Abstract
Objective:
The aim of this study was to determine the feasibility of sentinel lymph node (SLN) biopsy in patients with gastric cancer for the assessment of regional lymph node status.
Summary Background Data:
SLN is the first draining node from the primary lesion, and it is the first site of lymph node metastasis in malignancy. SLN mapping and biopsy are of great significance in the determination of the extent of lymphadenectomy, allowing patients with gastric cancer to have a better quality of life without jeopardizing survival.
Methods:
The SLN biopsy was performed in 46 consecutive patients having gastric cancer with a preoperative imaging stage of T1/T2, N0, or M0. Three hours prior to each operation, 99mTc tin colloid (2.0 mL, 1.0 mCi) was endoscopically injected into the gastric submucosa around the primary tumor. Subsequently, serial lymphoscintigraphy was performed using a dual-head gamma camera. After the SLN biopsy had been performed using a gamma probe, all patients underwent radical gastrectomy (D2 or D2+α). The SLN was cut and immediately frozen-sectioned. A paraffin block was then produced for permanent hematoxylin-eosin staining and immunohistochemistry (IHC).
Results:
SLNs were successfully identified in 43 of 46 patients (success rate, 93.5%). On average, 2 (range, 1–8) SLNs were identified per patient. The positive predictive value, negative predictive value, sensitivity, and specificity of SLN biopsy were 100% (11 of 11), 93.8% (30 of 32), 84.6% (11 of 13), and 100% (30 of 30), respectively. SLNs were located at the level I lymph nodes in 38 (88.4%), the level I+II nodes in 2 (4.7%), and the level II nodes in 3 (7.0%). No micrometastases of SLNs was found on IHC for cytokeratin.
Conclusions:
SLN biopsy using a radioisotope in patients with gastric cancer is a technically feasible and accurate technique, and it is a minimally invasive approach in the assessment of patient nodal status.
Sentinel lymph node (SLN) biopsy using a radioisotope is a technically feasible and minimally invasive approach for the assessment of nodal status in patients with gastric cancer. It may provide surgeons with information that allows the determination of the extent of lymphadenectomy in gastric cancer. The technique awaits a multicenter validation study.
The role of lymphadenectomy in gastric cancer has been debated for decades.1 Issues that D2 dissection may yield better outcome in gastric cancers than D1 dissection have been addressed, but still no prospective randomized trials proved the benefit of lymphadenectomy in D2 dissection.2 Nonetheless, the proponents for the value of more extended lymphadenectomy are increasing in number, and major centers in the United States favors D2 dissection.3 However, because preoperative diagnostic techniques, including computed tomography (CT) and ultrasonography, do not provide an accurate prediction of metastasis in the regional lymph node nodes, gastrectomy with extended lymphadenectomy (D2 or D2+α) is still considered as a standard surgical approach for early gastric cancer in Korea and in some other countries.4,5 In addition to prolonged operation time and hospitalization, the morbidities accompanying extended lymphadenectomy, such as bleeding, leakage, pancreatitis, subdiaphragmatic abscess, lymphorrhea, and chylous ascites cannot be trivialized.6 Recently, a wedge resection with limited lymphadenectomy was attempted by Ohgami et al7 to improve the quality of life in patients with early gastric cancer.
Miwa8 claimed that extended lymphadenectomy in early gastric cancer resulted in a significantly lower 10-year recurrence rate than limited lymphadenectomy and suggested 2 optimal methods of node dissection in early gastric cancer based on nodal status: for patients without metastatic regional lymph node to be treated by limited lymphadenectomy (D1) for quality of life considerations, and for those with metastatic regional lymph node to be treated by extended lymphadenectomy (D2 or D2+α) for survival. Therefore, if feasible, sentinel lymph node (SLN) mapping and biopsy would be of significant value in the management of early gastric cancer, in which extended lymphadenectomy may be unnecessary.
