Abstract
Objective:
To evaluate the role of regional lymphadenectomy in patients with liver tumors.
Background:
Lymph node status is 1 of the most important prognostic factors in oncologic surgery; however, the role of lymph node dissection remains unclear for hepatic tumors.
Methods:
A total of 120 consecutive patients undergoing liver resections for primary and secondary hepatic tumors were prospectively enrolled in the study. “Regional” lymphadenectomy was carried out routinely after specimen removal. Incidence, site, and influence on survival of node metastases were analyzed.
Results:
Only 1 postoperative complication (intra-abdominal bleeding) was related to lymph node excision. Median number of dissected nodes was 6.8 ± 3.6. Periportal, pericholedochal, and common hepatic artery stations were always removed. Lymph node metastases were found in 17 (16.5%) patients. The percentage rises to 20.3% when considering only noncirrhotic patients. The incidence of lymph node metastases was 7.5% for hepatocellular carcinoma, 14% for colorectal metastases, 40% for noncolorectal metastases, and 40% for intrahepatic cholangiocarcinoma (P < 0.002). Median survival time was 486 ± 93.2 days among all patients with node metastases and 725 ± 29.7 among patients without node metastases. The 2-year survival was 37.1% and 86.7%, in the 2 groups (P < 0.05). The 2-year recurrence rate was 77.6% and 45.3%, respectively (P < 0.05).
Conclusions:
Regional lymphadenectomy is a safe procedure after liver resection, and it should be routinely applied in patients with primary and secondary hepatic tumors, particularly in those without chronic disease. A careful evaluation of node status is nevertheless advisable also in patients with hepatocellular carcinoma on cirrhosis.
Regional lymphadenectomy was carried out prospectively in 120 patients undergoing liver resection for different primary and secondary hepatic tumors. Incidence of lymph node metastases was 20.3% in noncirrhotic patients. The 2-year survival and recurrence rates were significantly affected by the presence of lymph node metastases.
Lymph node status is a definite prognostic factor in oncologic surgery and significantly affects long-term survival, as reported by the tumor staging system of the International Union Against Cancer (IUCC), which is the most widespread classification of malignant tumors worldwide.1 The impact on survival of lymph node metastases has already been reported for lung cancer,2 esophageal cancer,3 and renal cancer.4 The prognostic value and the extent of lymph node dissection are strongly defined for breast carcinoma5 and other gastrointestinal neoplasms.6–8 Some authors have claimed that a minimum number of lymph nodes should be dissected in gastric and colorectal carcinoma to obtain a reliable staging of the tumor.9,10
Regional lymphadenectomy is already the standard procedure that completes hepatic resection in the case of carcinoma arising from the extrahepatic bile duct.11,12 However, the indication, extent, and role of lymph node excision are still a matter of discussion, and no clear guidelines exist in patients with other types of primary or secondary hepatic tumors. An increased operative risk of liver resection has been reported when lymph node dissection is performed in patients with liver tumors.13,14 Therefore, concerns still remain with regard to its routine application.
It is of great interest to clarify which patients with hepatic cancers should benefit from lymph node excision, in which cases this procedure should be mandatory and whether the operative risk is really increased by it.
We have prospectively evaluated the feasibility and safety of a routine regional lymphadenectomy and the incidence, site, and impact on survival of lymph node metastases in patients with primary and secondary liver tumors amenable to curative liver resection. Our results therefore refer to the most recent therapeutic strategies in the field of liver tumors.
MATERIALS AND METHODS
From April 1999 to November 2001, 120 patients were admitted to the Surgical Unit of the Department of Surgery and Transplantation, University of Bologna, Bologna, Italy, for the presence of hepatobiliary tumors and were prospectively enrolled in this study, which was approved by the local ethics committee. Informed consent, indicating the advantages and possible disadvantages of regional lymph node dissection and the expected results by the physicians, was signed by all the patients on admission to the hospital. No one refused to take part in the study.
The primary end point of the study was to analyze the incidence and site of lymph node metastases in patients with different liver tumors submitted to curative liver resection, to clarify in which of them lymph node dissection should be carried out as a part of the standard procedure.
Secondary end points were 1) the determination of the increased postoperative morbidity and/or mortality with lymph node dissection; 2) the evaluation of the relationship between the lymphadenomegaly seen at preoperative CT scan and the real presence of lymph node metastases at the pathologic examination; and 3) the influence of lymph node metastases on long-term survival.
