Abstract
Objective:
To assess the respective impact of surgical and immunologic factors on patient/graft outcome and rejection after pediatric liver transplantation.
Summary Background Data:
Orthotopic liver transplantation (OLT) constitutes a validated therapeutic modality for acute liver failure and end-stage liver disease in children. Only a few large studies of factors influencing outcome of pediatric OLT are available in the literature. Studies considering the impact of rejection on graft outcome are scarce in adult OLT and are not even available for pediatric recipients.
Methods:
Five hundred consecutive pediatric recipients (<15 years) of a primary OLT performed between March 1984 and July 2000 were retrospectively reviewed. The main indication was biliary atresia (n = 328). A living related donor graft was used from July 1993 onwards in 82 children (16%). Survival was calculated and multivariate analysis was performed.
Results:
Actuarial survival rates at 1, 5, and 10 years were 85%, 81%, and 79% for patients, and 76%, 71%, and 70% for grafts, respectively. At the multivariate analysis, only 3 factors were found to be independently correlated with better patient survival: year of transplantation (P = 0.001), pretransplant diagnosis (P < 0.001, worst results for liver tumors), and ABO matching (P < 0.001, worst results for ABO incompatibility). Similarly, 3 factors were independently correlated with better rejection-free graft survival: tacrolimus as primary immunosuppressant (P < 0.001), a negative T-cell crossmatch (P = 0.016), and younger age of the donor (P < 0.001).
Conclusions:
Pediatric OLT constitutes a complex undertaking with multifactorial impact on results: (1) a strong learning curve effect was shown to impact on overall results; (2) pediatric liver tumors still represent a challenging indication for OLT; (3) primary immunoprophylaxis with tacrolimus provided a lower rejection incidence; (4) the younger donor age effect deserves further immunologic investigations.
Orthotopic liver transplantation has become a validated therapeutic modality for liver failure in children. This single center study assesses the overall results of pediatric liver transplantation including graft acceptance. Particular emphasis is put on the risk factors specific to this group of patients and on the respective role of nonimmunologic and immunologic parameters.
Orthotopic liver transplantation (OLT) constitutes a validated therapeutic modality for acute liver failure, end-stage liver disease, and selected metabolic disorders in children. Particularly in small children, the shortage of size-matched postmortem grafts and subsequent high mortality on the waiting list led to the development of alternative techniques allowing the use of partial hepatic grafts from adult donors.1,2 Although these innovative surgical techniques and improved immunosuppression have broadened the application of liver replacement in this category of recipients, OLT in children remains a challenging undertaking and factors impacting on results are multifactorial.3 Only a few large studies of factors influencing outcome of pediatric OLT are available in the literature. The only multivariate analysis of a single center series is from Goss et al analyzing 440 patients.3–5 These authors discussed the impact of nonimmunologic factors on outcome, and they did not take into account immunologic parameters such as HLA compatibility and T-cell crossmatch. Moreover, studies considering the impact of rejection on graft outcome are scarce in adult OLT, and are not even available for pediatric recipients. Therefore, we performed a retrospective single center study to assess the overall results of pediatric liver transplantation including cellular rejection, with particular emphasis on the risk factors specific to this group of patients and on the respective role of nonimmunologic and immunologic parameters.
MATERIALS AND METHODS
Study Population
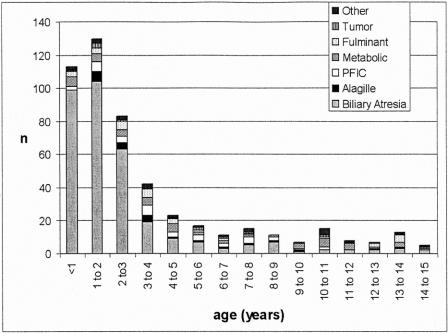
The medical records of 500 consecutive pediatric patients (<15-years old) who received a primary liver transplant between March 1984 and July 2000 at Saint-Luc University Clinics in Brussels (242 boys and 258 girls; median age at transplantation: 2.1 year; range: 0.2–14.5) were retrospectively reviewed. The most common pretransplant diagnoses were biliary atresia (66%), followed by the heterogeneous group of metabolic diseases (9%), and progressive familial intrahepatic cholestasis (PFIC) (7%) (Tables 1 and 2). Seventy-four percent of children were under 4 years of age at the time of transplantation (Fig. 1).
