of microvascular invasion is the histologic grade of the HCC, which can be determined preoperatively by percutaneous needle biopsy.8,9,15,22–24 The close relationship between these two parameters may explain why vascular invasion is often eliminated in multivariate models for analyzing tumor recurrence prognostic factors that include histologic grading, and vice versa.8,9,14–24 Our study confirmed this theory, showing a very low incidence of microscopic vascular invasion (4%) when only G1-G2 HCCs were considered for OLT. Nonetheless, the degree of differentiation of HCC can affect the recurrence risk irrespective of any vascular involvement, being an independent marker of the tumor's biologic aggressiveness.9,26 This is consistent with the lack of tumor recurrence in our 2 cases of vascular microinvasion.
As a consequence, many authors say that liver needle biopsy is essential in proper patient selection for transplantation, although they fear the risk of tumor seeding.9,18,26 There were no cases of tumor implantation attributable to needle biopsies in our experience, however.
HCC recurred in only 6% of our cases and was a minor cause of death after OLT (Table 5). Multifocal and bilobular lesions, occurring, respectively, in 50% and 27% of our patients, had no prognostic significance. The percentage of patients with tumors >5 cm was only 12% of the entire group and 18% of patients with pkHCC, but none of them had recurrent disease. The exclusion criteria (extrahepatic metastases, vascular invasion, poorly differentiated HCC) may have indirectly set a limit for the size rather than for the number of nodules, and this could explain the characteristics of our population. The 3 recurrent HCCs were all G2 unencapsulated tumors but <5 cm (Table 6). Moreover, the long median follow-up in our series (44 months) implies a low risk of underestimating tumor recurrence since the only 3 tumor relapses occurred within a year of transplantation.
The prevalence of iHCC in our study (31%) was comparable with other reports8,9 and related to the diagnostic limits of imaging techniques and the prolonged waiting time.36 Despite the difference in the size of the nodules (Table 4), the recurrence-free survival of patients with iHCC was not significantly better than in patients with pkHCC (Fig. 3), as confirmed elsewhere.9 We found a trend toward a statistically significant difference (P = 0.06) in overall survival rates between the 2 groups (iHCC vs. pkHCC, Fig. 2), but this does not seem to be justified by the tumor's characteristics and recurrences (Table 5). Overall, this suggests that iHCC should be considered in the same way as pkHCC for prognostic purposes and, consequently, also in terms of postoperative treatment and follow-up.9
Several papers10,37,38 have shown that complementary therapies (resection, TACE, CT, PEI) may help control tumor growth while awaiting transplantation and thus reduce the risk of micrometastatic deposits in the early postoperative phase (chemotherapy). In our series, using a multimodal approach both preoperatively (94% of pkHCC patients were treated while awaiting OLT) and postoperatively (44% of all HCC patients received CT) probably contributed toward the results in terms of overall and recurrence-free survival.
CONCLUSION
In the group studied with all cases of G1-G2 HCC, the usual HCC staging procedures (TNM, Milan criteria) applied in retrospect were unable to predict tumor recurrence, whereas using HCC grades (G1 and G2) based on preoperative FNAB to select candidates for OLT was associated with an extremely low rate of tumor recurrence comparable with that of incidentally detected HCC, and an overall long-term survival comparable with OLT for benign diseases (Fig. 2). These results are consistent with reports in the literature after OLT in carefully selected HCC patients,5–7 although nodule size and number were not used as selection criteria. Our experience confirms observations by some other authors9,18,26 that tumor differentiation may accurately reflect tumor aggressiveness and the consequent posttransplant risk of recurrence. It appears to be more accurate than macromorphological parameters and also reduces the risk of rejecting patients who would in fact benefit from OLT. These results support a new strategy for selecting HCC patients suitable for transplantation based on immunohistochemical or molecular biology techniques for identifying new biohumoral or pathologic factors relating to the invasiveness of HCC. In association with histologic grading, such new parameters could further increase prognostic precision in estimating the post-OLT risk of tumor recurrence, substantially improving the patient selection process.39–44 Further studies are needed in this direction.
Footnotes
Reprints: Prof. Umberto Cillo, MD, Clinica Chirurgica 1°, Department of Surgical and Gastroenterological Sciences, University of Padua, School of Medicine Via Giustiniani 2, Policlinico III piano, 35128 Padova, Italy. Email: cillo@unipd.it.
