Abstract
Objective:
To select suitable candidates for breast-conserving treatment (BCT) after neoadjuvant chemotherapy (NAC), based on the classification of tumors into localized or diffuse types using contrast-enhanced computed tomography (CE-CT).
Summary Background Data:
A relatively high rate of loco-regional failure after BCT has been reported with breast cancer downstaged by NAC. Accurate assessment of the suitability of BCT and the response to NAC, before the initiation of NAC, will allow the optimal selection of an appropriate therapeutic course.
Methods:
We evaluated 110 consecutive patients with operable breast carcinomas measuring 3-cm or more in diameter by CE-CT after NAC treatment with doxorubicin and docetaxel at National Cancer Center Hospital, Tokyo, from May 1998 to November 2001. Lesions were classified as either localized or diffuse types by mammography (MMG), ultrasonography (US), and CE-CT.
Results:
Tumors designated as localized type by MMG, US, and CE-CT were reduced to tumors less than 3.0 cm (P < 0.0001) in a concentric circle (P < 0.0001). Localized tumors by CE-CT were treated safely with BCT maintaining a negative margin status (P = 0.01). In contrast, diffuse type tumors shrunk into a mosaic pattern consisting of tumors larger than 3.1 cm. Tumors classified as localized by CE-CT responded better pathologically than diffuse tumors (P = 0.0365). Multivariate analysis demonstrated that morphologic type by CE-CT and histologic type were significant predictors of candidates for safe BCT.
Conclusions:
The classification of tumors into either localized or diffuse types, using CE-CT before NAC administration, accurately predicts which tumors will be suitable candidates for BCT after NAC.
We evaluated 110 large, operable breast carcinomas to predict the safety of breast-conserving treatment (BCT) after neoadjuvant chemotherapy (NAC). By classifying tumors into either localized or diffuse types using CT, we could accurately predict tumor shrinkage pattern, identify tumors small enough for BCT following NAC, and also predict response.
A large randomized clinical trial confirmed the efficacy of neoadjuvant chemotherapy (NAC) in downstaging breast carcinomas and permitting the increased use of breast-conserving treatment (BCT), although no survival advantage was demonstrated.1 The response rates of patients to NAC are generally high, ranging from 70 to 90%. Some patients, however, do not derive any benefit from NAC, and it would be advantageous to identify these patients before initiating NAC. In patients who were initially candidates for mastectomy but underwent BCT after NAC-mediated tumor downstaging, the incidence of ipsilateral breast tumor recurrence (IBTR) (14.5%) increased in comparison to patients undergoing BCT as initially planned (6.9%) (P = 0.04).1 This may be due to the wide mosaic satellites of residual viable tumor cells in the original tumor-bearing area, despite tumor downstaging following NAC administration.2,3 The reduction of a tumor into either a concentric circle or a wide mosaic-like pattern may be a critical factor for determining the suitability of BCT.4
The relationship between predictors of tumor shrinkage patterns and suitability of BCT has not been previously examined. Nakamura et al, however, reported in a small number of patients that papillotubular, estrogen receptor-positive, low nuclear grade, and negative c-erbB 2 tumors had a tendency to show mosaic-like patterns of residual tumor cells.6 The morphologic tumor type prior to NAC may be a strong predictor of tumor shrinkage pattern and suitability of BCT. In this study, we investigated whether classifying tumors into diffuse or localized types, through the use of diagnostic imaging, can predict the safety of BCT and the response to NAC.
PATIENTS AND METHODS
Patients
One hundred ten consecutive patients with operable breast carcinomas measuring greater than 3-cm in diameter who were evaluated by palpation and contrast-enhanced computed tomography (CE-CT) before and after NAC at the National Cancer Center Hospital (NCCH) in Tokyo from May 1998 to November 2001 were enrolled in this study. They were also evaluated by mammography (MMG) and ultrasonography (US). The treatment protocol consisted of 4 cycles of doxorubicin and docetaxel at doses of 50 and 60 mg/m2, respectively, with a 21-day cycle length, followed by mastectomy or breast-conserving surgery. Eligible women received initial pathologic confirmation of breast carcinoma by core needle biopsy. Complete staging was determined by chest x-ray, liver ultrasound, and bone scan for all patients prior to initiating NAC. All patients gave informed consent for study participation as approved by the institutional review board of NCCH.