TABLE 1. Pretransplant Diagnosis, Median Age at Transplantation, and Actuarial 5-year Patient Survival in 500 Pediatric Liver Recipients Grafted at Saint-Luc University Clinics Between March 1984 and July 2000
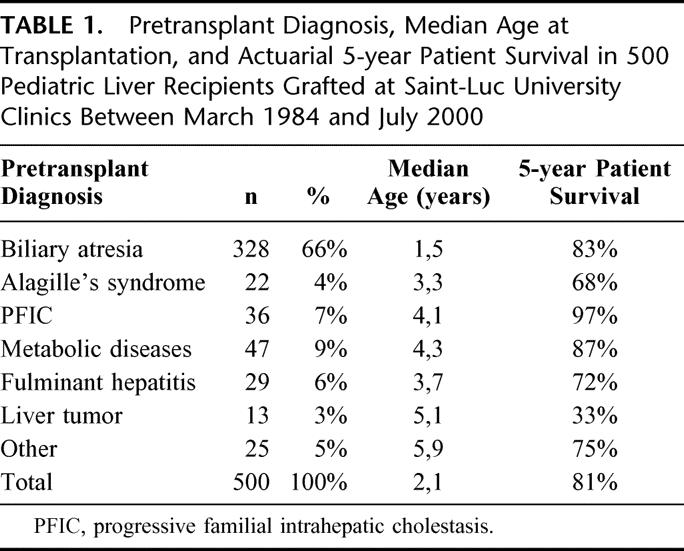
TABLE 2. Metabolic Diseases in 47 Pediatric Liver Transplant Recipients
FIGURE 1. Pretransplant diagnosis according to the age at transplantation. BA, biliary atresia; PFIC, progressive Familial intrahepatic cholestasis.
Donor Characteristics and Surgical Techniques
Median (range) donor age was 12.2 year (0.1–55.2). A postmortem liver was used in 418 patients (84%), the graft being a whole-size liver in 191 patients (38%), a reduced-size liver in 194 patients (40%), and a split liver in 33 cases (6%) (Table 3). From 1993 onwards, living related (LR) liver transplantation was introduced at our center, and this technique was used in 82 patients (16% of the whole series and 40% since 1993). Technical details were previously reported.2 In brief, in reduced-size grafts, the donor liver underwent a partial resection on a bench table, which discarded the right lobe and preserved either the left lateral segment (segments 2 and 3) or the full left lobe (segments 2, 3, and 4); split grafts were obtained by division of the liver parenchyma and of the vascular and biliary pedicles to obtain 2 grafts, the larger right lobe being transplanted into an adult recipient and the left lateral segment into a child. The operation in the LR donor included an intraoperative cholangiogram to define the biliary duct anatomy of the donor and consisted in the procurement of segments 2 and 3, or the entire left liver, according to the recipient size. No major complications were seen in living donors following the procurement.6 Euro-Collins solution was used for cold graft perfusion and preservation until February 1988. Thereafter, preservation was done with University of Wisconsin solution. The distribution of the type of graft according to the year of transplantation is given in Figure 2. The different types of grafts and their respective ischemia times are listed in Table 3.
TABLE 3. Liver Transplant Techniques and Corresponding Ischemia Times (Median, Range)
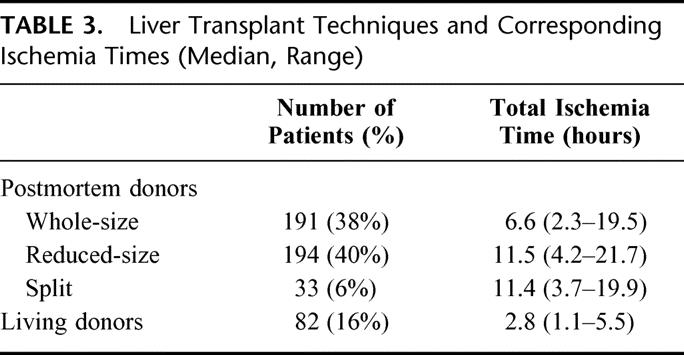
FIGURE 2. Liver transplantation techniques according to the year of transplantation.
