Abstract
Objective:
To critically review the theoretical and actual risks and benefits of accelerated partial breast irradiation (APBI) after breast-conserving surgery.
Summary Background Data:
Because of rapid evolution of radiation therapy techniques related to brachytherapy and three-dimensional conformal radiation therapy, APBI has very recently come to the forefront as a potential local treatment option for women with breast cancer. This review aims to give an overview of the biologic rationale for APBI techniques, and benefits and limitations of APBI techniques.
Methods:
The authors reviewed the currently available published world medical literature on breast-conserving surgery with and without postoperative irradiation; all studies involving partial breast irradiation, including brachytherapy, for breast cancer; and currently accruing and planned APBI trials. The focus of this review was the early results of treatment in terms of toxicity, complications, cosmesis, and local control.
Results:
On average, approximately 3% of patients treated with breast-conserving surgery will have an in-breast local recurrence away from the original lumpectomy site with or without postoperative standard whole-breast irradiation. The results of phase I-II studies involving approximately 500 patients treated with APBI after breast-conserving surgery have been published. Although many of the studies have limited long-term follow-up and potential selection bias, early results suggest that toxicity, cosmesis, and local control are comparable to outcomes seen after breast-conserving surgery followed by standard whole-breast irradiation.
Conclusions:
Recent advances in radiation delivery and published series of partial breast irradiation support large randomized trials comparing APBI with standard whole-breast irradiation after breast-conserving surgery.
Rapid evolution of radiation therapy techniques has brought accelerated partial breast irradiation to the forefront as a potential local treatment option for women with breast cancer who are treated with conservative surgery. This review gives an overview of the biological rationale, techniques, potential benefits, complications, and planned national trials evaluating accelerated partial breast irradiation after conservative surgery.
At present, radiation therapy after breast-conserving surgery is generally delivered to the whole breast over a period of 5 to 6 weeks. Recently, however, rapidly emerging technological advances in radiation delivery have allowed the concept of accelerated partial breast irradiation (APBI) to quickly come to the forefront as a potential alternative to standard whole-breast irradiation after breast-conserving surgery. APBI refers to radiation therapy that is delivered over a shorter period than the standard 5 to 6 weeks (“accelerated”) and is delivered to only a portion of the breast (“partial”). Since the late 1990s, investigators at multiple institutions have been investigating the efficacy and safety of APBI. Initial results of several clinical trials of APBI have now been published. In this article, we will review the potential advantages of APBI, the biologic rationale for APBI, and the various methods of delivering APBI, each of their associated benefits and limitations, and a potential large randomized multidisciplinary trial evaluating APBI after breast conserving surgery for breast cancer.
POTENTIAL ADVANTAGES OF ACCELERATED PARTIAL BREAST IRRADIATION
Increase in Proportion of Women Undergoing Postlumpectomy Irradiation
All oncologists have had patients who declined breast-conserving surgery because receiving 6 weeks of radiation therapy would not be possible. Patients cite reasons such as inability to find a radiation facility close to home, inability to bear the cost of relocating for radiation treatment at a facility far from home, difficulty finding transportation to and from a radiation facility on a daily basis, and extreme elderly age or physical handicap. APBI may allow some patients with barriers to standard treatment to receive appropriate adjuvant radiation therapy.
Malin et al1 recently reviewed the question of what proportion of patients do not receive radiation therapy after breast-conserving surgery. In their extensive review, they found that reported rates of radiation therapy after breast-conserving surgery vary greatly. In the late 1980s, data from the Surveillance, Epidemiology, and End Results registry showed that the rates of radiation use varied from region to region, with 60% of women in Iowa receiving radiation therapy after breast-conserving surgery, compared with 81% of women in Seattle. Overall, approximately 20% of women treated in the United States in the late 1990s still did not receive radiation therapy after breast-conserving surgery.1 The use of radiation therapy has also been shown to be age related2–4: elderly patients are much more likely than their younger counterparts not to receive radiation therapy after breast-conserving surgery. Because a significant minority of patients in the United States continue not to receive appropriate radiation therapy after conservative breast surgery, many health professionals believe that the use of APBI may increase rates of utilization of radiation therapy as a standard component of breast-conserving therapy for carcinoma.
Theoretical Potential for Breast Preservation After Local Recurrence
At present, the standard of care following in-breast local recurrences in women who underwent whole-breast irradiation is mastectomy. Because APBI involves irradiating only a portion of the breast, in-breast local recurrences after APBI could in theory potentially be treated with additional conservative surgery and radiation therapy. This approach might be most appropriate for women with recurrences other than at the lumpectomy site and having a larger amount of breast tissue. Theoretically, patients could receive either additional brachytherapy at the site of the recurrence or standard whole-breast irradiation. However, this concept would need to be tested in a well-thought-out prospective study.
Biologic Rationale for Accelerated Partial Breast Irradiation
There is no controversy concerning the need for adequate local therapy for women with breast cancer—it is common knowledge that inadequate local therapy can lead to local failure. There remains controversy, however, regarding the relationship between inadequate local therapy and the development of distant metastases and the subsequent death of women following a local recurrence. This controversy, which persists despite the fact that 11 published randomized trials comparing conservative surgery alone with conservative surgery plus whole-breast irradiation have failed to show a survival disadvantage for surgery alone, is the basis for concern among breast cancer clinical investigators who are skeptical of the widespread adoption of APBI without large-scale evidence that such an intervention does not adversely affect overall survival by increasing local failure rates.
Location of Local Recurrences After Breast Conservation
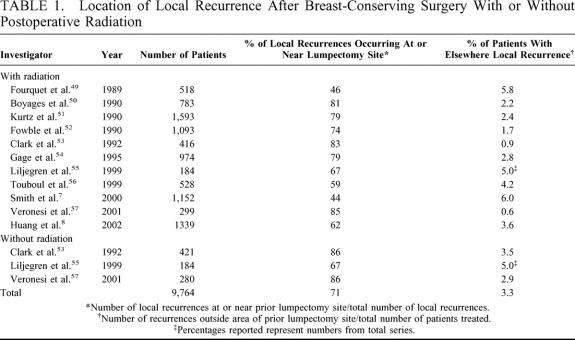
The purpose of breast irradiation after conservative breast surgery is to prevent local recurrence. The volume of breast irradiated is based on the theoretical volume of breast at risk for local recurrence. In general, the entire breast is considered at risk, and therefore the entire breast is included in the treatment volume. But where do local recurrences actually occur in the breast? Whether an in-breast recurrence is a true local recurrence or an “elsewhere” recurrence is sometimes difficult to determine. However, in general, the majority of in-breast local recurrences occur in the region of the prior lumpectomy site whether the patient undergoes postoperative breast irradiation or not. Table 1 lists reported rates of local recurrence in relation to the previous lumpectomy site from selected large studies of breast-conserving surgery with and without postoperative irradiation with mean follow-up time greater than 5 years. There are a couple of ways to look at the data. One is to look at the percentage of local recurrences that occur in the immediate vicinity of the lumpectomy site. This percentage ranged from 44% to 86%, and the mean percentage among the series, which together comprised nearly 10,000 patients, was about 71%. An update of the National Surgical Adjuvant Breast and Bowel Project B-06 trial, in which patients were treated with lumpectomy with or without radiation therapy with follow-up on 1039 patients, demonstrated that 75% of local recurrences occurred at or near the lumpectomy site.5 Another useful way of examining the data is to look at the percentage of patients treated with breast-conserving surgery who had elsewhere recurrences. On average, elsewhere recurrences occurred in approximately 3.3% of patients (range, 0.6% to 5.8%). Looking at the data in these terms suggests that treatment of the immediate vicinity of the lumpectomy site could theoretically result in inadequate local therapy in a maximum of about 3.3% of patients. It might be possible for these patients to have their recurrence treated with a second breast-conserving surgery and partial breast irradiation of a different region of the breast or even whole-breast irradiation. Interestingly, several investigators have found that elsewhere recurrences are associated with a better prognosis than are index breast carcinomas.6–8 Because the rate of development of breast tumors outside the area of the initial primary tumor—the so-called ipsilateral second primary breast cancer tumors—is similar to the rate of development of contralateral breast cancer whether a patient received whole-breast irradiation or not, it is unlikely that irradiation of the whole breast after breast-conserving surgery prevents such occurrences.
