Abstract
Rad51 can promote extensive strand exchange in vitro in the absence of ATP hydrolysis, and the Rad51-K191R mutant protein, which can bind but poorly hydrolyze ATP, also promotes strand exchange. A haploid strain expressing the rad51-K191R allele showed an equivalent sensitivity at low doses of ionizing radiation to rad51-K191A or rad51 null mutants and was defective in spontaneous and double-strand break-induced mitotic recombination. However, the rad51-K191R/rad51-K191R diploid sporulated and the haploid spores showed high viability, indicating no apparent defect in meiotic recombination. The DNA repair defect caused by the rad51-K191R allele was suppressed in diploids and by mating-type heterozygosity in haploids. RAD54 expressed from a high-copy-number plasmid also suppressed the γ-ray sensitivity of rad51-K191R haploids. The suppression by mating-type heterozygosity of the DNA repair defect conferred by the rad51-K191R allele could occur by elevated expression of factors that act to stabilize, or promote catalysis, by the partially functional Rad51-K191R protein.
The RAD51 gene encodes a structural and functional homologue of the Escherichia coli RecA protein (1, 4, 37). Rad51 exhibits ATP-dependent binding to single-stranded DNA and a weak ATPase activity (37, 41). Strand exchange by Rad51 requires a nucleotide cofactor but can occur in the presence of the nonhydrolyzable ATP analogs, AMP-PNP and ATPγS (41, 43). This observation has raised the issue of the role of ATP hydrolysis in Rad51-mediated recombination. The invariant lysine residue within the Walker A motif of Rad51 has been substituted with arginine or alanine to generate mutants able to bind but not hydrolyze ATP (Rad51-K191R), or unable to bind ATP (Rad51-K191A). The Rad51-K191R mutant protein was shown to promote strand exchange in vitro when the concentration of the mutant protein was about four times higher than normally used for the wild-type protein, but the Rad51-K191A protein was completely defective for DNA binding and strand exchange (43). The rad51-K191A allele expressed from the native promoter on a low-copy-number plasmid, or expressed from a strong constitutive promoter, was unable to complement the methyl methanesulfonate sensitivity or recombination defects of a rad51Δ strain (37, 43). However, the rad51-K191R allele showed partial complementation of the DNA repair and recombination defects of the rad51Δ strain when expressed from a low-copy-number plasmid (37) and full complementation when overexpressed (43). Thus, it appears that the requirement for ATP hydrolysis can be circumvented when the intracellular concentration of Rad51-K191R is elevated. In contrast, binding and hydrolysis of ATP are critical for RecA function in vivo, even though extensive strand exchange occurs in vitro in the absence of ATP hydrolysis (25, 32).
Deletion of RAD51 in vertebrates results in cell inviability and early embryonic lethality in mice (23, 44). A RAD51 conditional cell line has been made by deleting both copies of RAD51 in DT40 chicken cells in the presence of HsRAD51 regulated by a tetracycline-repressible promoter (38). Down regulation of the gene resulted in rapid depletion of HsRad51 concomitant with a G2/M phase arrest and the accumulation of cytologically visible chromosomal breaks and eventual cell death. These findings suggest that the essential role of RAD51 in vertebrates is to repair breaks generated during DNA replication. To assess the role of ATP hydrolysis by the human Rad51p for cell viability, Morrison et al. (29) examined the activities of various mutant alleles by monitoring the rescue of cell viability of the conditional rad51−/− cell line. No viable cell lines were recovered from cells transfected with the Hsrad51-K133A-expressing plasmid, but some cell lines were recovered following transfection with the Hsrad51-K133R plasmid. These cell lines were shown to have very high levels of expression of the mutant protein, suggesting that, as for Saccharomyces cerevisiae, ATP hydrolysis is not essential when the mutant allele is overexpressed. Expression of the Hsrad51-K133R allele in a wild-type mouse embryonic stem cell line resulted in increased sensitivity to mitomycin C and ionizing radiation, suggesting that this allele confers a dominant negative phenotype (39). This cell line also showed reduced spontaneous sister-chromatid exchange and a fivefold reduction in DSB-induced homologous recombination (39). The goal of this study was to determine whether ATP hydrolysis by Rad51 is essential for DNA repair proficiency when the protein is expressed at native levels. The rad51-K191R and rad51-K191A alleles were substituted for the wild-type locus in haploid and diploid yeast, and the phenotypes of the resulting strains in DNA repair, mitotic recombination, and meiosis were determined.
MATERIALS AND METHODS
Media, growth conditions, and genetic methods.
Rich medium, synthetic complete medium lacking the appropriate amino acid or nucleic acid base, and sporulation medium were prepared as described previously (36). Raffinose (2%) was substituted for glucose as a nonrepressing carbon source in synthetic complete medium that was used for induction of HO. Transcription from the GAL1 promoter was induced by the addition of 1/10 volume of 20% galactose to the growth medium. Yeast cells were grown at 30°C unless otherwise stated. Transformation, sporulation, and tetrad dissection were carried out as described previously (36). The percent sporulation was determined by microscopic analysis of cells scraped from sporulation plates and suspended in water. Percent sporulation values are averages of at least three independent cultures of each strain, and no fewer than 200 cells were counted for each sample. Tetrad numbers for the wild-type strain were obtained from the study by Kirkpatrick et al. (22). The measurement of recombination rates of the ade2-inverted repeat was as described previously (31).