SLNs, the first draining nodes from a tumor, should be theoretically the first site of lymph node metastases. Moreover, SLN biopsy has revolutionized the surgical management of malignant melanoma9,10 and breast cancer.11,12 However, its feasibility in gastric cancers has not been well established. The feasibility and reliability of SLN biopsy in gastric cancer remain to be settled because of the complicated lymphatic basin from the stomach and the presence of frequent skip metastases. Recently, Kitagawa et al13 have reported promising results about its feasibility in gastric cancer. The aim of current study was to determine its feasibility and reliability in gastric cancer.
METHODS
From November 2001 through December 2002, 46 consecutive patients were enrolled in this study. All patients were confirmed to have gastric adenocarcinoma by endoscopic biopsy preoperatively. Their preoperative stage by imaging studies, computed tomography (CT), and abdominal ultrasonography, was diagnosed to be T1 or T2 without lymph node (N0) or distant metastasis (M0). Written informed consent for lymphatic mapping and the SLN biopsy was obtained from every patient before operation. Three hours prior to each operation, 99mTc tin colloid (2.0–3.0 mL, 1.0 mCi) was endoscopically injected into 4 sites of the gastric submucosa around the primary tumor. Subsequently, serial lymphoscintigraphy was performed using a dual-head gamma camera (MultiSPECTII, Siemens, Germany). Planar scans were also obtained after 10, 30, 60, 90, and 120 minutes.
Patients arrived at the operating room 1 to 3 hours after the radioisotope injection. After a general anesthesia followed by the opening of the abdominal wall, radioactivity was monitored using a hand-held gamma probe (NEO2000TM Gamma Detection System; Neoprobe Co., Ltd, OH) as soon as possible and without significant manipulation of the stomach or greater omentum. SLN was defined by a radioactivity level 10 times higher than background.13,14 Excised SLNs were sent to a pathologist separately, and then routine radical gastrectomy (D2 or D2+α) was performed on all patients. Before completing the surgery, the absence of residual radioactivity in the abdomen was confirmed with a hand-held gamma probe. Excised SLNs were sent to department of pathology in a fresh state for single frozen section stained with hematoxylin and eosin (H&E). When SLN nodes were histologically interpreted as negative for metastasis at frozen biopsy, 2 additional sections were cut from the paraffin block for permanent H&E staining and immunohistochemical (IHC) for cytokeratin (1:100; Dako, CA). The remainder of the specimen was processed in the standard manner used for the pathologic diagnosis of gastric adenocarcinoma specimens.
RESULTS
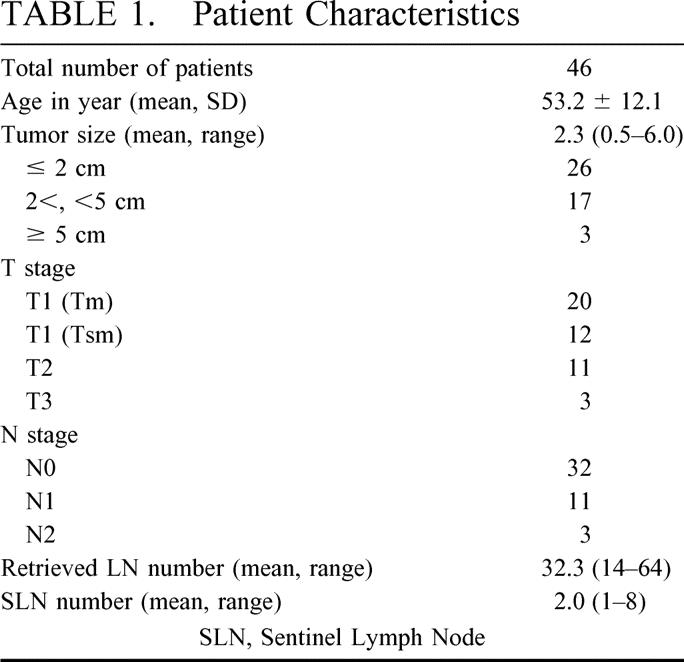
The clinicopathologic characteristics of patients enrolled in this study are summarized in Table 1. On average, 2 (range, 1–8) sentinel lymph nodes and 32.3 (range, 14–64) retrieved lymph nodes were identified per patient. A preoperative lymphoscintigraphy was performed only in 10 patients because of practical problems. Among 10 patients, SLNs of only 3 patients were lymphoscintigraphically visualized 10, 60, and 120 minutes after injection, respectively. In 1 of these 3 patients, a SLN was not detected by a gamma probe, although the remaining 7 patients whose SLN was not lymphoscintigraphically visualized were successfully detected by a gamma probe.