TABLE 1. Location of Local Recurrence After Breast-Conserving Surgery With or Without Postoperative Radiation
Where are local recurrences likely to occur in patients with ductal carcinoma in situ (DCIS)? The pattern is almost identical to that seen with recurrences after breast-conserving surgery for invasive breast cancer; approximately 3% of patients have elsewhere recurrences, and about 75% to 80% of all local recurrences occur within the original operative site with or without radiation therapy.9–12
Incidence of Simultaneous Occurrence of Carcinoma Within the Breast Away From the Primary Tumor Location
What is the actual incidence of multifocality and multicentricity associated with invasive breast carcinomas? Put another way, if only a portion of the breast is irradiated, what is the theoretical risk of leaving disease untreated? Answers to these questions come from carefully performed histologic studies of the distribution of cancer in the breast.
One key reference on this topic was published in 1985 by Holland et al13 In this classic study, 282 mastectomy specimens from women with localized T1 and T2 tumors were studied with a combination of radiologic and pathologic, techniques and the tumor distribution was mapped in relationship to the index tumor.13 The authors found that when the index tumor was 2 cm or smaller in largest diameter, the likelihood of finding a focus of residual in situ or invasive carcinoma more than 2 cm from the primary tumor was about 28%.13 Does this mean that 28% of patients treated with partial breast irradiation to 2 cm from the lumpectomy cavity are at risk for in-breast failure? Possibly, however, the clinical data from the initial studies of partial breast irradiation (see below) do not support this. Additionally, as almost all of these tumors were detected only by physical examination and were in the range of 3 to 4 cm, many clinical investigators suggest that the data from the Holland et al study are not applicable to today's smaller, mammographically detected tumors. Furthermore, Holland et al found that primary tumors larger than 2 cm were just as likely as much smaller tumors to be associated with residual carcinoma distant from the primary tumor. Later, the same investigators showed that the likelihood of finding residual tumor distant from the index invasive primary tumor was higher in the case of primary tumors with an extensive intraductal component (EIC).14 Forty-four percent of patients with an EIC had prominent residual intraductal carcinoma, compared with only 3% of patients without an EIC.14 Investigators from Japan also showed that women less than 40 years of age are more likely to have DCIS extending further from the index invasive carcinoma than are women older than 40 years.15
However, it is critical to note that these findings do not mean that patients with DCIS are more likely than patients with invasive breast cancer to have multicentric or multifocal disease. This is a common misconception not supported by evidence. Faverly et al16 investigated the concept of multifocality in patients with pure DCIS treated with mastectomy and found that only 8% of cases of DCIS were associated with a multifocal distribution with gaps greater than 1 cm.
Using similar histologic review and simulated breast-conserving surgery on mastectomy specimens, Faverly and colleagues recently identified a group of patients who would be very unlikely to have residual tumor foci greater than 2 cm from the index tumor.17 These investigators found that among women with a radiographic absence of calcifications or tumor density beyond the edge of the index tumor and a 1 cm microscopically tumor−free margin, only 11% of patients will have residual carcinoma greater than 2 cm from the primary tumor.
Taken together, these important studies suggest that patients older than 40 years of age with microscopically tumor-free margins of greater than 1 cm without invasive lobular carcinoma or an EIC as well as patients with pure DCIS lesions, may be the optimal candidates for APBI.
Equivalence of Accelerated-Fractionation and Standard-Fractionation Postlumpectomy Irradiation
In 2002, Canadian investigators reported the results a large randomized trial in which 1234 women were randomly assigned to receive whole-breast irradiation given over approximately 3 weeks or whole-breast irradiation given over the more traditional 5 to 6 weeks after lumpectomy (Fig. 1). 18 At a median follow-up of nearly 6 years, local control and cosmetic outcome were equivalent in the 2 groups. Reducing the period of radiation delivery to 3 weeks is more convenient for patients and less resource intensive. In fact, a 3-week fractionation schedule is becoming the standard for postlumpectomy radiation therapy in Canada. This concept also has a radiobiological basis: results from numerous clinical trials indicate that a larger dose per fraction, with treatment given over a briefer period, should be just as effective a lower dose per fraction with treatment given over a longer period.18,19 Simply stated, accelerated irradiation is radiation given over a shorter time period. The ultimate acceleration would therefore be a single-fraction, given only one time. Partial breast irradiation by definition means that the radiation dose is specifically targeted to include only a portion of the breast as opposed to the more or less standard of ‘whole-breast irradiation.’ It is generally agreed that by increasing the dose per fraction and giving a high enough total dose, APBI can be as effective as longer radiation schedules in eradicating microscopic residual tumor; however, toxicity in the form of excessive fibrosis and edema remains a concern.20
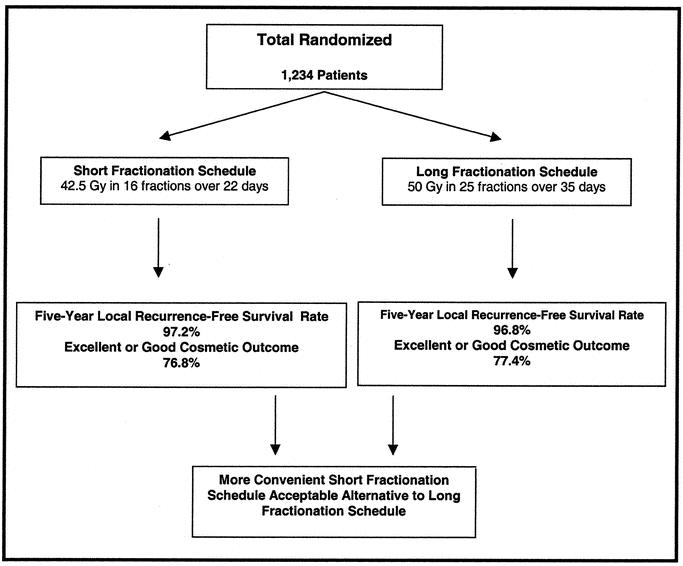
FIGURE 1. Canadian randomized trial of accelerated-fractionation versus traditional whole breast irradiation after lumpectomy for women with lymph node-negative breast cancer.18
METHODS OF DELIVERING PARTIAL BREAST IRRADIATION AND ASSOCIATED OUTCOMES
Partial breast irradiation can be delivered using brachytherapy, using hypofractionated conformal radiation therapy, or intraoperatively with either a linear accelerator or electrons. Each of these techniques has their own set of potential benefits and limitations. There is a growing literature evaluating the use of brachytherapy as the sole type of radiation therapy following breast-conserving surgery. At the present time, limited efficacy data are currently known for trials evaluating interstitial breast brachytherapy.
Brachytherapy
In the 1920s, the English surgeon Sir Geoffrey Keynes used interstitial radium needles to treat not only primary breast tumors but also the regional lymphatics.21 The use of brachytherapy—the placement of radioactive sources within (interstitial brachytherapy) or very close to the tumor bed (intracavitary brachytherapy)—has the main potential benefit of limiting toxicity to healthy tissue while delivering the maximum dose to the tissue at risk for disease. This potential benefit is the result of the inverse square law, which states that radiation intensity decreases as the inverse square of the distance from the point source of the radiation.
Generally, the radiation source is added to multiple catheters surrounding the tumor bed by automated afterloading technology that minimizes health care provider's potential radiation exposure. The optimal radiation dose and dose rate to maximize therapeutic effectiveness and minimize complications have yet to be determined. Brachytherapy can be delivered with either low-dose-rate (LDR) or high-dose-rate (HDR) radiation sources. With LDR techniques, a dose of 45 to 50 Gy is delivered to the clinical target volume at a rate of about 30 to 70 cGy/h. This technique requires that the patient be admitted to the hospital for about 4 days. HDR techniques are now much more common. With HDR brachytherapy, which is delivered on an outpatient basis, a total dose of 34 Gy is delivered in twice-daily fractions of 3.4 Gy over a total of 5 days.
Since the early studies of brachytherapy for breast cancer, the use of brachytherapy for boost treatment followed by standard whole-breast irradiation has been established as an effective strategy for achieving local control.22 However, the use of brachytherapy for boost treatment has largely been abandoned in the United States as most patients now receive a radiation boost with electron beams.23
At this time, the use of brachytherapy as the sole type of radiation therapy after breast-conserving surgery remains investigational. However, the use of brachytherapy has several theoretical potential advantages for patients. One very appealing potential advantage is reduction in treatment time. With brachytherapy, delivery of radiation therapy to the breast is generally completed over a period of 4 to 5 days, much shorter than the standard treatment of 5 to 6 weeks. The use of brachytherapy can allow all local therapy to be completed prior to the initiation of systemic therapy. This may be critical with regard to local control because it is not uncommon for women in the United States to receive 4 to 6 months of chemotherapy prior to definitive radiation therapy to the breast. At this point in time, it has also been suggested that there is a potential cost advantage in favor of brachytherapy compared with standard whole-breast irradiation.
