Abstract
Objective:
To highlight the current available evidence in antireflux surgery through a systematic review of randomized controlled trials (RCTs).
Summary Background Data:
Laparoscopic fundoplication is currently suggested as the gold standard for the surgical treatment of gastroesophageal reflux disease, but many controversies are still open concerning the influence of some technical details on its results.
Methods:
Papers related to RCTs identified via a systematic literature search were evaluated according to standard criteria. Data regarding the patient sample, study methods, and outcomes were abstracted and summarized across studies. Defined outcomes were examined for 41 papers published from 1974 to 2002 related to 25 RCTs. A meta-analysis was performed pooling the results as odds ratios (OR), rate differences (RD), and number needed to treat (NNT). Data given as mean and/or median values were pooled as a mean ± SD (SD).
Results:
No perioperative deaths were found in any of the RCTs. Immediate results showed a significantly lower operative morbidity rate (10.3% versus 26.7%, OR 0.33, RD −12%, NNT 8), shorter postoperative stay (3.1 versus 5.2 days, P = 0.03), and shorter sick leave (20.1 versus 35.8 days, P = 0.03) for laparoscopic versus open fundoplication. No significant differences were found regarding the incidence of recurrence, dysphagia, bloating, and reoperation for failure at midterm follow-up. No significant differences in operative morbidity (13.1% versus 9.4%) and in operative time (90.2 versus 84.2 minutes) were found in partial versus total fundoplication. A significantly lower incidence of reoperation for failure (1.6% versus 9.6%, OR 0.21, RD −7%, NNT 14) was found after partial fundoplication, with no significant differences regarding the incidence of recurrence and/or dysphagia. Routine division of short gastric vessels during total fundoplication showed no significant advantages regarding the incidence of postoperative dysphagia and recurrence when compared with no division. The use of ultrasonic scalpel compared with clips or bipolar cautery for the division of short gastric vessels showed no significant effect on operative time, postoperative complications, and costs.
Conclusions:
Laparoscopic antireflux surgery is at least as safe and as effective as its open counterpart, with reduced morbidity, shortened postoperative stay, and sick leave. Partial fundoplication significantly reduces the risk of reoperations for failure over total fundoplication. Routine versus no division of short gastric vessels showed no significant advantages. A word of caution is needed when implementing these results derived from RCTs performed in specialized centers into everyday clinical practice, where experience and skills may be suboptimal.
Evidence-based answers to current controversies in open versus laparoscopic surgery for gastroesophageal reflux disease, including the impact of technical details on outcomes, are given in this systematic review of randomized controlled trials.
The serendipitous discovery of the antireflux effect of wrapping the gastric fundus around the distal esophagus1 led Rudolph Nissen to perform the first fundoplication for gastroesophageal reflux disease (GERD) nearly half a century ago.2 Since then, various technical details of total fundoplication3–5 or partial fundoplications6–8 have been suggested. In 1991, the so-called “Nissen fundoplication” was performed for the first time through a laparoscopic approach.9,10 To date, various large series showed its safety, efficacy, good quality of life, short hospital stay, early return to work, and cost savings.11–14 However, little is known about the reproducibility of such results in nonspecialized centers,15 and about current indications and results faced by long-term acid suppression therapy.16,17 Gastroenterologists and surgeons definitely do not share the same enthusiasm in surgical referral of patients with GERD.18 The gastroenterological medical community appears at least skeptical about the efficacy of laparoscopic antireflux surgery,19–21 claiming also that too many technical modifications of fundoplication are performed and complications are often blamed on 1 type of modification or another.19,20 Furthermore, a recently introduced third party – endoscopic augmentation of lower esophageal sphincter pressure22,23 – might potentially compete in this arena.
When one of the authors, already experienced in laparoscopic antireflux surgery, moved to his current hospital, he needed to establish a new surgical referral of patients with GERD. He was asked to provide the available evidence on current status of antireflux surgery, and this need prompted this review.
MATERIALS AND METHODS
The first step of evidence-based medicine is to rephrase our problems or information needs into answerable questions.24 Therefore, we developed 4 questions:
Open or laparoscopic approach?
Partial or total wrap?
Division or no division of short gastric vessels?
Hiatoplasty and calibration of the wrap by an esophageal bougie?
We then performed a literature search of computer databases of all articles published through 2002 with no language limitation (MEDLINE 1966–2002, EMBASE 1980–2002, HealthSTAR 1975–2002, and the Cochrane Library 2/2002). A computer-assisted search was conducted using the following combination of Medical Subject Heading (MESH) terms: “Gastroesophageal reflux” and “fundoplication.” We also did a manual search using references from the articles retrieved and main review articles.11–14 For each citation, we downloaded the title, abstract, authors, institution, journal, and major and minor descriptors.
Prospective randomized controlled trials (RCTs) related to fundoplication for gastroesophageal reflux disease in adults were selected. Each study was independently reviewed by the authors, and methodological criteria and the results of each study were recorded. Studies were judged suitable for meta-analysis only if they met all the following criteria: (1) prospective randomized trial dealing with laparotomic and/or laparoscopic fundoplication for gastroesophageal reflux disease; (2) well-defined outcomes including at least 1 of the following: (a) perioperative mortality and morbidity rates, (b) details about the rates of specific postsurgical results (ie, recurrence, dysphagia, etc.). Only results fully reported in journal articles were considered. All the trials regarding already abandoned surgical techniques, such as the Angelchik device or the ligamentum teres gastroplasty, were excluded.
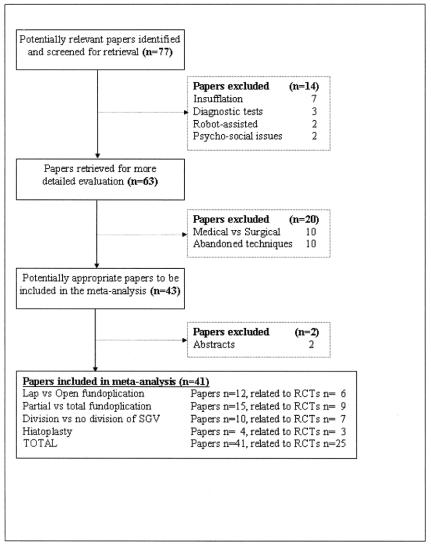
We found 77 papers reporting the results of RCTs; all these articles passed through a multilevel, systematic review by a team of 2 surgeons and 1 gastroenterologist, trained in epidemiology and health services research, according to the QUOROM statement.25 Forty-one papers met all the inclusion criteria. This review is therefore based on these 41 papers, reporting the results of 25 RCTs (Fig. 1). Full papers of all these trials were reviewed blindly and independently by all authors to tabulate subject demographics, study design, definition of outcomes, and frequencies of each end point, using a standardized data abstract form. Disagreement was resolved by consensus. Independent methodological quality assessment of each article using the Jadad scale,26 with scores ranging from 0 to 5, was also performed. Studies addressing each 1 of the 4 questions were separately analyzed.
FIGURE 1. Flow diagram of papers’ inclusion and exclusion according to the QUOROM statement.25
Open Versus Laparoscopic Fundoplication
The outcomes considered were: conversion rates in the laparoscopic group, overall morbidity and mortality rates, length of the operation, length of postoperative hospital stay, length of sick leave, incidence of postoperative recurrence of GERD (detected by either endoscopy and/or pH-metry, when available, or by the recurrence of symptoms), incidence of postoperative new-onset dysphagia of any grade, incidence of postoperative bloating syndrome of any grade, incidence of reoperation for any failure, and immune status.