Yeast strains and plasmids.
All strains are isogenic and in the W303 background (leu2-3,112 trp1-1 ura3-1 can1-100 ade2-1 his3-11,15) with the corrected RAD5 allele (Table 1). The chromosomal rad51-K191R and rad51-K191A alleles were generated by a PCR-based allele replacement method (13). The rad51-K191R and rad51-K191A alleles were amplified by PCR using primers YER095W-F and YER095W-R from Research Genetics and plasmids pR51.5 and pR51.4 as templates, respectively (43). The resulting products were fused to the fragments of the Kluyveromyces lactis URA3 gene by a second PCR using the primers described by Erdeniz et al. (13). The pairs of PCR products from the second PCR were then cotransformed into strain W1588-4C selecting for Ura+ transformants. Strains LSY977 and LSY982 were plated on medium containing 5-fluoroorotic acid to select for deletion of URA3 and one copy of the duplication. The presence of the correct mutation in strains LSY979 and LSY983 was verified by PCR amplification of the rad51 locus followed by DNA sequencing of the PCR products. The phenotype of the strains with the rad51-K191R-URA3-rad51-K191R allele was the same as that of strains with the rad51-K191R allele, presumably due to the lack of a promoter for the downstream allele. Other strains listed were made by crossing the appropriate haploids, sporulating the resulting diploids, and screening the haploid progeny by phenotype.
TABLE 1.
Yeast strains
| Strain | Genotypea | Source or reference |
|---|---|---|
| W1588-4C | MATa | R. Rothstein |
| W1588-4A | MATα | R. Rothstein |
| HKY595-3B | MATarad51::LEU2 | H. Klein |
| HKY595-1C | MATα rad51::LEU2 | H. Klein |
| YAR91 | MATα ade2-5′ Δ-TRP1-ade2-n spo13::hisG ade2::hisG-URA3-hisG | 31 |
| B403-1A | MATarad51::HIS3 | 2 |
| LSY410 | MATarad51::URA3 | 31 |
| LSY411 | MATα rad51::URA3 | 31 |
| LSY535 | MATα rad57::URA3 | 20 |
| LSY698 | MATamet17::ADE2 | 3 |
| LSY786 | MATα yku70::HIS3 | 28 |
| LSY919-10B | MATα met17::ADE2 rad51::HIS3 | This study |
| LSY956 | MATα met17::ADE2 | This study |
| LSY977 | MATarad51-K191R-URA3-rad51-K191R | This study |
| LSY979 | MATarad51-K191R | This study |
| LSY982 | MATarad51-K191A-URA3-rad51-K191A | This study |
| LSY983 | MATarad51-K191A | This study |
| LSY981-1A | MATα rad51-K191R-URA3-rad51-K191R met17::ADE2 | This study |
| LSY981-4B | MATα rad51-K191R-URA3-rad51-K191R | This study |
| LSY1012, LSY1013 | MATα rad51-K191R ade2-5′ Δ-TRP1-ade2-n ade2::hisG | This study |
| LSY1200-1D | MATarad51::LEU2 yku70::HIS3 | This study |
| LSY1201-3D | MATarad51-K191R-URA3-rad51-K191R yku70::HIS3 | This study |
| LSY1255-12B | MATα rad51-K191R rad57::URA3 | This study |
| LSY1255-12C | MATarad51-K191R rad57::URA3 | This study |
| LSY1039 | Diploid formed by crossing LSY977 with LSY1013 (rad51-K191R/rad51-K191R) | This study |
| LSY1040 | Diploid formed by crossing HKY595-1C with B403-1A (rad51::LEU2/rad51::HIS3) | This study |
| LSY1041 | Diploid formed by crossing HKY595-3B with LSY981-1A (rad51::LEU2/rad51-K191R) | This study |
| LSY1093 | Diploid formed by crossing W1588-4C with W1588-4A (RAD51/RAD51) | This study |
| LSY1104 | Diploid formed by crossing LSY919-10B with LSY982 (rad51::HIS3/rad51-K191A) | This study |
| LSY1196 | Diploid formed by crossing LSY979 with LSY919-10B (rad51::HIS3/rad51-K191R) | This study |
| LSY1212 | Diploid formed by crossing LSY979 with LSY411 (rad51::URA3/rad51-K191R) | This study |
All strains are of the W303 genotype (leu2-3, 112 trp1-1 ura3-1 can1-100 ade2-1 his3-11, 15 RAD5); only the mating type and differences from this genotype are shown.
Plasmids pR51.4 (rad51-K191A) and pR51.5 (rad51-K191R) were gifts from P. Sung, plasmids pRS414-MATa and pRS414-MATα were gifts from R. Rothstein, plasmid YEp13-RAD54 was a gift from D. Schild, and pFH800 (TRP1 ARS1 CEN4 GAL1p-HO) was provided by J. Nickoloff.
