Abstract
Objective:
Assess application and outcomes of split-liver transplantation within the United States.
Summary Background Data:
While a theoretically attractive mechanism to increase cadaver organ supply, split-liver transplantation has been infrequently applied. The American Society of Transplant Surgeons, in an attempt to gather preliminary data on split-liver transplantation, performed a data protected survey of transplant centers participating in the U.S. Scientific Registry for Transplant Recipients.
Methods:
Between April 2000 and May 2001, 89 surgical teams were surveyed. Elicited data included graft type, recipient status, procurement method, graft sharing, graft outcomes, recipient outcomes, and experience with cadaver, whole-organ transplantation.
Results:
Eighty-three surgical teams reported data on 207 left lateral segment, 152 right trisegment, 15 left lobe, and 13 right lobe grafts. The split procedure was performed ex vivo in 54% and in situ in 46% of grafts. Complications were frequent in all graft types with biliary and vascular complications equally distributed between grafts procured by either technique. Primary nonfunction, graft failure, and recipient death correlated with transplant status.
Conclusions:
Split-liver transplantation has been principally applied to adult-child pairs with at least one recipient critically ill. Biliary and vascular complications account for the majority of morbidity in grafts procured by either split technique with graft failure and recipient death observed more frequently in critically ill recipients. Enhanced utilization and improved results may be possible through improved information sharing and modification of allocation criteria.
The American Society of Transplant Surgeons conducted a survey of U.S. transplant centers to assess application and outcomes of split-liver transplantation. Split-liver transplantation is infrequently used but yields promising results as a mechanism to reduce organ scarcity. Enhanced utilization and improved outcomes may be possible through improved information sharing and the potential modification of current organ allocation criteria.
Split-liver transplantation (SLT), a procedure where 1 cadaver donor liver is divided to provide for 2 recipients, has been a surgical option for more than a decade.1–3 Despite an increasing cadaver organ shortage, a paucity of data exists from North American, European, and Asian transplant centers on the performance of SLT.4–6 Impediments to increased SLT utilization, including technical and logistic demands as well as a prohibitive organ allocation system, have been identified,5 however, the potential exists for SLT to significantly increase cadaver organ supply. The American Society of Transplant Surgeons (ASTS), recognizing the need to realize the greatest supply from a cadaver donor pool and the potential of SLT, attempted to gather preliminary data on SLT application and outcomes through a survey of transplant centers participating in the U.S. Scientific Registry for Transplant Recipients (SRTR).
METHODS
Survey Administration
In February 2000, the President of the ASTS requested the Standards of Organ Procurement Committee to perform a data-protected survey of transplant centers contributing to the SRTR to determine SLT application and outcomes. A survey was drafted and adopted by the Committee prior to distribution. Data collection was initiated in April 2000 and continued through May 2001.
Identification of U.S. Transplant Centers for Data Collection
The 1999 Annual Report of the SRTR6 identified 109 transplant centers that had performed at least one liver transplant procedure during the previous year. From this list, 89 surgical “teams” were identified as some perform transplant procedures at two or more facilities. Two contributing teams are located in Canada. The Director of each surgical team received an introductory letter describing the goals of the survey as well as a questionnaire. Self-reporting was encouraged through follow-up letters issued at monthly intervals. The data-protected survey did not disclose individual center information; rather, noncenter-specific data updates were periodically distributed to participating centers throughout the data collection period. Access to center-specific data was strictly limited to the President of the ASTS, the Standards of Organ Procurement Committee Chair, and the study coordinator.
Survey Format
The survey elicited data on graft type, United Network for Organ Sharing (UNOS) recipient status at the time of transplantation, method of graft procurement (in situ or ex vivo), recipient morbidity/mortality, graft sharing between centers, graft outcomes, and transplant center experience with whole-organ, cadaver liver transplantation.
The anatomic classification of the liver described by Couinaud7 and refined by Bismuth8 is the universally accepted reference system for the description of SLT grafts. This survey recognized two distinct surgical procedures: the adult/child SLT that yields a left lateral segment ([LLS] Couinaud segments II-III) and a right trisegment ([RTS] Couinaud segments I, IV-VIII) graft,9 and the adult/adult SLT that yields a left lobe (Couinaud segments I-IV or II-IV) and right lobe (Couinaud segments V-VIII, or I, V-VIII) graft.10,11
RESULTS
Application of SLT
Eighty-three surgical teams submitted data yielding a 93% response. Data on 387 SLT grafts included 207 LLS, 152 RTS, 15 left lobe, and 13 right lobe grafts. Of the 83 responding teams, only 36 (43%) reported experience with SLT. When stratified by SLT volume, 23 of the 36 teams with SLT experience (63%) had performed less than 5 procedures. The 13 teams reporting experience with 5 or more SLT procedures accounted for a total of 221 grafts (range10–75) or 57% of the cumulative data.
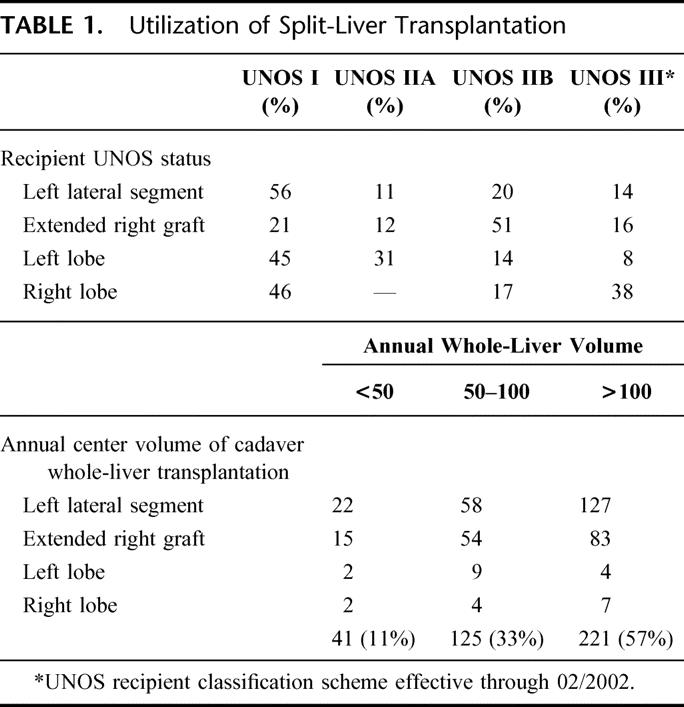
SLT was most frequently applied to at least one urgent recipient for both adult/pediatric and adult/adult pairs (Table 1) as the majority of LLS, right, and left lobe grafts were applied to UNOS status I and IIA recipients. SLT technique was ex vivo in 54% and in situ in 46% of overall procurements with 9 teams (25%) reporting experience with both techniques. When queried as to their preference, 48% of responders preferred in situ, 27% preferred ex vivo, and 25% expressed no preference.