TABLE 1. Patient Characteristics
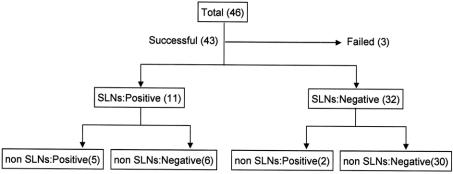
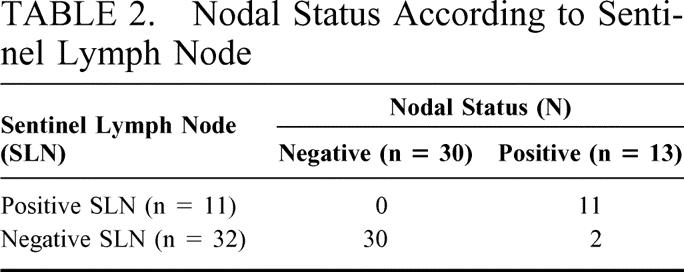
SLNs were successfully identified in 43 patients (93.5%) with gastric cancer. Among 32 patients in which SLNs were negative for metastasis, all non-SLNs were also negative except by 2 patients. Those 2 patients were false negative (6.3%, 2 of 32) by SLN biopsy. Among 11 patients with positive SLNs, 5 were positive and 6 negative in non-SLNs. In those 6 patients, the SLN was the only site of lymph node metastasis (Fig. 1). The positive and negative predictive values of the SLN to predict regional lymph node status were 100% (11 of 11) and 93.8% (30 of 32), respectively. Sensitivity and specificity of the SLN biopsy were 84.6% (11 of 13) and 100% (30 of 30) (Table 2).
FIGURE 1. Results of SLN biopsy in 46 patients with gastric cancer. The detection rate was 93.5% (43 of 46), and the false negative rate 6.3% (2 of 32). In 6 patients, SLN was the only site of lymph node metastasis.
TABLE 2. Nodal Status According to Sentinel Lymph Node
All of the SLNs were located in the perigastric lymph nodes (level I), such as LN #1, #3, #4, #5, and #6, except in 3 patients (7%) where, though not metastatic in pathology, these were skipped over the perigastric area and identified along left gastric artery (LN #7, level II, Fig. 2). In other 2 patients (4.7%), SLNs were located at levels II and I (Fig. 3). In 10 patients, SLNs were positive for metastasis by frozen biopsy, which was also confirmed by permanent H&E staining. Among 33 patients in whom SLNs were negative for metastasis on frozen biopsy, 32 were also negative either on permanent H&E staining or IHC for cytokeratin, and 1 patient was positive by permanent H&E staining. Therefore, the accuracy of the SLN frozen biopsy was 97.7% (42 of 43). No micrometastases of SLNs were found on IHC for cytokeratin.