Imaging Examinations
Helical CT scanning was performed using an X-Vigor scanner (Toshiba, Japan) at a current of 300 mA. Patients underwent one single spiral acquisition, during deep inspiratory apnea in the supine position, for up to 30 seconds. The first non-contrast-enhanced CT scan served as a baseline, imaged from the cranial end of the sternum to the inframammary fold. Subsequently, an enhanced zoomed scan visualized the whole breast, using a collimation of 5 mm and a pitch of 1 mm. One hundred milliliters of nonionic contrast material (300 mg I/mL) was injected at 2 mL/s. Fifty seconds elapsed between the administration of contrast material and the initiation of scanning. The reconstruction interval was 5 mm.6
A Mammomat 3000 (Siemens, Germany) was used for mammographic examination. In addition to standard oblique and cranio-caudal projections, cranio-caudal and medio-lateral spot views (5 cm in diameter) without magnification, were also obtained for most patients. Whole-breast US was performed using a SSA340A (Toshiba, Japan), possessing an annular array transducer. With all modalities in this study, tumor diameters were measured in the transverse direction.
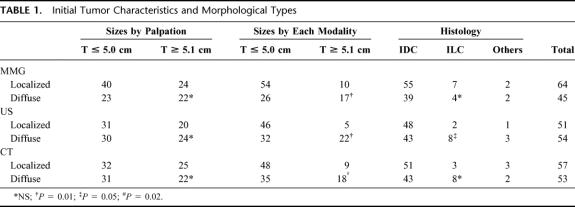
To predict the shrinkage pattern, the diagnostic image findings were used to classify tumor morphology into either localized type or diffuse type (Fig. 1). By mammography, localized tumors were either devoid of microcalcifications or possessed microcalcifications contained within the tumor. Diffuse type tumors included density elevation type tumors (tumors whose border is not clearly defined), tumors with widespread microcalcifications beyond their edges, and multiple tumors. US findings also identified both localized and diffuse tumor types, including tumors with ducts, noticeable shadow formation, and multiple foci (Fig. 2). Enhanced CE-CT images classified these 2 types, with localized tumors visualized as single spots, while diffuse type tumors included those with surrounding spots, multiple spots, and glandular spreading (whole or part of a gland occupied by enhanced lesion) (Fig. 3). 7
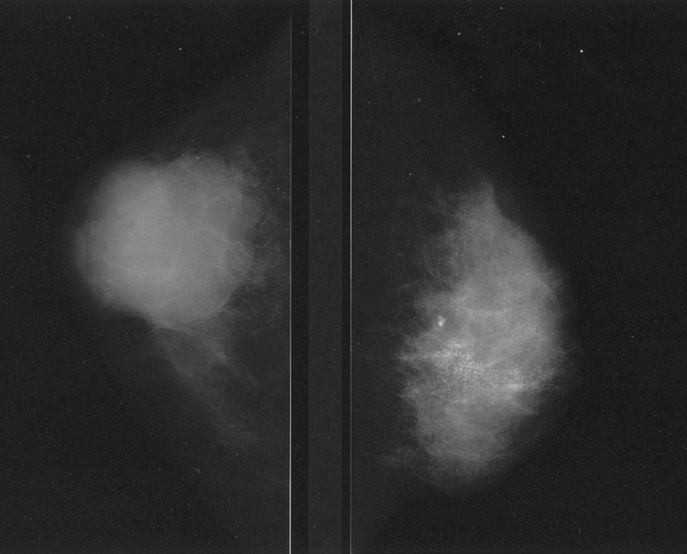
FIGURE 1. Localized tumor type (left) and diffuse type (right) by MMG.