TABLE 1. Utilization of Split-Liver Transplantation
Center Volume
Center volume of cadaver whole-liver transplantation correlated with SLT application (Table 1). Among transplant centers performing less than 50 cadaver whole-liver transplants annually, only 13 of 42 responding groups (31%) had performed an SLT versus 14 of 29 centers (48%) performing 50 to 100 cadaver whole-liver transplants annually. All 9 of the reporting transplant centers with annual volume greater than 100 cadaver whole-organ transplants had performed SLT.
Recipient Complications
Successful implementation of SLT mandates additional surgical and medical considerations as partial-liver grafts predispose to complications resulting from anatomic variation, technical considerations, and recipient physiology. Frequent complications include hemorrhage, bile leakage, anastomotic stricture, necrosis of the transected parenchyma, disparity of graft size (too small or too large), hepatic venous outflow obstruction, and complex vascular reconstructions.12–14 Reported morbidity varied with SLT graft type. Biliary and vascular complications were the principal source of morbidity in all graft types procured by either split technique; however, distinctions were apparent. Primary nonfunction, graft failure, and recipient death were observed more frequently in critically ill recipients of all SLT graft types.
Left Lateral Segment Grafts
Data on 207 LLS grafts indicated a 32% incidence of complications. Biliary complications were most frequent (13%), followed by vascular complications (9%) and postoperative hemorrhage (3%). Biliary complications included parenchyma surface leak (69%), anastomotic stricture (23%), and anastomotic leak (8%). Vascular complications were equally distributed between hepatic artery thrombosis and portal vein thrombosis with a single report of hepatic artery stenosis. A variety of other nonspecific complications including reoperation for peritonitis, bowel obstruction, hemorrhage from the Roux-limb jejunojejunostomy, and inability to provide surgical wound closure accounted for an additional 5% of reported complications.
LLS graft complications stratified by UNOS status are presented in Table 2. The incidence of LLS graft complications remained constant between UNOS recipient status; however, the complication type varied. Vascular complications were more frequent in critically ill recipients while biliary complications were more common among UNOS status IIB and III recipients. Primary nonfunction and graft failure were observed principally in UNOS status I recipients. The incidence of primary nonfunction was 8% and graft failure 12%, with all cases, except 1, occurring in UNOS status I recipients.
TABLE 2. Left Lateral Segment Complications by Recipient UNOS Status
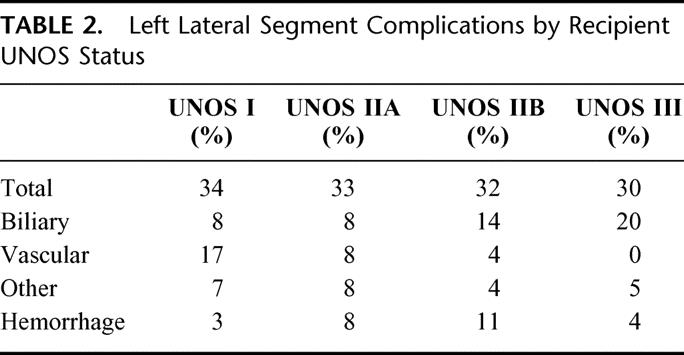
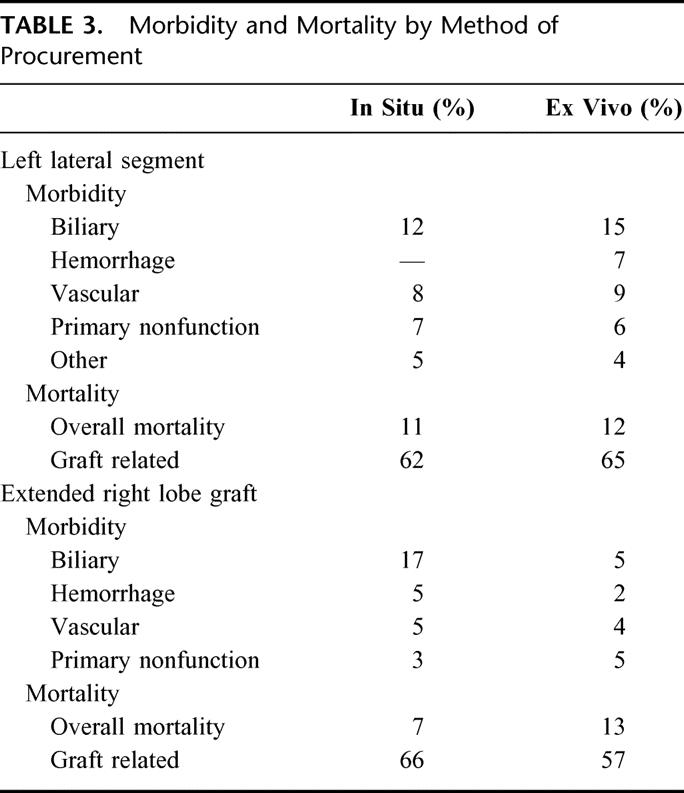
The incidence of LLS recipient death was 11% with two thirds graft-related. Death occurred 4 times more frequently among UNOS status I recipients. LLS morbidity and mortality by procurement technique (Table 3) are comparable, except for the incidence of postoperative hemorrhage. Postoperative hemorrhage requiring reoperation occurred in 7% of ex vivo LLS recipients versus no in situ LLS recipients.
