Abstract
Objective:
To determine the predictive value of the preoperative serum concentration of type IV collagen 7s domain (7s collagen) for postoperative hepatic failure in patients undergoing liver resection for hepatocellular carcinoma.
Summary Background Data:
Clear and reliable criteria for predicting hepatic failure after liver resection are needed. The serum 7s collagen concentration correlates with the histologic degree of active hepatitis and hepatic fibrosis and may predict the regenerative potential of the liver.
Methods:
Potential risk factors for postoperative hepatic failure, including the serum 7s collagen concentration, were evaluated in 251 patients who underwent liver resection for hepatocellular carcinoma. Prognostic significance was determined by univariate and multivariate analyses.
Results:
Hepatic failure developed postoperatively in 25 patients, 4 of whom died. The serum 7s collagen concentration correlated with the histologic degree of hepatitis activity and hepatic fibrosis. The serum 7s collagen concentration was a risk factor for postoperative hepatic failure by univariate analysis and was the only risk factor on multivariate analysis. No patient with a serum 7s collagen concentration <12 ng/mL died of postoperative hepatic failure, and all 4 patients who died had a serum 7s collagen concentration ≥12 ng/mL.
Conclusions:
The preoperative serum 7s collagen concentration correlated independently with hepatic failure following liver resection for hepatocellular carcinoma. Patients whose serum 7s collagen is ≥12 ng/mL are poor candidates for hepatic resection.
The serum concentration of type IV collagen 7s domain correlated with postoperative hepatic failure, and ≥12 ng/mL is a relative contraindication to liver resection for hepatocellular carcinoma. Some therapy other than resection should be chosen for those patients.
Improvements in the preoperative assessment of liver function and advances in surgical techniques have reduced morbidity and mortality of liver surgery. However, liver resection in patients with hepatocellular carcinoma (HCC) still results in postoperative hepatic failure and death because most patients with HCC also have chronic liver disease.1–9 When selecting the therapy for HCC, it is important to know the extent of resection that can be tolerated. Investigators have searched for risk factors that predict postoperative hepatic failure, and various methods have been based on preoperative liver function.4,5,9–18 Should a patient be deemed a poor operative candidate, locoregional treatment, including percutaneous ethanol injection, microwave coagulation therapy, and radiofrequency ablation therapy, has been developed and used for HCC, especially small HCCs.19
Previous studies have shown that hepatic fibrosis and active hepatitis are negative predictive factors for liver regeneration and risk factors for postoperative hepatic failure.3,9,20–26 It also has been reported that the serum concentration of type IV collagen correlates with the risk of hepatic failure in patients with chronic liver damage.27 Recently, the type IV collagen 7s domain (7s collagen), involved in connective tissue metabolism, has been identified as a biochemical marker for assessing fibrogenesis and fibrosis in cirrhosis.28–30 The serum concentration of 7s collagen has been reported to correlate with the severity of active hepatitis and hepatic fibrosis and the magnitude of abnormalities in liver function tests.29
In this study, we evaluated a correlation between the preoperative serum 7s collagen concentration and postoperative hepatic failure following hepatic resection, and to serve as a marker for identifying candidates for liver resection for HCC
PATIENTS AND METHODS
Since 1994, we have measured the serum 7s collagen concentration in 257 patients with HCC prior to liver resection. Five patients who underwent concomitant resection of a synchronous second tumor and 1 patient who underwent liver resection and the right atrial tumor thrombectomy requiring extracorporeal circulation were excluded. The remaining 251 patients were the subjects in this study.
The study was conducted in accordance with the Helsinki Declaration and the guidelines of the Ethics Committee of our institution.
Measurement of 7s Collagen
The serum 7s collagen concentration was measured preoperatively with a commercially available type IV collagen 7s domain radioimmunoassay kit (Diaiatron Co., Tokyo, Japan), which uses a polyclonal antibody against the 7s domain of type IV collagen isolated from human placenta. The reference range of the serum 7s collagen concentration is ≤6 ng/mL.