Western blot analysis.
Cells were grown in 50 ml of liquid medium to mid-log phase. Extracts were prepared as described previously, and 100 μg of protein was loaded onto each lane of a sodium dodecyl sulfate-10% polyacrylamide gel (40). The proteins were transferred onto Immobilon-P membranes (Millipore) and probed with affinity-purified α-Rad51 antibody (kindly provided by P. Sung). The antibody cross-reacts with several other proteins in yeast extracts that served as loading controls.
Gamma irradiation survival assays.
Cells were grown in liquid medium to mid-log phase. The cultures were serially diluted, and aliquots of each dilution were plated on solid medium. The plates were irradiated in a Gammacell-220 containing 60Co (Atomic Energy of Canada) for the designated dose. The plates were incubated for 3 days before surviving colonies were counted. Each strain was assayed at least three times, and mean values with standard deviations were calculated. It was noted that the rad51-K191R strains showed higher sensitivity to irradiation when pregrown in rich medium than in synthetic medium.
Physical analysis of mating-type switching.
Wild-type, rad51::LEU2 and rad51-K191R strains were transformed to Trp+ with plasmid pFH800. Growth of cultures and physical analysis of mating-type switching was performed as described previously (28).
RESULTS
The rad51-K191R allele confers sensitivity to ionizing radiation in haploids.
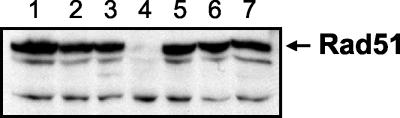
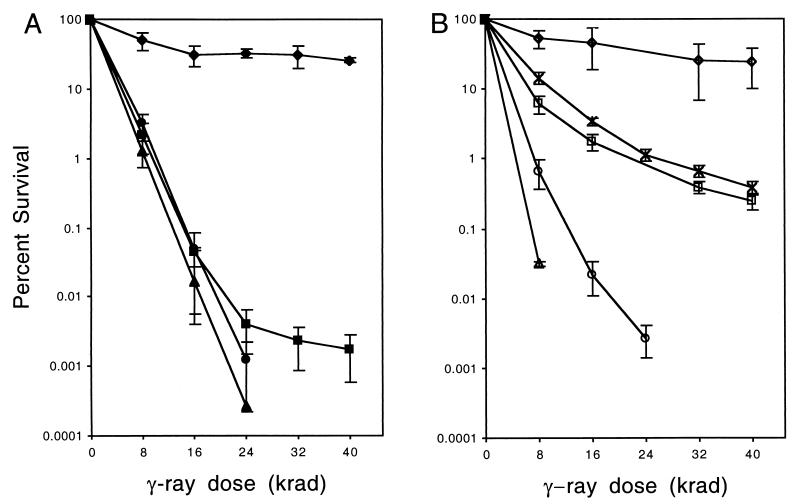
To determine whether the rad51-K191R allele confers DNA repair and recombination deficiencies when present in single copy, the chromosomal copy of RAD51 was replaced with the rad51-K191R allele in a haploid strain. A strain containing the rad51-K191A allele was also generated. By Western blot analysis, the Rad51-K191R and Rad51-K191A proteins were expressed at levels equivalent to that of the wild-type protein (Fig. 1). When treated with ionizing radiation, the rad51-K191R strain showed sensitivity similar to that of the null mutant at low doses of gamma irradiation but was more resistant than the null mutant to high doses (Fig. 2A). The reason for the resistant subpopulation is unclear but could be due to transient elevated expression of Rad51-K191R in a small fraction of cells. As anticipated, the γ-ray sensitivity of the rad51-K191A mutant was the same as that of the rad51 null strain (Fig. 2A).
FIG. 1.
Expression of the Rad51-K191R protein. Western blot analysis of extracts prepared from the indicated strains and probed with α-Rad51 antibodies. Lanes: 1, RAD51; 2, rad51-K191R; 3, rad51-K191A; 4, rad51::LEU2; 5, MATa rad51-K191R + pRS414; 6, MATa rad51-K191R + pRS414-MATa; 7, MATa rad51-K191R + pRS414-MATα.
FIG. 2.
Sensitivity of rad51-K191R haploid and diploid strains to gamma irradiation. (A) Percent survival of RAD51 (♦), rad51::LEU2 (▴), rad51-K191R (▪), and rad51-K191A (•) haploid strains to increasing dose of γ-rays. (B) Percent survival of RAD51/RAD51 (⋄), rad51-K191R/rad51-K191R (□), rad51-K191R/rad51::LEU2 (X), rad51::LEU2/rad51::HIS3 (▵), rad51-K191A/rad51::HIS3 (○).
The rad51-K191R mutant is defective for spontaneous and double-strand break (DSB)-induced mitotic recombination.