Seven patients (5.8%) were excluded because of the impossibility to radically remove all grossly visible tumor at the time of preoperative or intraoperative evaluation. Ten patients (8.3%) were excluded since the final diagnosis was a tumor arising from the extrahepatic bile duct (gallbladder carcinoma or Klatskin's tumor). The remaining 103 patients were included in this study.
There were 55 (53.4%) males and 48 (46.6%) females. The mean age was 61.8 ± 9.3 years (range 39–77 year). The reason for hospital admission was hepatocellular carcinoma (HCC) in 40 (38.9%) patients (29 of them on cirrhosis, 28.2%), metastases from colorectal cancer in 43 (41.7%), metastases from other tumors (noncolorectal and non-neuroendocrine cancer) in 10 (9.7%), and intrahepatic cholangiocarcinoma in 10 (9.7%).
Imaging workup for patients with liver tumors included the evaluation of liver function, measurement of the α-fetoprotein and/or carcinoembryonic antigen level, ultrasonography, chest x-ray film, and abdomen dual-phase spiral CT scan. CT scans were reviewed before surgery by a senior radiologist and by one of the principal investigators (G.E. and G.L.G.). Lymphadenomegaly suspicious for lymph node metastases was shown in 13 patients (12.6%).
In patients with metastatic disease, absence of local recurrence was investigated by the use of colonscopy or gastroscopy and CT scan.
The mean number ± sd of neoplastic nodules within the liver was 1.7 ± 1.1 (range 1–10). The mean diameter ± sd of the largest tumor was 4.9 ± 2.5 cm (range 1–20 cm).
The type of procedure was defined according to the segmental classification of the liver by Coinaud.15 Resection of 3 or more segments was defined as a major hepatectomy. Liver resection was defined “curative” when there was no extrahepatic disease present and all the macroscopically visible hepatic tumor could be completely removed with an adequate surrounding surgical margin, which had to be confirmed clear from cancer at microscopic examination. The surgical technique of liver resections as performed in our institution has already been described elsewhere.16
In all patients operated on for metastatic diseases, an adjuvant systemic postoperative chemotherapy based on 5-fluorouracil, folinic acid, and other anticancer drug (depending on the site of the primary tumors and patient compliance) was administered without randomization.
Surgical Procedures
Because the classification of the regional nodes of the liver is not definitive in the literature, the harvested lymph nodes were categorized on the basis of the topographic relations to the surrounding structures, following the rules of the Japanese Society of Biliary Surgery.17
The lymphatic system of the liver drains through: 1) superficial lymphatics from the convex surface, which run through the coronary ligament and cross the diaphragm to reach precardiac, phrenic, and juxtaesophageal lymph nodes; 2) superficial lymphatics from the concave surface, which run to lymph nodes in the hepatic pedicle; 3) deep lymphatics, which leave the liver at the porta hepatis and run around bile duct and proper hepatic artery. Secondary lymphatic dissemination from liver tumors can occur via these latter 2 pathways.18
On the basis of these considerations, liver resection was performed together with the so-called “regional lymphadenectomy,” meaning the lymph node excision around the hepatic pedicle (which comprehends the cystic duct, pericholedochal, hilar, periportal, and periarterial lymph nodes), the retropancreatic space (posterior pancreatic station), and the common hepatic artery as far as the celiac trunk. In the case of intrahepatic cholangiocarcinoma located in the left hemi-liver, lymph nodes were carefully dissected around the left gastric artery and in the lesser sac.19 Hilar, cystic duct, and pericholedochal nodes were lumped together as pericholedochal nodes. Lymph nodes around the portal vein and proper hepatic artery in the hepatic pedicle were considered together as periportal nodes.
A single representative section per node was microscopically examined with hematoxylin and eosin staining by the same group of pathologists.
Follow-up
Patients were followed up for recurrence every 3 months after surgery with α-fetoprotein and/or carcinoembryonic antigen level and ultrasonography for the first year and then every 6 months; with chest x-ray film every 6 months. A CT scan was performed whenever a local or distant recurrence was suspected from the other examinations.
The follow-up ended at April 30th, 2002. In all patients, the follow-up was at least 4 months.
Statistical Analysis
Operative features and postoperative morbidity were analyzed to reveal if they were modified by lymph node dissection, comparing them with retrospective series that have been already published.20,21
The incidence and site of lymph node metastases were evaluated in the overall groups, on the basis of the different diseases, and in relation to the number and diameter of the tumor. A relation between lymph node status and preoperative CT scan evaluation of lymph nodes was also investigated. The influence of lymph node status on 2-year overall survival and the recurrence rate were eventually analyzed.