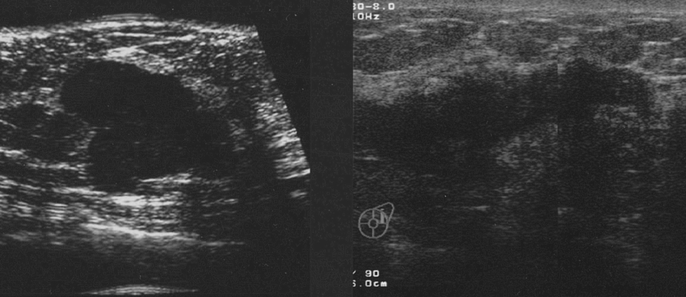
FIGURE 2. Localized tumor type (left) and diffuse type (right) by US.
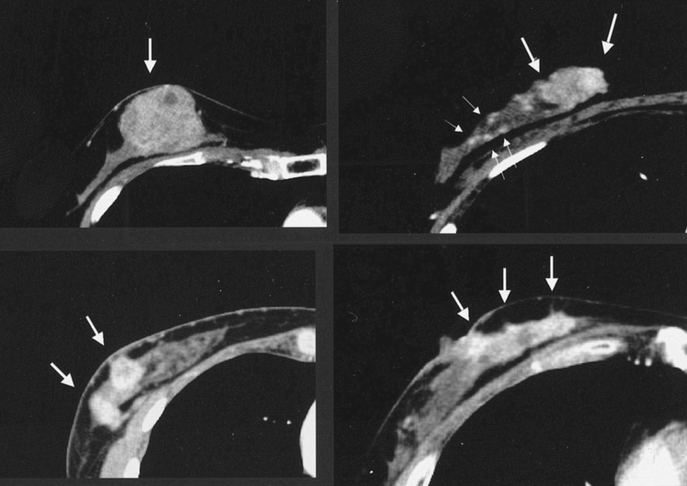
FIGURE 3. Localized tumor type (left upper) and diffuse type: tumor and spots (right upper), multiple spots (left lower), and glandular spreading type (right lower) by CE-CT.
MMG and CE-CT imaging studies were prospectively evaluated by 2 radiologists (K.M. and N.S.). US images were evaluated by 2 additional radiologists (H.M. and N.S.). Classifications of morphologic subtypes were made independently by N.S. and S.A. The coincidence rates of the classifications were 97% (107 of 110). Following discussion, cases without coincident interpretations were mutually agreed upon.
Histopathological Examinations
Surgical specimens were sectioned at approximately 7-mm to 10-mm intervals along a transverse axis, for analysis by breast pathologists. Margins were classified as negative if no invasive or in situ ductal carcinoma was observed within 1 mm of the margin and positive when cancerous cells were observed at the margin. The response to NAC was classified according to the general rules for the clinical and pathologic recording of breast cancer.8 For Grade 0, no response was observed; Grade 1a comprised those tumors with any degenerative change or severe degenerative change in less than one third of cancerous cells; for Grade 1b, severe degenerative changes were observed in one third to two thirds of the cancerous cells; Grade 2 tumors contained degeneration of more than two thirds of cancerous cells; Grade 3 tumors demonstrated a complete response, with no cancerous cells remaining.
Statistical Analysis
The χ2 test was used for comparisons of sizes, histology, changes, and responses among morphologic groups. Differences with P < 0.05 were considered to be significant. A logistic regression model was used for univariate and multivariate analysis.
