Abstract
Objectives:
To analyze the feasibility and safety of transhiatal approach for resection of corrosively scarred esophagus.
Background Summary Data:
The unrelenting corrosive strictures of esophagus merit esophageal substitution. Because of the risk of complications in the retained esophagus, such as malignancy, mucocele, gastroesophageal reflux, and bleeding, esophageal resection is deemed necessary. Transthoracic approach for esophageal resection is considered safe. The safety and feasibility of transhiatal resection of the esophagus is not established in corrosive injury of the esophagus.
Patients and Methods:
Transhiatal approach was used for resection of the scarred esophagus for all patients between January 1986 and December 2001. The intraoperative complications, indications for adding thoracotomy, and postoperative outcome were studied in 51 patients. Follow-up period varied from minimum of 6 months to 15 years.
Results:
Esophageal resection was achieved in 49 of 51 patients whereas thoracotomy was added in 2 patients. In 1 of the patients tracheal injury occurred whereas in other patient there were dense adhesions between tracheal membrane and esophagus. Gastric tube was used for esophageal substitution in 40 (78.4%) patients whereas colon was transplanted in 11 (21.6%) patients. Colon was used only when stomach was not available. One patient (1.9%) had tracheal membrane injury whereas 4 patients (7.8%) had recurrent laryngeal nerve palsy. One patient each had thoracic duct injury and intrathoracic gastric tube leak. There was no operative mortality. Anastomotic complications like leak were present in 19.6% and stricture in 58.8% patients. All the patients were able to resume their normal duties and swallow normal food within 6 months of the surgery.
Conclusion:
One-stage transhiatal esophageal resection and reconstruction could be safely used for the extirpation of scarred esophagus. Use of gastric conduit was technically simple, quicker, and offered good functional outcome. Postoperative anastomotic stricture amenable to dilatations was the commonest complication.
Transhiatal esophageal resection was safely used in 49 of 51 patients for corrosive injury without any mortality and low morbidity. Good functional outcome was achieved in all the patients.
Most of the patients of corrosive injury of esophagus that are referred to a surgeon in chronic stage have persistent dysphagia because of unrelenting esophageal strictures. These patients merit esophageal substitution for normal alimentation. However, the need for esophageal resection in these patients is debatable.1 The surgeons favoring resection prefer transthoracic approach because it allows the mobilization of esophagus under direct vision.2–5 Transhiatal approach did not gain popularity in this setting because it was considered a blind procedure and fraught with risks especially when dense periesophageal adhesions were expected.6,7
Since 1986, the senior author has practiced transhiatal approach without thoracotomy for extirpation of the diseased esophagus in both benign and malignant conditions.1,8 However, the safety and feasibility of this approach in corrosive injury of the esophagus is still not widely accepted.
In this retrospective review, we have analyzed our results of transhiatal resection of scarred esophagus with regard to its feasibility, complications and discussed some of the technical points that we believe can help avoid the procedure-related complications.
PATIENTS AND METHODS
In this retrospective consecutive review, all patients of corrosive injury of esophagus requiring esophageal substitution in an elective setting between January 1986 and December 2001 were included. Patients presenting to emergency services with esophageal perforation after instrumentation were excluded from this review. The patient details were retrieved from the hospital records.
All the patients were on endoscopic dilatation regimen. The patients presenting with absolute dysphagia or inadequate intake had feeding jejunostomy performed as the initial procedure for alimentation and build up. The preparation for surgery included correction of anemia, dehydration, treatment of respiratory tract infections, and chest physiotherapy. The Karnofsky performance status of at least 70 was achieved in all the patients with target preoperative serum albumin of 3.5 g%. All the patients with prolonged cough underwent screening for pulmonary tuberculosis before the symptoms were attributed to aspiration pneumonia.
Thin barium studies and esophagogastroduodenoscopy was performed whenever possible to assess the site of the lesion and status of the stomach. The colon was prepared in all the patients before surgery. Colonoscopy and barium enema was performed in those patients where use of stomach as conduit was considered doubtful.