Standard Brachytherapy
Table 2 lists the results of published studies of brachytherapy as the sole mode of radiation therapy after breast-conserving surgery for selected patients with breast cancer. One of the first and most often cited studies evaluating breast brachytherapy for unselected patients with breast cancer was the study by Fentimen et al25 performed at Guy's Hospital in London. At first glance, the report of this study, titled “Inadequacy of Iridium Implant as Sole Radiation Treatment of Operable Breast Cancer,” seems to suggest that the concept of partial breast irradiation with brachytherapy is doomed: the local recurrence rate was 37% at 6 years. However, one must evaluate the study more carefully before drawing conclusions. A total of 27 patients were treated in this series with “tumorectomy without any attempt to achieve wide excision either grossly or microscopically.”25 In fact, 56% of the patients had involved margins of resection. Tumorectomy was followed by LDR interstitial brachytherapy with iridium for a total dose of 55 Gy over 5 days. Although the lumpectomy bed plus a 2-cm margin was treated with radiation, no firm conclusions can be drawn with respect to the efficacy of this technique given the inadequacy of margin control. Two interesting reported findings from this study were that 90% of the local recurrences were within the same quadrant as the primary tumor that and no patient developed was observed to develop breast fibrosis at the dosage used.25
TABLE 2. Published Reports on Brachytherapy as the Sole Modality of Radiation Therapy After Conservative Surgery for Breast Cancer
In a study from the Ochsner Clinic,26 investigators treated 50 patients with DCIS or invasive carcinomas 4 cm, or smaller who underwent breast-conserving surgery with afterloading catheters for LDR and HDR brachytherapy designed to treat the lumpectomy cavity plus a 2 to 3 cm margin around it. In contrast to the patients in the Guy's Hospital study, all patients in the Ochsner Clinic study had negative margins of resection. The Ochsner group reported one patient with a local recurrence (2%) and 3 patients with nodal recurrences (6%) at a median follow-up of 75 months. Grade I and II side effects, which included skin erythema, moist desquamation, telangiectasia, pain, and fibrosis, occurred in 22% of the patients, and grade III complications requiring surgical intervention occurred in 8% of the patients. Among a similar group of patients treated with external-beam radiation therapy, grade I and II complications were more common and the rate of grade III complications was similar to that seen with brachytherapy. However, 2 patients receiving brachytherapy developed severe fat necrosis that necessitated extensive surgery. In a similar study from Ontario, Perera et al found a 13% rate of fat necrosis after brachytherapy, with 4 patients requiring surgical excision with a median follow-up time of only 1.7 years.27 These authors suggest that fat necrosis following brachytherapy may be detected more easily in patients who have only a portion of the breast irradiated than in patients who undergo whole-breast irradiation.
In a multicenter study reported by Wazer et al,28 at a median follow-up time of 24 months, fat necrosis was found in 8 (27%) of 30 patients treated with high-activity brachycatheters (iridium 192; 3 to 10 Ci) in 2 fractions per day for 5 consecutive days to a total dose of 34 Gy. However it should also be noted that these investigators reported complete resolution of a palpable mass associated with fat necrosis in 5 (63%) of these 8 patients and a documented decrease in the size of the involved area in the remaining 3 patients who developed fat necrosis. To decrease the rate of fat necrosis, Wazer et al now stratify patients into 3 groups on the basis of target volume—150 mL or less, 151 to 200 mL, and greater than 200 mL—and treat these volumes to a total dose of 34 Gy, 32 Gy, and 30 Gy, respectively. Wazer et al also noted in their conclusions that further investigation was needed to optimally define treatment parameters (dosimetric and volume effects) to ensure maximal local control and minimal complications. In an update of their study at a median follow-up of 2.8 years, there has been only one ipsilateral recurrence which appeared to be a new primary breast tumor 9 cm from the initial primary tumor.30
In a study with strict entry criteria, including tumor size less than 2 cm, negative microscopic margins, no axillary disease, and age greater than 60 years, investigators at the University of Kansas Medical Center found no local recurrences at a median follow-up of 47 months in patients receiving APBI.31,32 In this study, 24 patients were treated with LDR brachytherapy to a total dose of 20 to 25 Gy given over 24 to 48 hours. The cosmetic appearances after treatment ranged from very good to excellent, and no long-term complications were reported.
Investigators from the National Institute of Oncology in Hungary have recently reported their phase I-II study of 45 patients treated with brachytherapy as the sole mode of radiation therapy after breast-conserving surgery and the early results of a randomized study comparing brachytherapy (46 patients treated) with standard whole-breast irradiation.33 Patients were eligible for these studies if they had invasive tumors less than 2 cm, negative microscopic margins, and no axillary disease. At a median follow-up of 57 months, among the 45 patients treated in the phase I-II study with a margin of 2 cm using HDR brachytherapy catheters (total dose 30 to 36 Gy in 7 fractions), there were 2 local recurrences (4.4%) and 3 axillary recurrences (6.3%). At a median follow-up of 30 months, no local or regional recurrences were reported in the patients treated in the HDR arm (total dose 36 Gy in 7 fractions) of the phase III trial. Furthermore, there was no significant difference between the 2 treatment arms with respect to radiation-related side effects.33
In the largest reported study of interstitial brachytherapy as the sole modality of radiation therapy for invasive breast cancer, Vicini et al from William Beaumont Hospital reported the 3-year results in 174 patients treated over 4 to 5 days with both LDR brachytherapy with 50 Gy and HDR brachytherapy with 32 Gy to the lumpectomy cavity plus a margin of 1 to 2 cms.34 No local recurrences were reported, and the cosmetic results were judged to be excellent or good in 90% of the patients. Although much more mature follow-up will be needed before definitive conclusions can be drawn, differences in disease-free and overall survival between patients treated with brachytherapy and appropriately matched control patients treated with whole-breast irradiation were not statistically significant.
In 1997, the Radiation Therapy Oncology Group began accrual on protocol 95–17, a multi-institutional phase I-II trial designed to evaluate LDR and HDR brachytherapy for stage I and II breast cancer. Eligibility requirements for this study were stringent: patients had to have tumors less than 3 cm, no EIC, negative microscopic margins of resection, a finding of no residual microcalcifications on mammography performed after lumpectomy, and no more than 3 lymph nodes with metastatic carcinoma, none with extracapsular extension. At closure of the protocol in March 2000, 66 patients had been treated with 34 Gy delivered in 10 fractions, and 33 patients had received of 45 Gy using LDR brachytherapy.35 Planned end points to be evaluated include target volume coverage, cosmetic results, wound complication rates, ipsilateral breast cancer recurrence, and costs and benefits compared with standard external beam whole-breast treatment.
In summary, although the number of published reports on the use of brachytherapy as the sole type of radiation therapy after conservative surgery for breast cancer is small (approximately 450 patients) and although follow-up is limited, the results of the Vicini et al prospective study as well as the studies outlined in Table 2 would support a large North American phase III study comparing brachytherapy with whole-breast irradiation.
Balloon Intracavitary Brachytherapy
Potential disadvantages of standard catheter-based interstitial brachytherapy include the technical difficulty of learning to place the catheters correctly and the limited number of clinicians in the United States who routinely use this method. In addition, many patients and health care providers find the appearance of traditional brachytherapy catheters disturbing: when patients and their health care providers see photographs of the 10 to 25 brachytherapy catheters that are traditionally placed, they often describe them as appearing “barbaric” and “very painful looking.” This impression of brachytherapy catheters might have limited their more universal use in the United States.