Partial Versus Total Wrap
The outcomes considered were: overall morbidity and mortality rates, length of the operation, incidence of postoperative new-onset dysphagia of any grade, incidence of postoperative recurrence of GERD (detected by either endoscopy and/or pH-metry, when available, or by the recurrence of symptoms), and incidence of reoperation for any failure.
Division Versus No Division of Short Gastric Vessels
The outcomes considered were: overall morbidity and mortality rates, length of the operation, incidence of postoperative new-onset dysphagia of any grade, and incidence of postoperative recurrence of GERD (detected by either endoscopy and/or pH-metry, when available, or by the recurrence of symptoms). Furthermore, different devices for laparoscopic division of short gastric vessels were compared regarding postoperative morbidity rates, length of the operation, and costs.
Hiatoplasty and Calibration
The outcomes considered were incidence of disruption of hiatal repair, incidence of postoperative new-onset dysphagia of any grade, and incidence of adverse effects (ie, esophagogastric perforation by the calibrating bougie).
Statistical Analysis
Results were analyzed by the DerSimonian-Laird (random effects) method27 for comparing and summarizing outcomes of individual RCTs. Results were pooled as odds ratios (OR). Confidence intervals (CI) were always calculated at 95%. The alpha level was set at 0.05 for a two-tailed test. The rate difference (RD) (ie, the difference in event rates between the groups) was used as a measure of the therapeutic effect. A personally developed statistical program28 was used for this purpose. Results were also verified using another appropriate meta-analysis software.29 Intertrial heterogeneity in treatment effect was evaluated using the Q statistic of DerSimonian-Laird.27 To further detect heterogeneity, a visual display was obtained, representing the results on a L'Abbè plot.30 Final analyses were performed using the StatsDirect (version 1,9,8) and RevMan (version 4.2.2) statistical softwares. When significant differences were encountered, numbers needed to treat (NNT), that is the number of patients that need to be treated to obtain 1 therapeutic effect,31 were also calculated; mathematically, NNT is equivalent to the reciprocal of RD, and the 95% CI for the NNT are the reciprocal of the 95% CI for RD. Results of continuous variables given in the trials as a mean and/or median value (length of the procedure, hospital stay, sick leave, costs) were pooled as a mean ± SD, and differences between groups were analyzed by a paired two-tail t test. When a specific issue was addressed by a single trial, its results where analyzed calculating absolute risk reduction (ARR), that is the difference between control event rate (CER) and experimental event rate (EER), relative risk reduction (RRR), that is the same difference divided by the CER, and number needed to treat (NNT), that is equivalent to the reciprocal of ARR.
RESULTS
Open Versus Laparoscopic Fundoplication
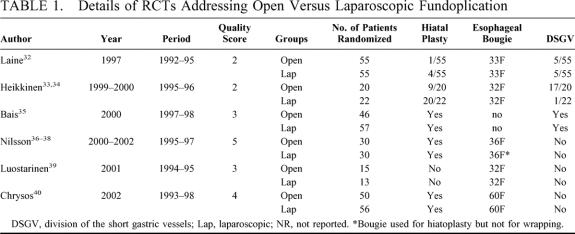
The results of open versus laparoscopic fundoplication were investigated in 6 RCTs: 1 from Turku, Finland,32 1 from Oulu, Finland,33,34 1 multicenter trial from the Netherlands,35 1 from Lund, Sweden,36–38 another 1 from Tampere, Finland,39 and the last 1 from Heraklion, Greece.40 All these trials compared open versus laparoscopic Nissen fundoplication with some technical variations (Table 1). They were published between 1997 and 2002, mean (range) quality score was 3.2 (2 to 5).
TABLE 1. Details of RCTs Addressing Open Versus Laparoscopic Fundoplication
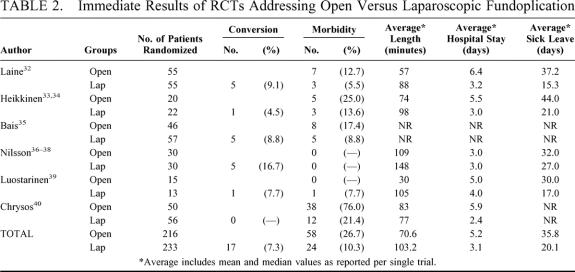
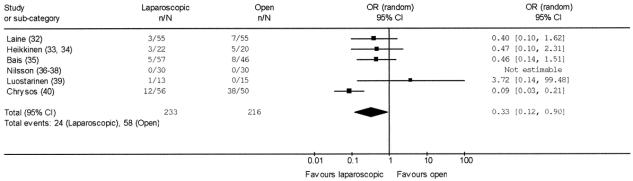
Considering immediate perioperative results (Table 2), no perioperative death was recorded. The need of conversion to open surgery in the laparoscopic arm arose in 17 cases (7.3%), all of which included in the original randomization arm according to an intention-to-treat analysis. Twenty-four of 233 patients submitted to laparoscopic fundoplication (10.3%) suffered at least 1 perioperative complication compared with 58 of 216 patients submitted to open fundoplication (26.7%). The pooled OR for perioperative complications in laparoscopic fundoplication was 0.33 (95% CI 0.12 to 0.90) (Fig. 2); no significant heterogeneity was found (Q = 10.35, df = 5, P = 0.07). The pooled RD was –12% (95% CI –30% to 6%), and pooled NNT was 8 (95% CI 3 to 16). The pooled length of the operative procedure was longer in the laparoscopic procedures (103.2 ± 27.2 minutes) than in the open ones (70.6 ± 37.5 minutes), although not significantly (P = 0.067). On the other hand, pooled postoperative hospital stay was significantly shorter (P = 0.03) in the laparoscopic group (3.1 ± 0.6 days) than in the open one (5.2 ± 1.3 days), as was pooled sick leave (20.1 ± 5.2 versus 35.8 ± 6.2 days, P = 0.03).
TABLE 2. Immediate Results of RCTs Addressing Open Versus Laparoscopic Fundoplication
FIGURE 2. Pooled OR of operative morbidity in laparoscopic versus open Nissen fundoplication.
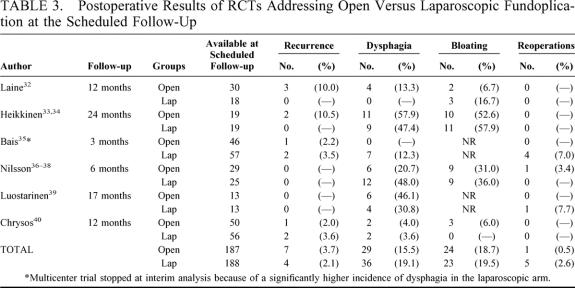
Concerning postoperative results (Table 3) at the scheduled follow-up (range 3–24 months), no significant differences were found in laparoscopic versus open fundoplication regarding recurrence (pooled OR 0.80, 95% CI 0.24 to 2.68; pooled RD –0.1%, 95% CI –5% to 3%), dysphagia (pooled OR 1.16, 95% CI 0.42 to 3.20; pooled RD 1%, 95% CI –10% to 13%), bloating (pooled OR 1.21, 95% CI 0.56 to 2.63; pooled RD 1%, 95% CI –11% to 13%), and reoperation rates (pooled OR 1.74, 95% CI 0.42 to 7.25; pooled RD 1%, 95% CI –2% to 4%).