Death occurring within 30 days after the surgical procedure was defined as an operative mortality. Death occurring after surgery and before discharge was defined as a hospital mortality. Survival was considered from the day of surgery to the day of death or the most recent follow-up visit.
Results are expressed as mean ± sd. The χ2 test was used for categorical variables. The T-student test was used to compare continuous variables. Survival curves were estimated by means of the Kaplan-Meier method (excluding operative deaths). Differences in survival curves between the groups were compared by the long-rank test. A P value less than 0.05 was defined as significant. Statistical analysis was carried out with the SPSS test.22
RESULTS
Operative Features and Early Outcome
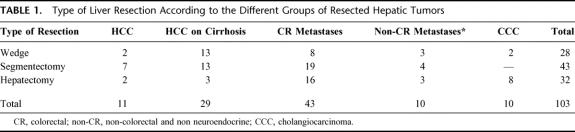
A wedge resection was performed in 28 patients (27.2%), a segmentectomy in 43 (41.7%), and a major hepatectomy in 32 (31.1%). The distribution of the type of liver resection within different etiological groups is reported in Table 1.
TABLE 1. Type of Liver Resection According to the Different Groups of Resected Hepatic Tumors
Mean operative time ± sd was 298 ± 143 minutes (range 90–800 minutes). Mean intraoperative blood transfusion ± sd was 230 ± 172 mL (range 0–1300 mL); mean fresh frozen plasma transfusion ± sd was 180 ± 299 mL (range 0–950 mL). Blood transfusion ± sd was required in 48 (30.8%) patients and fresh frozen plasma in 45 (27.9%) patients.
Mean postoperative stay ± sd was 8 ± 5.3 days. Postoperative complications appeared in 40 (39.2%) patients and all but 1 were resolved with medical therapy; the most frequent was ascites in 16 (15.7%) patients, followed by pleural effusion in 12 (11.8%). Nine cases of postoperative ascites (56.3%) appeared in patients operated on for HCC on cirrhosis (32.1% of patients in this group). The only surgical complication was the 1 case of postoperative intra-abdominal bleeding within retropancreatic space, which was directly related to lymph node dissection and required surgical revision through few transfixed stitches.
Operative mortality was 3.8% (4 of 103). There was no additional hospital mortality. Of these 4 patients, 2 underwent liver resection for hepatocellular carcinoma on cirrhosis: 1 died due to irreversible hyperglycemic coma 28 days after surgery; the second one died after the smallest wedge resection of this series because of liver failure. One patient, who had previously undergone cardiac surgery due to coronary heart disease, died of heart failure 6 days after a right hepatectomy for colorectal metastases. The fourth patient died 20 days after a left hepatectomy with resection and reconstruction of portal vein for intrahepatic cholangiocarcinoma involving the hilar plane due to hepatic failure following uncorrectable postoperative portal vein thrombosis.
Lymphadenectomy, Lymph Node Metastases, and Relation With CT Scan Lymphadenomegaly
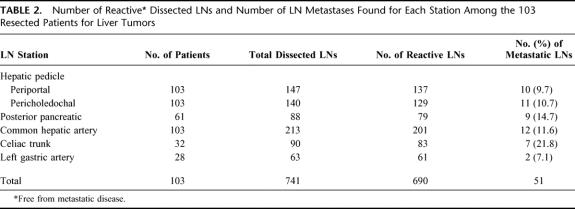
Among the 103 patients enrolled in this study, 741 lymph nodes were dissected and examined by the pathologist. The median number of dissected lymph nodes was 6.8 ± 3.6 per patient (range 4–30).
The lymph node stations that were constantly present were pericholedochal and periportal station, and common hepatic artery. The number of dissected lymph nodes found in each station is reported in Table 2. Furthermore, the site and overall number of lymph node metastases are reported in Table 2.
TABLE 2. Number of Reactive Dissected LNs and Number of LN Metastases Found for Each Station Among the 103 Resected Patients for Liver Tumors
Among the 13 patients with suspected lymph node invasion at the preoperative CT scan, only in 6 (46.1%) were lymph node metastases confirmed by pathologic examination. Among the remaining 90 patients without lymphadenomegaly, in 11 (12.2%) lymph node metastases were found by pathologists. The sensitivity, specificity, and diagnostic accuracy of CT scan to reveal lymph node metastases in patients with liver tumors were 35.2%, 91.8%, and 46.1%, respectively.