Postoperative Complications
The variables identifying postoperative hepatic failure included hepatic coma with hyperbilirubinemia (total serum bilirubin concentration >5 mg/dL for more than 5 days), intractable pleural effusion or ascites requiring use of diuretics or thoracocentesis, or abdominal paracentesis on 2 or more occasions or institution of continuous drainage, or variceal bleeding.30,31
Pathologic Examination
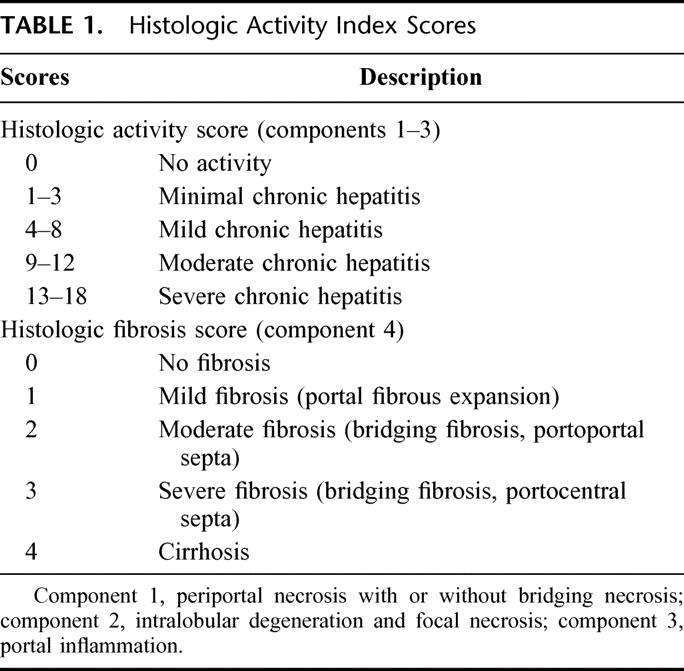
Surgical specimens were cut into serial slices 5 mm thick, fixed in 10% formalin, and stained with hematoxylin and eosin. The histologic grade of tumor differentiation was assigned using a modification of the classification by Edmonson-Steiner.32,33 The histologic activity index (HAI) with some modifications (Table 1) 34,35 was used to evaluate the severity of active hepatitis (histologic activity score) and the degree of fibrosis (histologic fibrosis score). HAI scores consist of 4 components; component 1, periportal necrosis with or without bridging necrosis; component 2, intralobular degeneration and focal necrosis; component 3, portal inflammation; component 4, fibrosis. HAI scores (components 1–3) of 0 indicated no activity (histologic activity score 0); scores of 1 to 3 indicated minimal activity (histologic activity score 1); scores of 4 to 8 indicated mild activity (histologic activity score 2); scores of 9 to 12 indicated moderate activity (histologic activity score 3); and score of ≥13 indicated severe activity (histologic activity score 4). The degree of fibrosis (histologic fibrosis score) was determined by component 4 of the HAI score. A histologic fibrosis score of 1 indicated portal fibrous expansion, a score of 2 indicated the presence of portal-portal septa without architectural distortion, a score of 3 indicated portocentral septa with architectural distortion, and a score of 4 indicated cirrhosis.
TABLE 1. Histologic Activity Index Scores
Statistics
Student t test was used to analyze differences in age and tumor size. The Mann-Whitney U test was used to analyze differences in laboratory test results. Fisher exact test or the χ2 test was used to compare categoric data between groups. The correlation between the serum 7s collagen concentration and results of other laboratory tests was determined by Pearson's correlation coefficient. The correlation between the serum 7s collagen concentration and the histologic activity score or fibrosis score in noncancerous liver was determined by Spearman's rank correlation. The odds ratio was used to estimate the relative risk for postoperative hepatic failure. Logistic regression analysis was used for univariate analysis. For multivariate analysis, multiple logistic regression analysis was used.
RESULTS
The 251 patients in this study included 204 men and 47 women. The age range was from 30 to 80 years (mean ± sd, 62.3 ± 7.7 years). A total of 180 patients were positive for anti-hepatitis C antibody (anti-HCV) alone, 36 patients were positive for hepatitis B surface antigen (HBsAg) alone, 4 patients were positive for both anti-HCV and HBsAg, and 31 patients were negative for both viral markers.
The serum 7s collagen concentration distributed from 2.6 to 15.0 ng/mL (mean ± sd, 7.4 ± 2.2 ng/mL, Fig. 1). Although the serum 7s collagen concentration correlated with serum concentration of total bilirubin and albumin, the indocyanine green retention test at 15 minutes (ICGR15), serum activity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count, the r values were very low (Table 2, Fig. 2).