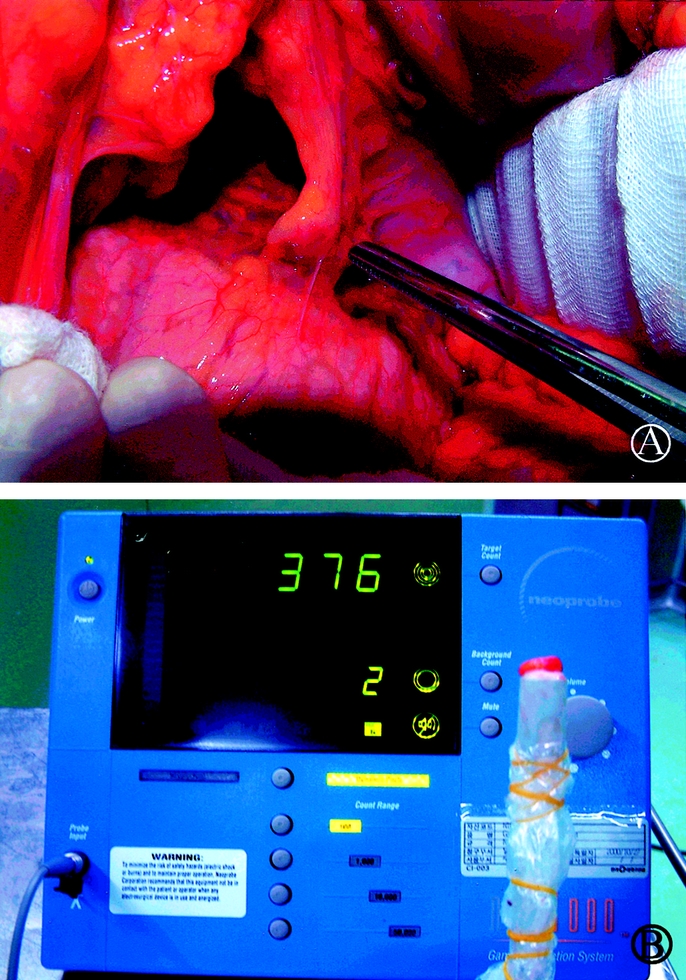
FIGURE 2. (A) Intraoperative findings of SLNs biopsy in a patient with gastric cancer: The SLN is shown along the left gastric artery (LN #7). (B) The ex vivo sentinel lymph node is placed on the tip of the gamma probe.
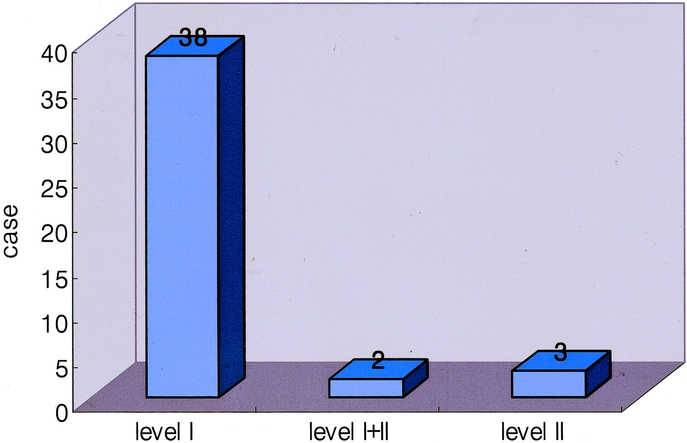
FIGURE 3. Distribution of the locations of SLNs in 43 patients with gastric cancer. Most SLNs were located at the perigastric lymph nodes (level I). SLNs were located along the left gastric artery (level II) in 3 patients (7.0%, 3 of 43); in another 2 patients (4.7%, 2 of 43), SLNs were located at levels II and I.
DISCUSSION
The concept of SLN characterization is of great interest to many surgical oncologists because it may be a guideline to the determination of the extent of cancer surgery. The feasibility of SLN biopsy has been studied extensively for cancers in the stomach,1,13–17 colon,18,19 head and neck,20 thyroid,21 prostate,22 cervix,23 breast,11,12 and skin.9,10 The clinical implications of SLN biopsy, however, in gastric cancer remain controversial. Maruyama et al5 asserted that SLN biopsy in gastric cancer could not be used for reducing the extent of lymphadenectomy because of the complicated lymphatic streams from the stomach and the presence of frequent skip metastases. Tsuburaya et al24 reported that the sensitivity of sentinel lymph node biopsy performed by exploring the adjacent basin would be very low, especially for the lesions in the lesser curvature and posterior wall. However, Kitagawa et al17 recently claimed that SLN mapping during laparoscopic surgery, and during laparotomy, is a sensitive and feasible intraoperative technique for identifying lymph node metastasis in patients with gastric cancer. The present study is a first trial of SLN biopsy using a radioisotope in patients with gastric cancer in a country other than Japan.