Esophageal resection was performed in all the patients by transhiatal approach as described by Orringer and Sloan9 and the senior author8 previously. Gastric tube was used for esophageal substitution as the first choice. In cases of nonavailability of stomach, an isoperistaltic transverse and left colon was used based on ascending branch of left colic artery and inferior mesenteric vein.4,10,11 All esophageal substitutes were placed in the posterior mediastinum. By gentle dissection from abdominal and cervical wounds, an adequate tunnel for the esophageal substitute was ensured. Postoperatively integrity of cervical anastomosis was tested by diatrizoate meglumine and diatrizoate sodium 60% on 9th or 10th postoperative day. If there was no leak, the patient was asked to swallow saliva and started on soft solid feeds followed by liquids as deglutition reflex improved. The anastomotic complications were noted. The neck leaks were managed conservatively by open drainage and lately by early endoscopic balloon dilatation.12 The anastomotic strictures were dealt by weekly endoscopic dilatations. The information about gain in weight, postoperative dysphagia, and daily work routine was recorded from the follow-up visit records. Any death within 30 days of surgery was taken as operative mortality. The follow up period varied from 6 months to 15 years.
RESULTS
During the period of 16 years, 51 patients underwent elective transhiatal esophagectomy for corrosive injury of esophagus. Associated gastric injury was seen in 12 patients. Mean age of the patients was 26.5 ± 8.6 years (range, 14–48 years). There were 34 males and 17 female patients in the study. Most of the patients (83.4%) had history of acid ingestion while remaining had history of alkali ingestion. Majority of patients (58.8%) had grade IV dysphagia at the time of surgery. Grade II and III dysphagia was present in 5.9% and 35.3% of patients.
The interval between corrosive ingestion and surgery was less than 6 months in 10 (19.6%); 6 month to 12 months in 24 (47%); 1 to 2 years in 11 (21.6%); 3 to 5 years in 3 (5.9%); and more than 5 years in 1 patient (1.9%). The indication for surgery was failure of endoscopic dilatation or undilatable stricture. Three patients were diagnosed as suffering from pulmonary Koch's and were treated by antitubercular chemotherapy for a period of at least 3–4 months before surgery.
Site of Lesion
Hypopharynx and cervical esophagus was involved in 2 (3.9%) patients whereas isolated middle third and lower third esophageal injuries were seen in 7 (13.7%) and 3 (5.9%) patients, respectively. Nine patients (17.6%) had lesion involving predominantly upper and middle third of esophagus whereas 12 (23.5%) had predominantly middle and lower third esophageal involvement. Panesophageal involvement was seen in 18 patients (35.3%).
To build up the poor nutritional status before surgery, feeding jejunostomy was carried out in 34 (66.7%) patients. Three patients had tracheostomy tube placed at the time of initial injury because of laryngeal edema and respiratory distress. Associated gastric injury was present in 12 (23.5%) patients in the form of antral strictures whereas one patient had near total gastric scarring. Two of these patients responded to endoscopic balloon dilatation and gastric tube could be used in these patients. Two patients had undergone antrectomy 1- and 2-year before esophageal substitution.
Transhiatal esophageal resection was attempted in all the patients and conducted successfully in 49 patients (96.6%). In 2 patients right anterolateral thoracotomy was added. One patient had tracheal injury that was detected on the table, and second had densely adherent esophagus to the tracheal membrane, which required dissection under vision to avoid injury to the tracheal membrane. Gastric tube was used as esophageal substitute in 40 patients (78.4%) while colonic interposition was performed in 11 (21.6%) patients. Colonic transplant was used only when stomach was not found suitable. In 10 patients stomach was scarred and contracted while in one patient previous feeding gastrostomy (done at other hospital) had injured the right gastroepiploic artery.
Operative Complications
One patient (1.9%) had tracheal injury while 4 patients (7.8%) had transient recurrent laryngeal nerve palsy. No case of abnormal intrathoracic bleed was encountered in the present study. One patient had thoracic duct injury, which was ligated on 5th postoperative day. Another patient had intrathoracic leakage of gastric tube that was repaired by thoracotomy. Postoperatively 10 patients (19.6%) had cervical anastomotic leak which recovered on conservative management. Thirty patients (58.8%) had anastomotic site stricture requiring dilatation. There was statistically no significant difference in the anastomotic complications in patients with gastric or colonic transpositions (Table 1). The mean hospital stay was 16.5 ± 6.7 days (range, 18–37). There was no operative mortality in our series.