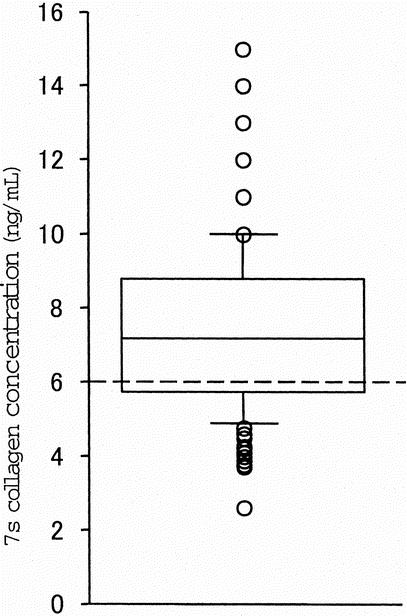
FIGURE 1. The serum 7s collagen concentration in patients who underwent liver resection for hepatocellular carcinoma. The serum 7s collagen concentration distributes from 2.6 to 15.0 ng/mL (mean ± sd, 7.4 ± 2.2 ng/mL). The reference range of the serum 7s collagen concentration is ≤6.0 ng/mL (dotted line).
TABLE 2. Correlation Between Serum 7s Collagen Concentration and Results of Other Laboratory Tests
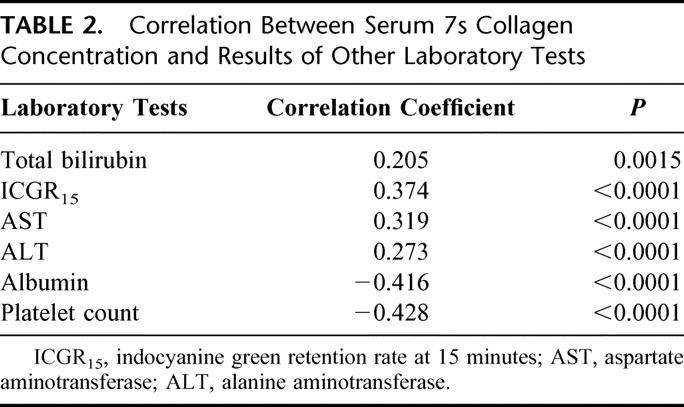
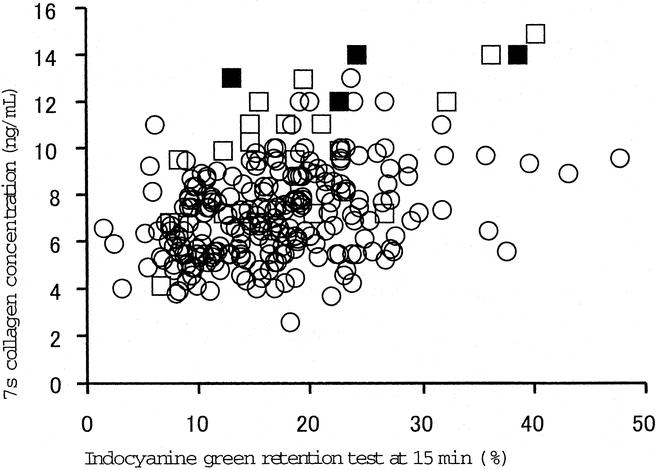
FIGURE 2. The serum 7s collagen concentration in patients with or without postoperative hepatic failure and operative procedure. Major hepatectomy, segmentectomy or greater; minor hepatectomy, smaller than segmentectomy.37 Circles, patients without postoperative hepatic failure; open squares, patients with postoperative hepatic failure; closed squares, patients who died of postoperative hepatic failure.
The serum 7s collagen concentration correlated with the histologic activity score (ρ = 0.271, P < 0.0001) and the histologic fibrosis score (ρ = 0.437, P < 0.0001).