Lymph Node Status in Relation to Disease, Site of Invasion, and Number and Diameter of Nodules
Tumor metastases in dissected lymph nodes were found in 17 (16.5%) patients. The percentage is 20.3% (15 of 74) if we consider only noncirrhotic patients.
The incidence of lymph node invasion was 7.5% for HCC (3 of 40, with 2 cases of the 29 resected for HCC on cirrhosis), 14.0% for colorectal metastasis (6 of 43), 40.0% for metastasis from other sites (4 of 10), and 40.0% for intrahepatic cholangiocarcinoma (4 of 10). The distribution of lymph node metastases among different etiological groups is reported in Table 3. A significant difference in lymph node invasion was shown among patients with different diseases (P < 0.002); in particular, noncolorectal, nonneuroendocrine metastases and biliary tumors showed an incidence of lymph node metastases significantly different compared with the other cancers.
TABLE 3. Number and Incidence of Patients With LN Metastases Divided According to Diagnosis
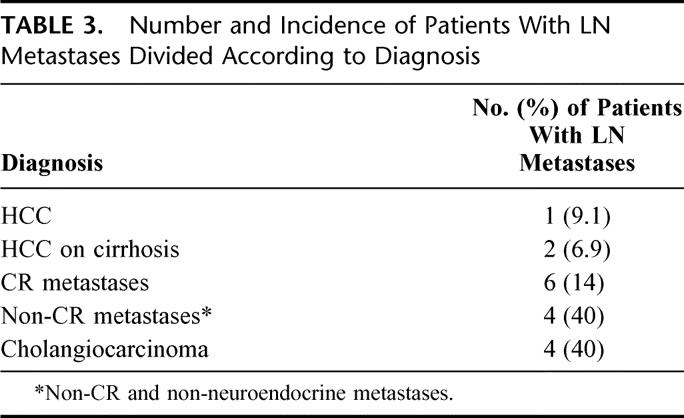
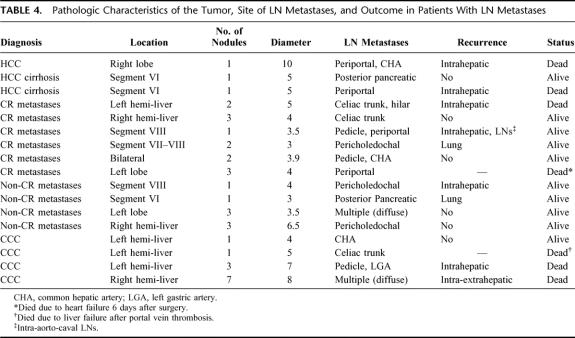
Table 4 describes the relation between location of the tumor, site of lymph node metastases, and outcome. There was no relationship between the position of the neoplasm inside the liver and the lymph node stations that were involved; only in the case of intrahepatic cholangiocarcinoma of the left hemi-liver was there a tendency to diffuse toward lymph nodes around the left gastric artery and celiac trunk (2 of the 3 cases), even if this could not be evaluated from a statistical significance point of view.
TABLE 4. Pathologic Characteristics of the Tumor, Site of LN Metastases, and Outcome in Patients With LN Metastases
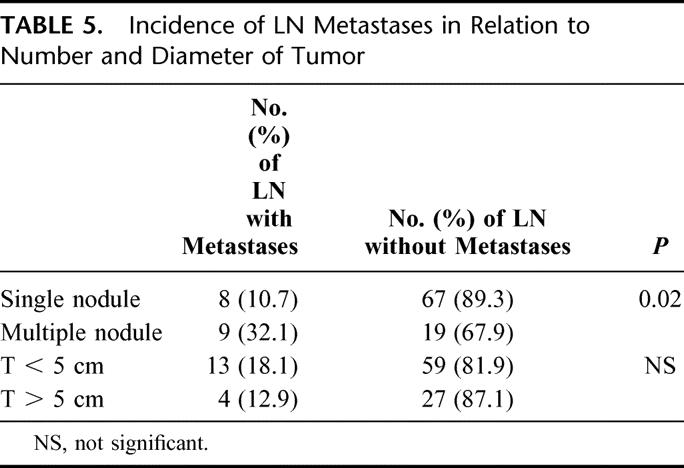
No difference was found in lymph node metastases depending on the diameter of the tumor; on the other hand, the number of nodules was significantly related to the incidence of lymph node metastases, as shown in Table 5.