TABLE 1. Anastomotic Complications in Gastric and Colonic Conduits
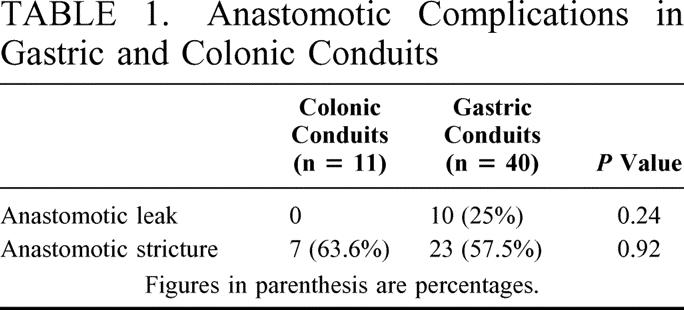
All the patients with anastomotic stricture recovered within 6 months after few sittings of endoscopic dilatation and were able to swallow solid food. Weight gain was noted in all the cases and all resumed normal daily routine postoperatively. There were 28 patients with more than 5-year follow up and 6 patients were more than 10 years postoperative. One of our patients with acid injury and gastric tube advancement had recurrence of dysphagia at 30 months after surgery. The esophagogram revealed a stricture in the midthoracic part, which responded to 5 sittings of endoscopic dilatation. The patient was symptom free at 52 months of follow up.
DISCUSSION
Although lye is the most frequently ingested caustic in the west, acid ingestion is a commoner cause of upper gastrointestinal tract injury in this part of the world. This is a result of the fact that concentrated sulfuric acid is readily available in the households as toilet cleaner and is much cheaper than alkaline toilet cleaners. Two previous reports published from our institute did not find any difference in the spectrum of esophageal or gastric injuries caused by acid or alkali ingestion.13,14 The acids ingestion led to esophageal injury in 87.8% patients in comparison to hundred percent in alkali ingestion. This finding was contrary to the general belief that esophagus is spared in acid ingestion. The reason attributed was the ingestion of large volume of concentrated sulfuric acid with suicidal intent.13
Furthermore, the endoscopic grade of the injury was also found to be similar in the acid versus alkali ingestion, ie, 39% versus 45.1% grade II esophageal injuries and 48.8% versus 54.8% grade III injuries.13,14 No difference was observed technically while performing esophageal resection in either type of corrosive injury in the present series.
Transhiatal esophageal resection is an accepted and established procedure for malignant lesions of the esophagus.8,9 Avoidance of thoracotomy, reduced surgical trauma and minimal need for postoperative ventilation make the procedure attractive as most of our patients are nutritionally depleted and have compromised pulmonary function.
Sweet15 introduced and popularized resection of entire esophagus for caustic strictures with reconstruction using the entire stomach. In the present series transhiatal resection of the scarred esophagus was carried out in 49/51 patients. We preferred gastric tube instead of whole stomach for esophageal substitutes. There was no mortality in the series and procedure related morbidity was low. The need for resection of the corrosively scarred esophagus is debatable. The risk of developing malignancy in a retained scarred esophagus after corrosive injury has been reported to be 2.4% that is around 1000 fold that of general population.16 Other authors have also reported a high risk of developing malignancy.17–20 However some of the workers believe that risk of damage to the membranous wall of the trachea and to the laryngeal nerves, an increased operative time and risk of postoperative bleeding outweigh any potential benefit of esophageal resection.21,22 In the study by Raffensperger et al22 none of the patients had any problem with the residual esophagus while Wu et al21 restricted esophagectomy only for severe gastric stricture and esophagorespiratory fistula. Orringer and Sloan9,23 also believed that small reported incidence of malignant transformation within caustic esophageal stricture were not great enough to warrant esophagectomy. However, the authors believed that gastroesophageal reflux into the bypassed strictured esophagus may be a potential source of gastrointestinal bleeding from esophagitis subsequently. Hence esophagectomy in such patients eliminated diseased organ. It has been observed that the risk of developing malignancy is considerable if follow up is long, as malignancy has been reported to develop several years after corrosive ingestion.3,7,17,18,20 In patients of corrosive injury who are young and otherwise expected to have normal life span, a lifelong surveillance would be required if such an esophagus is retained.10,16 Moreover malignancy has been reported to be in advanced stage at the time of detection and beyond the realms of cure.16
Retained and excluded esophagus has also been reported to develop mucocele in up to 50% of patients after 5 years. The mucocele may become infected and rupture at suture line or lead on to features of lung compression that may require thoracotomy and esophagectomy.11,24,25
The operative complications in the present series included tracheal membrane injury in 1 patient that was detected on the table and repaired by adding thoracotomy. Transient recurrent laryngeal nerve palsy occurred in 4 patients (7.8%). No patient had permanent recurrent laryngeal nerve paralysis or abnormal intrathoracic bleed. Our results compare well with other similar studies.6,7,26 Most of the complications in the present series occurred in the early part of the study.