REFERENCES
- 1.Iwatsuki S, Gordon RD, Shaw BW, et al. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease: results in 93 consecutive patients. Ann Surg. 1988;207:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olthoff KM, Millis JM, Rosove MH, et al. Is liver transplantation justified for the treatment of hepatic malignancies? Arch Surg. 1990;125:1261–1268. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. [DOI] [PubMed] [Google Scholar]
- 5.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–1577. [DOI] [PubMed] [Google Scholar]
- 8.Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. [DOI] [PubMed] [Google Scholar]
- 9.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of the tumor characteristics on outcome. Ann Surg. 1998;228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salizzoni M, Zamboni F, Lupo F, et al. Liver transplantation for early-detected, multifocal hepatocellular carcinoma. Br J Surg. 2001;88:1194–1195. [DOI] [PubMed] [Google Scholar]
- 12.Yao FY, Ferrel L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 13.Kirimlioglu H, Dvorchick I, Ruppert K, et al. Hepatocellular carcinomas in native livers from patients with orthotopic liver transplantation: biologic and therapeutic implications. Hepatology. 2001;34:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlitt HJ, Neipp M, Weimann A, et al. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17:324–331. [DOI] [PubMed] [Google Scholar]
- 15.Lauwers GY, Terris B, Balis KP, et al. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic index. Am J Surg Pathol. 2002;26:25–34. [DOI] [PubMed] [Google Scholar]
- 16.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. [DOI] [PubMed] [Google Scholar]
- 17.Margarit C, Charco R, Hidalgo E, et al. Liver transplantation for malignant disease: selection and pattern of recurrence. World J Surg. 2002;26:257–263. [DOI] [PubMed] [Google Scholar]
- 18.Hemming AW, Cattral MS, Reed AI, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh JW, Dvorichik I, Subotin M, et al. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444–450. [DOI] [PubMed] [Google Scholar]
- 20.Iwatsuki S, Dvorchik I, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh JW, Dvorchik I, Bonham CA, et al. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka J, Yamanaka N, Nakasho K, et al. Clinicopathologic analysis of stage II-III hepatocellular carcinoma showing early massive recurrence after liver resection. J Gastroenterol Hepatol. 2000;15:1192–1198. [DOI] [PubMed] [Google Scholar]
- 23.Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232. [DOI] [PubMed] [Google Scholar]
- 24.Park NH, Chung YH, Youn KH, et al. Close correlation of p53 mutation to microvascular invasion in hepatocellular carcinoma. J Clin Gastroenterol. 2001;33:397–401. [DOI] [PubMed] [Google Scholar]
- 25.Frilling A, Malago M, Broelsch CE. Current status of liver transplantation for treatment of hepatocellular carcinoma. Dig Dis. 2001;19:333–337. [DOI] [PubMed] [Google Scholar]
- 26.Tamura S, Kato T, Berho M, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30. [PubMed] [Google Scholar]
- 27.Okuda K, Noguchi T, Kubo Y, et al. A clinical and pathological study of diffuse type hepatocellular carcinoma. Liver. 1981;1:280–289. [DOI] [PubMed] [Google Scholar]
- 28.Kishi K, Shikata T, Makuuchi M, et al. Hepatocellular carcinoma: a clinical and pathologic analysis of 57 hepatectomy cases. Cancer. 1983;51:542–548. [DOI] [PubMed] [Google Scholar]
- 29.Hsu HC, Wu TT, Wu MZ, et al. Tumor invasiveness and prognosis in resected hepatocellular carcinoma: clinical and pathogenetic implications. Cancer. 1988;61:2095–2099. [DOI] [PubMed] [Google Scholar]
- 30.Kemeny F, Vadrot J, Franco D, et al. Morphological and histological features of resected hepatocellular carcinoma in cirrhotic patients in the west. Hepatology. 1989;9:253–257. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki Y, Imaoka S, Ishiguro S, et al. Clinical features of small hepatocellular carcinoma as assessed by histologic grades. Surgery. 1996;119:252–260. [DOI] [PubMed] [Google Scholar]
- 32.Edmonson H, Steiner P. Primary carcinoma of the liver: a study of 100 cases among 48900 necropsies. Cancer. 1954;1:462–503. [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusion of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35:421–430. [DOI] [PubMed] [Google Scholar]
- 34.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. [DOI] [PubMed] [Google Scholar]
- 35.Kaihara S, Kiuchi T, Ueda M, et al. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75(suppl):37–40. [DOI] [PubMed] [Google Scholar]
- 36.Donato MF, Arosio E, Del Ninno E, et al. High rates of hepatocellular carcinoma in cirrhotic patients with high liver cell proliferative activity. Hepatology. 2001;34:523–528. [DOI] [PubMed] [Google Scholar]
- 37.Olthoff KM. Surgical options for hepatocellular carcinoma: resection and transplantation. Liver Transpl Surg. 1998;4(5 suppl 1):98–104. [PubMed] [Google Scholar]
- 38.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701––703. [DOI] [PMC free article] [PubMed]
- 39.Ito Y, Matsuura N, Sakon M, et al. Expression and prognostic roles of the G1-S modulators in hepatocellular carcinoma: p27 independently predicts recurrence. Hepatology. 1999;30:90–99. [DOI] [PubMed] [Google Scholar]
- 40.King KL, Li AF, Chau GY, et al. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000;88:2464–2470. [DOI] [PubMed] [Google Scholar]
- 41.Poon RT, Wong J, Lau C, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furumoto K, Arii S, Mori A, et al. RECK gene expression in hepatocellular carcinoma: correlation with invasion-related clinicopathological factors and its clinical significance. Reverse-inducing-cysteine-rich protein with Kazal motifs. Hepatology. 2001;33:189–195. [DOI] [PubMed] [Google Scholar]
- 43.Gorrin-Rivas MJ, Arii S, Mori A, et al. Implications of human macrophage metalloelastase and vascular endothelial growth factor gene expression in angiogenesis of hepatocellular carcinoma. Ann Surg. 2000;231:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito Y, Takeda T, Sakon M, et al. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer. 2001;84:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]