TABLE 5. Incidence of LN Metastases in Relation to Number and Diameter of Tumor
Lymph Node Status and Survival
At the end of the study period, the median follow-up was 18 months (range 4–34 months); 11 patients died and the remaining 92 are alive.
Median survival time was 486 ± 93.2 days in patients with lymph node metastases and 725.2 ± 29.7 for patients without lymph node metastases. The 1- and 2-year survival was 48.4% and 43.8% in patients with positive lymph nodes, and 95.3% and 86.7% in patients with negative lymph nodes, respectively (P < 0.01; Fig. 1).
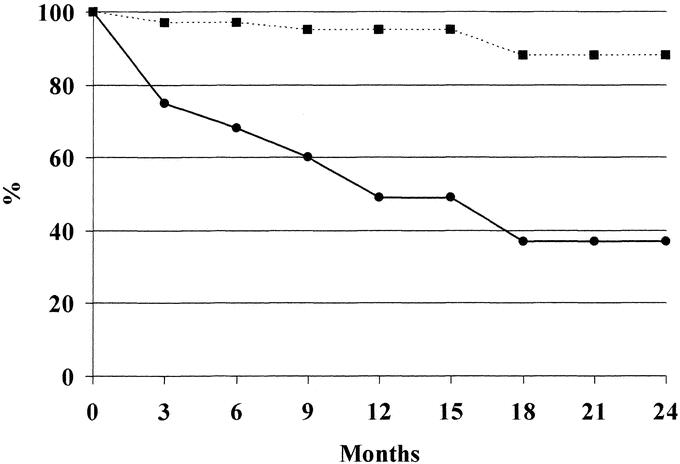
FIGURE 1. One- and two-year actuarial survival of resected patients for liver tumors with lymph node metastases (continue line) and without lymph node metastases (dotted line) (see “lymph node status and survival” of the “Results” section for description of designations).
The 1- and 2-year recurrence rate was 55.2% and 77.6% in the first group and 24.7% and 45.3% in second one, respectively (P < 0.01).
At the time of writing, tumor recurrence has appeared in 9 (52.9%) patients with lymph node metastases and in 17 (19.7%) without lymph node metastases (P < 0.05).
The most common site of tumor recurrence was intrahepatic (more than half of the cases), followed by lung, as can be seen from Table 4.
Table 4 summarizes the pathologic characteristics of the tumor, site of lymph node metastases, and outcome of the 17 patients with lymph node invasion according to the primary diagnosis (indication to resection).
DISCUSSION
We investigated the feasibility and the role of “regional lymphadenectomy” in patients with primary and secondary liver tumors and demonstrated that liver resection combined with the excision of lymph nodes around the hepatic pedicle, the retropancreatic space, and the common hepatic artery as far as the celiac trunk is a safe procedure that can be performed in all patients, without an increased risk of complications and with an acceptable increase of operative time.
Only 1 case of postoperative complication and no operative mortality were attributed to lymph node dissection. The relatively high rate of postoperative ascites is justified by the number of cirrhotic patients enrolled in this study, and the overall data are consistent with data from the literature.20,23 More than half the cases of postoperative ascites appeared in patients operated on for HCC on cirrhosis. In these patients, lymph node excision could impair lymphatic drainage, eventually leading to the dispersion of third space fluids into the abdominal cavity. The already reported finding of an increased risk of operative mortality caused by lymph node in cirrhotics13,14 was not confirmed from this study.
In the present series, at least 4 lymph nodes were dissected in all patients, and 3 stations were constantly present: the pericholedochal station, the periportal station, and the common hepatic artery. The most common sites of lymph node metastases were the pericholedochal node and common hepatic artery station. These nodes appear to be the key stations for lymphatic spread from liver tumors toward regional and more distant nodes. Elias et al demonstrated that there is no continuous chain progression from 1 lymph node to another lymph node station, by studying the rates of lymph nodes present in the different anatomic sites in the hepatic pedicle and celiac region, and of the nodal sites affected.24 It seems that “skip” lymph node metastases are the rule. We therefore believe that at least 4 regional lymph nodes should be dissected to obtain a reliable statement on lymph node status, as in surgery for colorectal cancer the minimum number of lymph nodes requested is 12.10 A final conclusion on lymph node status can not otherwise be expressed.