RESULTS
The mean age at surgery of 110 patients was 52 years, with a range from 29 to 69 years of age. Sixty patients (54.5%) presented with T2 tumors, 34 (30.9%) with T3 and 16 (14.5%) with T4b (skin involvement) tumors. Fifteen patients (13.6%) achieved a complete clinical response, while 68.1% (75 of 110) showed a partial clinical response. Pathologically, according to the general rules for the clinical and pathologic recording of breast cancer,8 6 tumors (5.5%) achieved a Grade 3 response, 33 (30%) achieved a Grade 2 response, 30 (27%) achieved a Grade 1b response, 35 (32%) achieved a Grade 1a response, and 6 (5.5%) did not demonstrate a response.
The relationships between the initial tumor characteristics and the morphologic types defined by diagnostic imaging are displayed in Table 1. Prior to NAC, 57 tumors were classified as localized by CE-CT, while 53 were designated as diffuse type. Based on physical examination, sizes of localized and diffuse tumors, as classified by all of the modalities, did not differ. When size was determined by each modality, however, localized tumors tended to be smaller. Invasive lobular carcinomas (ILC) tended to exhibit a diffuse morphology by US (P = 0.05). Of sixteen tumors showing skin involvement, 14 tumors were classified as localized by CE-CT. Five patients failed to be evaluated by breast US before NAC, and 2 were not evaluated after NAC. One patient failed to be evaluated by MMG before NAC, and another failed to be evaluated after NAC.
TABLE 1. Initial Tumor Characteristics and Morphological Types
We compared the morphologic changes in the images before and after NAC (Table 2). According to all 3 modalities, localized tumors maintained their localized phenotype (P < 0.0001) (Fig. 4). Furthermore, diffuse tumors maintained their diffuse phenotype (Fig. 5). This suggested that localized tumors shrink into a concentric circle, while diffuse tumors shrink into a mosaic-like pattern of tumor cells. The relationship between pathologic response and morphologic type is shown in Table 3. Localized tumors by CE-CT tended to respond better than diffuse type tumors (P = 0.036).
TABLE 2. Morphological Changes in the Imaging Before and After NAC
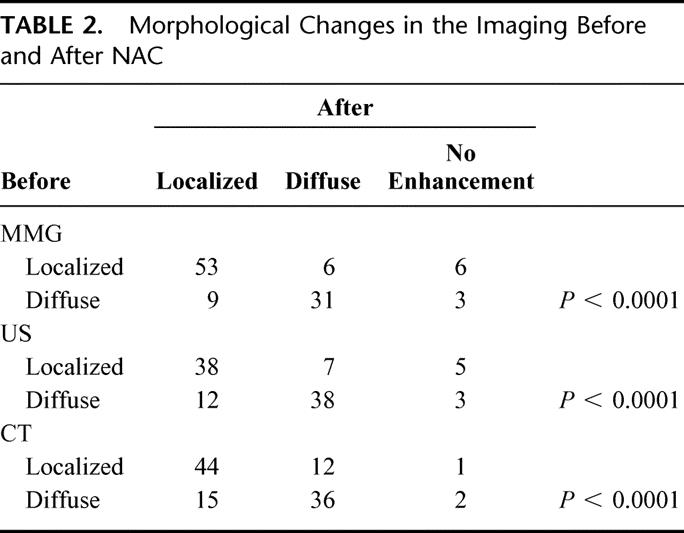
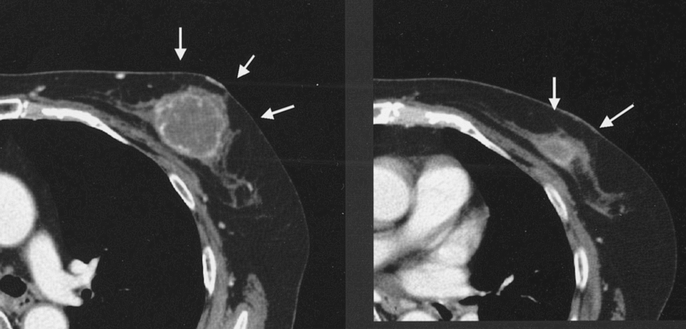
FIGURE 4. A typical case with localized type tumor by CE-CT (left) decreased in size to form a concentric circle (right).