The rate of spontaneous mitotic recombination was determined by using haploid strains containing two different mutant alleles of the ADE2 gene present as an inverted repeat (31). Both the rad51::LEU2 and rad51-K191R alleles conferred a 10-fold decrease in the rate of recombination (2.48 × 10−5 ± 0.7 × 10−5 and 2.48 × 10−5 ± 2.3 × 10−5/cell/generation, respectively) compared with the wild-type strain (2.8 × 10−4 ± 0.3 × 10−4/cell/generation). Mating-type switching in S. cerevisiae is a gene conversion event initiated by a DSB made by the HO endonuclease. The kinetics of DSB repair at the MAT locus can be monitored following induction of the HO gene under the control of the GAL1 promoter (11). When HO was induced, efficient cleavage of the MATa locus occurred in rad51-K191R, rad51::LEU2, and RAD51 strains. Completion of gene conversion and switching to MATα result in the formation of a novel DNA fragment of 1.8 kb. The 1.8-kb fragment was visible 2 h after HO induction in the wild-type strain, but no conversion product was detected in the rad51::LEU2 and rad51-K191R strains, even after 5 h (Fig. 3). The bands of slightly faster mobility than the 1.8-kb MATa band in the mutant strains at the 5-h time point are probably partial digestion products due to hyperresected ends. Thus, the rad51-K191R haploid strain is completely defective in the repair of a single DSB.
FIG. 3.
The rad51-K191R strain is defective for mating-type switching. A cartoon of the MATa locus showing the location of the HO cut site and probe for Southern blots is shown in the upper panel. Switching to MATα produces a novel 1.8-kb StyI fragment. Kinetic analysis of mating-type switching in RAD51, rad51-K191R, and rad51::LEU2 strains is shown in the lower panel. Galactose was added to the cultures for 1 h at time zero to induce expression of HO, and samples were removed every hour for DNA analysis.
Meiosis occurs in the absence of Rad51-mediated ATP hydrolysis.
Previous studies have shown that RAD51 is required during meiosis and rad51Δ/rad51Δ diploids rapidly lose viability during sporulation (16, 37). Surprisingly, a rad51-K191R/rad51-K191R diploid showed efficient sporulation and tetrad dissection revealed 75% viability of the haploid spores, compared with 94% for the wild-type diploid (Table 2). The rad51 null and rad51-K191A homozygous diploids produced only 1 to 2% asci, and none of the asci contained four spores. Reduced spore viability can be caused by decreased recombination resulting in nondisjunction of chromosome homologues during meiosis I (33). Meiosis I nondisjunction is characterized by a decrease in the class of tetrads with 4+:0− viable spores and a corresponding increase in the number of tetrads with 2+:2− and 0+:4− viable spores. However, the most common tetrad class derived from the rad51-K191R homozygous diploid was 3+:1− viable spores (Table 2). Thus, the inviability of the rad51-K191R spores is more consistent with random cell death than a defect in meiotic recombination and subsequent missegregation of chromosomes.
TABLE 2.
Sporulation and spore viability of rad51 mutants
| Relevant genotype | % Sporulation | No. of tetrads | % Tetrads with indicated spore viability pattern
|
% Spore viability | ||||
|---|---|---|---|---|---|---|---|---|
| 4:0 | 3:1 | 2:2 | 1:3 | 0:4 | ||||
| RAD51/RAD51a | 70.1 | 1012 | 82 | 14 | 3 | 0.5 | 0.5 | 94 |
| rad51-K191R/rad51-K191R | 56.2 | 171 | 36 | 39 | 17 | 6 | 3 | 75 |
| rad51Δ/rad51Δ | 1.7 | NAb | ||||||
Data for the wild-type diploid taken from Kirkpatrick et al. (22).
None of the asci found for the rad51Δ/rad51Δ diploid contained four spores, precluding tetrad analysis.
Because the rad51-K191R/rad51-K191R homozygous diploid was meiosis proficient, we considered the possibility that the defect conferred by the rad51-K191R mutation was suppressed in diploids. The rad51-K191R diploid was found to be far more resistant to radiation than the equivalent haploid strain (Fig. 2B). Since increased gene dosage suppresses the radiation sensitivity conferred by the rad51-K191R allele, it seemed possible that the increased survival of the rad51-K191R homozygous diploid was due to the presence of two copies of the mutant allele. However, a rad51-K191R/rad51::LEU2 diploid showed radiation sensitivity equivalent to that of the rad51-K191R/rad51-K191R strain. The rad51::LEU2/rad51::HIS3 diploid was even more sensitive to ionizing radiation than was the equivalent haploid strain (Fig. 2B). Although the rad51-K191A/rad51::HIS3 diploid was slightly more resistant to gamma irradiation than the rad51::LEU2/rad51::HIS3 diploid, it was no more resistant than the rad51-K191A haploid strain (Fig. 2B). Consistent with previous studies, the rad51-K191A allele conferred a dominant negative DNA repair defect in heterozygous diploids (8, 12), but this was not observed for the rad51-K191R/RAD51 diploid (data not shown).
Mating-type heterozygosity or RAD54 present in high copy suppresses the rad51-K191R phenotype.