Immunosuppressive Protocols and Definition of Rejection
The general postoperative management has been previously described.7 Primary baseline immunosuppression varied over time according to ongoing protocols. Basically, it consisted of a triple drug regimen including steroids, a calcineurin inhibitor, and azathioprine (until mid-1997, after which only double immunosuppression was administered). Intravenous methylprednisolone therapy was started during surgery at a dose of 10 mg/kg/d, and steroid dosage was subsequently tapered to reach 1 mg/kg/d at 2 weeks posttransplant, and 0.25 mg/kg/d at 3 months, with a progressive switch to alternate-day 0.5 mg/kg/2 days from 6 months to 1 year.8 The primary calcineurin inhibitor was cyclosporine-A (CyA; Sandimmun, Novartis, Basel, Switzerland) (n = 343, 69%), cyclosporine-A microemulsion (CyA-ME; Neoral, Novartis, Basel, Switzerland) (n = 52, 10%) or tacrolimus (Prograft; Fujisawa, Osaka, Japan) (n = 99, 20%). CyA and CyA-ME were adjusted to trough blood levels of 250–300 ng/mL in the immediate posttransplant period, 100–150 ng/mL at 1 year, and 50–100 ng/mL thereafter. From mid-1991 onwards, oral tacrolimus was progressively introduced as primary immunosuppressant and was used in all patients since 1999. We aimed at tacrolimus trough blood levels of 10–15 ng/mL during the first month and 5–10 ng/mL thereafter, adapted also to graft tolerance. In addition to baseline immunosuppression, 358 and 107 children received azathioprine and/or anti-T cell antibodies as an induction therapy, respectively, as parts of research protocols for rejection prophylaxis.9 Induction therapy with antilymphocyte antibodies was as follows: polyclonal rabbit antithymocyte antibody was administered in 28 children, whereas monoclonal antibody induction consisted in anti-CD3 monoclonal antibody OKT3 or anti-CD25 monoclonal antibody LO-Tact1 in 29 and 50 children, respectively.
Acute cellular rejection (AR) was diagnosed in cases with clinical and biochemical evidence, and confirmed by histologic examination of a core needle biopsy.10 Proven AR was managed with a 3-day scheme of IV methylprednisolone 10 mg/kg/d followed by recycling doses during the 3 following days (7.5, 5 and 2.5 mg/kg/d).6,9 Acute rejection was considered as steroid resistant when nonresponding to the above-mentioned regimen. In the CyA era, steroid resistant AR was treated in the early days with polyclonal antilymphocyte or monoclonal anti-CD3 antibodies, and later on by a switch from CyA or CyA-ME to tacrolimus; in the tacrolimus era, steroid resistant AR was treated by a temporary increase of the trough level and/or addition of mycophenolate mofetil (Cellcept, Roche, Basel, Switzerland). The main histologic features of chronic rejection were loss of the bile ducts in more then 50% of portal triads, with cholestasis and subsequent need for retransplantation. Results with respect to rejection were expressed as rejection-free graft survival rates according to time after the primary OLT. A diagnosis of cytomegalovirus (CMV) infection was considered in case of detection of circulating CMV antigen and/or IgM antibody as well as CMV-hepatitis proven by liver biopsy.