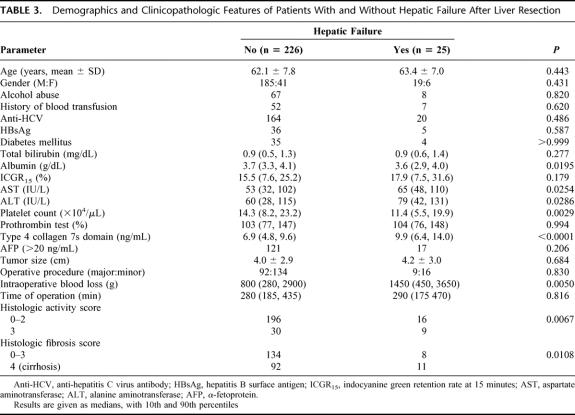
Postoperative hepatic failure developed in 25 patients (hepatic coma with hyperbilirubinemia, 6 patients; intractable pleural effusion or ascites, 24 patients; and variceal bleeding, 1 patient). Four of these patients died of the hepatic failure (hospital death). The clinicopathologic findings were compared in the 226 patients without and the 25 patients with postoperative hepatic failure (Table 3). The groups were similar in age, gender, percentage of patients with a history of alcohol abuse (an estimated daily intake of 86 g ethanol for at least 10 years, according to the criteria of the Liver Cancer Study Group of Japan36), diabetes mellitus, blood transfusion, and anti-HCV and HBsAg positivity. Although the serum total bilirubin concentration, the ICGR15, and prothrombin test were not different between groups, the serum albumin concentration and the platelet count were lower in patients with than without postoperative hepatic failure. Serum activity of AST and ALT and the serum 7s collagen concentration were higher in patients with than without postoperative hepatic failure. Although no difference was noted in tumor size, operative procedure, or time of operation, intraoperative blood loss was greater in patients who developed postoperative hepatic failure. The percentage of patients with moderate hepatitis activity (histologic activity score 3) or cirrhosis (histologic fibrosis score 4) was greater in patients who developed postoperative hepatic failure.
TABLE 3. Demographics and Clinicopathologic Features of Patients With and Without Hepatic Failure After Liver Resection
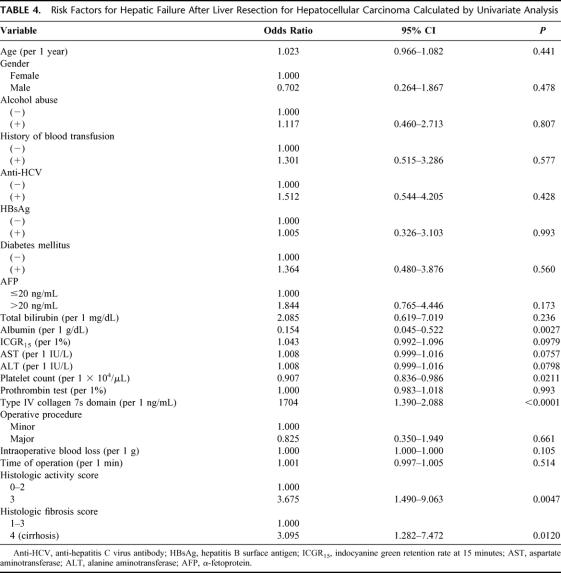
Table 4 shows the odds ratios (OR) of the possible risk factors for postoperative hepatic failure calculated by univariate analysis. The serum concentration of albumin (OR = 0.154) and 7s collagen (OR = 1.704), the platelet count (OR = 0.907), histologic activity score 3 (OR = 3.675), and cirrhosis (OR = 3.095) were risk factors for postoperative hepatic failure. Activities of AST and ALT were also possible risk factors (OR = 1.008). The serum 7s collagen concentration was significantly higher in patients with postoperative hepatic failure than in patients without the complication in both major hepatectomy (segmentectomy or greater)37 group and minor hepatectomy (smaller than segmentectomy) group (P = 0.0007 and P = 0.0021, respectively, Fig. 3). In both groups, the serum 7s collagen concentration in patients who died of the postoperative hepatic failure was ≥12 ng/mL. The risk of postoperative hepatic failure increased as the serum albumin concentration and platelet count decreased and as AST and ALT activity and the serum 7s collagen concentration increased. The risk of the postoperative hepatic failure was high in patients with moderately active hepatitis or cirrhosis on histopathology. Again, the risk of the postoperative hepatic failure closely correlated with both the severity of active hepatitis and the degree of hepatic fibrosis.