Recently, there has been extreme great interest on the part of physicians and patients alike in the technique of balloon intracavitary brachytherapy, which offers several potential advantages over traditional interstitial brachytherapy for patients with breast cancer. Balloon intracavitary brachytherapy after lumpectomy can be given with a new breast brachytherapy applicator called the MammoSite (MammoSite RTS: Proxima Therapeutics, Alpharetta, GA).36 The MammoSite device consists of a HDR source at the center of an inflatable balloon that can be placed into the lumpectomy cavity at the time of surgery or after surgery when the definitive margin status is known (Fig. 2). The current device looks similar to a Foley catheter, is 15 cm in length and approximately 6 mm in diameter, and is designed to be inflated to a diameter of 4 to 5 cm with a volume of 30 to 70 mL of normal saline with contrast agent for radiographic imaging of optimal positioning. The MammoSite device is pliable and can be worn within a bra making it potentially more appealing to both patients and clinicians than traditional brachytherapy applicators. The MammoSite device has an inflation channel and a central treatment channel that connects to a computerized afterloading device for delivery of the HDR radiation source. An initial experience with 12 patients enrolled at the William Beaumont Hospital as part of the manufacturer's sponsored multi-institutional device safety trial was recently reported.36 The authors reported that reproducible placement of the device was easily achieved. Compared with standard interstitial brachytherapy, the mean dose homogeneity index was less uniform (0.77 vs. 0.93), but coverage of the planning target volume was improved (90% vs. 69.8%).36 The authors noted that the balloon conforms to the lumpectomy cavity and that the dose prescribed is to 1 cm from the balloon. If the distance is less than 1 cm, the skin will receive greater than the prescribed dose, and skin injury can result. The authors also suggested that the breast parenchyma could be used to close the cavity with sutures to ensure a sufficient distance of the catheter from the skin, although the cosmetic effect of this maneuver may be suboptimal. Furthermore, although the prescribed dose is to 1 cm these investigators believe that the effective thickness of treatment may be closer to 2 cm because as the balloon is inflated beyond the volume of the lumpectomy cavity the surrounding tissue is circumferentially stretched with resulting thinning of the surrounding tissue.
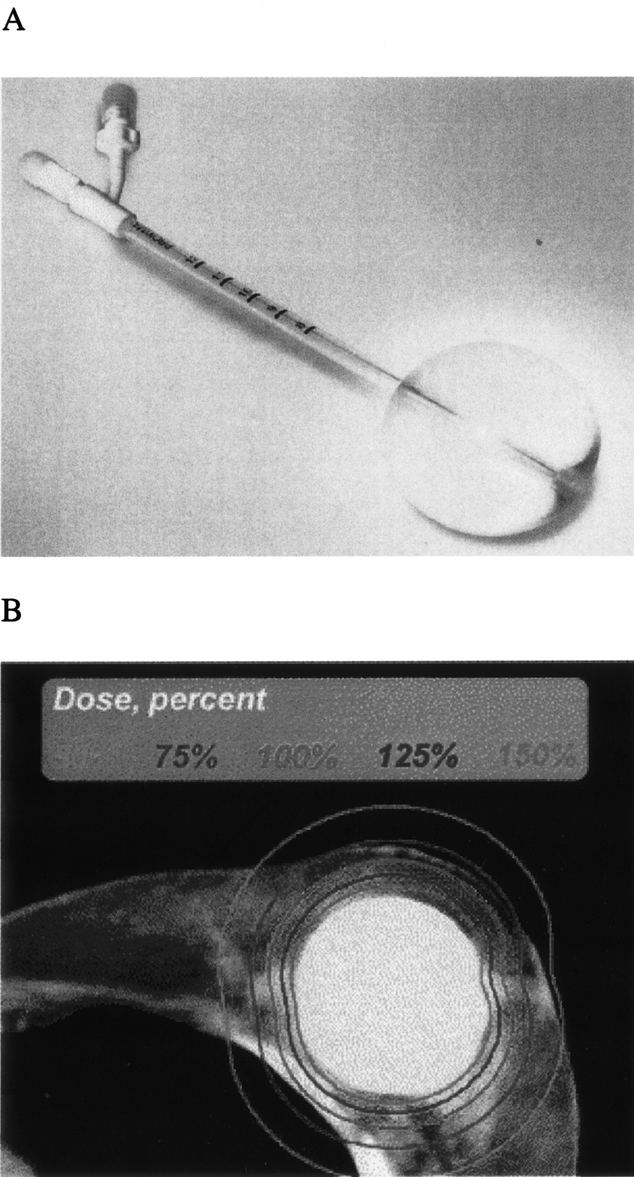
FIGURE 2. MammoSite applicator (A) and computed tomography scan of device within breast showing the dose distribution to the surrounding tissue (B; from Edmundson et al36; reprinted with permission).
The current MammoSite device has limitations with respect to conformance to the size and shape of the segmental mastectomy cavity. The current device is spherical and has a capacity volume of approximately 70 mL. It is very common for the shape of the lumpectomy cavity to be oblong rather than spherical. Furthermore, what is interesting to note is that the tumor is rarely just at the center of the lumpectomy specimen, such that margins may be 3 or 4 cm on one side yet only a few millimeters on the opposite side. An optimal lumpectomy for MammoSite technology would be performed such that the excision removed sufficient healthy tissue concentrically around the tumor. This type of excision is more directed and is best achieved utilizing intraoperative sonography (for tumors with mass lesions) and immediate margin assessment.37,38 Concerns related to increasing the size of the balloon include a considerably increased amount of time needed for treatment and the dose delivered to the heart and lungs.36 Edmundson and colleagues suggest that MammoSite-based therapy can be effective only if the volume of tissue removed at lumpectomy is 50 mL or less.36 As the volume of tissue removed for lumpectomy at M. D. Anderson Cancer Center and many other centers is usually far above this volume (median, approximately 65 mL), it is of considerable concern that the current device may be useful for only a small proportion of patients. A great mistake by the surgical community would be to decrease the standard volume of resection to better conform to the MammoSite's volume limitations, as this might likely be a source of increased recurrence rates. The manufacturer of the device has recently developed larger catheters in a variety of sizes, and this may increase the number of patients theoretically eligible for MammoSite therapy.
The results of the initial clinical experience with the MammoSite breast brachytherapy device in women with invasive ductal carcinomas smaller than 2 cm was recently reported.35 Seventy patients were enrolled in this 8-center prospective trial evaluating safety and applicator performance following device placement by 14 different investigators (Fig. 3). Patient eligibility criteria were as follows: age greater than 45 years, no axillary nodal metastases, surgical resection specimen with negative margins, and a minimum skin-to-balloon surface distance of 5 mm. Conformance and skin spacing were assessed by computed tomography. A dose of 34 Gy was delivered in 10 fractions over 5 days with HDR brachytherapy prescribed to 1 cm from the balloon surface. Of the 70 patients enrolled, 16 (23%) did not have the MammoSite device implanted because of a cavity that was too large in 10 patients, final pathology findings in 4 patients, and inadequate skin spacing in 2 patients. Fifty-four patients had the MammoSite device placed, and 43 patients (80%) completed brachytherapy. The MammoSite device was removed because of inadequate conformance to the cavity in 7 patients, patient age in one patient, pathology findings in one patient, and skin spacing in 2 patients. Therefore, even with the very stringent entry criteria used, only 61% of patients enrolled in the study actually completed brachytherapy with the MammoSite catheter.
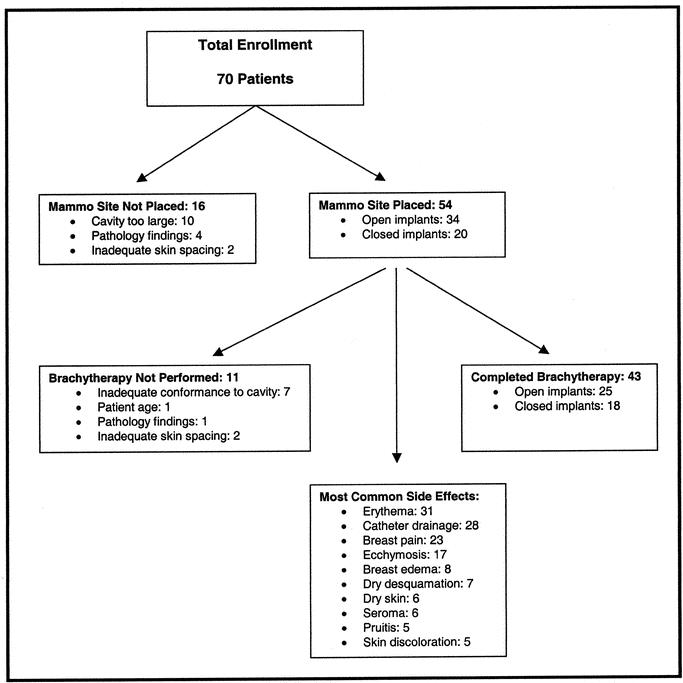
FIGURE 3. Initial performance and safety trial of MammoSite breast brachytherapy device in women treated with breast conserving surgery for Invasive Ductal Carcinoma less than 2 cm (From Keisch et al35; reprinted with permission).