Suppression of radiation sensitivity in diploids has been observed for a number of DNA repair mutants (34), and in the case of rad18 and rad55 diploids, the suppression is due to mating-type heterozygosity rather than ploidy (18, 26). We tested the effect of mating-type heterozygosity by introduction of a plasmid expressing MATα into the MATa rad51-K191R strain. In this event the resistance of the resulting strain to ionizing radiation was increased almost 100-fold (Fig. 4A). This finding, as well as the observed radiation resistance of the rad51-K191R/rad51::LEU2 diploid, indicates that suppression of the mutant phenotype is due to mating-type heterozygosity rather than gene dosage of the rad51-K191R allele. The radiation sensitivity of the haploid rad51::LEU2 and rad51-K191A strains was not suppressed by mating-type heterozygosity (Fig. 4A and data not shown). By Western blot analysis, the level of the Rad51-K191R protein was found to be the same in haploid strains homozygous or heterozygous for MAT, indicating that the suppression is not due to increased expression of the mutant protein (Fig. 1).
FIG. 4.
Mating-type heterozygosity or RAD54 in high copy suppresses the DNA repair defect of the rad51-K191R strain. A. Haploid rad51-K191R strains expressing both MATa and MATα information are more resistant to gamma irradiation than haploids expressing only one MAT allele. MATa RAD51 + pRS414 (♦), MATa RAD51 + pRS414-MATα (⋄), MATa rad51-K191R + pRS414 (▪), MATa rad51-K191R + pRS414-MATα (□), MATa rad51::HIS3 + pRS414 (▴), MATa rad51::HIS3 + pRS414-MATα (▵). (B) RAD54 in high copy suppresses the DNA repair defect of the rad51-K191R and rad57 strains. RAD51 + pRS425 (♦), RAD51 + YEp13-RAD54 (⋄), rad51-K191R + pRS425 (▪), rad51-K191R + YEp13-RAD54 (□), rad57::URA3 + pRS425 (•), rad57::URA3 + YEp13-RAD54 (○), rad51::HIS3 + pRS425 (▴), MATa rad51::HIS3 + YEp13-RAD54 (▵). (C) Overexpression of the rad51-K191R allele suppresses the γ-ray sensitivity of rad51 strains. RAD51 + pRS425 (♦), RAD51 + p51.5 (⋄), rad51-K191A + pRS425 (•), rad51-K191A + p51.5 (○), rad51::HIS3 + pRS425 (▴), MATa rad51::HIS3 + p51.5 (▵). (D) RAD54 in high copy is additive with mating-type heterozygosity. RAD51/RAD51 (♦), rad51-K191R/rad51::URA3 + pRS425 (▪), rad51-K191R/rad51::URA3 + YEp13-RAD54 (□).
Analysis of the published microarray data did not reveal any known recombination genes that are expressed more highly in diploid strains expressing both mating-type alleles than diploids expressing only one mating-type allele (15). However, the expression of a RAD54-lacZ fusion is reported to be twofold higher in MATa/α diploids than MATa/a diploids (9). Therefore, we tested a high-copy-number plasmid expressing RAD54 for suppression of the γ-ray sensitivity of the rad51-K191R strain. RAD54 present in high copy reduced the γ-ray sensitivity of the rad51-K191R strain almost 100-fold compared with the vector alone, but it did not suppress the γ-ray sensitivity of the rad51::URA3 or rad51-K191A strain (Fig. 4B and data not shown). RAD52 in high copy, or simultaneous overexpression of RAD55 and RAD57, failed to suppress the γ-ray sensitivity of the rad51-K191R strain (data not shown). The suppression of the rad51-K191R DNA repair defect by mating-type heterozygosity or by RAD54 in high copy is less than overexpression of the rad51-K191R allele itself (Fig. 4C). To determine whether mating-type heterozygosity and high-copy-number RAD54 have additive effects in suppressing γ-ray sensitivity, the high-copy-number RAD54 plasmid was introduced into a rad51-K191R/rad51::URA3 diploid. The resulting strain showed a significant increase in radiation resistance, indicating additive effects (Fig. 4D).
The γ-ray sensitivity of strains deleted for RAD55 or RAD57 is also suppressed by mating-type heterozygosity (26), suggesting that RAD54 in high copy might also suppress the DNA repair defect of rad55 and rad57 strains. RAD54 present in high copy increased the γ-ray resistance of the rad57 strain about 10-fold compared with the vector alone (Fig. 4B). To determine whether the suppression of the rad51-K191R DNA repair defect by mating-type heterozygosity, or high-copy-number RAD54, is dependent on RAD57, a rad51-K191R rad57 double mutant was made. This strain was slightly more sensitive to gamma irradiation than either of the single mutants and was not suppressed by mating-type heterozygosity or by overexpression of RAD54 (data not shown).
We considered the possibility that DNA repair mediated by the Rad51-K191R mutant protein is inefficient and could be in competition with the end-joining pathway for DSB repair. End joining is repressed in diploids by MATa1/α2 transcriptional repression of LIF2/NEJ1 (14, 21, 30, 45), and this repression could possibly result in the increased efficiency of repair observed in rad51-K191R diploids. Expression of LIF2/NEJ1 from a high-copy-number plasmid did not increase the γ-ray sensitivity of a rad51-K191R diploid, and rad51-K191R yku70 haploids showed the same sensitivity to irradiation as did rad51-K191R strains (data not shown). Thus, suppression of the γ-ray sensitivity of the rad51-K191R strain by MAT heterozygosity does not occur by repression of end joining.