FIGURE 5. A typical case with diffuse type tumor by CE-CT (left), which diminished to mosaic residue (right).
TABLE 3. Pathological Response to NAC and Morphological Types by Diagnostic Imaging Before NAC
The results of univariate analysis on the initial histologic characteristics of the tumor on core biopsy, pathologic response to NAC, morphologic category, and prechemotherapy size based on diagnostic imaging and physical examination associated with safe BCT are listed in Table 4. Fifty-eight tumors (53%) measured less than 3.0 cm pathologically after NAC, while 52 (47%) remained larger than 3.0 cm. For BCT, we defined a residual tumor size of 3.0 cm as a safe upper limit. Significant associations were observed between almost all variables, except for initial size based on US. Localized tumors diminished in size to less than 3.0 cm (P = 0.002 for MMG, 0.0007 for US, and <0.0001 for CE-CT, respectively). Diffuse tumors did not consistently demonstrate reductions in size to less than 3.0 cm.
TABLE 4. Univariate Analysis on Tumor Characteristics Associated With Safe BCT (Achieving t ≤ 3.0 cm)
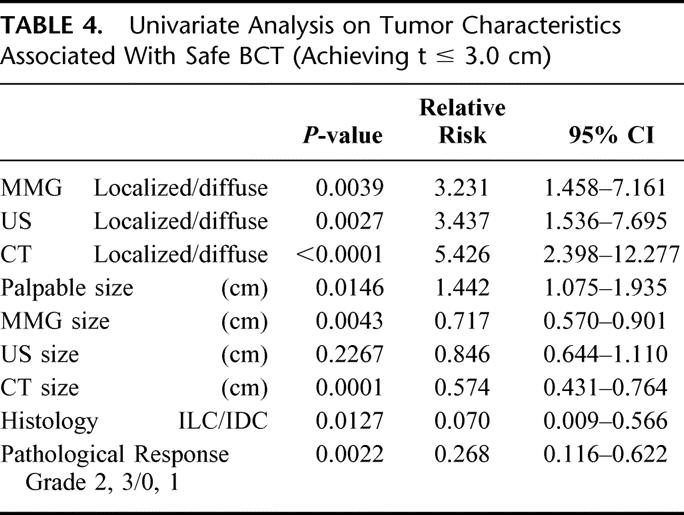
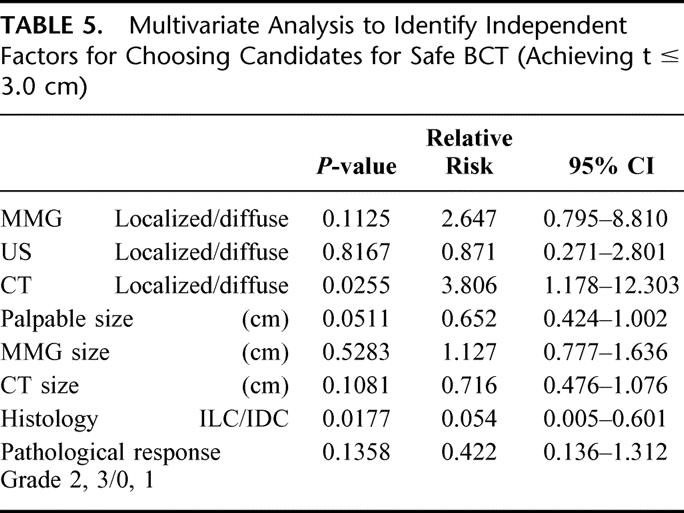
Multivariate regression analyses using a logistic regression model were conducted to identify independent factors for choosing candidates for safe BCT (Table 5). At the end of the multivariate analyses, the morphologic categories by CE-CT (P = 0.0255) and histology (P = 0.0175) remained significantly related to safe BCT following NAC.