During the follow-up, no recurrence was found in thoracic lymph nodes. In this series, secondary lymphatic dissemination from liver tumors did not follow lymphatics of the convex surface, which cross the diaphragm and reach phrenic and juxtaesophageal stations, as already reported by others.18 This allowed us to consider the dissection of the lymph node stations in the thorax or into the mediastinum, which could substantially increase postoperative morbidity without any rational justification, to be an unjustified overtreatment.
In the present series, the number of nodules, but not the diameter of the tumor, significantly affected the presence of lymph node metastases. Furthermore, lymph node status was strongly related to the different tumor types.
The Liver Cancer Study Group of Japan reported a prevalence of lymph node metastases from HCC ranging from 25% to 33% in autopsy series, and 2.2% in series of resected patients.23
In the present prospective study, the incidence of lymph node metastases was 6.1% and 8.3% in resected patients for HCC with or without cirrhosis, respectively. In a recent review from the International Registry of Hepatic Tumors in Liver Transplantation (OLT), the incidence of lymph node metastases in cirrhotic patients with HCC undergoing OLT was 6.5%.25
The prognosis of patients with lymph node metastases from HCC is generally very poor, even if hepatic resection with lymph node dissection is performed.23,26 We believe that systematic regional lymph node dissection is necessary in patients for HCC without cirrhosis undergoing surgical resection for a correct staging of the disease and its subsequent clinical implications. On the contrary, in patients carrying HCC on cirrhosis, the possible benefit of lymph node excision should be balanced with the definite increase of medical complications, such as ascites, which could prolong the postoperative medical efforts. The prognosis after liver transplantation for HCC on cirrhosis is quite dismal in the presence of lymph node metastases.25,27 Therefore, to optimize patient selection, frozen section evaluation of at least 4 lymph nodes should be performed in patients with HCC on cirrhosis at the time of transplantation and a back up patient should be always present in the hospital to allow an optimal use of the procured graft.
The role of hepatic resection in the case of colorectal liver metastases has already been well established, while the indication of concomitant lymph node dissection is still unclear.28–30 Colorectal hepatic metastases can lead to infiltration of regional lymph nodes via the lymphatic route of the liver, the so-called “lymphatic remetastasis” of liver metastases.29 In this prospective study, the incidence of lymph node metastases was 14%, which is slightly lower than the 20% to 30% described in a recent review where lymph node metastases were evaluated among 15 retrospective studies.30 Since in patients with colorectal metastases it appears that the rate of lymph node invasion varies from 14% to 30%, we believe that regional lymphadenectomy should be performed as a standard procedure to achieve a reliable stage of the disease. In the present series, the median survival time was 16 months and the 2-year survival was 31.5% when lymph node metastases were found. This result should be considered encouraging compared with the natural history of unresected colorectal hepatic metastases, which carries a median survival time of less than 1 year and no survivors 3 years after diagnosis.31
Among the patients resected for noncolorectal, non-neuroendocrine liver metastases, and intrahepatic cholangiocarcinoma, the incidence of lymph node invasion was 40%. No clear guidelines exist regarding the indication and role of lymphadenectomy in these patients.32,33 However, in view of the high rate of lymph node metastases reported in this prospective series, we believe that lymph node dissection should be always performed to obtain a precise stage of the disease. The influence on survival has to be defined with further studies.
Further considerations must be drawn from this study. The present prospective series of more than 100 cases includes patients with “limited” disease, still amenable to be treated with partial hepatectomy. Lymph node invasion was found in 16.5% of patients, and this value increases to 20.3% when considering only noncirrhotic patients. The definition of lymph node status is important to assess a definitive stage of the disease. Patients with more advanced diseases will need closer observations, and more aggressive adjuvant therapies, whenever feasible, could be more appropriate.
These considerations should be kept in mind when facing with the widespread diffusion of techniques of percutaneous ablation of liver tumors. Initially proposed only for the treatment of unresectable cirrhotic patients with HCC, these ablative procedures recently included in their indications also primary and secondary tumors arising in patients without any underlying chronic liver disease.34,35 Often, the indication for an ablative treatment is made for “unresectable diseases,” even if the decision as to the feasibility of a partial hepatectomy is made by a physician other than a liver surgeon.