TABLE 4. Risk Factors for Hepatic Failure After Liver Resection for Hepatocellular Carcinoma Calculated by Univariate Analysis
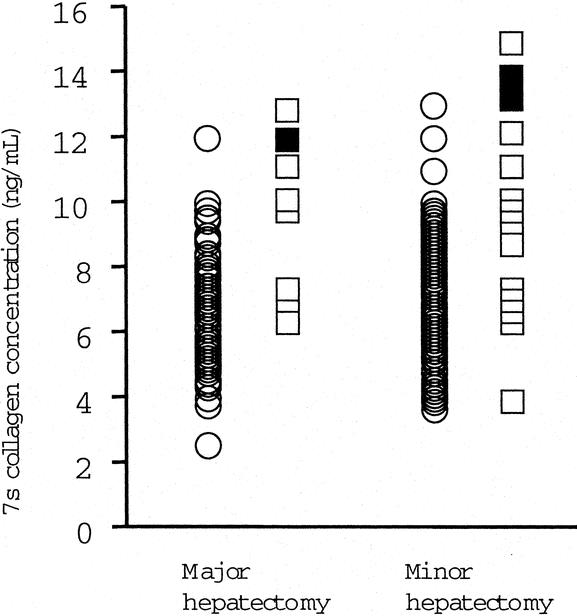
FIGURE 3. The correlation between serum 7s collagen concentration and indocyanine green retention rate at 15 minutes. Circles, patients without postoperative hepatic failure; open squares, patients with postoperative hepatic failure; closed squares, patients who died of postoperative hepatic failure.
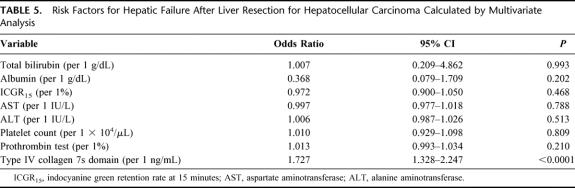
Multivariate analysis was used to estimate the adjusted odds ratio for postoperative hepatic failure based on preoperative data (Table 5). The serum 7s collagen concentration alone was an independent risk factor for postoperative hepatic failure (adjusted OR = 1.727).
TABLE 5. Risk Factors for Hepatic Failure After Liver Resection for Hepatocellular Carcinoma Calculated by Multivariate Analysis
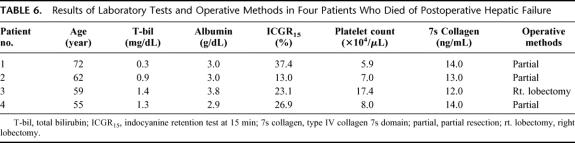
The ICGR15, which has been used as guidelines for hepatic reserve, ranged from 6.5% to 40.8% in patients with postoperative hepatic failure,3,5,7,10,17,18,23,26,38–42 whereas the serum 7s collagen concentration in the patients distributed mainly in a relatively high serum concentration of 7s collagen (Fig. 2). The serum 7s collagen concentration was ≥12 ng/mL in 4 patients who died. The serum concentrations of total bilirubin and albumin, the ICGR15, and the platelet count were distributed over wide ranges in these 4 patients (Table 6).
TABLE 6. Results of Laboratory Tests and Operative Methods in Four Patients Who Died of Postoperative Hepatic Failure
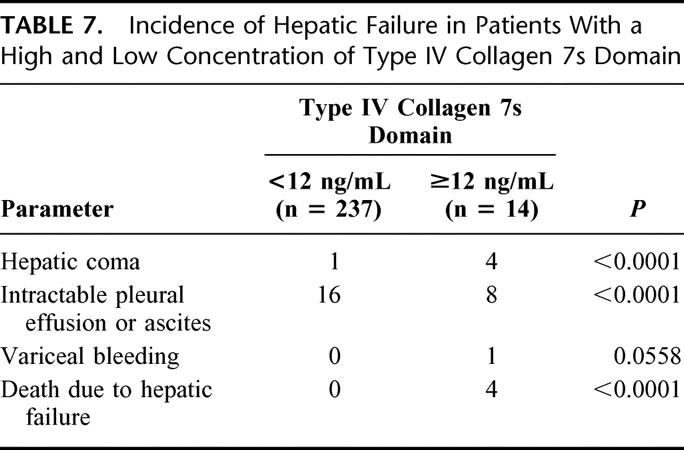
We next divided the subjects into two groups: 1 group included the 237 patients with a low serum 7s collagen concentration (<12 ng/mL) and the other group consisted of the 14 patients with a high serum 7s collagen concentration (≥12 ng/mL, Table 7). The incidences of hepatic coma and intractable pleural effusion or ascites were higher among patients with a high serum 7s collagen concentration than patients with a low serum 7s collagen concentration. Variceal bleeding developed in only 1 patient, but this patient had an elevated serum 7s collagen concentration (≥12 ng/mL). No patient with a low serum 7s collagen concentration died of postoperative hepatic failure, and all 4 of the patients who died had a high serum 7s collagen concentration.