Careful attention to the details of surgery will avoid these complications and some of the technical points merit emphasis here. The authors feel that dissection in mediastinum should begin posteriorly and then proceed laterally and anteriorly as has been emphasized earlier also.8,9 The risk of injury to thoracic duct and azygos vein is greater if the surgeon leaves the company of esophagus and strays posteriorly. One has to realize that unlike carcinoma, the periesophageal adhesions are usually present all along the length of esophagus in corrosive injury. Hence the progress in blunt esophageal dissection is often slow and requires greater patience on part of the surgeon. Greater care is necessary at the upper third of the esophagus especially anteriorly where esophagus abuts the tracheal membrane. The periesophageal lysis of the adhesions at this area should be performed using gentle force. The greater part of this dissection can be accomplished under vision through cervical incision. If in this area, the surgeon makes no substantial progress, authors recommend addition of right anterolateral thoracotomy and sharp dissection under vision. Use of excessive blunt force can lead on to tracheal membrane tear. This approach was adopted in 1 patient in the present series.
The commonest injury of recurrent laryngeal nerve is neuropraxia that is caused by traction on the nerve during medial retraction. By avoiding use of retractors for medial retraction and instead using gentle retraction with the finger can help prevent traction injury to the nerve.
The esophageal substitution in the present series was preferably done by gastric conduits whenever available. In case the stomach was not found suitable, transverse and left colonic conduits were used. Mere presence of antral stricture was not considered an indication for colonic transplant if the size of the stomach was normal. Two patients in the present series responded to endoscopic balloon dilatation and gastric tube could be used as esophageal substitute. We had discussed the advantages of gastric advancement in the previous communication.1 The excellent blood supply and thick wall of the stomach minimize the chance of graft necrosis. The stomach can be mobilized to reach the base of the tongue and its use warrants only one anastomosis. In comparison to colonic interposition, the extent of operative dissection and resultant physiologic trauma has also been reported to be less when preparing stomach for advancement.10,26 Moreover colonic interposition can be complicated by delayed gastric emptying or graft tortuosity and redundancy, which mandates revision surgery.10 Risk of colonic necrosis is also higher and procedure of colonic interposition is technically more demanding.4,10,11 It has been observed that colonic conduits did not offer any inherent advantage to outweigh the operative time, complexity, and technical demands of their preparation.
Lower incidence of neck anastomotic leaks has been reported with colonic tubes in comparison to gastric tubes.22,27–29 Similar findings were observed in the present study also. However rate of anastomotic stricture formation was similar with both the conduits in the present series (Table 1). In a recent communication,6 cervical anastomotic leaks and strictures after transhiatal esophageal resection and gastric advancement were reported as 20% and 30% respectively. Ein29 reported anastomotic leak in 9 of 11 patients (81.8%) and anastomotic stricture in 8 of 11 (72.7%). In the present series anastomotic leak was present in 19.6% and stricture in 56.6%. These figures are higher when compared with surgery for carcinoma esophagus where we have earlier reported anastomotic leak rate of 4.3% and stricture in 8.5% of patients.30 The reason for higher anastomotic leak could be due to the poor healing reaction in the residual scarred esophagus. On the other hand higher stricture rate represents the other extreme, ie, florid fibrotic response by the dynamic fibrous tissue of the injured esophagus.
It has been reported that patients undergoing cervical esophagogastrostomy for benign disease can develop problems associated with anastomosis in the 4th or 5th postoperative year, which may be severe enough to require anastomotic revision.10 In the present series, 28 patients had more than 5-year follow up and none of them required surgical revision. However, 1 patient did require endoscopic dilatations at 30 months after surgery. The incidence of regurgitation also declined drastically with passage of time and was not seen beyond 1 year of surgery. In the study by Orringer,26 no clinically significant reflux was observed. In the study by Ein et al,31 normal swallowing was reported in 29 of the 33 patients with gastric tubes at the time of follow up. Long-term function of stomach conduit was reported to be better than the colonic conduit by Orringer et al also.26
All the esophageal substitutes were placed in the posterior mediastinum in the present study. We prefer posterior mediastinum as it is the shortest route and the orthotopic position of the substitutes facilitates postoperative endoscopy. Moreover lung compression is avoided and operative dissection is minimized by placing substitutes in this position and is preferred by other authors also.2,6,7,10,32
Aspiration during swallowing has been the major cause of concern in these patients postoperatively. Loss of sensation in the hypopharynx and supraglottic larynx, concomitant injury to glottic mechanism and dyscoordinate swallowing following long periods of absolute dysphagia are the factors reported to affect oral alimentation.11,33 Reconstruction of pharynx and hypopharynx with an innervated flap had been used to help return some sensation to the injured area.33–35 The site of anastomosis ie, pharynx or hypopharynx and cervical esophagus had not been found to alter the incidence of aspiration.28 We used cervical esophagus for construction of anastomosis in the present series. In patients where tight stricture was present at the site of anastomosis, end esophagostomy was spatulated proximally towards the hypopharynx to achieve wider anastomotic lumen. To prevent aspiration and augment swallowing reflex, patients were started on soft solid feeds (eg, banana) and encouraged to swallow saliva. The authors believe that use of soft solid diet helps prevent aspiration and acts as dilator of the anastomotic site. The patients were started on liquids after they could swallow saliva and solid food. By following this schedule the swallowing difficulties were overcome in the present series.