TABLE 3. Postoperative Results of RCTs Addressing Open Versus Laparoscopic Fundoplication at the Scheduled Follow-Up
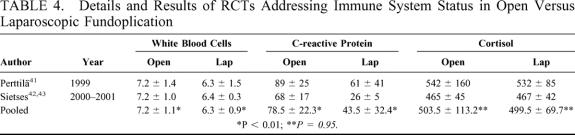
The immune system status was investigated in 3 papers related to the original trials from Turku41 and from the Netherlands,42,43 showing significantly reduced white blood cells counts and serum C-reactive protein levels after laparoscopic fundoplication, with no significant differences in serum cortisol levels (Table 4).
TABLE 4. Details and Results of RCTs Addressing Immune System Status in Open Versus Laparoscopic Fundoplication
Partial Versus Total Wrap
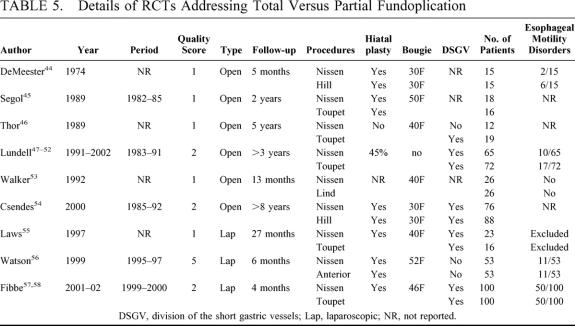
The effects of partial versus total wrap were investigated in 9 RCTs. Six dealt with open fundoplication: 1 from Honolulu, USA;44 1 multicenter from France;45 1 from Stockolm,46 and another from Goteborg,47–52 Sweden; 1 from Liverpool, UK;53 and 1 from Santiago, Chile.54 The remaining 3 trials dealt with laparoscopic fundoplication: from Birmingham, USA;55 from Adelaide, Australia;56 and from Hamburg, Germany.57,58 The details of these studies are shown in Table 5, and their results are shown in Table 6. They were published between 1974 and 2002, mean (range) quality score was 1.8 (1 to 5). Concerning the partial fundoplication arm, Toupet posterior fundoplication was evaluated in 5 of these RCTs,45–52,55,57,58 Hill repair in 2,44,54 Lind procedure in 1,53 and anterior fundoplication in one.56
TABLE 5. Details of RCTs Addressing Total Versus Partial Fundoplication
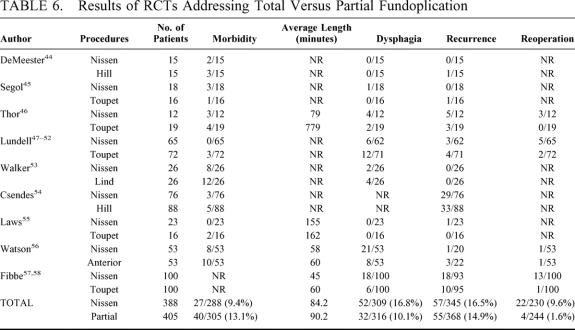
TABLE 6. Results of RCTs Addressing Total Versus Partial Fundoplication
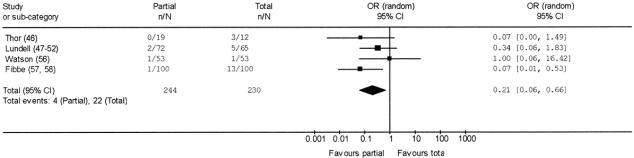
There were no perioperative deaths. No significant differences were found concerning operative morbidity in partial (40/305, 13.1%) versus total (27/288, 9.4%) fundoplication (pooled OR 1.47, 95% CI 0.84 to 2.57; pooled RD 4%, 95% CI −0.1 to 7%). The pooled length of the operative procedure was not significantly different in partial (90.2 ± 48.7 minutes) versus total fundoplication (84.2 ± 49.2 minutes). At the scheduled postoperative follow-up (range 4 months to 8 years), no significant differences were found in partial versus total fundoplication about new-onset dysphagia (9.3% versus 16.8%; pooled OR 0.56, 95%CI 0.25 to 1.22; pooled RD −5%, 95% CI −12% to 3%) and recurrence (15.1% versus 16.5%; pooled OR 0.82, 95% CI 0.53 to 1.27; pooled RD 1%, 95% CI −4% to 6%). A reoperation for failure was necessary in 4 out of 244 patients submitted to partial fundoplication (1.6%) compared with 22 out of 230 patients submitted to total fundoplication (9.6%). The pooled OR for reoperation in partial fundoplication was 0.21 (95% CI 0.06 to 0.66) (Fig. 3); no significant heterogeneity was found (Q = 3.41, df = 3, P = 0.33). The pooled RD was −7% (95% CI −16% to 1%) and pooled NNT was 14 (95% CI 6 to 100).
FIGURE 3. Pooled OR of reoperation for failure in partial versus total fundoplication.
Division Versus No Division of Short Gastric Vessels
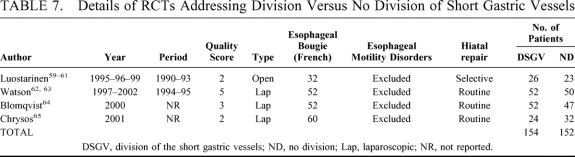
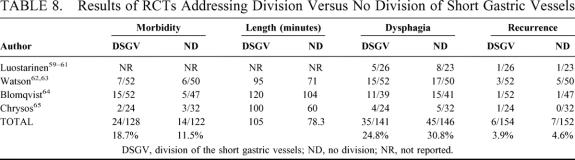
The effects of fundic mobilization by division of short gastric vessels (SGV) were investigated in 4 RCTs. One RCT related to open fundoplication was from the Tampere University in Finland,59–61 whereas, concerning laparoscopic fundoplication, we found a trial from Adelaide, Australia;62,63 1 from Goteborg, Sweden;64 and 1 from Heraklion, Greece.65 The details of these studies are shown in Table 7, and their results are shown in Table 8.
TABLE 7. Details of RCTs Addressing Division Versus No Division of Short Gastric Vessels
TABLE 8. Results of RCTs Addressing Division Versus No Division of Short Gastric Vessels
There were no perioperative deaths. No significant differences were found in division versus no division of SGV concerning morbidity (18.7% versus 11.5%; pooled OR 1.74, 95% CI 0.76 to 3.99; pooled RD 6%, 95% CI −6% to 18%). The pooled length of the operative procedure was longer after division (105 ± 13.2 minutes) versus no division (78.3 ± 22.9 minutes) of SGV, albeit with borderline significance (P = 0.06). No significant differences were found in division versus no division of SGV concerning the incidence of dysphagia (24.8% versus 30.8%; pooled OR 0.72, 95% CI 0.42 to 1.21; pooled RD −6%, 95% CI −16% to 4%), and recurrence (3.9% versus 4.6%; pooled OR 0.81, 95% CI 0.27 to 2.50; pooled RD −0.2%, 95% CI −4% to 4%).
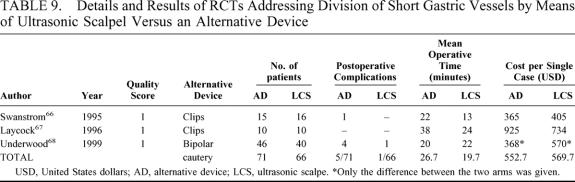
We found 3 trials comparing the use of different laparoscopic devices to divide short gastric vessels. In the first 2 trials the ultrasonic scalpel was compared with multifire clip applier,66,67 whereas in the last trial,68 it was compared with bipolar coagulating forceps. The details and results of these trials are shown in Table 9. No significant differences were found using the alternative device versus the ultrasonic scalpel in postoperative complications (7.0% versus 1,5%; pooled OR 2.89, 95% CI 0.5 to 15.45; pooled RD 5%, 95% CI −2% to 12%), in length of the operation (26.6 ± 9.9 versus 19.7 ± 5.9 minutes) and in costs (552.6 ± 322.4 versus 569.7 ± 164.5 USD per single case).