TABLE 5. Multivariate Analysis to Identify Independent Factors for Choosing Candidates for Safe BCT (Achieving t ≤ 3.0 cm)
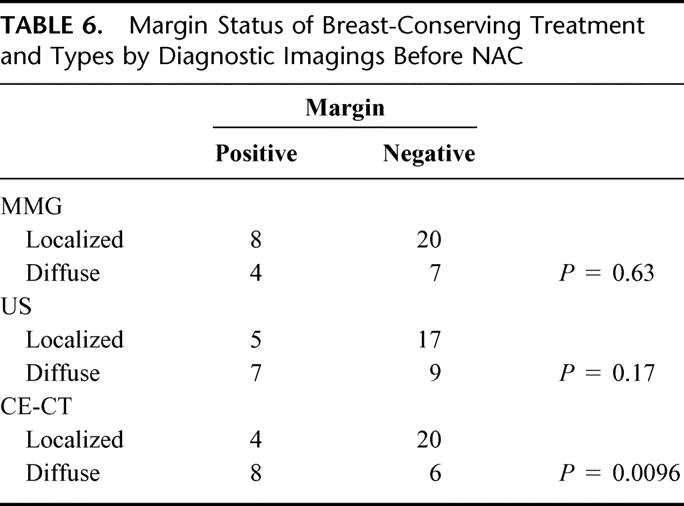
Of 67 patients eligible for BCT (T< 3 cm), 38 patients chose BCT. Twelve patients had positive resection margins following BCT. Margin status was correlated with tumor type, as determined by CE-CT (Table 6). Diffuse tumors consistently exhibited positive margins (P = 0.0096). Of 7 patients eligible for BCT who had had skin involvement prior to NAC, 2 patients with localized tumor by CE-CT chose BCT and obtained negative margin status. No IBTRs were observed after a 2 year median follow-up.
TABLE 6. Margin Status of Breast-Conserving Treatment and Types by Diagnostic Imagings Before NAC
DISCUSSION
Appropriate use of NAC may allow BCT in patients who would otherwise be treated by mastectomy. Accurate prediction of the suitability of BCT and the response to NAC, prior to initiating NAC downstaging, would help in the optimal selection of treatment. The classification of tumors into either localized or diffuse types using diagnostic imaging provides a basis for making this determination. Localized tumors responded well to NAC and were reduced into smaller, concentric tumors that could be safely treated by wide excision, giving a negative margin status. Diffuse tumors, however, diminished into a mosaic pattern of residual tumor cells, giving a positive margin status when treated with BCT. Thus, they most likely would be more suitably treated by mastectomy or wide excision of the area corresponding to the original tumor size. Multivariate analysis demonstrated that classification by CE-CT was a powerful predictor of the safety of BCT. ILC was also an independent predictor of safe BCT, but the number of ILC was so small that the 95% confidence interval became wide.
In this study, we defined a residual tumor size of 3.0 cm as a safe upper limit for BCT. Although larger tumor size is not an absolute contraindication for BCT,9 tumors smaller than 3 or 4 cm by physical examination are eligible for BCT to facilitate cosmetically acceptable results and good local control.10 As determined by the Japanese Breast Cancer Society, the current guidelines recommend tumors smaller than 3 cm as being safe for BCT.11 Complete removal of tumor has been confirmed as crucial for BCT with good local control. Therefore, we have applied the guideline of 3 cm as a safe size limit for patients undergoing BCT.
Prediction of response to NAC has recently focused on expression of potential biologic markers such as estrogen receptor, p53, HER2/c-erbB-2, and Ki67 in the primary breast tumor.12,13 ER-negative, poorly differentiated tumors were more likely to be associated with a higher response to NAC. The present study indicated that the morphologic category based on CE-CT was a significant predictor of pathologic tumor response to NAC. There are no reports that have associated morphology with the response to chemotherapy.