ABO and HLA Compatibility
Most donor-recipient pairs were ABO-identical (n = 442, 88%) or compatible (n = 49, 10%). HLA typing was done retrospectively, using the complement fixation technique for HLA class I typing and molecular biology for HLA class II typing from 1994 onwards. For calculation of HLA-mismatches, only the broad antigen specificities were taken into account without considering the split ones. T- and B-cell crossmatch was also done retrospectively by testing pretransplant sera for donor-specific lymphocytotoxic antibodies against donor splenic tissue or lymphocytes obtained for routine tissue typing. Crossmatch was available for 373 donor-recipient pairs and was positive for T cells only (after E Rosette isolation of T cells) in 27 of them (7%).
HLA A, B, and DR matching was available for 383, 386, and 364 donor-recipient pairs, respectively. Zero, 1, and 2 HLA-A mismatches were observed in 57 (11%), 160 (32%), and 166 (33%) patients (%), respectively. The corresponding figures for HLA-B mismatches were 15 (3%), 127 (25%), and 244 (49%) patients (%), respectively. Zero, 1, and 2 mismatches for HLA-DR were observed in 32 (6%), 158 (32%), and 174 (35%) patients (%), respectively.
Statistical Analysis
Numerical variables are expressed by median and range. Patient and graft survival, as well as rejection-free graft survival curves, were estimated by Kaplan-Meier product limit method. The impact of various factors on survival was studied in univariate way by log-rank test, linear trend test, and Cox-Wald test for categorical, ordinal and numerical variables, respectively. A multivariate analysis of survival was performed using the Cox proportional hazards model, with a backward selection of variables with the Wald test. The variables taken into account in the multivariate analysis of patient survival, graft survival, and rejection-free survival are listed in table 5. The assumption of proportional hazards was checked by graphing log-minus-log survival plots. The relationship of rejection with patient and graft survival as well as of retransplantation with patient survival was studied by the time-dependent Cox regression model, including a time-dependent indicator switching from 0 to 1 at the time of rejection or retransplantation. P < 0.05 was considered statistically significant. The analysis was performed with SPSS 10.0 software (SPSS Inc., Chicago, Ill).
TABLE 5. Results of the Multivariate Analysis Assessing the Impact of Recipient, Graft, and Transplant Variables on Patient and Graft Outcome, Including Rejection and CMV-Infection
RESULTS
Overall Results
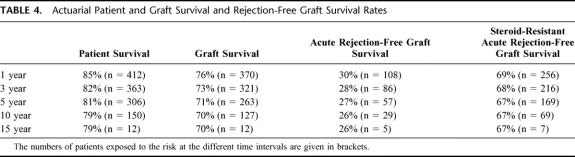
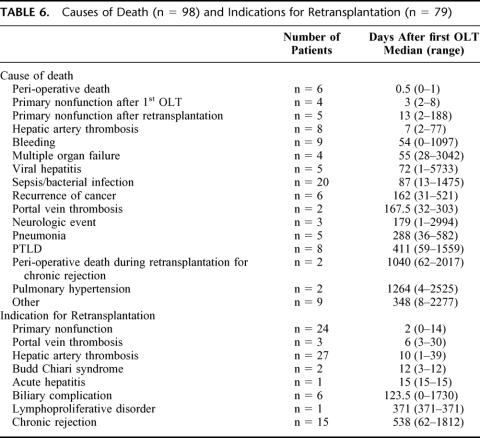
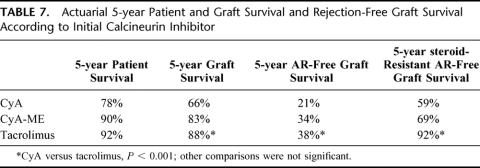
Actuarial patient and graft survival rates are given in Table 4. In the LR donor subgroup (n = 82), 5-year patient survival was 90%, compared with 84% for postmortem grafts transplanted during the same period (n = 110)(NS). Ninety-eight patients (20%) died during follow-up (median post-OLT interval = 0.2 year; range: 0–15.7), and 79 patients (16%) needed a retransplantation (median post-OLT interval = 11 days; range: 0–15.6 years). The causes of mortality and the indications for retransplantation are listed in Table 6. Three hundred and 10 patients (62%) presented at least one episode of AR, of which 139 (45%) were steroid resistant. Actuarial AR-free graft survival rates are listed in Table 7, according to the type of calcineurin inhibitor administered as primary immunosuppressant. Most AR occurred early after transplantation (53% within 3 weeks after transplantation, and 64% within 3 months). Chronic rejection occurred in 15 patients (3%), all of them being retransplanted at a median (range) post-OLT interval of 1.5 years (0.2–5.0). CMV-infection was encountered in 95 patients (19%).