TABLE 3. Morbidity and Mortality by Method of Procurement
Right Trisegment Grafts
The RTS graft is the complement of the LLS graft. A total of 152 RTS grafts were reported. The 55 graft deficit between the number of LLS grafts and RTS grafts is unaccountable. The overall incidence of RTS complications was 26%. Biliary complications were most frequent (11%), followed by a 5% incidence of vascular complications, posttransplant hemorrhage (4%), and other complications (3%) including acute rejection, intra-abdominal hematoma, aspergillosis from a paraspinous abscess, candidial sepsis from a necrotic segment IV, and delayed graft function. There was a 4% incidence of nongraft-related complications that included respiratory failure, pulmonary edema, posttransplant neuropathy, and perforated duodenal ulcer. As observed in LLS grafts, the most frequent biliary complication was parenchyma leak from the transected liver surface (82%) with a smaller incidence of biliary stricture (18%). Vascular complications of RTS grafts were distinct from LLS grafts. Hepatic arterial thrombosis was most frequently reported, but portal and hepatic venous thromboses were not observed in RTS grafts. Furthermore, there were five reported cases of hepatic artery pseudoaneurysm with one hepatic artery disruption. At least three of the reported psuedoaneurysms were infectious in etiology.
Analysis by method of procurement (Table 3) revealed distinct differences in RTS outcomes. Complications were comparable with respect to hemorrhage, vascular, and primary nonfunction between grafts procured by either technique; however, biliary complications were more frequent among in situ SLT recipients while recipient death was more frequent among ex vivo SLT recipients. Recipient death was usually from graft failure in each group.
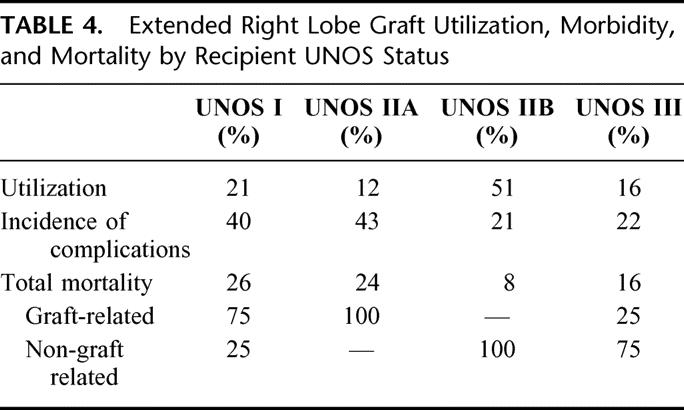
The effect of recipient UNOS status at SLT is summarized in Table 4. While the majority of RTS grafts were used in nonurgent, UNOS status IIB recipients, morbidity and mortality were concentrated among urgent, UNOS status I and IIA recipients. The overall incidence of reported mortality among RTS recipients was 15% with more than one half of deaths attributed to graft-related complications. The distribution of graft-related mortality is highlighted by stratification. Mortality among UNOS status I and IIA recipients was related to graft function. The opposite is true of mortality among UNOS status IIB and III recipients. The overall incidence of primary nonfunction was 4%.
TABLE 4. Extended Right Lobe Graft Utilization, Morbidity, and Mortality by Recipient UNOS Status
Left and Right Lobe Grafts
The number of left and right lobe grafts resulting from adult/adult SLT is too few for meaningful analysis. Of the 15 left lobe and 13 right lobe grafts, 76% of left lobe and 46% of right lobe grafts were for urgent status recipients. The incidence of complications for adult/adult SLT was 26% for left lobe versus 22% for right lobe grafts. Biliary complications were most frequent in 18% of left lobe and 10% of right lobe grafts. Vascular complications occurred in 4% of left lobe and 9% of right lobe grafts. Primary nonfunction and graft failure were reported as 7% and 9% for left lobe versus 9% and 14% for right lobe grafts, respectively. Recipient death was observed in 7% of left versus 8% of right lobe grafts.
Graft Sharing Between Centers
Five centers reported sharing 18 grafts, or 5% of total graft number. All shared grafts were the result of adult/child SLT with the distance between shared-graft centers ranging from <10 miles to across the United States. Only one shared-graft complication was reported: primary nonfunction requiring retransplantation of an RTS graft with a cross-country transport.
Comparison of Survey Data to Published Outcomes of SLT
Sans a dedicated SLT registry in Europe, North America, or Asia, it is exceedingly difficult to accurately determine outcomes as detailed data do not exist outside of individual reports. Data on 39,246 grafts from the European Liver Transplant Registry suggest SLT represented 3.7% of the total reported transplants; however, SLT data have not been stratified and outcomes were not reported.4 This report of 387 SLT grafts is the largest collection of data to date, but it is subject to the natural limitations of voluntary participation and self-reporting. Given these limitations, we compared these data to the English-language published literature as well as a review of recent abstracts.
Adult/Child SLT
The initial series of SLT reported by Broelsch in 1990 demonstrated recipient survival that was inferior to cadaver, whole-liver recipients.3 A later, expanded series failed to improve SLT outcomes resulting in early skepticism.15 The Europeans were the first to demonstrate comparable whole-organ results when de Ville reported an experience of 100 grafts collected from the European Split Liver Registry in 1995.16 Graft and recipient 6-month survival were stratified by status at SLT with elective pediatric graft and recipient survival of 80% and 89% versus 61% and 61%, respectively, for children transplanted urgently. Elective adult graft and recipient survival were 72% and 80% versus 56% and 68%, respectively, for urgent adult status. Twenty percent of grafts were lost to complications including hepatic artery thrombosis (11%), portal vein thrombosis (4%), and a 19% incidence of biliary complications. These data were equivalent to the European Liver Transplant Registry survival data for cadaver, whole-organ liver transplantation.