TABLE 9. Details and Results of RCTs Addressing Division of Short Gastric Vessels by Means of Ultrasonic Scalpel Versus an Alternative Device
Hiatoplasty and Calibration
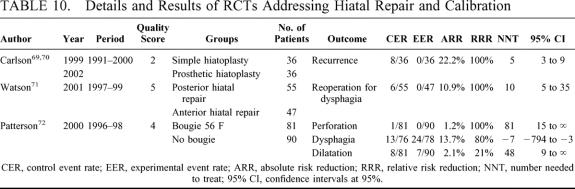
We found no RCT comparing hiatal repair versus no repair. In one RCT from USA69,70 the standard posterior hiatal repair was compared with a prosthetic reinforced repair in patients with large (>8 cm) hiatal hernias. In another RCT from Adelaide, Australia, the standard posterior hiatal repair was compared with an anterior repair.71 Concerning calibration of hiatoplasty and wrapping by means of an esophageal bougie, there was 1 trial from Portland, Oregon.72 The details and results of these trials are shown in Table 10.
TABLE 10. Details and Results of RCTs Addressing Hiatal Repair and Calibration
DISCUSSION
The first finding of this systematic review is the complete absence of postoperative deaths in any of the RCTs, dealing either with open or laparoscopic fundoplication. Actually, both open and laparoscopic antireflux surgery entail a low but definite risk of operative mortality.14,73,74 Actually, this rate was 0.008% (8 out of 10,489 cases) in a review of 41 laparoscopic series published between 1993 and 2000;14 it increased to 0.09% (1 out of 1162 cases) for laparoscopic and 0.2% (9 out of 3933 cases) for open surgery in a population-based study in Finland from 1987 to 1996,73 and to 0.8% (168 out of 20,004 cases) in a population-based study in USA from 1992 to 1997.74 In the latter study, a volume/outcome relationship was identified, with mortality rates ranging from 1.3% among surgeons with <5 cases to 0% among surgeons with >50 cases treated during the study period. Looking at these figures, there is no doubt that postoperative mortality rates after antireflux surgery reported in case-series are affected by a publication bias. The results achieved in the RCTs analyzed in this review come from specialized centers with high caseload volumes and/or very well selected populations of patients. Whether these results are determined by a “practice makes perfect” effect or by a “selective referral” effect,75 the possibility of postoperative mortality should be kept in mind and anticipated for the patient candidate for antireflux surgery within the everyday clinical practice of a nonspecialized surgical center.
The overall conversion rate from laparoscopic to open fundoplication in these RCTs was 7.3% and operative morbidity rate for the laparoscopic arm was 10.3%. These figures are both higher than those reported (conversion 3.1%; morbidity 6.4%) in the review of laparoscopic case-series,74 confirming that a publication bias may be present as well.
Open Versus Laparoscopic Fundoplication
Laparoscopic fundoplication showed a significant reduction of operative morbidity rates, hospital stay, sick-leave period (Table 1 and Fig. 1), and activation of the immune system (Table 4) when compared with its open counterpart. Only the duration of the operation seemed to be prolonged, albeit with borderline significance. It can be concluded that the immediate results of laparoscopic fundoplication are equal to or better than those of open fundoplication, confirming what was already reported in many nonrandomized comparative studies.76–79 A short-term analysis of postoperative results at the scheduled follow-up (range 3 to 24 months) failed to show any differences concerning recurrence, dysphagia, bloating, and reoperation for failure, suggesting that, while waiting for longer follow-up, the laparoscopic approach reproduces the same results as its open counterpart. However, the only multicenter RCT35 had to be stopped at its interim analysis due to an unacceptable rate of postoperative dysphagia in the laparoscopic arm (Table 3). The publication of this trial triggered many critiques,80 mainly related to the low volume of cases treated per surgeon per year (about 2.7), and its authors had to admit that their results were biased by the existence of a learning curve and a maintenance curve.80 Actually, many studies previously investigated the learning curve for the surgeon and for the institution dealing with laparoscopic fundoplication.81–83 The issue of laparoscopic versus open fundoplication was also covered in several consensus conferences,84–88 all reaching the conclusion that fundoplication should possibly be performed through a laparoscopic approach provided there is an expert surgeon in charge of the operation. Little or nothing is known concerning the optimal volume of cases to be treated to maintain this expert status, as no volume-outcome analysis in laparoscopic antireflux surgery is available. These considerations prompt a word of caution about the widespread application of laparoscopic antireflux surgery in nonspecialized centers.
Partial Versus Total Wrap
Before the advent of laparoscopic surgery, the Nissen procedure was considered the most successful in terms of reflux control4,5 and was therefore more often performed than partial fundoplications. The last decade witnessed a strong debate about partial versus total fundoplication, shifting the attention to postoperative failures due to mechanical problems (ie, dysphagia), rather than worries about the recurrence of disease. It appeared that there was a special risk for dysphagia in patients with preoperative evidence of esophageal motility disorders, and the choice between total or partial fundoplication was suggested to be tailored on the absence or presence of impaired esophageal peristalsis at the preoperative manometric assessment.8,89,90 Surgeons began to perform partial fundoplications more frequently,11,91 and the results of several nonrandomized trials did not confirm this hypothesis.92–94 On the other hand, some authors still cast serious doubts regarding the effectiveness of partial fundoplication on the control of reflux,93,95–97 and others suggest that a floppy Nissen can be effective even in patients with defective esophageal peristalsis.98,99 In this analysis (Table 6), partial fundoplication appeared to be a better procedure than total fundoplication, showing similar operative time, morbidity and recurrence rates, but a significantly reduced rate of reoperations for failure (1.6% versus 9.6%), mainly due to postoperative dysphagia (10.1% versus 16.8%). All but 3 RCTs45,46,54 showed an equal distribution of patients with esophageal motility disorders in this analysis (Table 5). In the most recent trial,58 a subgroup analysis in patients with esophageal motility disorders failed to detect any difference in the occurrence of postoperative new-onset dysphagia and/or endoscopic evidence of recurrent disease. Actually, the etiology of dysphagia is multifactorial, and an abnormal preoperative manometric pattern is a poor predictor of postoperative new-onset dysphagia.100 Should we perform more partial fundoplications based on these results? It is hard to find a final answer to this question based on current data, as several potential sources of bias are present in this analysis: first of all, many RCTs included in the analysis of this specific issue were published in the 1980s or in the early 1990s, when surgical techniques were not as well developed and standardized as they are now; second, a large body of this evidence derives from a very short follow-up period (4 to 27 months in laparoscopic RCTs); and third, it can be incorrect to pool together results of different partial repairs relying on different pathophysiologic mechanisms, such as Toupet partial fundoplication or Hill's or Lind's repairs. While there is no way to overcome the first 2 potential sources of bias, we performed a subgroup analysis including only RCTs dealing with Toupet posterior fundoplication in the partial fundoplication arm. The results (data not shown) are not different from those deriving from the entire group analysis, confirming a significantly lower rate of reoperation in the partial fundoplication arm. At the moment it seems wiser to delay the search for a final answer and wait for the results of longer follow-up.101
Division Versus No Division of Short Gastric Vessels
All RCTs dealing with division versus no division of short gastric vessels excluded patients with esophageal motility disorders (Table 7) to prevent any possible bias regarding the incidence of postoperative new-onset dysphagia. No significant differences were detected regarding morbidity, dysphagia, and recurrence (Table 8); routine division of short gastric vessels cannot therefore be supported anymore. As twisting deformities resulting from an unskilled attempt to wrap an immobile gastric fundus and/or a mobile gastric body around the esophagus are a major cause of failure,102,103 it is advisable to perform complete fundal mobilization during the learning curve and in case of any doubt concerning mobility of gastric fundus.104
In a nonrandomized comparison, the harmonic scalpel seemed to be an extremely useful tool for the division of short gastric vessels, reducing operative time, morbidity, and costs.105 We failed to detect any significant advantage pooling the results of 3 RCTs (Table 9), although a trend in reduction of morbidity rates was noted (1.5 versus 7.0%).