As noted in Figure 3, the most commonly reported device and radiation-related side effects were erythema catheter-site drainage, breast pain, and ecchymosis. Catheter drainage is necessary in some patients to ensure that the balloon maintains contact with the lumpectomy cavity wall as a seroma is formed. With catheter drainage, gauze had to be placed around the catheter within the patient's bra. Most complications were self-limiting and resolved without intervention, but one patient developed an abscess that required drainage. No long-term follow-up data are available with respect to cosmesis or local control after treatment with the MammoSite brachytherapy catheter. The results of this study led the United States and Drug Administration to approve the catheter for clinical use and led the authors to conclude that the applicator provides a simpler method for performing reproducible breast brachytherapy. The indication for use of the MammoSite Catheter is to provide brachytherapy when the physician chooses to deliver intracavitary radiation therapy to the surgical margins following lumpectomy for breast cancer. However, it is critical that the surgical community understand that that the Food and Drug Administration also required a warning stating, “the safety and effectiveness of the MammoSite RTS as a replacement for whole breast irradiation in the treatment of breast cancer has not been established.” This statement has important ethical and legal considerations that at the minimum would need to be part of the informed consent process preceding treatment of patients with this new technique. The MammoSite brachytherapy device might actually prove to be more effective than standard whole-breast irradiation with respect to local control. However, this would need to be proven in a well-designed phase III study.
Intraoperative Radiation Therapy (IORT)
Delivery of a single dose of radiation at the time of surgery for definitive local treatment would be an extremely attractive alternative for patients undergoing lumpectomy for breast cancer. IORT has been used in patients with breast cancer to give an intraoperative boost dose of 9 Gy to the local tumor bed, with IORT followed by additional external-beam radiation therapy to the whole breast over 6 weeks.39 Investigators utilizing IORT for the boost dose state that directed application of the radiation dose can avoid the problem of a geographical miss and can ensure that treatment of the skin is avoided such that cosmesis may be improved. However, complications, primarily at the skin and the chest wall and ribs, are a major concern and limit the intraoperative dose. IORT can be delivered using a mobile linear accelerator or using a min-electron-beam low-energy x-ray source.
Linear Accelerator
Investigators at the European Institute of Oncology in Milan, Italy, have devised several methods for minimizing the toxicity of IORT and have recently performed a dose-escalation study of definitive IORT as the sole radiation treatment following conservative surgery for invasive breast cancer (Fig. 4). 40,41 To be eligible for the study, patients had to have invasive breast cancer 2.5 cm or less in larger diameter without an extensive intraductal component. Patients underwent resection of the primary tumor plus a 1-cm margin of normal tissue based on intraoperative evaluation. An aluminum-lead disk of an appropriate size (various sizes were available) was placed just above the pectoralis muscle to minimize thoracic radiation exposure, and the skin was retracted completely away from the radiation applicator using a novel retractor device to completely spare the skin from the radiation dose. Retraction was facilitated by dissection of the skin from the surrounding breast parenchyma circumferentially for 3 to 5 cm. Additionally, the deep aspect of the breast was mobilized above the pectoralis muscle for 5 to 10 cm around the tumor bed. IORT was delivered using a mobile dedicated linear accelerator device. Doses up to 21 Gy were delivered without major acute side effects. The total dose of 21 Gy is approximately biologically equivalent to 60 Gy given over 5 weeks and was chosen for all subsequent IORT studies. Although late side effects were not assessed because median follow-up was limited to 8 months, only 10 patients of the 84 patients who received a dose of 17 to 21 Gy had experienced mild or moderate side effects at the time of the investigators’ report, including hematoma (3 patients), mild breast pain (2 patients), infection (2 patients), and transitory edema (3 patients). To investigate whether external radiation therapy can be safely replaced by IORT in breast cancers no greater than 2.5 cm in diameter, an ongoing randomized trial at the European Institute of Oncology is comparing IORT at a total dose of 21 Gy (prescribed at the 90% isodose) with the standard external radiation therapy regimen of 50 Gy to the whole breast and 10 Gy as a boost to the tumor bed. The recruitment phase for this study is expected to finish in 3 years.
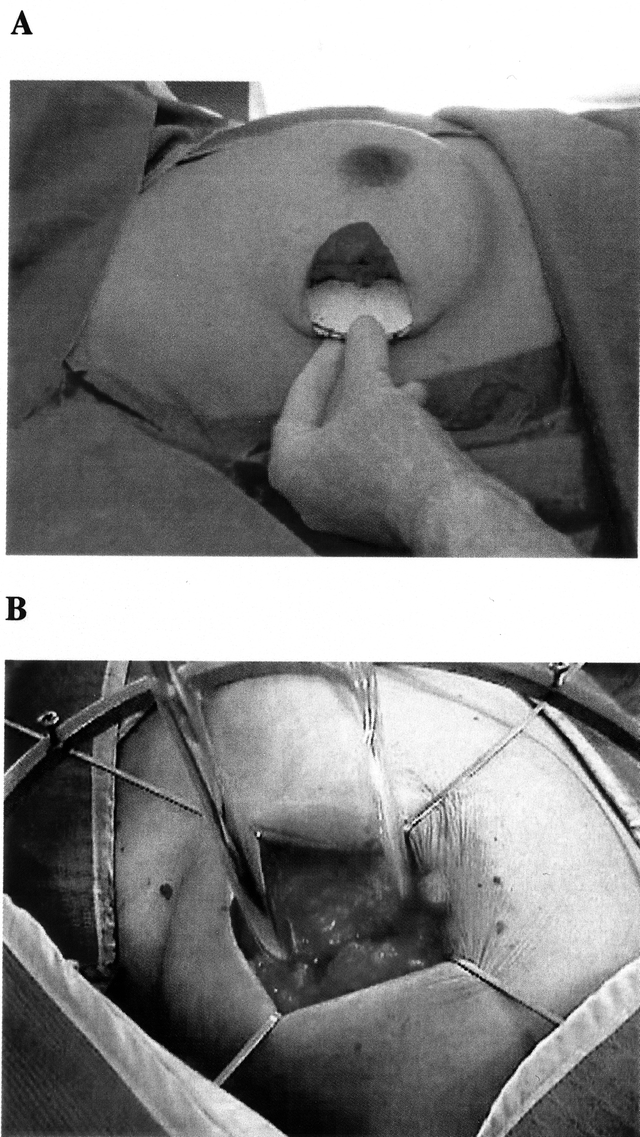
FIGURE 4. Intraoperative radiation therapy for breast cancer using a linear accelerator at the Instituto Europe di Oncologia in Milan, Italy. A, To minimize radiation to the thoracic wall, an aluminum-lead disk is placed between the deep fascia of the breast and the pectoralis muscle prior to start of radiation therapy. B, Placement of the linear accelerator applicator with complete retraction of the skin to prevent radiation injury. Photos from Veronesi et al41; reprinted with permission.
Low-Energy X-rays
Currently, investigators at University College Hospitals in the United Kingdom are evaluating definitive IORT with low-energy x-rays as the sole form of radiation therapy after segmental mastectomy. With this approach, IORT is delivered utilizing a mini-electron-beam-driven x-ray source called INTRABEAM (PeC) (Fig. 5). Low energy x-rays (50 kV maximum) are emitted from the tip of a 10-cm long, to 3.2-mm diameter probe that is enclosed in a spherical applicator (available in sizes ranging from 2.5 to 5 cm in diameter) that in turn is inserted into the tumor bed. IORT is delivered in about 25 minutes.42 The prescribed doses at 1 cm and 0.2 cm, respectively, are 5 Gy and 20 Gy. Vaidya and colleagues conceived of the partial breast irradiation project in 1996 and in March 2000 began a randomized trial of IORT utilizing their mini-electron-beam source verses standard whole-breast irradiation following lumpectomy for women with operable invasive breast cancer (T1-3, N0-1, M0). Patients with an EIC, invasive lobular subtype, or positive histologic margins are either excluded or given additional external-beam radiation therapy to the whole breast. Patients in this study have tungsten impregnated rubber sheets placed on the chest wall to protect the heart and lungs and placed over the wound to stop stray radiation, and the skin dose is monitored with thermoluminescent detectors. Several other centers in Europe, Australia, and the United States are now collaborating to accrue patients on this trial, which will need to enroll approximately 1000 patients over the next 3 to 4 years, in to achieve the power to prove equivalence.