It is expected that a tumor showing a better response to NAC and/or an initially smaller size would shrink into a smaller tumor that is more suitable for BCT. Multivariate analysis, however, suggested that the pathologic response and initial tumor size, as determined by palpation and diagnostic imaging, were not significant predictors of candidates for BCT. The morphologic type proved to be the more powerful predictor, probably due to shrinkage patterns.
After NAC, the major problem in the management of breast cancer is defining the extent of residual disease so that appropriate treatment may be undertaken. We reported that CE-CT could determine the extent of the residual cancerous lesions following NAC administration more accurately than other conventional diagnostic methods, such as MMG and US.7 MRI may also prove to be valuable in defining the extent of residual disease after NAC.3,14–17 Many institutions, however, lack the appropriate expertise in breast MRI. CE-CT is advantageous over MRI, as it requires only approximately 5 min for examination. In addition, CE-CT breast images are obtained in the supine position used during surgery, providing precise information about the extent of cancerous tissue in the breast, in a context most useful to the surgeon. In MRI studies, on the other hand, patients are examined in the prone position, minimizing the motion of the breast during breathing. The breast can easily change shape in different positions. The image quality of MRI depends on the performance of the machine and additional complicated technical parameters, making this technique difficult to use as a universal diagnostic procedure. CE-CT also has higher spatial resolution and is less expensive. Finally, the presence of contraindications to MRI, such as patients with pacemakers or serious claustrophobia, makes CE-CT a preferable technique.18
Fisher et al showed that patients with IBTR exhibit more distant metastases than patients without IBTR and considered IBTR to be a marker of distant metastasis, rather than a cause.19 Fortin et al, however, suggested that IBTR should be considered as a source for new distant metastases and an indicator of subsequent mortality by showing the difference in the time distribution of distant metastases for patients with and without IBTR.20 Recently, a long follow-up retrospective study reported that a high frequency of IBTR occurrence (21.5% at 10 years) after BCT in downstaged patients, as well as IBTR following NAC, is a strong predictor of distant metastases.21 Multiple re-excision after IBTR may increase the cost and associated trauma in women with breast cancer.22 To achieve local control, careful selection of patients who may eligible for BCT was suggested. In this study, none of the patients developed IBTR, probably due to the short follow-up time and appropriate preoperative management using CE-CT.
In conclusion, the classification of tumors into either localized or diffuse types using CE-CT prior to NAC administration accurately predicts which tumors will be suitable candidates for BCT following NAC.
ACKNOWLEDGMENTS
This work was supported in part by a Grant for Scientific Research from the Expenses for Health and Welfare Program (13–9) from the Ministry of Health, Labor and Welfare in Japan.
Footnotes
This work was supported in part by a Grant for Scientific Research from the Expenses for Health and Welfare Program (13–9) from the Ministry of Health, Labor and Welfare in Japan.
Reprints: Sadako Akashi-Tanaka, MD, Division of Breast Surgery National Cancer Center Hospital, 1–1 Tsukiji 5-chome, Chuo-ku, Tokyo 104–0045, Japan. E-mail: sakashi@gan2.ncc.go.jp.