In the resent study, SLN mapping and biopsy in patients with gastric cancer using 99mTc tin colloid proved satisfactory. The mean number of SLNs in patients was 2 (range, 1–8), which is lower than previously reported13 in patients with gastric cancer. Unlike breast cancer and malignant melanoma, a preoperative lymphoscintigraphy in cases of SLN biopsy for gastric cancer may be not available. A preoperative lymphoscintigraphy was performed only in 10 patients because of practical problems. In the remaining 7 patients, whose SLN was not lymphoscintigraphically visualized, the SLNs were successfully detected, although in 1 of 3 patients, whose SLN was lymphoscintigraphically visualized, the SLN was not detected by a gamma probe. Our lymphoscintigraphic results are consistent with those of Aikou et al,14 who visualized only 2 of 61 SLNs preoperatively by lymphoscintigraphy. They suggested that the failure of preoperative lymphoscintigraphy to detect SLNs might reflect the proximity of these nodes to the injection site. Mean time required for the SLNs biopsy was 20–30 minutes, and no any adverse complications related to this procedure were encountered.
In terms of the predictive value of our study, SLNs accurately predicted metastasis in the regional lymph nodes of each gastric cancer patient. The positive and negative predictive values of SLN were 100% and 93.8%, respectively. The skip metastasis in gastric cancer has been considered an obstacle to the utilization of the SLN concept. The incidence of skip metastasis in gastric cancer was found to be 0–10% in other retrospective studies14,25 and 5.1% in other SLN biopsy study.13 In our study, the potential skip metastasis occurred in 7%. Skip metastasis in gastric cancer, however, is not an obstacle in the use of SLN concept because a SLN biopsy can localize and identify such metastasis. Micrometastasis in gastric cancer using antibodies to cytokeratin was found in 4–6.3% of cases in other studies.14,26 In the present study, no micrometastasis of SLNs by IHC for cytokeratin was found in 32 of 33 patients in whom the SLNs were negative for metastasis on frozen biopsy. It is a shortcoming of our study that only 52 of the SLNs were examined for micrometastasis. If number of the SLNs examined for IHC had been higher, SLNs showing micrometastasis may have been detected.
In conclusion, SLN biopsy in gastric cancer using a radioisotope proved technically feasible for the detection of SLN located at level II or I and accurately predicted metastasis in the regional lymph nodes in each patient. This technique may be of a great benefit to surgeons for the determination of the extent of lymphadenectomy in gastric cancer. A multicenter validation study of SLN biopsy should establish standard guidelines for deciding the extent of lymphadenectomy in gastric cancer.
Footnotes
Reprints: Hyung-Ho Kim, MD, Department of Surgery, Dong-A University College of Medicine, 3–1 Dongdaeshin-Dong, Seo-Gu, Busan 602–715, South Korea. E-mail: hhkim@snubh.org.
REFERENCES
- 1.Hundley JC, Shen P, Shiver SA, et al. Lymphatic mapping for gastric adenocarcinoma. Am Surg. 2002;8:931–935. [PubMed] [Google Scholar]
- 2.Walter L Jr. Surgical management of gastric cancer. In: Silberman H, Silberman AW, eds. Surgical Oncology: Multidisciplinary Approach to Difficult Problems. New York: Arnold; 2002:525–540. [Google Scholar]
- 3.Ajani J, Brand R, Burak WE, et al. Practice Guidelines in Oncology-v. 1. 2002. Gastric cancer. Available at: http://www.nccn.org/physician-gls/index.html. Accessed Dec. 28, 2002.