Hiatoplasty and Calibration
There are no RCTs evaluating the role of routine hiatal closure, and probably there will never be, as nonrandomized studies already show an intolerable rate of paraesophageal herniation in patients not undergoing crural repair.106,107 Concerning the type of hiatal repair (anterior versus posterior; primary versus prosthetic) and the routine use of a bougie for calibration of the repair and of wrapping, we found only results deriving from single RCTs. Any result, therefore, should be interpreted with caution, keeping in mind that the amount of data gathered is largely insufficient to find a definitive answer to these questions, especially when faced with a large amount of data deriving from retrospective analyses and/or case-series.
Most surgeons are used to the standard posterior hiatoplasty; the group from Adelaide, Australia, described the possibility that standard posterior repair displaces the esophagus too anteriorly, therefore contributing to postoperative dysphagia.108 The results of their RCT seem to confirm this hypothesis, with an anterior repair eliminating the risk of reoperation for dysphagia (Table 10). However, the same authors admit a potential major bias in such trial, as the incidence of reoperation in the arm treated by standard posterior repair is far too high from that previously reported in other RCTs by the same group of surgeons.56,62
The rates of hiatal hernia recurrence after hiatal repair during primary laparoscopic fundoplication vary between 1 and 7%, but can reach 50% when facing large and/or paraesophageal hernias. There was little doubt that any repair of large hernias should be performed with a prosthetic reinforcement,109 and the results of one RCT69,70 seem to confirm this concept, with 5 patients to be treated to avoid 1 hernia recurrence (Table 10). Further research is needed to find which cut-off in hiatal hernia size mandates a prosthetic repair.110
The use of an esophageal bougie during hiatal closure and wrapping has long been one of the basic tenets of Nissen fundoplication4,5 to reduce postoperative dysphagia. Moreover, we found a time-trend towards an increase in its size throughout the RCTs analyzed in this review (Tables 1, 3, and 7). However, its routine use carries the risk of iatrogenic perforation, varying around 1% of cases.111,112 Some authors, therefore, do not recommend its routine use.113 Looking at the only available RCT,72 it appears that routine use of a bougie significantly reduces the rate of severe postoperative dysphagia, as defined by the authors, by 13.7%, with a 1.2% risk of iatrogenic perforation (Table 10). On the other hand, if we look at something more clinically relevant, such as the rate of postoperative dilatation, the results were higher in the group treated by routine use of the bougie. Therefore, the results of this trial have to be interpreted with caution, as they are strongly dependent upon the definition of postoperative dysphagia, and further research on this point is desirable.
CONCLUSIONS
Laparoscopic fundoplication is as effective as its open counterpart, allowing a reduced morbidity rate, shorter hospital stay, and recovery, with no significant differences in early functional results. Long-term (>5 years) follow-up, however, is needed. Partial fundoplication reduces the rate of reoperation due to mechanical failure, but longer follow-up is needed to evaluate its effectiveness in the control of reflux. There is no evidence to support routine division of short gastric vessels. Further RCTs are needed to determine the best way to perform hiatal closure (anterior versus posterior, simple versus prosthetic) and the benefit/risk ratio of routine calibration by means of an esophageal bougie. All of this evidence derives from specialized centers and from selected populations, and particular caution is therefore suggested when implementing it into everyday clinical practice.
ADDENDUM
During the review process of this manuscript, another prospective randomized trial suitable for inclusion into this meta-analysis (Chrysos E, Tsiaoussis J, Zoras OJ, et al. Laparoscopic surgery for gastroesophageal reflux disease patients with impaired esophageal peristalsis: total or partial fundoplication? J Am Coll Surg 2003;197:8-15) was published. The inclusion of data gathered from such trial into the data pooling and reanalysis did not significantly change any of the results presented in this manuscript.
Footnotes
Reprints: Marco Catarci, MD, UOC Chirurgia Generale & Oncologica; ACO San Filippo Neri, Via G. Martinotti, 20-00135 Roma, Italy. E-mail: m.catarci@sanfilipponeri.roma.it.
REFERENCES
- 1.Nissen R. Die transpleurael resektion der kardia. Dtsch Chir. 1937;249:311–316. [Google Scholar]
- 2.Nissen R. Eine einfache operation zur beeinflussung der refluxoesophagitis. Schweiz Med Wochenschr. 1956;86:590–592. [PubMed] [Google Scholar]
- 3.Rossetti M, Hell K. Fundoplication for the treatment of gastroesophageal reflux in hiatal hernia. World J Surg. 1977;1:439–444. [DOI] [PubMed] [Google Scholar]
- 4.Donahue PE, Samuelson S, Nyhus LM, et al. The floppy Nissen fundoplication: effective long-term control of pathologic reflux. Arch Surg. 1985;120:663–667. [DOI] [PubMed] [Google Scholar]
- 5.DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesphageal reflux disease: evaluation of primary repair in 100 consecutive patients. Ann Surg. 1986;204:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dor J, Humbert P, Dor V, et al. L'intéret de la technique de Nissen modifié pour la prevention du reflux après cardiomyotomie extra muqueuse. Mem Acad Clin. 1962;88:877–883. [Google Scholar]
- 7.Toupet A. Technique d'esophagogastroplastie et de phrenogastropexie appliqué dans la cure radicale des hernies hiatales et comme complement de l'operation de Heller dans le cardiospasmus. Mem Acad Clin. 1963;89:374–379. [Google Scholar]
- 8.Watson A, Jenkinson LR, Ball CS, et al. A more physiologic alternative to total fundoplication for the surgical correction of resistant gastroesophageal reflux. Br J Surg. 1991;78:1088–1094. [DOI] [PubMed] [Google Scholar]
- 9.Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1991;1:138–143. [PubMed] [Google Scholar]
- 10.Geagea T. Laparoscopic Nissen fundoplication: preliminary report on ten cases. Surg Endosc. 1991;5:170–173. [DOI] [PubMed] [Google Scholar]
- 11.Watson DI, Jamieson GG. Antireflux surgery in the laparoscopic era. Br J Surg. 1998;85:1173–1184. [DOI] [PubMed] [Google Scholar]
- 12.Hinder RA, Libbey JS, Gorecki P, et al. Antireflux surgery. Indications, preoperative evaluation and outcome. Gastroenterol Clin North Am. 1999;28:987–1005. [DOI] [PubMed] [Google Scholar]