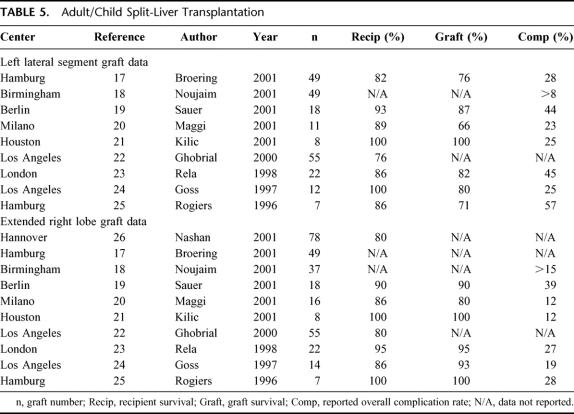
Adult/child SLT expanded during the late 1990s with single-center outcomes listed in Table 5. The data presented in this report, with respect to LLS grafts, compare favorably to existing literature. The overall 32% incidence of complications is similar to that reported by individual centers, as is the classification of complications, with biliary complications most common,17 followed by a 5% to 9% incidence of vascular complications.17,18,21 Vascular complications of hepatic artery, portal vein, and hepatic veins were reported,17–19,21 as observed in our survey. Exploration for sepsis from intestinal perforation has been reported23 outside our survey with poorer outcomes in the setting of urgent SLT.22
TABLE 5. Adult/Child Split-Liver Transplantation
Improved results in RTS recipients versus LLS recipients in our survey, with respect to the incidence and nature of complications, is similar to single-center reports (Table 5). Our 26% incidence of overall complications is similar to reported data, as are the 11% incidence of biliary and 5% incidence of vascular complications. Segment IV necrosis has been reported by other centers19,23,25,26 with nongraft-related complications more frequent among RTS recipients.22
SLT has been performed in Asia with organ sharing among Asian centers.27 While the total number of SLT procedures performed is low secondary to scarce cadaver donation, logistic, cultural, and manpower constraints, de Villa summarized Asian SLT data on 26 grafts from 5 centers. Complications were not reported by graft type but did include parenchymal surface bile leak, portal vein thrombosis, portal vein stenosis, hepatic artery insufficiency, and T-tube dislodgement requiring celiotomy. Four deaths were reported yielding an 85% overall recipient survival and at least 1 other recipient required retransplantation.
Graft sharing was infrequent accounting for 5% of total grafts. Ghobrial et al have reported the largest sharing experience of one LLS and 16 RTS grafts,22 Azoulay et al have reported sharing 4 of 36 SLT grafts,28 and Rela et al have shared 3 of 41 grafts obtained from ex vivo SLT.23
Adult/Adult SLT
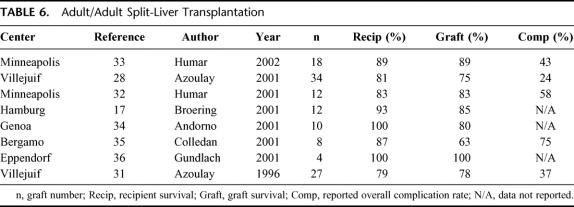
Sparse data exist on SLT between 2 adults as these techniques have been cautiously implemented among select transplant centers, particularly in Europe (Table 6). The initial series from the University of Chicago yielded patient and graft survival of 67% and 50%, respectively.29 Outcomes were less than matched recipients of cadaver whole grafts over the same time period; however, the difference did not achieve statistical significance.30 The Paul Brousse group has reported the largest SLT series for 2 adults. In 1996, Azoulay et al reported 1-year patient and graft survival of 79% and 78%, respectively, on 27 SLT grafts in 26 adults.31 The routine application of SLT during the study period increased overall graft availability by 28%. Biliary complications were most frequent with an incidence of 22%, followed by a 15% incidence of arterial complications and one reported primary nonfunction. All SLT procedures were performed ex vivo with an average benching time of 2 to 3 hours. An expanded series reported in 2001 of 34 SLT (ex vivo 30, in situ 4) comparing 1- and 2-year SLT patient and graft survival to 88 adults receiving cadaver whole-organ grafts over the same time period demonstrated right- and left-SLT graft 1-year recipient survival of 74% versus 88%, respectively, with 1-year graft survival of 74% for right-SLT versus 75% for left-SLT recipients.28 The incidence of SLT vascular complications was 6% with a 22% incidence of biliary complications. Graft complications were not uniformly distributed among graft types with a significantly higher incidence of biliary complications observed in left grafts and a higher incidence of arterial complications in right grafts.28 While no significant difference between whole and SLT recipient 1-year survival was identified, the increased incidence of complications observed in SLT grafts contributed to a significantly decreased incidence of graft survival at 1 year, particularly among left-SLT recipients. The authors performed SLT in 15% of available donors and reported a 62% net increase in adult recipients through the routine application of SLT. This led the Paris group to conclude that SLT between 2 adults was technically feasible with good outcomes, provided recognition of donor and recipient limitations as well as surveillance for complications unique to SLT.28
TABLE 6. Adult/Adult Split-Liver Transplantation
Humar et al reported the first North American series of 6 adult/adult SLT procedures demonstrating patient and graft survival of 83% at a mean follow-up of 9 months.32 Mean recipient weight was 89 kg in right graft versus 60 kg for left graft recipients. Right lobe recipient complications included hepatic artery thrombosis, celiotomy for hemorrhage, and a cut surface bile leak. Left lobe recipient complications included hepatic artery thrombosis, an anastomotic leak requiring hepatojejunostomy, and an incisional hernia. An updated abstract by the same group reports 9 procedures yielding 18 grafts with a mean follow-up of 18 months.33 Seventeen of the 18 recipients were UNOS status IIB and 1 was UNOS status IIA. Patient and graft survival was 89% for right lobe versus 78% for left lobe grafts. Biliary complications were most frequent (27%), followed by an 11% incidence of vascular complications that resulted in 2 deaths. There was one reported primary nonfunction in an unspecified graft.
The data herein are in agreement with these published reports (Table 6). Complications were observed in 26% of left graft and 22% of right graft SLT recipients. The same pattern of complications emerged from our survey data as reported by the Paris group with vascular complications more frequent in right lobe grafts and biliary complications more frequent in left lobe grafts.28 Graft survival was higher in right lobe SLT recipients with a similar incidence of primary nonfunction and recipient death.