In conclusion, esophageal resection of the scarred esophagus using transhiatal approach was performed safely in 49 of the 51 patients. Stomach was the preferred conduit whenever available. Presence of antral stricture alone was not considered a contraindication for the use of stomach as esophageal substitute as some of these strictures were amenable to endoscopic balloon dilatation. All the conduits were placed in posterior mediastinum. No significant difference was observed in the anastomotic complications between gastric and colonic conduits. Good functional outcome was noted in all the patients.
Footnotes
Reprints: Dr Rajesh Gupta, Assistant Professor, Department of Surgery, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh-160012, India. E-mail: rajsarakshi@yahoo.co.in.
REFERENCES
- 1.Gupta NM, Goenka MK, Behera A, et al. Transhiatal oesophagectomy for benign obstructive conditions of the oesophagus. Br J Surg. 1997;84:262–264. [PubMed] [Google Scholar]
- 2.Canty TG, LoSasso BE. One stage esophagectomy and in situ colon transposition for esophageal replacement in children. J Pediatr Surg. 1997;32:334–337. [DOI] [PubMed] [Google Scholar]
- 3.Ti TK, Sivaloganathan V. Oesophageal resection and cervical oesophagogastrostomy for corrosive oesophageal stricture. Br J Surg. 1978;65:256–258. [DOI] [PubMed] [Google Scholar]
- 4.DeMeester TR, Johansson KE, Franze I, et al. Indications, surgical technique, and long-term functional results of colon interposition or bypass. Ann Surg. 1988;208:460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarfati E, Gossot D, Assens P, et al. Management of caustic ingestion in adults. Br J Surg. 1987;74:146–148. [DOI] [PubMed] [Google Scholar]
- 6.Adegboye VO, Brimmo A, Adebo OA. Transhiatal esophagectomy in children with corrosive esophageal stricture. Afr J Med Sci. 2000;29:223–226. [PubMed] [Google Scholar]
- 7.Bassiouny IE, Bahnassy AF. Transhiatal esophagectomy and colonic interposition for caustic esophageal stricture. J Pediatr Surg. 1992;27:1091–1096. [DOI] [PubMed] [Google Scholar]
- 8.Gupta NM. Oesophagectomy without thoracotomy: first 250 patients. Eur J Surg. 1996;162:55–62. [PubMed] [Google Scholar]
- 9.Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg. 1978;76:643–654. [PubMed] [Google Scholar]
- 10.Watson TJ, Peters JH, DeMeester TR. Esophageal replacement for end stage benign disease. Surg Clin N Am. 1997;77:1099–1113. [DOI] [PubMed] [Google Scholar]
- 11.Wain JC, Wright CD, Kuo EY, et al. Long-segment colon interposition for acquired esophageal disease. Ann Thorac Surg. 1999;67:313–318. [DOI] [PubMed] [Google Scholar]
- 12.Bhasin DK, Sharma BC, Gupta NM, et al. Endoscopic dilation for treatment of anastomotic leaks following transhiatal esophagectomy. Endoscopy. 2000;32:469–471. [DOI] [PubMed] [Google Scholar]
- 13.Zargar SA, Kochhar R, Nagi B, et al. Ingestion of corrosive acids: spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97:702–707. [PubMed] [Google Scholar]
- 14.Zargar SA, Kochhar R, Nagi B, et al. Ingestion of strong corrosive alkalies: spectrum of injury to upper gastrointestinal tract and natural history. Am J Gastroenterol. 1992;87:337–341. [PubMed] [Google Scholar]
- 15.Sweet RH. Subtotal esophagectomy with high intrathoracic anastomosis in the treatment of extensive cicatricial obliteration of the esophagus. Surg Gynecol Obstet. 1946;83:417–420. [PubMed] [Google Scholar]