- 13.Soper NJ. Laparoscopic management of hiatal hernia and gastroesophageal reflux. Curr Probl Surg. 1999;36:767–838. [DOI] [PubMed] [Google Scholar]
- 14.Carlson MA, Frantzides CT. Complications and results of primary minimally invasive antireflux procedures: a review of 10, 735 reported cases. J Am Coll Surg. 2001;193:428–439. [DOI] [PubMed] [Google Scholar]
- 15.Rantanen TK, Halme TV, Luostarinen ME, et al. The long term results of open antireflux surgery in a community-based health care center. Am J Gastroenterol. 1999;94:1777–1781. [DOI] [PubMed] [Google Scholar]
- 16.El-Serag HB, Sonnenberg A. Outcome of erosive reflux esophagitis after Nissen fundoplication. Am J Gastroenterol. 1999;94:1771–1776. [DOI] [PubMed] [Google Scholar]
- 17.Spechler SJ, Lee E, Anhen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331–2338. [DOI] [PubMed] [Google Scholar]
- 18.Sarani B, Scanlon J, Jackson P, et al. Selection criteria among gastroenterologists and surgeons for laparoscopic antireflux surgery. Surg Endosc. 2002;16:57–63. [DOI] [PubMed] [Google Scholar]
- 19.Kahrilas PJ. Laparoscopic antireflux surgery: silver bullet or the emperor's new clothes? Am J Gastroenterol. 1999;94:1721–1723. [DOI] [PubMed] [Google Scholar]
- 20.Hogan WJ, Shaker R. Life after antireflux surgery. Am J Med. 2000;108:1815–1819. [DOI] [PubMed] [Google Scholar]
- 21.Kahrilas PJ. Surgical therapy for reflux disease. JAMA. 2001;285:2376–2378. [DOI] [PubMed] [Google Scholar]
- 22.Katz PO. Gastroesophageal reflux disease: new treatments. Rev Gastroenterol Disord. 2002;2:66–74. [PubMed] [Google Scholar]
- 23.Galmiche JP, Barouk J. Endoscopic treatment of gastroesophageal reflux disease–fact or fancy? Curr Gastroenterol Rep. 2002;4:177–178. [DOI] [PubMed] [Google Scholar]
- 24.Sackett DL, Straus SE, Richardson WS, et al. Evidence-Based Medicine: How to Practice and Teach EBM, 2nd ed. Edinburgh: Churchill Livingstone, 2000. [Google Scholar]
- 25.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–1900. [DOI] [PubMed] [Google Scholar]
- 26.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers TC, Blum A, Buyse M, et al. Data Analysis for Clinical Medicine. The Quantitative Approach to Patient Care in Gastroenterology. Rome: International University Press, 1988. [Google Scholar]
- 29.Messori A, Rampazzo R. Meta-analysis of clinical trials based on censored end-points: simplified theory and implementation of the statistical algorithms on a microcomputer. Comput Methods Programs Biomed. 1993;40:261–267. [DOI] [PubMed] [Google Scholar]
- 30.L'Abbé KA, Detsky AS, O’ Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–233. [DOI] [PubMed] [Google Scholar]
- 31.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–1733. [DOI] [PubMed] [Google Scholar]
- 32.Laine S, Rantala A, Gullichsen R, et al. Laparoscopic vs conventional Nissen fundoplication. A prospective randomized study. Surg Endosc. 1997;11:441–444. [DOI] [PubMed] [Google Scholar]
- 33.Heikkinen TJ, Hakipuro K, Koivukangas P, et al. Comparison of costs between laparoscopic and open Nissen fundoplication: a prospective randomized study with a 3-month followup. J Am Coll Surg. 1999;188:368–376. [DOI] [PubMed] [Google Scholar]
- 34.Heikkinen TJ, Hakipuro K, Bringman S, et al. Comparison of laparoscopic and open Nissen fundoplication 2 years after operation. A prospective randomized trial. Surg Endosc. 2000;14:1019–1023. [DOI] [PubMed] [Google Scholar]
- 35.Bais JE, Bartelsman JFWM, Bonjer HJ, et al. Laparoscopic or conventional Nissen fundoplication for gastroesophageal reflux disease: randomised clinical trial. Lancet. 2000;355:170–174. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson G, Larsson S, Johnsson F. Randomized clinical trial of laparoscopic versus open fundoplication: blind evaluation of recovery and discharge period. Br J Surg. 2000;87:873–878. [DOI] [PubMed] [Google Scholar]
- 37.Wenner J, Nilsson G, Öberg S, et al. Short-term outcome after laparoscopic and open 360° fundoplication. A prospective randomized clinical trial. Surg Endosc. 2001;15:1124–1128. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson G, Larsson S, Johnsson F. Randomized clinical trial of laparoscopic versus open fundoplication: evaluation of psychological well-being and changes in everyday life from a patient perspective. Scand J Gastroenterol. 2002;37:385–391. [DOI] [PubMed] [Google Scholar]
- 39.Luostarinen M, Virtanen J, Matikainen M, et al. Dysphagia and oesophageal clearance after laparoscopic versus open Nissen fundoplication. A randomized, prospective trial. Scan J Gastroenterol. 2001;36:565–571. [DOI] [PubMed] [Google Scholar]
- 40.Chrysos E, Tsiaoussis J, Athanasakis E, et al. Laparoscopic vs open approach for Nissen fundoplication. Surg Endosc. 2002;16:1679–1684. [DOI] [PubMed] [Google Scholar]
- 41.Perttilä J, Salo M, Ovaska J, et al. Immune response after laparoscopic and conventional Nissen fundoplication. Eur J Surg. 1999;165:21–28. [DOI] [PubMed] [Google Scholar]
- 42.Sietses C, Wiezer MJ, Eijsbouts QAJ, et al. A prospective randomized study of the systemic immune response after laparoscopic and conventional Nissen fundoplication. Surgery. 1999;126:5–9. [DOI] [PubMed] [Google Scholar]
- 43.Sietses C, Wiezer MJ, Eijsbouts QAJ, et al. The influence of laparoscopic surgery on polymorphonuclear leukocyte function. Surg Endosc. 2000;14:812–816. [DOI] [PubMed] [Google Scholar]
- 44.DeMeester TR, Johnson LF, Kent AH. Evaluation of current operations for the prevention of gastroesophageal reflux. Ann Surg. 1974;180:511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segol P, Hay JM, Pottier D. Surgical treatment of gastroesophageal reflux: which operation to choose: Nissen, Toupet or Lortat-Jacob? A multicenter randomized trial. Gastroenterol Clin Biol. 1989;13:873–879. [PubMed] [Google Scholar]
- 46.Thor KBA, Silander T. A long-term randomized prospective trial of the Nissen procedure versus a modified Toupet technique. Ann Surg. 1989;210:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundell L, Abrahamsson H, Ruth M, et al. Lower esophageal sphincter characteristics and esophageal acid exposure following partial or 360° fundoplication: results of a prospective, randomized, clinical study. World J Surg. 1991;15:115–121. [DOI] [PubMed] [Google Scholar]
- 48.Lundell L, Abrahamsson H, Ruth M, et al. Long-term results of a prospective randomized comparison of total fundic wrap (Nissen-Rossetti) or semifundoplication (Toupet) for gastro-oesophageal reflux. Br J Surg. 1996;83:830–835. [DOI] [PubMed] [Google Scholar]
