Abstract
Objective:
To define the natural history of resected intraductal papillary mucinous neoplasms (IPMN) of the pancreas and to identify clinical and pathologic prognostic features.
Summary Background Data:
IPMN of the pancreas is a recently described pancreatic tumor. Because of a limited number of cases, prognostic factors and the natural history of resected cases have not been well defined.
Materials and Methods:
A prospective pancreatic database was reviewed to identify patients with IPMN who were surgically managed. Pathologic re-review of each case was performed, and the clinicopathologic features were examined. Log rank and χ2 analysis were used to identify factors predictive of survival and recurrence.
Results:
Over a 17-year period, 63 patients were identified. One patient was unresectable, 6 (10%) underwent a total pancreatectomy, and 56 (89%) had a partial pancreatectomy. Invasive carcinoma was present in 30 patients (48%). Transection margins were involved with atypia or carcinoma in 32 patients (51%). The median follow-up for survivors was 38 months. Disease-specific 5- and 10-year survival were 75% and 60%, respectively. Significant predictors of poor outcome included presentation with elevated bilirubin, presence of invasive carcinoma, increasing size and percentage of invasive carcinoma, histologic type of invasive carcinoma, positive lymph nodes, and vascular invasion. The presence of atypia or carcinoma in situ at the ductal resection margin was not associated with a poor outcome.
Conclusions:
Overall, IPMN has a favorable prognosis. Poor outcome in a subset of patients is largely the result of the presence, extent, and type of an invasive component, lymph node metastases, and vascular invasion.
Intraductal papillary mucinous neoplasms of the pancreas is a rare tumor whose natural history and prognostic features have not been well defined. Although the majority of patients fair well, poor outcome in a subset of patients can be predicted by the presence of adverse pathologic factors.
Intraductal papillary mucinous neoplasms of the pancreas (IPMN) have recently been defined and classified by the World Health Organization. Originally described in the 1980s, they are characterized by mucin production, cystic dilation of the pancreatic ducts, and intraductal papillary growth.1 They may exhibit a spectrum of dysplasia ranging from minimal mucinous hyperplasia to invasive carcinoma. Reports on these tumors have been sparse and have used a variety of confusing names.2–9 In recent years, however, a number of publications have helped to clarify the nomenclature and pathologic characteristics of this tumor, which is now uniformly referred to as IPMN.10–14 A rare related tumor type is intraductal oncocytic papillary neoplasm, an intraductal tumor with histologically distinctive oncocytic cells.15
Recently, IPMN has been increasingly recognized and is now being reported more frequently with larger case series.11–13 The reason for the increasing recognition of the disease is unclear, but is at least partially the result of a rising awareness of the disease and an increasing use of cross-sectional imaging. Despite the more frequent reporting of IPMN, the natural history of this disease is not well understood, and a number of controversies regarding its treatment persist. Although it is clear that overall, IPMN has a more favorable prognosis than standard ductal pancreatic carcinoma,13 some cases of IPMN have a poor outcome. Because case series have largely presented small numbers of patients with limited follow-up, the factors that determine outcome in these patients are not well understood. Insufficient emphasis has been placed on the presence and extent of any invasive carcinoma, a factor that intuitively should dictate the prognosis. The potential for this neoplasm to diffusely involve the pancreatic duct has been recognized, making treatment decisions difficult. The crux of the issue is whether a partial pancreatectomy is adequate treatment and what effect the histologic status of the margin has on outcome.
The purpose of this study was to review and characterize our experience with resected pancreatic tumors found to be IPMN. Our aim was to define clinical and pathologic factors associated with outcome as well as to define the natural history of completely resected tumors.