TABLE 7. Incidence of Hepatic Failure in Patients With a High and Low Concentration of Type IV Collagen 7s Domain
DISCUSSION
In this study, the preoperative serum 7s collagen concentration was a risk factor for postoperative hepatic failure by univariate analysis and an independent risk factor by multivariate analysis.
The risk of postoperative hepatic failure correlated closely with both the severity of active hepatitis and the degree of hepatic fibrosis. It is well established that cirrhosis is a risk factor for postoperative hepatic failure.3,10,13,20,24,43 The degree of hepatic fibrosis is useful as a predictor of liver regeneration and restoration of liver function after liver resection,44 and it correlates with the risk of postoperative ascites and the duration of postoperative hepatic failure.9 Several investigators have commented on the role of active hepatitis as a potential risk factor for hepatic failure following liver resection.9,24 In previous studies, serum transaminase activity was used to determine hepatitis activity. However, the histologic activity of hepatitis does not always correlate with serum transaminase activity, and neither AST and ALT activity was a risk factor by univariate or multivariate analysis. Recently, Eguchi et al25 emphasized the importance of using histology to evaluate the severity of active hepatitis as a predictor of postoperative hepatic failure. However, it is difficult to obtain noncancerous hepatic tissue from all patients preoperatively, so other less invasive markers are needed. The serum 7s collagen concentration has been reported to correlate with the severity of active hepatitis, as well as the degree of hepatic fibrosis.29 We confirmed that the serum 7s collagen concentration correlates with the histologic activity score and the fibrosis score in patients with HCC. Preoperative measurement of the serum 7s collagen concentration informs the surgeon about the severity of active hepatitis and the degree of fibrosis and provides a measure of the risk of postoperative hepatic failure.
Although the indocyanine green clearance test has been used to develop guidelines for hepatic resection, the recommended limit for safe liver resection is different in different series.3,5,7,10,17,18,23,26,38–42 In this study, the correlation between the serum 7s collagen concentration and the ICGR15 was weak, and the ICGR15 ranged widely in patients with postoperative hepatic failure. In addition, the ICGR15 ranged from 13.0% to 37.4% in patients who died of postoperative hepatic failure, and the ICGR15 was not a risk factor for the postoperative hepatic failure by either univariate or multivariate analysis. On the other hand, the incidence of hepatic coma and intractable pleural effusion and ascites was higher in patients with an elevated serum 7s collagen concentration. Additionally, the serum 7s collagen concentration was ≥12 ng/mL in all patients who died of postoperative hepatic failure. No other laboratory tests were risk factors for postoperative hepatic failure by multivariate analysis, although the serum albumin concentration and the platelet count were risk factors by univariate analysis. Thus, preoperative serum 7s collagen concentration seems to be the best available laboratory test for assessing hepatic reserve.
Other reported risk factors for postoperative hepatic failure include age,10,24 diabetes mellitus,13 and excessive intraoperative blood loss.7,13,18,26,43–45 Although excessive intraoperative blood loss was a risk factor by univariate analysis, age and diabetes mellitus were not risk factors in this study.
Limited liver resection has been recommended for patients with cirrhosis.3,27,45,47 In this study, 4 patients with a serum 7s collagen concentration ≥12 ng/mL who underwent major hepatectomy (1 patient) or limited resection (partial resection, 3 patients) died of postoperative hepatic failure. Recently, it has been reported that microwave coagulation therapy administered via laparotomy is a less invasive alternative to partial resection.47 The usefulness of other locoregional therapies, including percutaneous ethanol injection therapy, microwave coagulation therapy, and radiofrequency ablation therapy, has also been reported.19 Based on our data, patients with a serum 7s collagen concentration ≥12.0 ng/mL should undergo some treatment other than liver resection. Liver transplantation is another alternative in selected patients with a high serum 7s collagen concentration.
CONCLUSION
The preoperative serum 7s collagen concentration was correlated with the postoperative hepatic failure, and a concentration ≥ 12 ng/mL is a relative contraindication to liver resection. Such patients should receive alternative treatment.
Footnotes
Reprints: Shoji Kubo, MD, Department of Gastroenterological and Hepato-Biliary-Pancreatic Surgery, Osaka City University Graduate School of Medicine, 1-4-3 Asahimachi, Abeno-ku, Osaka 545-8585, Japan. E-mail:m7696493@msic.med.osaka-cu.ac.jp.