DISCUSSION
The Rad51-K191R mutant protein, which hydrolyzes ATP poorly, promotes extensive strand exchange in vitro when present at high concentration (43). When expressed from a constitutive promoter on a high-copy-number plasmid, the rad51-K191R allele completely suppresses the methyl methanesulfonate and γ-ray sensitivity of a rad51 null strain (Fig. 4) (43). We show here that haploid yeast strains expressing the rad51-K191R allele from the native chromosomal location are almost as defective in spontaneous mitotic recombination, DSB-induced recombination, and DNA repair as rad51 null mutants. Thus, the requirement for ATP hydrolysis by Rad51 is dependent upon the expression level of the mutant protein.
Diploids homozygous for the rad51-K191R mutation, or rad51-K191R haploids expressing both MATa and MATα, are much more resistant to ionizing radiation than haploids expressing only one MAT allele (Fig. 2 and 4). This mating-type heterozygosity-dependent suppression of radiation sensitivity is specific for the rad51-K191R allele and is not observed for the rad51 null or rad51-K191A alleles. Suppression of the DNA repair defects of several rad mutants by mating-type heterozygosity has previously been demonstrated. In the case of rad6 and rad18 mutants, suppression is thought to occur by channeling lesions from error-prone repair pathways into error-free recombinational repair (18, 46). Similarly, inhibition of end joining by MATa1/α2 transcriptional repression of LIF2/NEJ1 is likely to channel lesions from end-joining repair to recombinational repair (14, 21, 30, 45). Suppression of the γ-ray sensitivity of the rad51-K191R strain by MAT heterozygosity does not appear to occur by repression of end joining.
MAT heterozygosity is a cellular signal that usually indicates the presence of a homologous template for recombinational repair throughout the cell cycle. This situation contrasts with MATa and MATα haploids, which can use recombinational repair only during the S phase and G2 phases. The γ-ray sensitivity of rad50, xrs2, and mre11 mutants is suppressed in diploids, and this is thought to occur by preferential use of a homologue over a sister chromatid to template repair in these mutants (7, 19, 28). Haploids are thought to be more sensitive to radiation than diploids because they are unable to utilize a sister chromatid to template repair.
Diploid rad55 and rad57 null mutants are also more resistant to ionizing radiation than the corresponding haploids, and, in the case of rad55, this has been shown to be due to mating-type heterozygosity instead of ploidy (20, 26). In addition, the mitotic recombination and DNA repair defects of rad55 and rad57 mutants are suppressed by overexpression of RAD51 (17, 20). Similarly, the γ-ray sensitivity conferred by the rad52-20 allele, but not the rad52 null allele, is suppressed by mating-type heterozygosity or by overexpression of RAD51 (35). These findings, together with the observation that the phenotype conferred by the rad51-K191R allele is sensitive to the expression level of the mutant allele, suggested that MAT heterozygosity might result in increased levels of RAD51 expression. However, by Western blot analysis we were unable to detect a significant increase in Rad51 protein levels in haploids expressing both MAT alleles (Fig. 1). We cannot rule out the possibility that a subtle increase in protein levels is sufficient to suppress the mutant phenotype, or that a MAT-regulated posttranslational modification activates Rad51.
The suppressive effects of MAT heterozygosity and RAD54 in high copy on the γ-ray sensitivity of the rad51-K191R strain require RAD57. Rad57 acts as an obligate heterodimer with Rad55 to promote assembly of the Rad51 nucleoprotein filament in the presence of RPA (42). We assume that in the absence of Rad57, the Rad51-K191R mutant protein is unable to compete with RPA or is unable to form stable complexes with single-stranded DNA. The γ-ray sensitivity of the rad57 strain is suppressed about 10-fold by RAD54 in high copy. This could be due to partially overlapping functions of Rad55/57 and Rad54 in stabilization of the Rad51 nucleoprotein filament or a later step in repair.
The role of ATP hydrolysis by recombinases of the RecA family is currently unclear. It has been suggested that ATP hydrolysis is required for disassembly of the RecA filament to allow more-efficient recycling of the protein (6, 24, 27). This model attractively explains the phenotype conferred by the rad51-K191R allele. Overexpression of the mutant protein circumvents the requirement for ATP hydrolysis in vivo as expected because the presence of more of the mutant protein would obviate the requirement for recycling. In meiotic cells, expression of both RAD51 and RAD54 is increased about 20-fold and this could be sufficient for suppression of the rad51-K191R phenotype in meiotic recombination (10, 37). Alternatively, the meiosis-specific RecA homologue, Dmc1, might be able to substitute for some of the functions of Rad51 (5). Increased expression of proteins that promote recycling of Rad51 by disassembly of the Rad51 nucleoprotein filament might also be expected to suppress the DNA repair defect conferred by the rad51-K191R allele. The suppression conferred by RAD54 in high copy could be due to stabilization of the Rad51-K191R nucleoprotein filament (A. Mazin and S. Kowalczykowski, personal communication), or due to more-efficient recycling of the mutant protein by Rad54. Rad54 is a member of the Snf2 family of chromatin remodeling factors and could potentially utilize ATP-dependent translocation on duplex DNA to displace other proteins. Thus, suppression of the rad51-K191R allele by mating-type heterozygosity could occur by increased activity of Rad51 itself, or by interacting partners that increase the activity of Rad51 by promoting stability or catalytic turnover of Rad51/DNA complexes.