Comparison of Survey Data to Outcomes of the UNOS Scientific Registry of Transplant Recipients
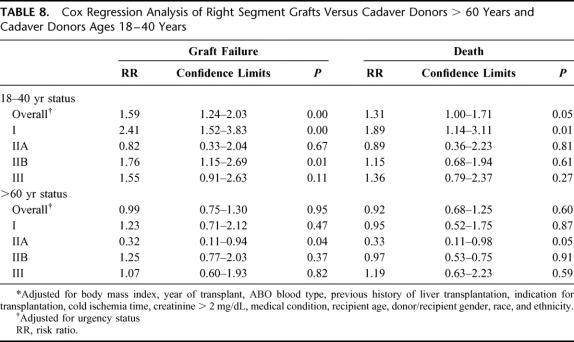
The UNOS SRTR responded to a data request from the OPTN Liver and Intestinal Transplantation Committee37 to provide outcomes of SLT right lobe grafts in adults. Graft-specific coding does not exist in the database; therefore, a data search was performed for allografts classified as “partial right liver segments” that had corresponding left segments also transplanted or right “split liver segments” prepared either ex vivo or in situ. Recognizing that SLT occurs in optimal donors, partial right graft outcomes were compared with two groups: a comparable group of whole-organ cadaver donors between 18 and 40 years of age and a surrogate “marginal donor” group consisting of cadaver whole-organ donors >60 years of age. Between January 1, 1994 and March 1, 2001, a total of 215 SLT right grafts, including 33 partial, 42 in situ, and 140 ex vivo grafts, were compared with 2901 grafts procured in donors >60 years of age and 9802 grafts procured from donors ages 18 to 40 years. Outcomes, measured by graft failure and recipient death, were stratified by medical urgency status and adjusted for body mass index, year of transplant, cause of death, ABO blood compatibility, history of previous liver transplantation, indication for transplantation, cold ischemia time, creatinine >2 mg/dL, medical condition, and donor/recipient age, gender, and ethnicity. Graft failure and death (Table 7) occurred in 68 (32%) and 56 (26%) of the 215 recipients, respectively. Data from right lobe grafts were generally comparable to outcomes of whole-organ grafts from donors >60 years of age and inferior to outcomes from cadaver whole-organ donors 18 to 40 years of age. When stratified by medical urgency, UNOS status I recipients demonstrated the poorest outcomes. Cox regression analysis of right graft outcomes compared with the 2 reference groups (Table 8) demonstrated a significantly increased overall relative risk of graft failure and death among right graft recipients versus cadaver donors 18 to 40 years of age. When stratified by UNOS status at liver transplantation, UNOS status I recipients of right grafts demonstrated a significantly increased risk of graft failure and death versus cadaver whole-organ donors 18 to 40 years of age (Table 8). Significantly increased graft failure was observed in UNOS status IIB and III right graft recipients as well (Table 8) but did not translate into significantly increased deaths.
TABLE 7. Outcomes of Right Segment Grafts Versus Cadaver Donors > 60 Years and Cadaver Donors Ages 18–40 Years
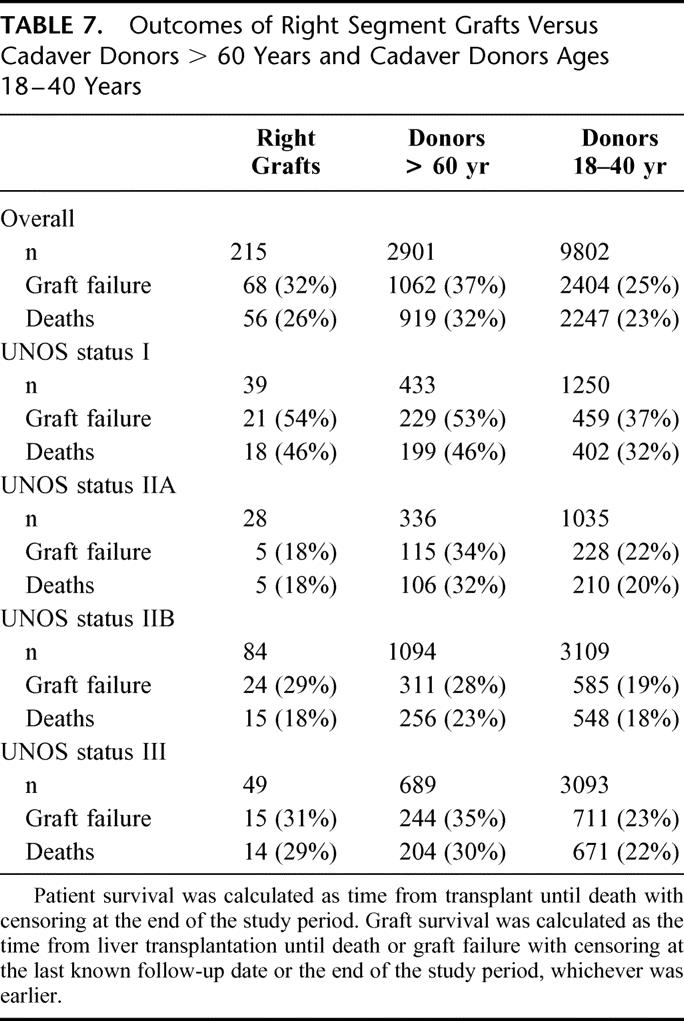
TABLE 8. Cox Regression Analysis of Right Segment Grafts Versus Cadaver Donors > 60 Years and Cadaver Donors Ages 18–40 Years
Right graft data were comparable to the “marginal donor” group with overall graft failure and death not statistically different (Table 8). Significantly lower graft failure and death were observed among UNOS status IIA right graft recipients versus marginal donors but were equal in all other groups (Table 8). Thus, the splitting of presumptive optimal donors yielded right grafts with outcomes similar to cadaver whole-organs procured from “marginal donors.”
The SRTR data demonstrate markedly poorer outcomes than our survey data or data from the literature. The SRTR's failure to distinguish RTS from right lobe grafts and to detail complications limits interpretation; however, the data do confirm that SLT is an infrequent procedure subject to significant complications. The 215 grafts identified from the UNOS database exceeds the number of RTS and right lobe grafts identified in this survey by 22%. Numeric discrepancy occurred in the SRTR that could not identify a corresponding SLT graft recipient in 12% of donors versus our observed 13% discrepancy. No data were available to demonstrate a “learning curve” or evaluate the progression of SLT with respect to technique. Furthermore, the limited number and imbalanced distribution of in situ and ex vivo right lobe grafts prevented stratification by SLT technique.
DISCUSSION
This early attempt to assess SLT application and outcomes in the United States is a data-protected survey that relied upon voluntary participation and self-reporting. The data are not verifiable and there are obvious gaps in organ accountability. Our reporting gap is 57 grafts, or 13% of total graft number, a value similarly observed in the SRTR. The discrepant data are likely the result of multiple factors including graft sharing with nonpaired graft reporting, misinterpretation of survey instructions, and the reporting of graft outcomes obtained through reduced-liver or living-donor transplantation. In the absence of an organized, verifiable, data collection instrument, information on these procedures will be extremely limited and dissemination inefficient.