REFERENCES
- 1.Lai ECS, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma: an audit of 343 patients. Ann Surg. 1995;221:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takenaka K, Kawahara N, Yamamoto K, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71–76. [DOI] [PubMed] [Google Scholar]
- 3.Wu CC, Ho WL, Yeh DC, et al. Hepatic resection of hepatocellular carcinoma in cirrhotic livers: is it unjustified in impaired liver function? Surgery 1996;120:34–39. [DOI] [PubMed] [Google Scholar]
- 4.Cohnert TU, Rau HG, Buttler E, et al. Preoperative risk assessment of hepatic resection for malignant disease. World J Surg. 1997;21:396–401. [DOI] [PubMed] [Google Scholar]
- 5.Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- 6.Lise M, Bacchetti S, De Pian P, et al. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998;82:1028–1036. [DOI] [PubMed] [Google Scholar]
- 7.Nonami T, Nakao A, Kurokawa T, et al. Blood loss and ICG clearance as best prognostic markers of post-hepatectomy liver failure. Hepatogastroenterology. 1999;46:1669–1672. [PubMed] [Google Scholar]
- 8.Pol B, Campan P, Hardwigsen J, et al. Morbidity of major hepatic resections: a 100-case prospective study. Eur J Surg. 1999;165:446–453. [DOI] [PubMed] [Google Scholar]
- 9.Farges O, Malassagne B, Fleijou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka N, Okamoto E, Kuwata K, et al. A multiple regression evaluation for prediction of posthepatectomy liver failure. Ann Surg. 1984;200:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didolkar MS, Fitzpatrick JL, Elias EG, et al. Risk factors before hepatectomy, hepatic function after hepatectomy and computed tomographic changes as indicators of mortality from hepatic failure. Surg Gynecol Obstet. 1989;169:17–26. [PubMed] [Google Scholar]
- 12.Noguchi T, Imai T, Mizumoto R. Preoperative estimation of surgical risk of hepatectomy in cirrhotic patients. Hepatogastroenterology. 1990;37:165–171. [PubMed] [Google Scholar]
- 13.Shimada M, Matsumata T, Akazawa K, et al. Estimation of risk of major complications after hepatic resection. Am J Surg. 1994;167:399–403. [DOI] [PubMed] [Google Scholar]
- 14.Kwon AH, Ha-kawa SK, Uetsuji S, et al. Use of technetium 99m diethypenetriamine-pentaacetic acid-galactosyl-human serum albumin liver scintigraphy in the evaluation of preoperative and postoperative hepatic functional reserve for hepatectomy. Surgery. 1995;117:429–434. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Castells Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann H, Reichen J. Hepatectomy: preoperative analysis of hepatic function and postoperative liver failure. Dig Surg. 1998;15:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. [DOI] [PubMed] [Google Scholar]
- 18.Miyagawa S, Makuuchi M, Kawasaki S, et al. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. [DOI] [PubMed] [Google Scholar]
- 19.Poon RTP, Fan ST, Tsang FHF, et al. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagasue N, Yukaya H, Ogawa Y, et al. Human liver regeneration after major hepatic resection: a study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki S, Takasaki K, Yamamoto M, et al. Liver regeneration and restoration of liver function after partial hepatectomy: the relation of fibrosis of the liver parenchyma. Hepatogastroenterology. 1999;46:2919–2924. [PubMed] [Google Scholar]
- 22.Tanaka H, Hirohashi K, Kubo S, et al. Influence of histological inflammatory activity on regenerative capacity of liver after percutaneouos transhepatic portal vein embolization. J Gastroenterol. 1999;34:100–104. [DOI] [PubMed] [Google Scholar]
- 23.Fan ST, Lai ECS, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. [DOI] [PubMed] [Google Scholar]
- 24.Nagasue N, Kohno H, Tachibana M, et al. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with Child-Tucotte class B and C cirrhosis. Ann Surg. 1999;229:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi H, Umeshita K, Sakon M, et al. Presence of active hepatitis associated with liver cirrhosis is a risk factor for mortality caused by posthepatectomy liver failure. Dig Dis Sci. 2000;45:1383–1388. [DOI] [PubMed] [Google Scholar]