- 49.Rydberg L, Ruth M, Lundell L. Does oesophageal motor function improve with time after successful antireflux surgery? Results of a prospective, randomised clinical study. Gut. 1997;41:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rydberg L, Ruth M, Lundell L. Mechanism of action of antireflux procedures. Br J Surg. 1999;86:405–410. [DOI] [PubMed] [Google Scholar]
- 51.Rydberg L, Ruth M, Abrahamsson H, et al. Tailoring antireflux surgery: a randomized clinical trial. World J Surg. 1999;23:612–618. [DOI] [PubMed] [Google Scholar]
- 52.Hagedorn C, Lonroth H, Rydberg L, et al. Long-term efficacy of total (Nissen-Rossetti) and posterior partial (Toupet) fundoplication. Results of a randomized clinical trial. J Gastrointest Surg. 2002;6:540–545. [DOI] [PubMed] [Google Scholar]
- 53.Walker SJ, Holt S, Sanderson CJ, et al. Comparison of Nissen total and Lind partial transabdominal fundoplication in the treatment of gastro-oesophageal reflux. Br J Surg. 1992;79:410–414. [DOI] [PubMed] [Google Scholar]
- 54.Csendes A, Burdiles P, Korn O, et al. Late results of a randomized clinical trial comparing total fundoplication versus calibration of the cardia with posterior gastropexy. Br J Surg. 2000;87:289–297. [DOI] [PubMed] [Google Scholar]
- 55.Laws HL, Clements RH, Swillie CM. A randomized, prospective comparison of the Nissen fundoplication versus the Toupet fundoplication for gastroesophageal reflux disease. Ann Surg. 1997;225:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson DI, Jamieson GG, Pike GK, et al. Prospective randomized double-blind trial between laparoscopic Nissen fundoplication and anterior partial fundoplication. Br J Surg. 1999;86:123–130. [DOI] [PubMed] [Google Scholar]
- 57.Fibbe C, Layer P, Keller J, et al. Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical and manometric study. Gastroenterology. 2001;212:5–14. [DOI] [PubMed] [Google Scholar]
- 58.Zornig C, Strate U, Fibbe C, et al. Nissen vs Toupet laparoscopic fundoplication. A prospective randomized study of 200 patients with and without preoperative esophageal motility disorders. Surg Endosc. 2002;16:758–766.11997817 [Google Scholar]
- 59.Luostarinen M, Koskinen M, Reinikainen P, et al. Two antireflux operations: floppy versus standard Nissen fundoplication. Ann Med. 1995;27:199–205. [DOI] [PubMed] [Google Scholar]
- 60.Luostarinen MES, Koskinen MO, Isolauri JO. Effect of fundal mobilization in Nissen-Rossetti fundoplication on esophageal transit and dysphagia. A prospective, randomized trial. Eur J Surg. 1996;162:37–42. [PubMed] [Google Scholar]
- 61.Luostarinen MES, Isolauri JO. Randomized trial to study the effect of fundic mobilization on long-term results of Nissen fundoplication. Br J Surg. 1999;86:614–618. [DOI] [PubMed] [Google Scholar]
- 62.Watson DI, Pike GK, Baigrie RJ, et al. Prospective double-blind randomized trial of laparoscopic Nissen fundoplication with division and without division of short gastric vessels. Ann Surg. 1997;226:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Boyle CJ, Watson DI, Jamieson GG, et al. Division of short gastric vessels at laparoscopic Nissen fundoplication. A prospective double-blind randomized trial with 5-year follow-up. Ann Surg. 2002;235:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blomqvist A, Dalenbäck J, Hagedorn C, et al. Impact of complete gastric fundus mobilization on outcome after laparoscopic total fundoplication. J Gastrointest Surg. 2000;4:493–500. [DOI] [PubMed] [Google Scholar]
- 65.Chrysos E, Tzortzinis A, Tsiaoussis J, et al. Prospective randomized trial comparing Nissen to Nissen-Rossetti technique for laparoscopic fundoplication. Am J Surg. 2001;182:215–221. [DOI] [PubMed] [Google Scholar]
- 66.Swanstrom LL, Pennings JL. Laparoscopic control of short gastric vessels. J Am Coll Surg. 1995;181:347–351. [PubMed] [Google Scholar]
- 67.Laycock WS, Trus TL, Hunter JG. New technology for the division of short gastric vessels during laparoscopic Nissen fundoplication. A prospective randomized trial. Surg Endosc. 1996;10:71–73. [DOI] [PubMed] [Google Scholar]
- 68.Underwood RA, Dunnegan DL, Soper NJ. Prospective randomized trial of bipolar electrosurgery vs ultrasonic coagulation for division of short gastric vessels during laparoscopic Nissen fundoplication. Surg Endosc. 1999;13:763–768. [DOI] [PubMed] [Google Scholar]
- 69.Carlson MA, Richards CG, Frantzides CT. Laparoscopic prosthetic reinforcement of hiatal herniorraphy. Dig Surg. 1999;16:407–410. [DOI] [PubMed] [Google Scholar]
- 70.Frantzides CT, Madan AK, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002;137:649–652. [DOI] [PubMed] [Google Scholar]
- 71.Watson DI, Jamieson GG, Devitt PG, et al. A prospective randomized trial of laparoscopic Nissen fundoplication with anterior versus posterior hiatal repair. Arch Surg. 2001;136:745–751. [DOI] [PubMed] [Google Scholar]
- 72.Patterson EJ, Herron DM, Hansen PD, et al. Effect of an esophageal bougie on the incidence of dysphagia following Nissen fundoplication. A prospective, blinded, randomized clinical trial. Arch Surg. 2000;135:1055–1062. [DOI] [PubMed] [Google Scholar]
- 73.Rantanen TR, Salo JA, Sipponen JT. Fatal and life-threatening complications in antireflux surgery: analysis of 5, 502 operations. Br J Surg. 1999;86:1573–1577. [DOI] [PubMed] [Google Scholar]
- 74.Flum DR, Koepsell T, Heagerty P, et al. The nationwide frequency of major adverse outcomes in antireflux surgery and the role of surgeon experience, 1992–1997. J Am Coll Surg. 2002;195:611–618. [DOI] [PubMed] [Google Scholar]
- 75.Black N, Johnston A. Volume and outcome in hospital care: evidence, explanations and implications. Health Serv Manage Res. 1990;3:108–114. [DOI] [PubMed] [Google Scholar]
- 76.Frantzides CT, Carlson MA. Laparoscopic versus conventional fundoplication. Surg Laparosc Endosc. 1995;5:137–143. [DOI] [PubMed] [Google Scholar]
- 77.Champault G, Volter F, Rizk N, et al. Gastroesophageal reflux: conventional surgical treatment versus laparoscopy. A prospective study of 61 cases. Surg Laparosc Endosc. 1996;6:434–440. [PubMed] [Google Scholar]
- 78.Stein HJ, Feussner H, Siewert JR. Antireflux surgery: a current comparison of open and laparoscopic approaches. Hepato-Gastroenterology. 1998;45:1328–1337. [PubMed] [Google Scholar]
- 79.Lind T. Changing surgical principles for gastro-oesophageal reflux disease: is laparoscopic fundoplication justified in the light of surgical complications? Eur J Surg. 2000;585:31–33. [DOI] [PubMed] [Google Scholar]
- 80.Gastro-oesophageal reflux disease – correspondence. Lancet 2000;356:69–73. [DOI] [PubMed] [Google Scholar]