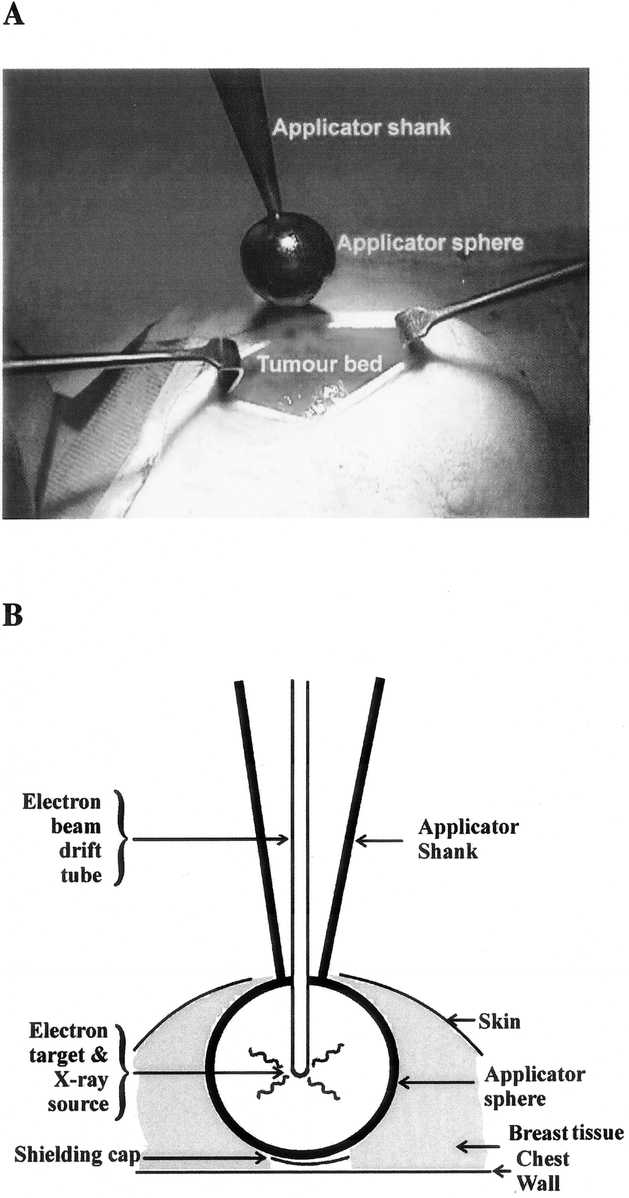
FIGURE 5. Targeted intraoperative radiation therapy at University College London. A, The applicator being placed in the tumor bed immediately after excision of the tumor. B, Photon-Radiosurgery System diagrammatic components. Photo courtesy of Dr Jayant Vaidya, University College London, United Kingdom.
The use of the low-energy x-ray source has several advantages as well as potential disadvantages related to dose attenuation. Because the biologic effective dose attenuates so rapidly, the specially designed operating rooms required in the case of radiation treatment with linear accelerators are not needed. However, the device has been criticized because the dose at 1 cm from the margin is only 5 Gy, a dose that may be ineffective in eradicating occult carcinoma. It is estimated that an effective dose can only be achieved up to 2 to 3 mm from the x-ray source. Many investigators are therefore concerned that many patients might not receive adequate local therapy with this technique.
Three-dimensional Conformal External-Beam Radiation Therapy
Formenti and colleagues have studied three-dimensional (3D) conformal partial breast irradiation using external-beam radiation therapy with the patient imaged and treated on a dedicated computed tomography scanner (Fig. 6). 43 The planning target volume used by these investigators was the tumor bed plus a 1- to 2-cm margin defined at postlumpectomy computed tomography. Nine patients in this study received a total dose of 25 to 30 Gy in 5 fractions. At a median follow-up of 3 years, all patients had good-to-excellent cosmesis. The authors concluded that hypofractionated conformal breast irradiation is feasible and that further studies are warranted. Recently, the technological advance of intensity-modulated radiation therapy (IMRT) has also been explored as a potential method for delivering a more uniform and standardized radiation dose in patients treated with breast conserving surgery.44,45 IMRT makes unnecessary the usual reliance on flat radiation fields and replaces that simple paradigm with a variable-intensity pattern that is determined with the aid of a computerized optimization algorithm.46 The main goal of much IMRT and optimization work is the delivery of more conformal plans to the patient.46
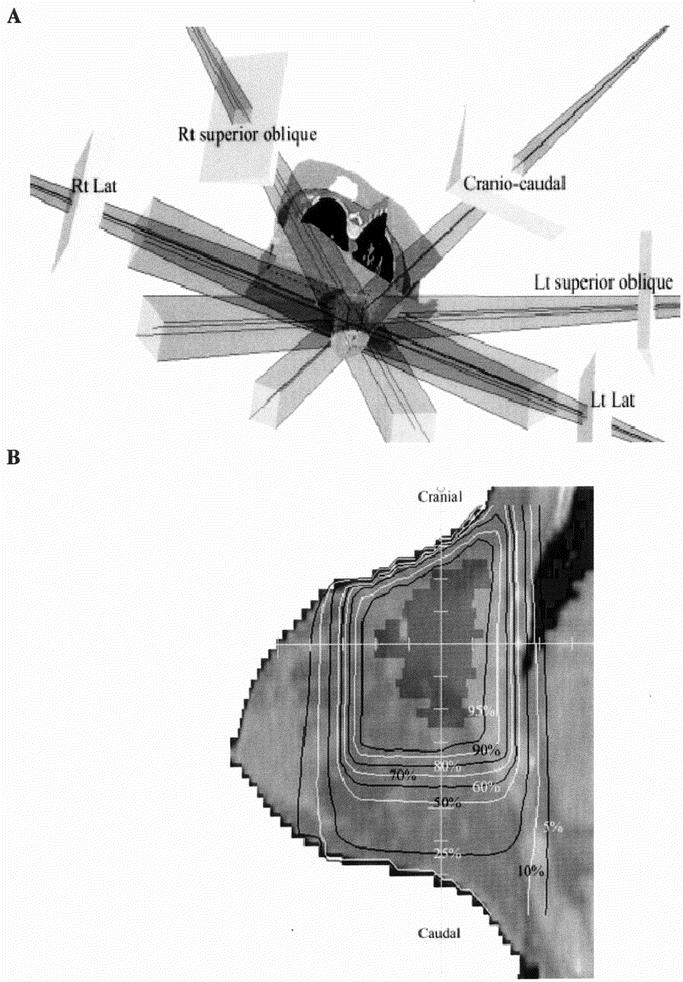
FIGURE 6. Three-dimensional Conformal Breast Irradiation. A, Reconstruction of radiation beams used in treatment of a patient in prone position on dedicated computed tomography table. B, Sagittal display of planning target volume and isodose distributions. Images courtesy of Formenti et al43; reprinted with permission.
One limitation of the developing 3D conformal external-beam radiation as a method for APBI is controlling movement associated with deep breathing.47 The advantage of using 3D conformal external-beam radiation therapy is that it is less invasive than brachytherapy and most radiation facilities in the United States already have the tools required for this method of radiation delivery. Furthermore, as the overwhelming majority of practicing radiation oncologists do not have experience with brachytherapy, APBI using external-beam therapy might be a more feasible alternative to brachytherapy techniques. However, more large-scale studies are needed to ensure that the 3D method will deliver a radiation dose adequate to ensure low breast recurrence rates.
Baglan et al recently reported on a novel 3D conformal radiation therapy technique to treat the lumpectomy cavity plus a 1.5-cm margin in patients with early-stage breast cancer.48 Nine patients in this pilot study were treated with a prescribed dose of 34 Gy (5 patients) or 38.5 Gy (4 patients) delivered in 10 fractions over 5 consecutive days. The impact of breathing motion on clinical target volume was studied. The authors found that 98% to 100% of the clinical target volume was covered by the 95% isodose surface at the extremes of inhalation and exhalation when a 5-mm additional breathing margin was added in the planning target volume. They concluded that APBI using 3D conformal radiation therapy is technically feasible with acute side effects being minimal and that additional studies are warranted to address long-term toxicity, cosmesis, and tumor control. A multi-institutional phase II study designed to address some of these factors is planned by the Radiation Therapy Oncology Group. One important aspect of all methods of delivering partial breast irradiation is determining what exactly represents the optimal treatment volume necessary to obtain similar or better local control rates compared with whole-breast irradiation following conservative surgery.