- 16.Stiff G, Alwafi A, Rees BI, et al. Corrosive injuries of esophagus and stomach: experience in management in regional pediatric center. Ann R Coll Surg Engl. 1996;78:119–123. [PMC free article] [PubMed] [Google Scholar]
- 17.Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer. 1980;45:2655–2658. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins R, Postlethwait R. Caustic burns and carcinoma of the esophagus. Ann Surg. 1981;194:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers BM, Ryckman FC, Talbeet JL. Blunt transmediastinal total esophagectomy with simultaneous substernal colon transplant for esophageal caustic strictures in children. J Pediatr Surg. 1981;16:184–189. [DOI] [PubMed] [Google Scholar]
- 20.Kim YT, Sung SW, Kim JH. Is it necessary to resect the diseased esophagus in performing reconstruction for corrosive esophageal stricture? Eur J Cardiothorac Surg. 2001;20:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Wu M-h, Lai W-W. Esophageal reconstruction for esophageal strictures or resection after corrosive injury. Ann Thorac Surg. 1992;53:798–802. [DOI] [PubMed] [Google Scholar]
- 22.Raffensperger JG, Luck SR, Reynolds M, et al. Intestinal bypass of the esophagus. J Pediatr Surg. 1996;31:38–47. [DOI] [PubMed] [Google Scholar]
- 23.Orringer MB, Kirsh MM, Sloan H. New trends in esophageal replacement for benign disease. Ann Thorac Surg. 1977;23:409–416. [DOI] [PubMed] [Google Scholar]
- 24.Chien KY, Wang PY, Lu KS. Esophagoplasty for corrosive stricture of the esophagus: an analysis of 60 cases. Ann Surg. 1974;179:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambon JP, Robert Y, Remy J, et al. Esophageal mucoceles complicating double exclusion of the esophagus after ingestion of the caustics. Ann Chir. 1989;43:724–730. [PubMed] [Google Scholar]
- 26.Orringer MB. Transhiatal esophagectomy for benign diseases. J Thorac Cardiovasc Surg. 1985;90:649–655. [PubMed] [Google Scholar]
- 27.Ahmad SA, Sylvester KG, Hebra A, et al. Esophageal replacement using the colon: is it a good choice? J Pediatr Surg. 1996;31:1026–1031. [DOI] [PubMed] [Google Scholar]
- 28.Hendren WH, Hendren WG. Colon interposition for esophagus in children. J Pediatr Surg. 1985;20:829–839. [DOI] [PubMed] [Google Scholar]
- 29.Ein SH. Gastric tubes in children with caustic esophageal injury: a 32-year review. J Pediatr Surg. 1998;33:1363–1365. [DOI] [PubMed] [Google Scholar]
- 30.Gupta NM, Gupta R, Manikyam SR, et al. Minimizing cervical esophageal anastomotic complications by a modified technique. Am J Surg. 2001;181:534–539. [DOI] [PubMed] [Google Scholar]
- 31.Ein S, Shandling B, Stephens C. 21-year experience with the pediatric gastric tubes. J Pediatr Surg. 1987;22:77–81. [DOI] [PubMed] [Google Scholar]
- 32.Splitz L. Gastric transposition for esophageal substitution in children. J Pediatr Surg. 1992;22:252–259. [DOI] [PubMed] [Google Scholar]
- 33.Choi RS, Lillehei CW, Lund DP, et al. Esophageal replacement in children who have caustic pharyngo-esophageal strictures. J Pediatr Surg. 1997;32:1083–1088. [DOI] [PubMed] [Google Scholar]
- 34.Wu M-H, Lai W-W, Lin MY, et al. Prevention and management of strictures after hypopharyngocolostomy or esophagocolostomy. Ann Thorac Surg. 1994;18:108–111. [DOI] [PubMed] [Google Scholar]
- 35.Ananthakrishanan N, Nachiappan M, Subba Rao KS. Island pectoralis major myocutaneous flap for pharyngo-oesophageal strictures prior to oesophagocoloplasty. J R Coll Surg Edinb. 2001;46:202–204. [PubMed] [Google Scholar]