- 26.Das BC, Isaji S, Kawarada Y. Analysis of 100 consecutive hepatectomies: risk factors in patients with liver cirrhosis or obstructive jaundice. World J Surg. 2001;25:266–273. [DOI] [PubMed] [Google Scholar]
- 27.Shimahara Y, Yamamoto N, Uyama N, et al. Significance of serum type IV collagen level of hepatectomized patients with chronic liver damage. World J Surg. 2002;26:451–456. [DOI] [PubMed] [Google Scholar]
- 28.Ueno T, Inozuka S, Torimura T, et al. Significance of serum type-IV collagen levels in various liver diseases: measurement with a one-step sandwich enzyme immunoassay using monoclonal antibodies with specificity for pepsin-solubilized type-IV collagen. Scand J Gastroenterol. 1992;27:513–520. [DOI] [PubMed] [Google Scholar]
- 29.Murawaki Y, Ikuta Y, Koda M, et al. Serum type III procollagen peptide, type IV collagen 7s domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: relationship to liver histology. Hepatology. 1994;20:780–787. [DOI] [PubMed] [Google Scholar]
- 30.Suou T, Yamada S, Hosho K, et al. Relationship between serum and hepatic 7s fragments of type IV collagen in chronic liver disease. Hepatology. 1996;23:1154–1158. [DOI] [PubMed] [Google Scholar]
- 31.Horii K, Kubo S, Hirohashi K, et al. Changes in erythrocyte deformability after liver resection for hepatocellular carcinoma associated with chronic liver disease. World J Surg. 1999;25:85–90. [DOI] [PubMed] [Google Scholar]
- 32.Liver Cancer Study Group of Japan. Classification of Primary Liver Cancer, 1st English ed. Toyko: Kanehara, 1997. [Google Scholar]
- 33.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 34.Knodell RG, Ishak KG, Black WC, et al. Formation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. [DOI] [PubMed] [Google Scholar]
- 35.Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 36.Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathological features and results of surgical treatment. Ann Surg 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 37.Healey JEJr, Schroy PC. The intrahepatic distribution of the hepatic artery in man. J Int Col Surg. 1953;20:133–148. [PubMed] [Google Scholar]
- 38.Matsumata T, Kanematsu T, Yoshida Y, et al. The indocyanine green test enables prediction of postoperative complications after hepatic resection. World J Surg. 1987;11:678–681. [DOI] [PubMed] [Google Scholar]
- 39.Fujio N, Sakai K, Kinoshita H, et al. Results of treatment of patients with hepatocellular carcinoma with severe cirrhosis of the liver. World J Surg. 1989;13:211–218. [DOI] [PubMed] [Google Scholar]
- 40.Hemming AW, Scudamore CH, Shackleton CR, et al. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163:515–518. [DOI] [PubMed] [Google Scholar]
- 41.Yasui M, Harada A, Torii A, et al. Impaired liver function and long-term prognosis after hepatectomy for hepatocellular carcinoma. World J Surg. 1995;19:439–443. [DOI] [PubMed] [Google Scholar]
- 42.Midorikawa Y, Kubota K, Takayama T, et al. A comparative study of postoperative complications after hepatectomy in patients with and without chronic liver disease. Surgery. 1999;126:484–491. [PubMed] [Google Scholar]
- 43.Takenaka K, Kanematsu T, Fukuzawa K, et al. Can hepatic failure after surgery for hepatocellular carcinoma in cirrhotic patients be prevented? World J Surg. 1990;14:123–127. [DOI] [PubMed] [Google Scholar]
- 44.Miyazaki S, Takasaki K, Yamamoto M, et al. Liver regeneration and restoration of liver function after partial hepatectomy: the relation of fibrosis of the liver parenchyma. Hepatogastroenterology. 1999;46:2919–2924. [PubMed] [Google Scholar]
- 45.Paquet KJ, Koussouris P, Mercado MA, et al. Limited hepatic resection for selected cirrhotic patients with hepatocellular or cholangiocellular carcinoma: a prospective study. Br J Surg. 1991;78:459–462. [DOI] [PubMed] [Google Scholar]
- 46.Kanematsu T, Takenaka K, Matsumata T, et al. Limited hepatic resection effective for selected cirrhotic patients with primary liver cancer. Ann Surg. 1984;199:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamanaka N, Tanaka K, Oriyama T, et al. Microwave coagulonecrotic therapy for hepatocellular carcinoma. World J Surg. 1996;20:1076–1081. [DOI] [PubMed] [Google Scholar]