SUMMARY AND FUTURE DIRECTIONS
The gold standard for testing a new medical technique or procedure is a randomized clinical trial. Over the past 3 decades, a series of systematically performed randomized trials by the National Surgical Adjuvant Breast and Bowel Project and the European Institute of Oncology have provided unequivocal scientific evidence that survival after modified radical mastectomy is equivalent to survival after radical mastectomy and that breast-conserving surgery followed by whole-breast irradiation is equivalent to modified radical mastectomy. On average, approximately 3% of patients treated with breast-conserving surgery will have an in-breast local recurrence away from the original lumpectomy site whether or not they undergo postoperative standard whole-breast irradiation. Additionally, most local recurrences (75%) after whole-breast irradiation occur in or near the site of the original index noninvasive or invasive tumor. Given these findings, the use of APBI may be appropriate in patients with carcinoma of the breast.
The results of phase I-II studies involving approximately 500 patients treated with APBI after breast-conserving surgery have been published. Although many of the studies have limited long-term follow-up and potential selection bias, early results suggest that toxicity, cosmesis, and local control are comparable to outcomes with standard breast irradiation after breast-conserving surgery. APBI has several potential benefits for our patients. These include treatment duration of 4 to 5 days versus 6 weeks, less exposure of normal breast tissue, greater accessibility for elderly or frail patients, a more convenient schedule for working patients, the ability to deliver radiation before chemotherapy without a potential delay in local therapy, and possibly less cost. However, several questions regarding these therapies still need to be scientifically addressed, including optimal patient selection, appropriate treatment volumes, and long-term safety and efficacy.
Brachytherapy as the sole type of radiation therapy after breast-conserving surgery has generally not been widely adopted because of the special expertise required for catheter insertion by radiation oncologists and the perceived discomfort associated with catheter placement and keeping the catheters within the breast for 4 to 5 days. However, a new, much simpler, more comfortable brachytherapy device, the MammoSite has recently been approved by the Food and Drug Administration for delivering partial breast irradiation and may prove to be effective in reducing the rate of local recurrence after breast-conserving surgery.
Large randomized prospective trials of APBI, supported by multiple cooperative trials groups, are necessary to confirm the toxicity of APBI and the short- and long-term efficacy of this technique with respect to local recurrence, survival, cosmesis, and patient satisfaction. Towards this end, the National Surgical Adjuvant Breast and Bowel Project is actively considering a randomized phase III trial comparing whole-breast irradiation and APBI for stage 0, I, or II breast cancer. The primary hypothesis of this study is that local tumor control in the breast following APBI is similar to that with whole-breast irradiation. Secondary hypotheses would address cosmesis, quality of life, survival, and cost of treatments. Eligibility requirements include noninvasive or invasive adenocarcinoma of the breast, tumor size of 3 cm or less, treatment by lumpectomy, microscopic negative margins, and no more than 3 positive lymph nodes; patients of any age are eligible. APBI would consist of multicatheter brachytherapy, MammoSite brachytherapy, or 3D conformal radiation therapy delivered over 5 to 7 days to a total dose of 34 Gy. Randomization would occur after lumpectomy and nodal surgery (Fig. 7). For appropriate statistical power, approximately 3000 patients will be required. Training for participating centers and continuous case review and oversight will be performed to ensure limited variability in treatment and to provide quality assurance.
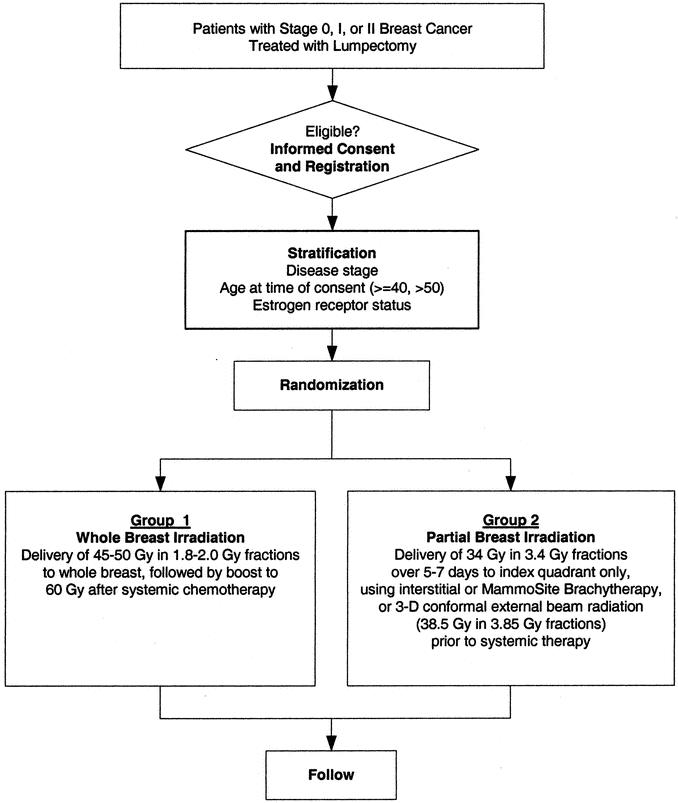
FIGURE 7. Proposed National Surgical Adjuvant Breast and Bowel Project Randomized Trial Evaluating Partial Breast Irradiation after Lumpectomy for Breast Cancer
To determine whether APBI is equivalent to whole-breast irradiation and to confirm the potential benefits of APBI, this well-controlled randomized trial is clearly needed at this time. To proceed without a phase III trial evaluating this new technology could lead to the loss of an extremely important opportunity to logically and scientifically advance the treatment of women with early-stage breast cancer.
Footnotes
Reprints: Henry M. Kuerer, MD, PhD, Department of Surgical Oncology, Box 444, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030. E-mail: hkuerer@mdanderson.org.