TABLE 4. Actuarial Patient and Graft Survival and Rejection-Free Graft Survival Rates
TABLE 6. Causes of Death (n = 98) and Indications for Retransplantation (n = 79)
TABLE 7. Actuarial 5-year Patient and Graft Survival and Rejection-Free Graft Survival According to Initial Calcineurin Inhibitor
Impact of Immunologic and Nonimmunologic Parameters on Patient and Graft Survival
Among the recipient, donor, and transplant variables tested in multivariate analysis (Table 5), only the pretransplant diagnosis, the year of transplantation (P = 0.001), and ABO compatibility (P < 0.001) were found to be independently correlated with patient survival. As outlined in Table 1, transplantation for liver tumors was related to a significantly lower survival (P < 0.001). As shown in Figure 3, 5-year patient survival improved from 71% in the 1984–1987 interval to 91% in the 1996–2000 interval. Five-year survival was 84% in case of ABO identity, compared with 71% and 22% for ABO compatibility and incompatibility, respectively (P < 0.001). No independent effect on patient survival could be demonstrated regarding recipient age, donor age, type of graft, ischemia time, gender match, HLA-matching, T-cell crossmatch and primary immunosuppression.
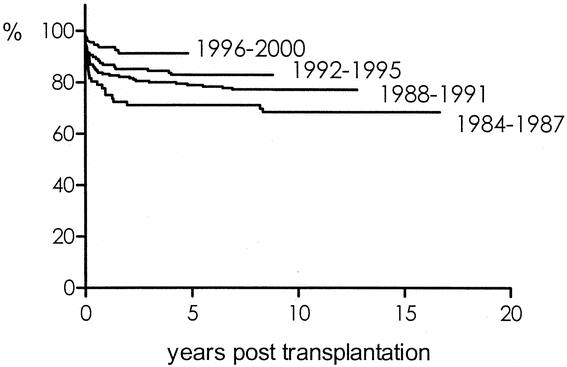
FIGURE 3. Actuarial patient survival according to era of transplantation. Univariate analysis, P = 0.001; multivariate analysis: P = 0.001.
Regarding graft survival, only ABO compatibility (P < 0.001) and the primary calcineurin inhibitor (P < 0.001) were shown to have a significant impact in the multivariate analysis. Use of tacrolimus and CyA-ME significantly improved graft survival compared with CyA (Table 7). This improvement is not solely due to time bias, since it remained true even after correction for the era of transplantation in the multivariate analysis. No independent effect on graft survival could be demonstrated regarding recipient age, pretransplant diagnosis, donor age, type of graft, ischemia time, the year of transplantation, gender match, HLA-matching or T-cell crossmatch.
Impact of Immunologic and Nonimmunologic Parameters on Rejection
As summarized in Table 5, only 3 variables tested in the multivariate analysis were shown to independently impact on AR free graft survival: the younger age of the donor (P < 0.001), tacrolimus as primary immunosuppressant (P < 0.001; Table 7), and T-cell crossmatch. In case of a negative crossmatch, 1, 5, and 10 year AR-free graft survival was 29, 26 and 25%, respectively, compared with 0, 0 and 0% in case of a positive crossmatch (P = 0.016). No significant effect of gender match or HLA compatibility was observed on AR.
Regarding variables affecting steroid-resistant AR-free graft survival, only donor age, the era of transplantation, and primary calcineurin inhibitor were found as significant variables at multivariate analysis (P = 0.001, P = 0.002, and P < 0.001, respectively), with better results along the learning curve, or when a younger donor was used, or when tacrolimus was used as primary immunosuppressant (Table 5). A likely explanation for decrease of steroid resistant rejection over time is a change in attitude over time to treat rejection less invasively. No significant effect of HLA compatibility or T-cell crossmatch was observed on steroid resistant AR.