- 4.Hayes N, Karat D, Scott DJ, et al. Radical lymphadenectomy in the management of early gastric cancer. Br J Surg. 1996;83:1421–1423. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama K, Sasako M, Kinoshita T, et al. Can sentinel node biopsy indicate rational extent of lymphadenectomy in gastric cancer surgery? Fundamental and new information on lymph-node dissection. Langenbecks Arch Surg. 1999;384:149–157. [DOI] [PubMed] [Google Scholar]
- 6.Smith JW, Shiu MH, Kelsey L. Morbidity of radical lymphadenectomy in the curative resection of gastric carcinoma. Arch Surg. 1991;126:1469–1473. [DOI] [PubMed] [Google Scholar]
- 7.Ohgami M, Otani Y, Kumai K, et al. Curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg. 1999;23:187–193. [DOI] [PubMed] [Google Scholar]
- 8.Miwa K. Optimal nodal dissection for early gastric cancer. Nippon Geka Gakkai Zasshi. 2001;102:484–489. [PubMed] [Google Scholar]
- 9.Morton DL, Wen DR, Wong JH. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. [DOI] [PubMed] [Google Scholar]
- 10.Buonomo O, Felici A, Granai AV, et al. Sentinel lymphadenectomy in cutaneous melanoma. Tumori. 2002;88:S49–S51. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards MJ, Whitworth P, Tafra L, et al. The details of successful sentinel lymph node staging for breast cancer. Am J Surg. 2000;180:257–261. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa Y, Fujii M, Kubota Y, et al. Radio-guided sentinel node detection for gastric cancer. Br J Surg. 2002;89:604–608. [DOI] [PubMed] [Google Scholar]
- 14.Aikou T, Higashi H, Natsugoe S, et al. Can sentinel node navigation surgery reduce the extent of lymph node dissection in gastric cancer? Ann Surg Oncol. 2001;8:90–93. [PubMed] [Google Scholar]
- 15.Hiratsuka M, Miyashiro I, Ishikawa O, et al. Application of sentinel node biopsy to gastric cancer surgery. Surgery. 2001;129:335–340. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda S, Shimada H, Ogoshi K, et al. Preliminary study for sentinel lymph node identification with Tc-99m tin colloid in patients with esophageal or gastric cancer. Tokai J Exp Clin Med. 2001;26:15–18. [PubMed] [Google Scholar]
- 17.Kitagawa Y, Ohgami M, Fujii H, et al. Laparoscopic detection of sentinel lymph nodes in gastrointestinal cancer: a novel and minimally invasive approach. Ann Surg Oncol. 2001;8:86–89. [PubMed] [Google Scholar]
- 18.Thorn M. Lymphatic mapping and sentinel node biopsy: is the method applicable to patients with colorectal and gastric cancer? Eur J Surg. 2000;166:755–758. [DOI] [PubMed] [Google Scholar]
- 19.Feig BW, Curley S, Lucci A, et al. A caution regarding lymphatic mapping in patients with colon cancer. Am J Surg. 2001;182:707–712. [DOI] [PubMed] [Google Scholar]
- 20.Pastore A, Turetta GD, Tarabini A, et al. Sentinel lymph node analysis in squamous carcinoma of the oral cavity and oropharyynx. Tumori. 2002;88:S58–S60. [DOI] [PubMed] [Google Scholar]
- 21.Fukui Y, Yamakawa T, Taniki T, et al. Sentinel lymph node biopsy in patients with papillary thyroid carcinoma. Cancer. 2001;92:2868–2874. [DOI] [PubMed] [Google Scholar]
- 22.Rudoni M, Sacchetti GM, Leva L, et al. Recent applications of the sentinel lymph node concept: preliminary experience in prostate cancer. Tumori. 2002;88:S16–S17. [DOI] [PubMed] [Google Scholar]
- 23.Rhim CC, Park JS, Bae SN, et al. Sentinel node biopsy as an indicator for pelvic nodes dissection in early stage cervical cancer. J Korean Med Sci. 2002;17:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuburaya A, Noguchi Y, Yoshikawa T, et al. Solitary lymph node metastasis of gastric cancer as a basis for sentinel lymph node biopsy. Hepatogastroenterology. 2002;49:1449–1452. [PubMed] [Google Scholar]
- 25.Santoro E, Carlini M, Carboni F, et al. Anatomico-surgical contribution to the knowledge of the lymphatic spread of gastric adenocarcinoma. Chir Ital. 2002;54:259–265. [PubMed] [Google Scholar]
- 26.Yasuda K, Adachi Y, Shiraishi N, et al. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771–774. [DOI] [PubMed] [Google Scholar]