REFERENCES
- 1.Malin JL, Schuster MA, Kahn KA, et al. Quality of breast cancer care: what do we know? J Clin Oncol. 2002;20:4381–4393. [DOI] [PubMed] [Google Scholar]
- 2.Ballard-Barbash R, Potosky AL, Harlan LC, et al. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88:716–726. [DOI] [PubMed] [Google Scholar]
- 3.Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. 2001;19:2254–2262. [DOI] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Hadley J, Kerner JF, et al. Patterns of breast carcinoma treatment in older women: patient preference and clinical and physical influences. Cancer. 2000;89:561–573. [PubMed] [Google Scholar]
- 5.Fisher ER, Anderson S, Tan-Chiu E, et al. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001;91(8 Suppl):1679–1687. [PubMed] [Google Scholar]
- 6.Fortin A, Larochelle M, Laverdiere J, et al. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J Clin Oncol. 1999;17:101–109. [DOI] [PubMed] [Google Scholar]
- 7.Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–1289. [DOI] [PubMed] [Google Scholar]
- 8.Huang E, Buchholz TA, Meric F, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–2067. [DOI] [PubMed] [Google Scholar]
- 9.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86:429–438. [DOI] [PubMed] [Google Scholar]
- 10.Hetelekidis S, Collins L, Silver B, et al. Predictors of local recurrence following excision alone for ductal carcinoma in situ. Cancer. 1999;85:427–431. [PubMed] [Google Scholar]
- 11.Kestin LL, Goldstein NS, Martinez AA, et al. Mammographically detected ductal carcinoma in situ treated with conservative surgery with or without radiation therapy: patterns of failure and 10-year results. Ann Surg. 2000;231:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura S, Woo C, Silberman H, et al. Breast-conserving therapy for ductal carcinoma in situ: a 20-year experience with excision plus radiation therapy. Am J Surg. 2002;184:403–409. [DOI] [PubMed] [Google Scholar]
- 13.Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979–990. [DOI] [PubMed] [Google Scholar]
- 14.Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol. 1990;8:113–118. [DOI] [PubMed] [Google Scholar]
- 15.Imamura H, Haga S, Shimizu T, et al. Relationship between the morphological and biological characteristics of intraductal components accompanying invasive ductal breast carcinoma and patient age. Breast Cancer Res Treat. 2000;62:177–184. [DOI] [PubMed] [Google Scholar]
- 16.Faverly DR, Burgers L, Bult P, et al. Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol. 1994;11:193–198. [PubMed] [Google Scholar]
- 17.Faverly DR, Hendriks JH, Holland R. Breast carcinomas of limited extent: frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer. 2001;91:647–659. [DOI] [PubMed] [Google Scholar]
- 18.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. [DOI] [PubMed] [Google Scholar]
- 19.Baglan KL, Martinez AA, Frazier RC, et al. The use of high-dose-rate brachytherapy alone after lumpectomy in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2001;50:1003–1011. [DOI] [PubMed] [Google Scholar]
- 20.Sartor CI, Tepper JE. Is less more? Lessons in radiation schedules in breast cancer. J Natl Cancer Inst. 2002;94:1114–1115. [DOI] [PubMed] [Google Scholar]
- 21.Keynes G. The treatment of primary carcinoma of the breast with radium. Acta Radiol. 1929;10:393–402. [Google Scholar]
- 22.Mansfield CM, Komarnicky LT, Schwartz GF, et al. Perioperative implantation of iridium-192 as the boost technique for stage I and II breast cancer: results of a 10-year study of 655 patients. Radiology. 1994;192(1):33–36. [DOI] [PubMed] [Google Scholar]
- 23.Shank B, Moughan J, Owen J, et al. The 1993–94 patterns of care process survey for breast irradiation after breast-conserving surgery–comparison with the 1992 standard for breast conservation treatment. The Patterns of Care Study, American College of Radiology. Int J Radiat Oncol Biol Phys. 2000;48:1291–1299. [DOI] [PubMed] [Google Scholar]
- 24.Polgar C, Fodor J, Major T, et al. Radiotherapy confined to the tumor bed following breast conserving surgery current status, controversies, and future projects. Strahlenther Onkol. 2002;178:597–606. [DOI] [PubMed] [Google Scholar]
- 25.Fentiman IS, Poole C, Tong D, et al. Inadequacy of iridium implant as sole radiation treatment for operable breast cancer. Eur J Cancer. 1996;32A:608–611. [DOI] [PubMed] [Google Scholar]
- 26.King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is, 1, 2) breast cancer. Am J Surg. 2000;180:299–304. [DOI] [PubMed] [Google Scholar]
- 27.Perera F, Engel J, Holliday R, et al. Local resection and brachytherapy confined to the lumpectomy site for early breast cancer: a pilot study. J Surg Oncol. 1997;65:263–267; discussion 267–268. [DOI] [PubMed]
- 28.Wazer DE, Lowther D, Boyle T, et al. Clinically evident fat necrosis in women treated with high-dose-rate brachytherapy alone for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2001;50:107–111. [DOI] [PubMed] [Google Scholar]
- 29.Samuel LM, Dewar JA, Preece PE, et al. A pilot study of radical radiotherapy using a perioperative implant following wide local excision for carcinoma of the breast. Breast. 1999;8:95–97. [Google Scholar]
- 30.Wazer DE, Berle L, Graham R, et al. Preliminary results of a phase I/II study of HDR brachytherapy alone for T1/T2 breast cancer. Int J Radiat Oncol Biol Phys. 2002;53:889–897. [DOI] [PubMed] [Google Scholar]
- 31.Jewell WR, Krishnan L, Reddy EK, et al. Intraoperative implantation radiation therapy plus lumpectomy for carcinoma of the breast. Arch Surg. 1987;122:687–690. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan L, Jewell WR, Tawfik OW, et al. Breast conservation therapy with tumor bed irradiation alone in a selected group of patients with stage I breast cancer. Breast J. 2001;7:91–96. [DOI] [PubMed] [Google Scholar]
- 33.Polgar C, Sulyok Z, Fodor J, et al. Sole brachytherapy of the tumor bed after conservative surgery for T1 breast cancer: five-year results of a phase I-II study and initial findings of a randomized phase III trial. J Surg Oncol. 2002;80:121–128; discussion 129. [DOI] [PubMed]
- 34.Vicini FA, Baglan KL, Kestin LL, et al. Accelerated treatment of breast cancer. J Clin Oncol. 2001;19:1993–2001. [DOI] [PubMed] [Google Scholar]
- 35.Keisch M, Vicini V, Kuske RR, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;55:289–293. [DOI] [PubMed] [Google Scholar]
- 36.Edmundson GK, Vicini FA, Chen PY, et al. Dosimetric characteristics of the MammoSite RTS, a new breast brachytherapy applicator. Int J Radiat Oncol Biol Phys. 2002;52:1132–1139. [DOI] [PubMed] [Google Scholar]
- 37.Fornage BD, Sneige N, Edeiken BS. Interventional breast sonography. Eur J Radiol. 2002;42:17–31. [DOI] [PubMed] [Google Scholar]
- 38.Rahusen FD, Bremers AJ, Fabry HF, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: a randomized clinical trial. Ann Surg Oncol. 2002;9:994–998. [DOI] [PubMed] [Google Scholar]
- 39.Reitsamer R, Peintinger F, Sedlmayer F, et al. Intraoperative radiotherapy given as a boost after breast-conserving surgery in breast cancer patients. Eur J Cancer. 2002;38:1607–1610. [DOI] [PubMed] [Google Scholar]
- 40.Intra M, Gatti G, Luini A, et al. Surgical technique of intraoperative radiotherapy in conservative treatment of limited-stage breast cancer. Arch Surg. 2002;137:737–740. [DOI] [PubMed] [Google Scholar]
- 41.Veronesi U, Orecchia R, Luini A, et al. A preliminary report of intraoperative radiotherapy (IORT) in limited-stage breast cancers that are conservatively treated. Eur J Cancer. 2001;37:2178–2183. [DOI] [PubMed] [Google Scholar]
- 42.Vaidya JS, Baum M, Tobias JS, et al. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol. 2002;28:447–454. [DOI] [PubMed] [Google Scholar]
- 43.Formenti SC, Rosenstein B, Skinner KA, et al. T1 stage breast cancer: adjuvant hypofractionated conformal radiation therapy to tumor bed in selected postmenopausal breast cancer patients–pilot feasibility study. Radiology. 2002;222:171–178. [DOI] [PubMed] [Google Scholar]
- 44.Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336–1344. [DOI] [PubMed] [Google Scholar]
- 45.Strom EA. Breast IMRT: new tools leading to new vision. Int J Radiat Oncol Biol Phys. 2002;54:1297–1298. [DOI] [PubMed] [Google Scholar]
- 46.Krueger EA, Fraass BA, Pierce LJ. Clinical aspects of intensity-modulated radiotherapy in the treatment of breast cancer. Semin Radiat Oncol. 2002;12:250–259. [DOI] [PubMed] [Google Scholar]
- 47.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. [DOI] [PubMed] [Google Scholar]
- 48.Baglan KL, Sharpe MB, Jaffray D, et al. Accelerated partial breast irradiation using 3D conformal radiation therapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2003;55:302–311. [DOI] [PubMed] [Google Scholar]
- 49.Fourquet A, Campana F, Zafrani B, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys. 1989;17:719–725. [DOI] [PubMed] [Google Scholar]
- 50.Boyages J, Recht A, Connolly JL, et al. Early breast cancer: predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol. 1990;19:29–41. [DOI] [PubMed] [Google Scholar]
- 51.Kurtz JM, Spitalier JM, Amalric R, et al. The prognostic significance of late local recurrence after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 1990;18:87–93. [DOI] [PubMed] [Google Scholar]
- 52.Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990;19:833–842. [DOI] [PubMed] [Google Scholar]
- 53.Clark RM, McCulloch PB, Levine MN, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992;84:683–689. [DOI] [PubMed] [Google Scholar]
- 54.Gage I, Recht A, Gelman R, et al. Long-term outcome following breast-conserving surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33:245–251. [DOI] [PubMed] [Google Scholar]
- 55.Liljegren G, Holmberg L, Bergh J, et al. 10-year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–2333. [DOI] [PubMed] [Google Scholar]
- 56.Touboul E, Buffat L, Belkacemi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys. 1999;43:25–38. [DOI] [PubMed] [Google Scholar]
- 57.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. [DOI] [PubMed] [Google Scholar]