- 81.Watson DI, Baigrie RJ, Jamieson GG. A learning curve for laparoscopic fundoplication. Definable, avoidable, or a waste of time? Ann Surg. 1996;224:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson DI, Jamieson GG, Baigrie RJ, et al. Laparoscopic surgery for gastroesophageal reflux: beyond the learning curve. Br J Surg. 1996;83:1284–1287. [PubMed] [Google Scholar]
- 83.Luostarinen MES, Isolauri JO. Surgical experience improves the long-term results of Nissen fundoplication. Scand J Gastroenterol. 1999;34:117–120. [DOI] [PubMed] [Google Scholar]
- 84.Beck IT, Champion MC, Lemire S, et al. The second Canadian consensus conference on the management of patients with gastroesophageal reflux disease. Can J Gastroenterol. 1997;11(suppl B):7–27. [PubMed] [Google Scholar]
- 85.Fuchs KH, Feussner H, Bonavina L, et al. Current status and trends in laparoscopic antireflux surgery: results of a consensus meeting. Endoscopy. 1997;29:298–308. [DOI] [PubMed] [Google Scholar]
- 86.Eypasch E, Neugebauer E, Fischer F, et al. Laparoscopic antireflux surgery for gastroesophageal reflux disease (GERD). Results of a consensus development conference. Surg Endosc. 1997;11:413–426. [PubMed] [Google Scholar]
- 87.Moss SF, Arnold R, Tytgat GN, et al. Consensus Statement for Management of Gastroesophageal Reflux Disease: results of a workshop meeting at Yale University School of Medicine, Department of Surgery, November 16 and 17, 1997. J Clin Gastroenterol. 1998;27:6–12. [DOI] [PubMed] [Google Scholar]
- 88.Société Nationale Française de Gastro-Entérologie. Conférence de consensus: reflux gastro-œsophagien de l'adulte: diagnostic et traitement. Gastroenterol Clin Biol. 1999;23:56–65. [PubMed] [Google Scholar]
- 89.Fuchs KH, Heimbucher J, Freys SM, et al. Management of gastroesophageal reflux disease 1995: tailored concept of anti-reflux operations. Dis Esophagus. 1994;7:250–254. [Google Scholar]
- 90.Kauer WKH, Peters JH, DeMeester TM, et al. A tailored approach to antireflux surgery. J Thorac Cardiovasc Surg. 1995;110:141–147. [DOI] [PubMed] [Google Scholar]
- 91.Windsor JA, Yellapu S. Laparoscopic anti-reflux surgery in New Zealand: a trend towards partial fundoplication. Aust NZ J Surg. 2000;70:184–187. [DOI] [PubMed] [Google Scholar]
- 92.Coster DD, Bower WH, Wilson VT, et al. Laparoscopic partial fundoplication vs. laparoscopic Nissen-Rossetti fundoplication: short-term results of 231 cases. Surg Endosc. 1997;11:625–631. [DOI] [PubMed] [Google Scholar]
- 93.Bell RC, Hanna P, Mills MR, et al. Patterns of success and failure with laparoscopic Toupet fundoplication. Surg Endosc. 1999;13:1189–1194. [DOI] [PubMed] [Google Scholar]
- 94.Pessaux P, Arnaud JP, Ghavami B, et al. Laparoscopic antireflux surgery: comparative study of Nissen, Nissen-Rossetti, and Toupet fundoplication. Surg Endosc. 2000;14:1024–1027. [PubMed] [Google Scholar]
- 95.Horvath KD, Jobe BA, Herron DM, et al. Laparoscopic Toupet fundoplication is an inadequate procedure for patients with severe reflux disease. J Gastrointest Surg. 1999;3:583–591. [DOI] [PubMed] [Google Scholar]
- 96.Farrell TM, Archer SB, Galloway KD, et al. Heartburn is more likely to recur after Toupet fundoplication than Nissen fundoplication. Am Surg. 2000;66:229–237. [PubMed] [Google Scholar]
- 97.Klapow JC, Wilcox CM, Mallinger AP, et al. Characterization of long-term outcomes after Toupet fundoplication: symptoms, medication use, and health status. J Clin Gastroenterol. 2002;34:509–515. [DOI] [PubMed] [Google Scholar]
- 98.Baigrie RJ, Watson DI, Myers JC, et al. Outcome of laparoscopic Nissen fundoplication in patients with disordered preoperative peristalsis. Gut. 1997;40:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oleynikov D, Eubanks TR, Oelschlager BK, et al. Total fundoplication is the operation of choice for patients with gastroesophageal reflux and defective peristalsis. Surg Endosc. 2002;16:909–913. [DOI] [PubMed] [Google Scholar]
- 100.Wills VL, Hunt DR. Dysphagia after antireflux surgery. Br J Surg. 2001;88:486–499. [DOI] [PubMed] [Google Scholar]
- 101.Fernando HC, Luketich JD, Christie NA, et al. Outcomes of laparoscopic Toupet compared to laparoscopic Nissen fundoplication. Surg Endosc. 2002;16:905–908. [DOI] [PubMed] [Google Scholar]
- 102.Hunter JG, Swanstrom LL, Waring JP. Dysphagia after laparoscopic antireflux surgery. The impact of operative technique. Ann Surg. 1996;224:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watson DI, de Beaux AC. Complications of laparoscopic antireflux surgery. Surg Endosc. 2001;15:344–352. [DOI] [PubMed] [Google Scholar]
- 104.Soper NJ. Fundoplication and the short gastric vessels: divide and conquer. Ann Surg. 2002;235:171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bischof G, Zacherl J, Imhof M, et al. Erfahrungen mit dem harmonischen Skalpell (Ultracision) in der laparoskopischen Antirefluxchirurgie. Zentralbl Chir. 1999;124:163–166. [PubMed] [Google Scholar]
- 106.Watson DI, Jamieson GG, Devitt P, et al. Paraesophageal hiatus hernia: an important complication of laparoscopic Nissen fundoplication. Br J Surg. 1995;82:521–523. [DOI] [PubMed] [Google Scholar]
- 107.Seelig MH, Hinder RA, Klingler PJ, et al. Paraesophageal herniation as a complication following laparoscopic antireflux surgery. J Gastrointest Surg. 1999;3:95–99. [DOI] [PubMed] [Google Scholar]
- 108.Watson DI, Jamieson GG, Mitchell PC, et al. Stenosis of the esophageal hiatus following laparoscopic fundoplication. Arch Surg. 1995;130:1014–1016. [DOI] [PubMed] [Google Scholar]
- 109.Basso N, Rosato P, De Leo A, et al. “tension-free” hernioplasty, gastrophrenic anchorage, and 360 degrees fundoplication in the laparoscopic treatment of paraesophageal hernia. Surg Laparosc Endosc Percutan Tech. 1999;9:257–262. [PubMed] [Google Scholar]
- 110.Granderath FA, Schweiger UM, Kamolz T, et al. Laparoscopic antireflux surgery with routine mesh-hiatoplasty in the treatment of gastroesophageal reflux disease. J Gastrointest Surg. 2002;6:347–353. [DOI] [PubMed] [Google Scholar]
- 111.Lowham AS, Filipi CJ, Hinder RA, et al. Mechanisms and avoidance of esophageal perforation by anesthesia personnel during laparoscopic foregut surgery. Surg Endosc. 1996;10:979–982. [DOI] [PubMed] [Google Scholar]
- 112.Schauer PR, Meyers WC, Eubanks S, et al. Mechanisms of gastric and esophageal perforations during laparoscopic Nissen fundoplication. Ann Surg. 1996;223:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Novitsky YW, Kercher KW, Callery MP, et al. Is the use of a bougie necessary for laparoscopic Nissen fundoplication? Arch Surg. 2002;137:402–406. [DOI] [PubMed] [Google Scholar]