REFERENCES
- 1.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. [DOI] [PubMed] [Google Scholar]
- 2.El-Didi MH, Moneer MM, Khaled HM, et al. Pathological assessment of the response of locally advanced breast cancer to neoadjuvant chemotherapy and its implications for surgical management. Surg Today. 2000;30:249–254. [DOI] [PubMed] [Google Scholar]
- 3.Abraham D, Jones R, Jones S, et al. Evaluation of neoadjuvant chemotherapeutic response and locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996;78:91–100. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda T, Jinno H, Matui A, et al. The role of neoadjuvant chemotherapy for breast cancer treatment. Breast Cancer. 2002;9:8–14. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S, Kenjo H, Nishio T, et al. Efficacy of 3D-MR mammography for breast conserving surgery after neoadjuvant chemotherapy. Breast Cancer. 2002;9:15–19. [DOI] [PubMed] [Google Scholar]
- 6.Akashi-Tanaka S, Fukutomi T, Miyakawa K, et al. Diagnostic value of contrast-enhanced computed tomography for diagnosing the intraductal component of breast cancer. Breast Cancer Res Treat. 1998;49:79–86. [DOI] [PubMed] [Google Scholar]
- 7.Akashi-Tanaka S, Fukutomi T, Watanabe T, et al. Accuracy of contrast-enhanced computed tomography in the prediction of residual breast cancer after neoadjuvant chemotherapy. Int J Cancer (Radiat Oncol Invest). 2001;96:66–73. [DOI] [PubMed] [Google Scholar]
- 8.The Japanese Breast Cancer Society: General Rules for Clinical and Pathological Recording of Breast Cancer. 14th ed. Tokyo: Kanehara; 2000. [Google Scholar]
- 9.Morrow M, Harris JR: Local management of invasive breast cancer. In: Harris JR, Lippman ME, Morrow M, et al., eds. Diseases of the Breast, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:526. [Google Scholar]
- 10.Heys D. Evaluation of breast cancer management: focus on neoadjuvant chemotherapy. Breast Cancer. 2001;8:339–350. [DOI] [PubMed] [Google Scholar]
- 11.Fukutomi T. Breast cancer [in Japanese]. In: Kakizoe T, ed. New Surgical Oncology Operative Techniques. Tokyo: Medical View, 2001:57. [Google Scholar]
- 12.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. [DOI] [PubMed] [Google Scholar]
- 13.Makris A, Powles T, Dowsett M, et al. Prediction of response to neoadjuvant chemoendocrine therapy in primary breast carcinomas. Clin Cancer Res. 1997;3:593–600. [PubMed] [Google Scholar]
- 14.Gilles R, Guinebretiere J-M, Toussaint C, et al. Locally advanced breast cancer: contrast-enhanced subtraction MR Imaging of response to preoperative chemotherapy. Radiology. 1994;191:633–638. [DOI] [PubMed] [Google Scholar]
- 15.Mumtaz H, Davidson T, Spittle M, et al. Breast surgery after neoadjuvant treatment. Is it necessary? Eur J Surg Oncol. 1996;22:335–341. [DOI] [PubMed] [Google Scholar]
- 16.Trecate G, Ceglia E, Stabile F, et al. Locally advanced breast cancer treated with primary chemotherapy: comparison between magnetic resonance imaging and pathologic evaluation of residual disease. Tumori. 1999;85:220–228. [DOI] [PubMed] [Google Scholar]
- 17.Tsuboi N, Ogawa Y, Inomata T, et al. Changes in the findings of dynamic MRI by preoperative CAF chemotherapy for patients with breast cancer of stage II and III: pathologic correlation. Oncology. 1999;6:727–732. [DOI] [PubMed] [Google Scholar]
- 18.Akashi-Tanaka S, Fukutomi T, Miyakawa K, et al. Contrast-enhanced computed tomography diagnosing the intraductal component and small invasive foci of breast cancer. Breast Cancer. 2001;8:10–15. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Anderson S, Fisher E, et al. Significance of ipsilateral breast tumor recurrence after lumpectomy. Lancet. 1991;338:327–331. [DOI] [PubMed] [Google Scholar]
- 20.Fortin A, Larochelle M, Laverdiere J, et al. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J Clin Oncol. 1999;17:101–109. [DOI] [PubMed] [Google Scholar]
- 21.Rouzier R, Extra J-M, Carton M, et al. Primary chemotherapy for operable breast cancer: Incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828–3825. [DOI] [PubMed] [Google Scholar]
- 22.Esserman L, Hylton N, Yassa L, et al. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol. 1999;17:110–119. [DOI] [PubMed] [Google Scholar]