Acknowledgments
We thank H. Klein, S. Marcand, J. Nickoloff, R. Rothstein, D. Schild, and P. Sung for generous gifts of yeast strains, plasmids, and antibodies. We also thank members of the Symington lab, W. K. Holloman, and the reviewers for helpful comments and suggestions.
The research reported herein was supported by grants from the National Institutes of Health (GM54099 to L.S. and T32 AI07161 to N.S.)
REFERENCES
- 1.Aboussekhra, A., R. Chanet, A. Adjiri, and F. Fabre. 1992. Semidominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA proteins. Mol. Cell. Biol. 12:3224-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, Y., A. P. Davis, and L. S. Symington. 1999. A novel allele of RAD52 that causes severe DNA repair and recombination deficiencies only in the absence of RAD51 or RAD59. Genetics 153:1117-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartsch, S., L. E. Kang, and L. S. Symington. 2000. RAD51 is required for the repair of plasmid double-stranded DNA gaps from either plasmid or chromosomal templates. Mol. Cell. Biol. 20:1194-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basile, G., M. Aker, and R. K. Mortimer. 1992. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol. Cell. Biol. 12:3235-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop, D. K., D. Park, L. Xu, and N. Kleckner. 1992. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69:439-456. [DOI] [PubMed] [Google Scholar]
- 6.Bork, J. M., M. M. Cox, and R. B. Inman. 2001. RecA protein filaments disassemble in the 5′ to 3′ direction on single-stranded DNA. J. Biol. Chem. 276:45740-45743. [DOI] [PubMed] [Google Scholar]
- 7.Bressan, D. A., B. K. Baxter, and J. H. Petrini. 1999. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7681-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanet, R., M. Heude, A. Adjiri, L. Maloisel, and F. Fabre. 1996. Semidominant mutations in the yeast Rad51 protein and their relationships with the Srs2 helicase. Mol. Cell. Biol. 16:4782-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, G. M., D. Schild, S. T. Lovett, and R. K. Mortimer. 1987. Regulation of RAD54- and RAD52-lacZ gene fusions in Saccharomyces cerevisiae in response to DNA damage. Mol. Cell. Biol. 7:1078-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, G. M., D. Schild, and R. K. Mortimer. 1989. Two DNA repair and recombination genes in Saccharomyces cerevisiae, RAD52 and RAD54, are induced during meiosis. Mol. Cell. Biol. 9:3101-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly, B., C. I. White, and J. E. Haber. 1988. Physical monitoring of mating type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:2342-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan, J. W., G. T. Milne, and D. T. Weaver. 1994. Homotypic and heterotypic protein associations control Rad51 function in double-strand break repair. Genes Dev. 8:2552-2562. [DOI] [PubMed] [Google Scholar]
- 13.Erdeniz, N., U. H. Mortensen, and R. Rothstein. 1997. Cloning-free PCR-based allele replacement methods. Genome Res. 7:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank-Vaillant, M., and S. Marcand. 2001. NHEJ regulation by mating type is exercised through a novel protein, Lif2p, essential to the Ligase IV pathway. Genes Dev. 15:3005-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galitski, T., A. J. Saldanha, C. A. Styles, E. S. Lander, and G. R. Fink. 1999. Ploidy regulation of gene expression. Science 285:251-254. [DOI] [PubMed] [Google Scholar]
- 16.Game, J. C., and R. K. Mortimer. 1974. A genetic study of x-ray sensitive mutants in yeast. Mutat. Res. 24:281-292. [DOI] [PubMed] [Google Scholar]
- 17.Hays, S. L., A. A. Firmenich, and P. Berg. 1995. Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc. Natl. Acad. Sci. USA 92:6925-6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heude, M., and F. Fabre. 1993. a/alpha-control of DNA repair in the yeast Saccharomyces cerevisiae: genetic and physiological aspects. Genetics 133:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov, E. L., V. G. Korolev, and F. Fabre. 1992. XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics 132:651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, R. D., and L. S. Symington. 1995. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol. Cell. Biol. 15:4843-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kegel, A., J. O. Sjostrand, and S. U. Astrom. 2001. Nej1p, a cell type-specific regulator of nonhomologous end joining in yeast. Curr. Biol. 11:1611-1617. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick, D. T., J. R. Ferguson, T. D. Petes, and L. S. Symington. 2000. Decreased meiotic intergenic recombination and increased meiosis I nondisjunction in exo1 mutants of Saccharomyces cerevisiae. Genetics 156:1549-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim, D. S., and P. Hasty. 1996. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol. 16:7133-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsley, J. E., and M. M. Cox. 1990. Assembly and disassembly of RecA protein filaments occur at opposite filament ends. Relationship to DNA strand exchange. J. Biol. Chem. 265:9043-9054. [PubMed] [Google Scholar]