Impact of Rejection on Graft Outcome
From the time dependent Cox proportional hazard model, AR did not significantly influence patient or graft survival. However, occurrence of steroid resistant AR significantly reduced graft survival (P = 0.016), but not patient survival. The occurrence of a chronic rejection significantly reduced patient and graft survival (P < 0.001).
Impact of Retransplantation on Patient Survival
From the time dependent Cox proportional hazard model, retransplantation significantly influenced patient survival (P < 0.001). Five-year patient survival after the first OLT was 81%, compared with 66% after a second OLT.
DISCUSSION
Since the first pediatric liver transplant performed by T.E. Starzl in 1963, remarkable progress has been achieved and OLT has become the established treatment of liver insufficiency in children.11 Nevertheless, few multivariate analysis are available in the pediatric OLT literature,3 and only one from a large single center study.5 Pediatric OLT patients differ from adult patients for 2 main reasons:1,2 the most common indications for OLT in the pediatric population are specific to this age group and particular technical challenges are associated with pediatric OLT. Biliary atresia constitutes the most common indication for liver transplantation in children; 30% and 81% of the children with biliary atresia in the present series were younger than 1 year and 3 years, respectively. Consequently, the number of pediatric candidates largely exceeds the number of small-sized pediatric donors of similar weight allowing for a whole-size OLT to be performed. Therefore, and due to the rapidly progressive character of pediatric liver diseases, small children account for a high mortality on the waiting list, up to 17% according to recent UNOS data.1 Scarcity of size-matched donors meanwhile has led to the development of innovative techniques such as reduced-size liver, split liver, and LR donor OLT, which contributed to decrease the mortality of pediatric patients awaiting OLT without affecting the results in terms of patient and graft survival, as shown in the literature and confirmed in this series.1,2,12 Moreover, in spite of the well-known technical difficulties in children younger than one year of age, the data do not show different patient or graft survival in this age group, when compared with older patients. Obviously, the experience of the transplant center, in particular availability of a pediatric oriented program, has a great influence in the achievement of such results.
Although the present multivariate analysis did not reveal any significant difference between OLT from LR or postmortem grafts either in posttransplant patient survival or in the rejection incidence, LR donors constitute for their pediatric recipients a unique opportunity to escape the uncertainty of the waiting time on the cadaveric list. In 1999, Reding et al reported a significantly lower pretransplant mortality rate in the LR group (2%) compared with the postmortem group (15%).6 Beside the main advantage of improved overall survival from the time of listing as estimated according to Whitington,13 LR donor OLT also provides higher quality grafts, with significantly reduced posttransplant hepatocyte and endothelial cell injury, compared with matched cadaveric reduced-sized grafts.14 Moreover, the technique allows for elective planning of the procedure, which may be important in case of OLT for primary liver tumors following chemotherapy. As could be expected, in this latter indication OLT was shown in the present series to provide a poorer patient survival compared with OLT for other indications.
This series showed a tremendous learning curve effect over years, which may not only be due to improvements of surgical skills but also to the overall management, including the pretransplant nutritional support, the refinements of immunosuppressive therapies as well as better prophylaxis and management of medical complications such as viral illnesses and related lymphoproliferative disorders.15 Primary immunosuppression with tacrolimus was shown to be independently associated with a lower incidence of AR and of steroid resistant AR. These retrospective results were recently confirmed by the prospective European multicenter randomized study comparing tacrolimus and CyA-ME in 181 pediatric OLT recipients: both AR and steroid resistant AR incidences were significantly lower in the tacrolimus group.16
The current study confirmed that ABO incompatibility significantly affects patient and graft survival.17 In contrast, the results do not suggest that HLA matching constitutes a determinant factor for the outcome of liver transplantation. Previous studies have mainly analyzed either adult18,19 or combined adult-pediatric populations20,21 and their conclusions were discordant. Only few published studies, and mainly with small numbers of patients, have analyzed pediatric series separately from the adult population. Francavilla et al concluded that HLA mismatches are not detrimental in primary pediatric liver transplantation.22 Regarding the role of anti-HLA antibodies, a positive crossmatch was shown to increase the incidence of acute cellular rejection, with however no impact on long-term graft survival in contrast with results from Hathaway et al.23 According to our results, crossmatch positive transplant recipients may require reinforced immunosuppressive measures to reduce the 100% incidence of AR observed in this group.