The diffusion of ablative treatments proceeds in spite of the absence of any randomized or controlled study on their efficacy, even in cirrhotics, and of any definitive improvement in results over surgery.20,36,37 The claimed benefits of these techniques are well known: the reduction in operative risk, postoperative pain and hospital stay, and even the limited impact on the cosmetic aspects, with the prospective of the same long-term results.
Our study recalls that 20.3% of patients with primary or secondary tumors arising in livers without any underlying disease will be undertreated by these techniques or, at least, understaged, and this is quite important for their final outcome. The categorization of intrahepatic or extrahepatic recurrence after percutaneous ablation would be inappropriate in one fifth of these cases since oncological radicality was not achieved.
It should be remembered that the surgical removal of carcinomas arising in most of the other organs of the body without the concomitant performance of lymphadenectomy is not accepted as an appropriate treatment of cure and staging.2–10 On the contrary, the debate remains open on the extent of such lymphadenectomy, including extended versus regional criteria.8,10 The new technique of thorascopic and laparoscopic surgery is gaining success precisely because it has been demonstrated that the extent of the lymphadenectomy is the same as the corresponding open procedure.38,39
In addition, we have also demonstrated that the conventional imaging techniques used in the preoperative evaluation of these tumors do not yet have enough power to reveal the neoplastic involvement of the regional lymph nodes. Due to the low sensitivity and diagnostic accuracy of double-phase CT scan, regional lymph nodes can be correctly explored only during laparotomy and frozen section samples have to be taken to correctly evaluate the lymph node status.
We thus believe that a reconsideration on the use of those technique of percutaneous ablation should be made by physicians involved in the field of hepatobiliary tumors. It should also be taken into consideration that the surgical risk for resection performed in nondiseased livers is around 0.5% when performed in specialized centers, even including major procedures for advanced disease.21
CONCLUSION
Liver resection with regional lymphadenectomy is a safe procedure in patients with liver malignancies, without an increased risk of morbidity and mortality. This procedure should be routinely applied for primary and secondary hepatic tumors in noncirrhotic patients. In patients with HCC on cirrhosis, a careful evaluation of lymph node status is advisable, in particular if they are candidates for liver transplantation. New adjuvant treatments are needed to improve long-term results in resected patients with lymph node metastases.
ACKNOWLEDGMENTS
The authors thank Giovanni Varotti, MD, Antonio Mazzeo, MD, and Gaetano Vetrone, MD, Department of Surgery and Transplantation, and Antonia D'Errico, MD, and Michelangelo Fiorentino, MD, Department of Pathology.
Footnotes
Reprints: Gian Luca Grazi, MD, Department of Surgery and Transplantation, University of Bologna, Sant'Orsola-Malpighi Hospital, Via Massarenti, 9, 40138 Bologna, Italy. E-mail:glgrazi@unibo.it.
REFERENCES
- 1.Sobin LH, Wittekind C, eds. International Union Against Cancer (UICC): TNM Classification of Malignant Tumors, 5th ed. New York: John Wiley and Sons, 1997. [Google Scholar]
- 2.Mitchell JD, Mathinsen DJ, Wright CD, et al. Resection for bronchogenetic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc Surg. 2001;121:465–471. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CP, Chen CY, Hsia JY, et al. Prediction of prognosis by the extent of lymph node involvement in squamous cell carcinoma of the thoracic esophagus. Eur J Cardio Thorac Surg. 2001;19:10–13. [DOI] [PubMed] [Google Scholar]
- 4.Miyao N, Masumori N, Takahashi A, et al. Lymph node metastasis in patients with carcinomas of the renal pelvis and ureter. Eur Urol. 1998;33:180–185. [DOI] [PubMed] [Google Scholar]
- 5.Mincey BA, Bammer T, Atkinson EJ, et al. Role of axillary node dissection in patients with T1a and T1b breast cancer: Mayo clinic experience. Arch Surg. 2001;136:779–782. [DOI] [PubMed] [Google Scholar]
- 6.Manzoni G, Verlato G, Guglielmi A, et al. Prognostic significance of lymph node dissection in gastric cancer. Br J Surg. 1996;83:1604–1607. [DOI] [PubMed] [Google Scholar]
- 7.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. [DOI] [PubMed] [Google Scholar]
- 8.Farnell MB, Nagorney DM, Sarr MG. The Mayo clinic approach to the surgical treatment of adenocarcinoma of the pancreas. Surg Clin North Am. 2001;81:611–623. [DOI] [PubMed] [Google Scholar]
- 9.Kodera Y, Yamamura Y, Shimizu Y, et al. The number of metastatic lymph nodes: a promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg. 1998;187:597–603. [DOI] [PubMed] [Google Scholar]
- 10.Prandi M, Lionetto R, Bini A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg. 2002;235:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada H, Endo I, Togo S, et al. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79:892–899. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada S, Yamashita Y, Aishima S, et al. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463–1466. [DOI] [PubMed] [Google Scholar]
- 14.Rassi E, Partensky C, Scoazec JY, et al. Peripheral cholangiocarcinoma: presentation, diagnosis, pathology and management. Eur J Surg Oncol. 1999;25:375–380. [DOI] [PubMed] [Google Scholar]
- 15.Coinaud C. Surgical Anatomy of the Liver Revisited. Paris, 1989.