- 25.Logan, K. M., and K. L. Knight. 1993. Mutagenesis of the P-loop motif in the ATP binding site of the RecA protein from Escherichia coli. J. Mol. Biol. 232:1048-1059. [DOI] [PubMed] [Google Scholar]
- 26.Lovett, S. T., and R. K. Mortimer. 1987. Characterization of null mutants of the RAD55 gene of Saccharomyces cerevisiae: effects of temperature, osmotic strength and mating type. Genetics 116:547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menetski, J. P., and S. C. Kowalczykowski. 1985. Interaction of recA protein with single-stranded DNA. Quantitative aspects of binding affinity modulation by nucleotide cofactors. J. Mol. Biol. 181:281-295. [DOI] [PubMed] [Google Scholar]
- 28.Moreau, S., J. R. Ferguson, and L. S. Symington. 1999. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol. 19:556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison, C., A. Shinohara, E. Sonoda, Y. Yamaguchi-Iwai, M. Takata, R. R. Weichselbaum, and S. Takeda. 1999. The essential functions of human Rad51 are independent of ATP hydrolysis. Mol. Cell. Biol. 19:6891-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooi, S. L., D. D. Shoemaker, and J. D. Boeke. 2001. A DNA microarray-based genetic screen for nonhomologous end-joining mutants in Saccharomyces cerevisiae. Science 294:2552-2556. [DOI] [PubMed] [Google Scholar]
- 31.Rattray, A. J., and L. S. Symington. 1994. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics 138:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehrauer, W. M., and S. C. Kowalczykowski. 1993. Alteration of the nucleoside triphosphate (NTP) catalytic domain within Escherichia coli recA protein attenuates NTP hydrolysis but not joint molecule formation. J. Biol. Chem. 268:1292-1297. [PubMed] [Google Scholar]
- 33.Roeder, G. S. 1997. Meiotic chromosomes: it takes two to tango. Genes Dev. 11:2600-2621. [DOI] [PubMed] [Google Scholar]
- 34.Saeki, T., I. Machida, and S. Nakai. 1980. Genetic control of diploid recovery after gamma-irradiation in the yeast Saccharomyces cerevisiae. Mutat. Res. 73:251-265. [DOI] [PubMed] [Google Scholar]
- 35.Schild, D. 1995. Suppression of a new allele of the yeast RAD52 gene by overexpression of RAD51, mutations in srs2 and ccr4, or mating-type heterozygosity. Genetics 140:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman, F., G. Fink, and J. Hicks. 1986. Methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Shinohara, A., H. Ogawa, and T. Ogawa. 1992. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69:457-470. [DOI] [PubMed] [Google Scholar]
- 38.Sonoda, E., M. S. Sasaki, J. M. Buerstedde, O. Bezzubova, A. Shinohara, H. Ogawa, M. Takata, Y. Yamaguchi-Iwai, and S. Takeda. 1998. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17:598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stark, J. M., P. Hu, A. J. Pierce, M. E. Moynahan, N. Ellis, and M. Jasin. 2002. ATP hydrolysis by mammalian RAD51 has a key role during homology-directed DNA repair. J. Biol. Chem. 277:20185-20194. [DOI] [PubMed] [Google Scholar]
- 40.Strahl-Bolsinger, S., A. Hecht, K. Luo, and M. Grunstein. 1997. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 11:83-93. [DOI] [PubMed] [Google Scholar]
- 41.Sung, P. 1994. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265:1241-1243. [DOI] [PubMed] [Google Scholar]
- 42.Sung, P. 1997. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11:1111-1121. [DOI] [PubMed] [Google Scholar]
- 43.Sung, P., and S. A. Stratton. 1996. Yeast Rad51 recombinase mediates polar DNA strand exchange in the absence of ATP hydrolysis. J. Biol. Chem. 271:27983-27986. [DOI] [PubMed] [Google Scholar]
- 44.Tsuzuki, T., Y. Fujii, K. Sakumi, Y. Tominaga, K. Nakao, M. Sekiguchi, A. Matsushiro, Y. Yoshimura, and T. Morita. 1996. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl. Acad. Sci. USA 93:6236-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valencia, M., M. Bentele, M. B. Vaze, G. Herrmann, E. Kraus, S. E. Lee, P. Schar, and J. E. Haber. 2001. NEJ1 controls non-homologous end joining in Saccharomyces cerevisiae. Nature 414:666-669. [DOI] [PubMed] [Google Scholar]
- 46.Yan, Y. X., R. H. Schiestl, and L. Prakash. 1995. Mating-type suppression of the DNA-repair defect of the yeast rad6 delta mutation requires the activity of genes in the RAD52 epistasis group. Curr. Genet. 28:12-18. [DOI] [PubMed] [Google Scholar]