The greater than 90% response to our survey demonstrates a keen interest in SLT. Clearly, the crisis in organ donation that currently exists is universal and has heightened awareness of potential avenues for increased donation. Despite this interest, our data confirm that SLT accounts for a very small fraction of cadaver grafts. Of the 83 responding teams, only 45% reported any experience with SLT and two thirds of centers with SLT experience had performed less than 5 procedures. The 13 groups reporting an experience of 5 or more SLT procedures accounted for 57% of the cumulative data. Transplant center volume correlated with SLT experience as all reporting centers with a volume of >100 transplants annually had experience with SLT versus 31% of centers with volume of <50 annual transplants. This observation may result from increased performance of pediatric transplantation among larger centers and, while a “learning curve” was not assayed by this survey, almost certainly exists as demonstrated in liver living-donation.38–40 The unequal distribution of experience, coupled with a required “learning curve,” forecast greater information sharing must occur prior to SLT achieving a larger role in alleviating the current organ crisis. Presently, graft sharing is an uncommon event with only 5 centers reporting a total of 18 shared grafts (4%). Improved information sharing has the potential for greater center graft sharing that would increase experience and decrease reluctance to accept partial grafts for transplantation.
The survey data are promising. The results are superior to the SRTR and similar to isolated center outcomes. A decade of data on transplantation of LLS grafts obtained from SLT or living-donors for children has validated the applicability of this technique through patient and graft survival that meets or exceeds whole-organ cadaver data.5 The benefits of SLT, without donor risk, make it the preferred technique for the transplantation of infants and small children without access to cadaver whole-organs.
The fate of adults who receive RTS grafts remains controversial. Two series examined RTS outcomes by comparing the split cohort with adults receiving cadaver whole-organs during the same time period22 or via case-matched controls.17 In each study, 1-year patient survival was comparable between RTS and whole-organ recipients. While excellent single-center data exist, our data and the SRTR demonstrate inferior outcomes when applied to adults in urgent need of liver transplantation. This question is extremely important for the future of SLT as equal outcomes between RTS grafts and cadaver whole-organs in recipients across categories of urgency and indication are prerequisite for SLT to be further applicable. The SRTR suggests the performance of SLT, presumably in “optimal” donors, delivers right graft outcomes (precise type of right graft not specified) similar to “marginal grafts” and inferior to whole-organs derived from cadavers 18 to 40 years of age. This difference may be justifiable if access is increased through a recipient's participation and complementary graft outcomes are considered; however, SLT will never significantly impact organ availability on a wide scale if these data prove true. These data currently do not exist outside of single center reports and underscore the necessity for a dedicated, multicenter study to define the role of RTS grafts in decompensated cirrhotics and fulminant hepatic failure to predict the ultimate applicability of SLT.
The inability of the SRTR to approach the data of select individual centers could be explained by the failure of these data to demonstrate a “learning curve,” corruption of these data with early attempts at SLT, and the predominance of grafts obtained by ex vivo dissection.
The in situ technique that incorporates hilar dissection and parenchymal transection in the donor prior to aortic cross clamp reduces cold ischemia time, simplifies identification of biliary and vascular structures, and avoids the mandatory benching period and rewarming necessary to perform ex vivo SLT.26,42 The technique can be performed without special equipment or impedance of thoracic or additional abdominal organ procurement.28 Speculation that the prolonged dissection required to prepare the in situ grafts prior to aortic cross clamp may be associated with increased blood loss and volume replacement prompted early concerns from cardiac-thoracic teams that the quality of hearts and lungs may be affected;43 however, data from centers with a commitment to in situ SLT suggest the effect is negligible.44
In situ SLT promotes organ sharing by decreasing cold ischemia and completing the dissection in the donor prior to removal and shipping of the 2 grafts. Comparison of in situ and ex vivo SLT left lateral segment grafts suggests a higher incidence of primary nonfunction among grafts prepared ex vivo versus in situ and greater applicability of in situ grafts to urgent status recipients24,26,31,41,45,46; however, other transplant centers have reported excellent results using the standard ex situ technique in nonurgent recipients.23 Our data indicate relative parity between the 2 techniques, except for the incidence of postoperative hemorrhage; however, no conclusions can be drawn due to small graft numbers in each group and nonstandard performance of each procedure under variable conditions.
The possibility of SLT complications distinct from standard, cadaver whole-organ grafts inevitably compounds the potential morbidity of an SLT recipient. In children, the enhanced possibility of morbidity resulting from SLT complications is offset by first quality hepatic parenchyma of abundant supply and the technical demands of pediatric whole-organ grafts. While parenchymal quality remains in the adult SLT recipient, supply is compromised and technical demands are greater than cadaver whole-organ transplant yielding comparatively lower SLT outcomes. Complications and reoperations are poorly tolerated by the decompensated cirrhotic deconditioned by an extended waiting period. Ironically, the U.S. patient-driven system of allocation to the sickest first without performance incentives for SLT allocates premium organs to these patients, further discouraging the utilization of SLT.