MATERIALS AND METHODS
Using a prospectively maintained pancreatic surgery database, all patients identified as having IPMN or those cases suspicious for having IPMN were identified. After pathologic re-review by a single senior pathologist (DK), 63 cases of IPMN were identified that were surgically managed from October 1983 through December 2000. A retrospective review of clinical and pathologic factors of these cases was then conducted. Information on demographics, preoperative workup, operative therapy, postoperative course, long-term outcome, and recurrence patterns was recorded. The pathology of all cases, including resection margins when possible, was re-reviewed and recorded. We have previously published a pathologic analysis of 28 of these patients.16
Cases were defined pathologically as examples of IPMN if they met the following criteria: grossly identifiable cystic structures at least 1.0 cm in greatest diameter documented to represent cystically dilated ducts on the basis of connection to the remainder of the ductal system, lined by flat or papillary mucinous or oncocytic epithelium exhibiting variable degrees of cytoarchitectural atypia, with or without a component of invasive adenocarcinoma. Cases were classified as main duct or branch duct type depending on the distribution of ductal involvement. In main duct type cases, the major pancreatic ducts were dilated and lined by neoplastic epithelium. Branch duct cases were confined to the secondary ducts, with minimal or no involvement of the major ducts. The amount of intraductal papilla formation was roughly categorized as minimal, moderate or marked.
All microscopic slides were reviewed to determine the nature of the epithelial lining and were classified as intestinal type, pancreatobiliary type, or oncocytic type using established criteria.15,16 Based on the most significant degree of cytoarchitectural atypia, the intraductal components of each tumor were classified as IPM adenoma, IPM, borderline, and IPM carcinoma in situ (IPMC) using criteria defined in the World Health Organization classification of pancreatic neoplasms.17,18 The examples with oncocytic epithelium all showed severe cytoarchitectural atypia and were therefore classified as intraductal oncocytic papillary carcinomas (IOPCs). The presence, histologic type, and extent of any associated invasive carcinoma were determined. Invasive carcinomas were classified as tubular if they resembled conventional infiltrating ductal adenocarcinomas, with predominately tubular neoplastic glands associated with a desmoplastic stroma in the absences of significant stromal mucin. Invasive carcinomas were classified as colloid if they consisted of more than 80% of large pools of extracellular mucin containing relatively scant strips, clusters, and individual neoplastic cells.19 In cases exhibiting an invasive component, the proportion of the overall tumor consisting of invasive carcinoma was estimated, as was the greatest microscopic dimension of the invasive component. Sections were also examined for the presence of perineural invasion, vascular invasion, and lymph node metastases.
In all cases with available material, the distal (for pancreatoduodenectomy specimens) or proximal (for distal pancreatectomy specimens) resection margins were re-evaluated (total pancreatectomy specimens having no parenchymal resection margin). Each en face margin section was examined for any intraductal proliferative lesions. In many instances, individual IPMNs extended diffusely throughout medium sized as well as smaller ducts. In the smaller ducts, the appearance was indistinguishable from independent foci of pancreatic intraepithelial neoplasia (PanIN).20 Because controversy exists regarding criteria to distinguish independent foci of PanIN from peripheral extension of IPMNs, all intraductal lesions were considered when evaluating the parenchymal margins. Each margin was scored based on the greatest degree of atypia, with foci of IPM adenoma or PanIN1 recorded as “positive, mild atypia,” foci of IPM, borderline or PanIN2 recorded as “positive borderline atypia,” and foci of IPMC or PanIN3 recorded as “positive, carcinoma in situ.” Parenchymal margins were also evaluated for the presence of invasive carcinoma in cases containing an invasive component. Only the margins exhibiting no proliferative lesions within the ducts were recorded as “negative.”
Clinical data were obtained from a combination of medical record review, reports of outside medical records and communication with patients and their other physicians. For radiologic data, no specific review of the actual radiographs was undertaken. Survival analysis was performed by the methods of Kaplan and Meier with log rank univariate comparisons. All deaths from causes other than IPMN were censored at the time of last follow-up. Interrelationships between factors and associations of factors with a recurrence episode and presence of invasive disease were performed using χ2 and Fisher exact test as appropriate. Statistical analysis was carried out with SPSS for Windows, version 10.0 (Statistical Package for the Social Sciences, SPSS, Inc., Chicago, IL). P values less than 0.05 were considered statistically significant.