The identification of a “younger donor effect” associated with a better graft acceptance in terms of AR constituted an unexpected finding of the multivariate analysis. It may be related to a higher content of bone marrow-derived cells in young donor livers, when compared with organs from older donors. This difference may be responsible for an increased systemic microchimerism in recipients of a young donor liver, with putatively an enhancement of the protolerogenic effect.24
Considering the influence of rejection on patient and graft survival, liver allografts have been considered as immunologically privileged in contrast with kidneys and hearts with regard to the low incidence of hepatic graft loss to rejection. The current study confirmed that AR does not significantly impact on patient and graft survival.
In conclusion, the power of a large single center study was used in this work to delineate the significance of nonimmunologic and immunologic impact factors in pediatric liver transplantation. Although a strong learning curve effect was shown to impact on the overall results, pediatric OLT remains a considerable undertaking. The data presented demonstrate that recipients below one year should no longer be considered as a contraindication for OLT, since no difference in patient or graft outcome was observed in this age group. Primary immunoprophylaxis with tacrolimus was demonstrated to provide a lower rejection incidence and better graft survival. However, many challenges are still ahead in the field of pediatric OLT, including the use of more specific immunosuppressive strategies with steroid avoidance, tolerance facilitation or induction, and the surgical strategy for hepatoblastomas not amenable to partial liver resection.
ACKNOWLEDGMENTS
The authors would like to acknowledge JP. Buts, O. Ciccarelli, Ph. Clapuyt, S. Clément de Cléty, T. Detaille, D. Hermans, M. Janssen, J. Lerut, D. Moulin, J. Rahier, C. Saint-Martin, C. Sempoux, L. Van Obbergh and F. Veyckemans, for their daily devoted involvement in the treatment of the pediatric OLT recipients as well as the surgical contribution of B. de Hemptinne and J. de Ville de Goyet in the first part of the series presented.
Footnotes
This work was supported in part by a grant from the Fonds de la Recherche Scientifique Medicale (N° 3.4557.00), Brussels, Belgium.
Reprints: Prof R. Reding, Department of Pediatric Surgery and Liver Transplantation, Université Catholique de Louvain, Saint-Luc University Clinics, 10 Hippocrate Avenue, B-1200, Brussels. E-mail:reding@chex.ucl.ac.be.