Most of SLT data reported to date are adult/child pairs where the split produces one RTS and an LLS graft. While this is the most successful combination, <10% of the wait-list is comprised of small children and one half are satisfied by pediatric donors.5 To further implement SLT, centers contemplating SLT must have access to a diversity of recipients of different sizes that can be paired. This flexibility is best achieved through center collaboration or “shared-lists” to benefit the overall donor pool. The previous UNOS policy permitting the unrestricted use of the remaining SLT graft by the performing center provided a direct incentive for the application of SLT; however, in regions where multiple centers are competing for each donor, strict allocation to the sickest recipient on the wait-list limits the flexibility of adapting the donor to the optimal recipients. The European allocation scheme of cadaver organs to centers rather than specific patients affords greater flexibility in the performance of SLT and standing agreements between centers that provide for sharing have been quite successful.47
ACKNOWLEDGMENTS
The authors thank the following transplant centers for their participation: Baylor University (Dallas, TX), Boston University (Boston, MA), California Pacific Medical Center (San Francisco, CA), Carolinas Medical Center (Charlotte, NC), Cedars Sinai Medical Center (Los Angeles, CA), Cleveland Clinic Hospitals (Cleveland, OH), Colombia-Presbyterian Medical Center (New York, NY), Deaconess Hospital (Boston, MA), Duke University (Durham, NC), Albert Einstein University (Philadelphia, PA), Emory University (Atlanta, GA), Froedtert Memorial Hospital (Milwaukee, WI), Good Samaritan Hospital (Phoenix, AZ), Hartford Hospital (Hartford, CT), Henry Ford (Detroit, MI), Johns Hopkins Hospital (Baltimore, MD), Howard University (Washington, DC), Indiana University (Indianapolis, IN), Inova Fairfax Hospital (Falls Church, VA), Integris Baptist Medical Center (Oklahoma City, OK), Jewish Hospital (Louisville, KY), Latter Day Saints Hospital (Salt Lake City, UT), Loma Linda University (Loma Linda, CA), London Health Sciences Center (London, Ontario, Canada), Louisiana State University (New Orleans, LA), Massachusetts General Hospital (Boston, MA), Medical College of South Carolina (Charleston, SC), Medical College of Virginia (Richmond, VA), Medical College of Wisconsin (Milwaukee, WI), Mount Sinai Medical Center (New York, NY), Northwestern University (Chicago, IL), New York University (New York, NY), Ochsner University (New Orleans, LA), Oregon Health Sciences (Portland, OR), Pennsylvania State University (Hershey, PA), Rush-Presbyterian Hospital (Chicago, IL), Scripps Clinic (La Jolla, CA), St. Francis Medical Center (Honolulu, HI), St. Louis University (St. Louis, MO), St. Luke Hospital (Houston, TX), St. Vincent Medical Center (Los Angeles, CA), Stanford University (Palo Alto, CA), Tampa General (Tampa, FL), Temple University (Philadelphia, PA), Thomas Jefferson University (Philadelphia, PA), Toronto General Hospital (Toronto, Ontario, Canada), Tulane University (New Orleans, LA), University of Alabama (Birmingham, AL), University of Arizona (Tucson, AZ), University of California Davis (Sacramento, CA), University of California Los Angeles (Los Angeles, CA), University of California San Francisco (San Francisco, CA), University of California Irvine (Irvine, CA), University of California San Diego (San Diego, CA), University of Cincinnati (Cincinnati, OH), University of Cleveland (Cleveland, OH), University of Colorado (Denver, CO), University of Florida (Gainesville, FL), University of Illinois (Chicago, IL), University of Iowa (Iowa City, IA), University of Kansas (Kansas City, KS), University of Kentucky (Lexington, KY), University of Maryland (Baltimore, MD), University of Medicine and Dentistry of New Jersey (Newark, NJ), University of Miami (Miami, FL), University of Michigan (Ann Arbor, MI), University of Minnesota (Minneapolis, MN), University of Nebraska (Omaha, NE), University of New Mexico (Albequerque, NM), University of Pennsylvania (Philadelphia, PA), University of Pittsburgh (Pittsburgh, PA), University of Rochester (Rochester, NY), University of Southern California (Los Angeles, CA), University of Tennessee (Memphis, TN), University of Texas (Galveston, TX), University of Texas (Houston, TX), University of Texas (San Antonio, TX), University of Virginia (Charlottesville, VA), University of Washington (Seattle, WA), Vanderbilt University (Nashville, TN), Washington University (St. Louis, MO), Willis-Knighton Medical Center (Shreveport, LA), and Yale University (New Haven, CT).
Footnotes
Reprints: John F. Renz, MD, PhD, Center for Liver Disease and Transplantation, Room PH 14C, New York Presbyterian Hospital, 622 W. 168th Street, New York, NY 10032. E-mail: jfr2103@columbia.edu.
REFERENCES
- 1.Pichlmayr R, Ringe B, Gubernatis G. Transplantation of a donor liver to 2 recipients (splitting transplantation): a new method in the further development of segmental liver transplantation. Langenbecks Archiv Chir. 1989;373:127–130. [PubMed] [Google Scholar]
- 2.Bismuth H, Morino M, Castaing D, et al. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989;76:722–724. [DOI] [PubMed] [Google Scholar]
- 3.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver: analysis of a preliminary experience with “Split Liver” grafting. Ann Surg. 1990;212:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Karam V, Delvart V, et al. Evolution of liver transplantation in Europe. Report of the European Liver Transplant Registry. Joint Meeting of the International Liver Transplantation Society, European Liver Transplantation Association, and the Liver Intensive Care Group of Europe. Berlin, Germany, July 11–13, 2001.
- 5.Emond JC, Freeman RB, Renz JF, et al. Optimizing the use of donated cadaver livers: analysis and policy development to increase the application of split-liver transplantation. Liver Transpl. 2002;8:863–872. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. 1999 Annual Report of the U.S. Scientific Registry for Transplant Recipients and the Organ Procurement and Transplantation Network: Transplant Data: 1990–1998. U.S. Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation, Rockville, MD; United network for Organ Sharing, Richmond, VA (www.UNOS.org).
- 7.Couinaud C. Le Foie. Paris: Masson, 1957. [Google Scholar]
- 8.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 9.Yersiz H, Renz JF, Hisatake GM, et al. The conventional technique of in-situ split-liver transplantation. J Hepatobiliary Pancreatic Surg. 2003;10:11–15. [DOI] [PubMed] [Google Scholar]
- 10.Yersiz H, Renz JF, Hisatake GM, et al. Technical and logistical considerations of in situ split-liver transplantation for two adults: I. Creation of a left segment II, III, IV and right segment I, V-VIII grafts. Liver Transpl. 2001;7:1077–1080. [DOI] [PubMed] [Google Scholar]
- 11.Yersiz H, Renz JF, Hisatake GM, et al. Technical and logistical considerations of in situ split-liver transplantation for two adults: II. Creation of a left segment I-IV and right segment V-VIII grafts. Liver Transpl. 2002;8:78–81. [DOI] [PubMed] [Google Scholar]
- 12.Renz JF, Reichert PR, Emond JC. Biliary anatomy as applied to pediatric living donor and split-liver transplantation. Liver Transpl. 2000;6:801–804. [DOI] [PubMed] [Google Scholar]
- 13.Renz JF, Reichert PR, Emond JC. Hepatic arterial anatomy as applied to living-donor and split-liver transplantation. Liver Transpl. 2000;6:367–369. [DOI] [PubMed] [Google Scholar]
- 14.Emond JC, Heffron TG, Whitington PF, et al. Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg, Gynecol Obstet. 1993;76:11–17. [PubMed] [Google Scholar]
- 15.Emond JC, Heffron TG, Thistlethwaite JR, et al. Innovative approaches to donor scarcity: a critical comparison between split liver and living related liver transplantation. Hepatology. 1991;14:92A. [Google Scholar]
- 16.de Ville de Goyet J. Split liver transplantation in Europe: 1988 to 1993. Transplantation. 1995;59:1371–1376. [DOI] [PubMed] [Google Scholar]
- 17.Broering D, Mueller L, Ganschow R, et al. Is there still a need for living-related liver transplantation in children. Ann Surg. 2001;234:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noujaim HM, Mayer DA, Buckels JAC, et al. Division of vascular pedical and vascular complications after ex vivo split liver transplantation. Report of the European Liver Transplant Registry, Joint Meeting of the International Liver Transplantation Society, European Liver Transplantation Association, and the Liver Intensive Care Group of Europe. Berlin, Germany, July 11–13, 2001.