- 16.Mazziotti A, Cavallari A. Techniques in Liver Surgery. London: Greenwich Medical Media, 1997. [Google Scholar]
- 17.Japanese Society of Biliary Surgery. General Rules for Surgical and Pathological Studies on Cancer of Biliary Tract, 4th ed. Tokyo: Kanehara, 1997. [Google Scholar]
- 18.Kokudo N, Sato T, Seki M, et al. Hepatic lymph node involvement in resected cases of liver metastases from colorectal cancer. Dis Colon Rectum. 1999;42:1285–1291. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji T, Hiraoka T, Kanemitsu K, et al. Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 2001;129:401–407. [DOI] [PubMed] [Google Scholar]
- 20.Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for colorectal metastases. Influence of parechymal involvement and total tumor volume, vs number and location, of long-term survival. Arch Surg. 2002;137:1187–1192. [DOI] [PubMed] [Google Scholar]
- 22.Norusis MJ. SPSS/PC+ User's Guide, version 8. 0, Chicago, 1998.
- 23.Liver Cancer Study Group of Japan. Primary liver cancer of Japan: clinicopathological features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 24.Elias D, Saric J, Jaeck D, et al. Prospective study of microscopic lymph node involvement of the hepatic pedicle during curative hepatectomy for colorectal metastases. Br J Surg. 1996;83:942–945. [DOI] [PubMed] [Google Scholar]
- 25.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uenishi T, Hirohashi K, Shuto T, et al. The clinical significance of lymph node metastases in patients undergoing surgery for hepatocellular carcinoma. Surg Today. 2000;30:892–895. [DOI] [PubMed] [Google Scholar]
- 27.Marsh JW, Dvorchik I, Bonham CA, et al. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Yokoi Y, Suzuki S, et al. Results of extensive surgery for liver metastases in colorectal carcinoma. Br J Surg. 1992;79:35–38. [DOI] [PubMed] [Google Scholar]
- 29.Beckurts KTE, Holscher AH, Thorban S, et al. Significance of lymph node involvement at the hepatic hilum in the resection of colorectal liver metastases. Br J Surg. 1997;84:1081–1084. [PubMed] [Google Scholar]
- 30.Rodgers MS, McCall JL. Surgery for colorectal liver metastases with hepatic lymph node involvement: a systematic review. Br J Surg. 2000;87:1142–1155. [DOI] [PubMed] [Google Scholar]
- 31.Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. [DOI] [PubMed] [Google Scholar]
- 32.Harrison LE, Brennan MF, Newmann E, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–632. [DOI] [PubMed] [Google Scholar]
- 33.Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. [DOI] [PubMed] [Google Scholar]
- 34.Livraghi T, Bolondi L, Buscarini L, et al. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Italian cooperative HCC study group. J Hepatol. 1995;22:522–526. [DOI] [PubMed] [Google Scholar]
- 35.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. [DOI] [PubMed] [Google Scholar]
- 36.Hoshida Y, Shiratori Y, Omata M. Difficulties in conducting controlled trials in radical therapies for nonadvanced hepatocellular carcinoma. Hepatology. 2000;32:877–880. [DOI] [PubMed] [Google Scholar]
- 37.Arii S, Futugawa S, Inoue K, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology. 2000;32:1224–1229. [DOI] [PubMed] [Google Scholar]
- 38.Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg. 2002;73:900–904. [DOI] [PubMed] [Google Scholar]
- 39.Nelson H. Laparoscopic colectomy for colon cancer: a trial update. Swiss Surg. 2001;7:248–251. [DOI] [PubMed] [Google Scholar]