REFERENCES
- 1.McDiarmid SV, Davies DB, Edwards EB. Improved graft survival of pediatric liver recipients transplanted with pediatric-aged liver donors. Transplantation. 2000;70:1283–1291. [DOI] [PubMed] [Google Scholar]
- 2.Otte JB, de Ville de Goyet J, Reding R, et al. Pediatric liver transplantation: from the full-size liver graft to reduced, split, and living related liver transplantation. Pediatr Surg Int. 1998;13:308–318. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric liver transplantation registry. Studies of Pediatric Liver Transplantation (SPLIT): year 2000 outcomes. Transplantation. 2001;72:463–476. [DOI] [PubMed]
- 4.McDiarmid SV. Management of the pediatric liver transplant patient. Liver Transpl. 2001;7:S77–S86. [DOI] [PubMed] [Google Scholar]
- 5.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg. 1998;228:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reding R, de Ville de Goyet J, Delbeke I, et al. Pediatric liver transplantation with cadaveric or living related donors: comparative results in 90 elective recipients of primary grafts. J Pediatr. 1999;134:280–286. [DOI] [PubMed] [Google Scholar]
- 7.Reding R, Gennari F, Janssen M, et al. The pediatric liver transplant program at the Université Catholique de Louvain, Cliniques Saint-Luc, Brussels: overall results in 444 children (1984–1997). Acta Gastroenterol Belg. 1999;62:285–289. [PubMed] [Google Scholar]
- 8.Sokal EM, Veyckemans F, de Ville de Goyet J, et al. Liver transplantation in children less than 1 year of age. J Pediatr. 1990;117:205–210. [DOI] [PubMed] [Google Scholar]
- 9.Reding R, Feyaerts A, Vraux H, et al. Prophylactic immunosuppression with anti-interleukin-2 receptor monoclonal antibody LO-Tact-1 versus OKT3 in liver allografting. A two- year follow-up study. Transplantation. 1996;61:1406–1409. [DOI] [PubMed] [Google Scholar]
- 10.Hubscher SG, Clements D, Elias E, et al. Biopsy findings in cases of rejection of liver allograft. J Clin Pathol. 1985;38:1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Koep LJ, Schroter GP, et al. Liver replacement for pediatric patients. Pediatrics. 1979;63:825–829. [PMC free article] [PubMed] [Google Scholar]
- 12.de Ville de Goyet J, Hausleithner V, Reding R, et al. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation. 1993;56:1130–1136. [DOI] [PubMed] [Google Scholar]
- 13.Whitington PF. Living donor liver transplantation: ethical considerations. J Hepatol. 1996;24:625–627. [DOI] [PubMed] [Google Scholar]
- 14.Reding R, Wallemacq P, Moulin D, et al. Early hepatocyte, endothelial, and bile duct cell injury after pediatric liver transplantation from cadaveric or living-related donors. Transplantation. 1998;65:681–685. [DOI] [PubMed] [Google Scholar]
- 15.Smets F, Bodeus M, Goubau P, et al. Characteristics of Epstein-Barr virus primary infection in pediatric liver transplant recipients 4. J Hepatol. 2000;32:100–104. [DOI] [PubMed] [Google Scholar]
- 16.Kelly D, Jara P, Rodeck B, et al. Tacrolimus dual therapy versus cyclosporin-microemulsion triple therapy in pediatric liver transplantation: results from a multicentre randomized trial. Am J Transplant. 2002;2:351. [Google Scholar]
- 17.Reding R, Veyckemans F, de Ville de Goyet J, et al. ABO-incompatible orthotopic liver allografting in urgent indications. Surg Gynecol Obstet. 1992;174:59–64. [PubMed] [Google Scholar]
- 18.Gunson BK, Hathaway M, Buckels JA, et al. HLA matching in liver transplantation: a retrospective analysis. Transplant Proc. 1992;24:2434–2435. [PubMed] [Google Scholar]
- 19.Nikaein A, Backman L, Jennings L, et al. HLA compatibility and liver transplant outcome. Improved patient survival by HLA and cross-matching. Transplantation. 1994;58:786–792. [PubMed] [Google Scholar]
- 20.Thorogood J. Relationship between HLA compatibility and first liver allograft survival. l'ESPRIT Study Group. Transplant Proc. 1993;25:2655–2656. [PubMed] [Google Scholar]
- 21.Markus BH, Duquesnoy RJ, Gordon RD, et al. Histocompatibility and liver transplant outcome. Does HLA exert a dualistic effect? Transplantation. 1988;46:372–377. [PMC free article] [PubMed] [Google Scholar]
- 22.Francavilla R, Hadzic N, Underhill J, et al. Role of HLA compatibility in pediatric liver transplantation. Transplantation. 1998;66:53–58. [DOI] [PubMed] [Google Scholar]
- 23.Hathaway M, Gunson BK, Keogh AC, et al. A positive crossmatch in liver transplantation–no effect or inappropriate analysis? A prospective study. Transplantation. 1997;64:54–59. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Murase N. Microchimerism, macrochimerism, and tolerance. Clin Transplant. 2000;14:351–354. [DOI] [PubMed] [Google Scholar]