- 19.Sauer IM, Pascher A, Steinmuller T, et al. Split liver and living donation liver transplantation: the Berlin experience. Transplant Proc. 2001;33:1459–1460. [DOI] [PubMed] [Google Scholar]
- 20.Maggi U, Rossi G, Reggiani P, et al. Graft loss and retransplantation rate after split in situ liver transplantation. Joint Meeting of the International Liver Transplantation Society, European Liver Transplantation Association, and Liver Intensive Care Group of Europe. Berlin, Germany, July 11–13, 2001, Liver Transplantation 2001:7.
- 21.Kilic M, Seu P, Goss JA. Maintaining the celiac trunk with the left graft for in situ split liver transplantation. Joint Meeting of the International Liver Transplantation Society, European Liver Transplantation Association, and Liver Intensive Care Group of Europe. Berlin, Germany, July 11–13, 2001, Liver Transplantation 2001:7.
- 22.Ghobrial RM, Yersiz H, Farmer DG, et al. Predictors of survival after in vivo split liver transplantation: analysis of 110 consecutive patients. Ann Surg. 2000;232:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rela M, Vougas V, Muiesan P, et al. Split liver transplantation. Ann Surg. 1998;227:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goss JA, Yersiz H, Shackleton CR, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation. 1997;64:871–877. [DOI] [PubMed] [Google Scholar]
- 25.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers: the ultimate expansion of a limited donor pool. Ann Surg. 1996;224:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nashan B, Lueck R, Becker T, et al. Outcome of split liver transplantation using ex-situ or in-situ splits from cadaver donors. Transplant 2001, Chicago, IL, May 12–16, 2001.
- 27.de Villa VH, Chen CL, Chen YS, et al. International sharing of split liver grafts in Asia: initial experience. Clin Transplant. 2000;14:355–359. [DOI] [PubMed] [Google Scholar]
- 28.Azoulay D, Castaing D, Adam R, et al. Split-liver transplantation for two adult recipients: feasibility and long-term outcomes. Ann Surg. 2001;233:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver: analysis of a preliminary experience with ‘split-liver’ grafting. Ann Surg. 1990;212:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368–375; discussion 375––377. [DOI] [PMC free article] [PubMed]
- 31.Azoulay D, Astarcioglu I, Bismuth H, et al. Split-liver transplantation: the Paul Brousse policy. Ann Surg. 1996;224:737–746; discussion 746––748. [DOI] [PMC free article] [PubMed]
- 32.Humar A, Ramcharan T, Sielaff T, et al. Split liver transplantation for two adult recipients: an initial experience. Am J Transplant. 2001;1:366–372. [DOI] [PubMed] [Google Scholar]
- 33.Humar A, Kandaswamy R, Sielaff T, et al. Split-liver transplants for 2 adult recipients: an initial experience. Transplant 2001, Chicago, IL, May 12–16, 2001. [DOI] [PubMed]
- 34.Andorno E, Antonucci A, Bottino G, et al. Arterial reconstruction in adult recipients during split liver transplantation, Joint Meeting of the International Liver Transplantation Society, European Liver Transplantation Association, and Liver Intensive Care Group of Europe. Berlin, Germany, July 11–13, 2001.
- 35.Colledan M, Andorno E, Segalin A, et al. Alternate split liver technique: the equal size split. Transplant Proc. 2001;33:1335–1336. [DOI] [PubMed] [Google Scholar]
- 36.Gundlach M, Broering D, Topp S, et al. Split-cava technique: liver splitting for two adult recipients. Liver Transpl. 2000;6:703–706. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe R, Merion R, Rush S, et al. Analysis Response for Data Request from the OPTN Liver and Intestinal Committee Meeting of January 24, 2002. Submitted by the Scientific Registry of Transplant Recipients. Dated February 1, 2002.
- 38.Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl. 2000;6:296–301. [DOI] [PubMed] [Google Scholar]
- 39.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301–311; discussion 311––312. [DOI] [PMC free article] [PubMed]
- 40.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7:680–686. [DOI] [PubMed] [Google Scholar]
- 41.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers: the ultimate expansion of a limited donor pool. Ann Surg. 1996;224:331–339; discussion 339–341. [DOI] [PMC free article] [PubMed]
- 42.Reyes J, Gerber D, Mazariegos GV, et al. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg. 2000;35:283–289; discussion 289––290. [DOI] [PubMed]
- 43.Karbe T, Kutemeier R, Rogiers X, et al. Logistical aspects and procedures in split-liver transplantation. Transplant Proc. 1996;28:43–44. [PubMed] [Google Scholar]
- 44.Ramcharan T, Glessing B, Lake JR, et al. Outcome of other organs recovered during in situ split-liver procurements. Liver Transpl. 2001;7:853–857. [DOI] [PubMed] [Google Scholar]
- 45.Reyes J, Gerber D, Mazariegos GV, et al. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg. 2000;35:283–289. [DOI] [PubMed] [Google Scholar]
- 46.Rogiers X, Malago M, Gawad KA, et al. One year of experience with extended application and modified techniques of split liver transplantation. Transplantation. 1996;61:1059–1061. [DOI] [PubMed] [Google Scholar]
- 47.Gawad KA, Topp S, Gundiach M, et al. Sharing of split livers between centers is easily feasible. Transplant Proc. 2000;32:59. [DOI] [PubMed] [Google Scholar]