RESULTS
Demographics
From October 1983 through June of 2000, 63 patients with IPMN were managed surgically with curative intent. The median age was 70 (range, 41–87). There were 32 females (51%) and 31 males (49%). Eighteen patients (29%) had a history of a previous extrapancreatic malignancy. There was a significant family history of cancer (excluding nonmelanoma skin cancers), defined as at least 1 first-degree relative afflicted, in 27 of 55 (49%) evaluatable patients. A complete history regarding alcohol consumption and smoking could be obtained in 62 patients. There were 6 (10%) heavy or former heavy alcohol users, 24 (39%) social drinkers, and 32 (51%) patients that denied alcohol use. There were 10 (16%) current and 18 (29%) former smokers. Thirty-four (55%) patients denied any history of smoking.
Presentation
The majority of patients (81%) were symptomatic at presentation (Table 1). Six patients (10%) presented with pancreatic exocrine insufficiency requiring oral pancreatic enzymes. One patient presented with a palpable abdominal mass. Nine patients (14%) presented with new onset diabetes or had a recent worsening of their diabetes at presentation. A serum bilirubin greater than 1 mg/dL was found at presentation in 15 (24%) patients.
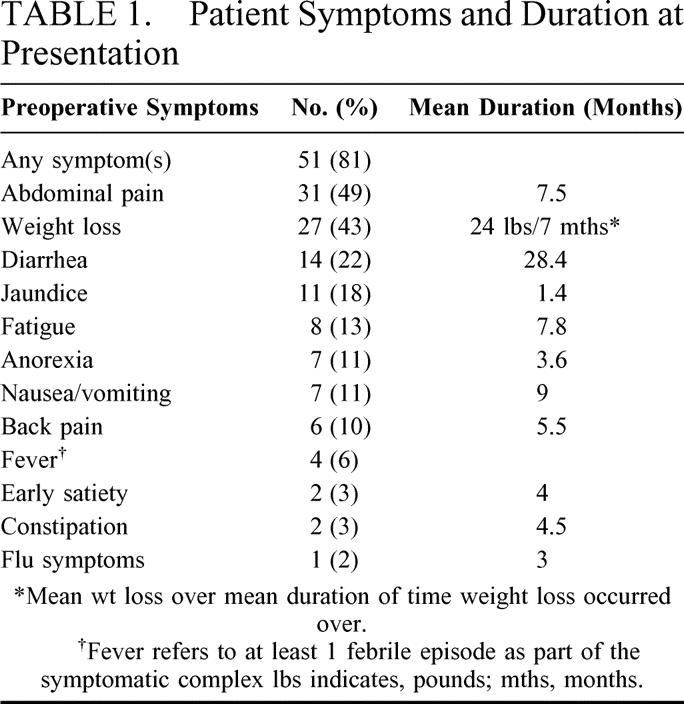
TABLE 1. Patient Symptoms and Duration at Presentation
Endoscopic retrograde cholangio-pancreatography was attempted in 36 patients and successful in 32. A dilated pancreatic duct was noted in 22 of 27 cases (81%) where the size of the duct was commented on. Mucin at the papilla was noted in 7 of 29 cases (24%) where this was commented on. All patients had at least 1 cross-sectional imaging study in the form of magnetic resonance cholangio-pancreatography or computed tomography. Computed tomography was used most often and performed in 62 cases (98%). There was a distinct mass reported on 55 of the scans (88%), of which 45 (75%) were considered cystic in appearance.
Operative Therapy
Pancreaticoduodenectomy was the most common operation and was performed in 41 cases (66%), 6 of which were pylorus preserving. A distal pancreatectomy was performed in 14 cases (23%) and was considered to be a subtotal pancreatectomy in 8 of these cases (pancreas divided to the right of the porto-mesenteric vessels). Six patients (10%) underwent a total pancreatectomy. An enucleation was performed in 1 patient (2%) and 1 patient (2%) had a locally unresectable tumor. Of the 20 cases where a distal or total pancreatectomy was performed, 12 had concomitant splenectomy. A resection of the portal vein or superior mesenteric vein was required in 4 cases, 1 of which included a colectomy. Three patients were treated with postoperative chemo-radiation.
Frozen section of the pancreatic margin was not routine and was performed in 17 cases (27%). In 14 of these 17 cases, the frozen section was negative (82%). One patient who had undergone a subtotal pancreatectomy had IPMN with mild atypia at the margin and the resection was not extended to a total pancreatectomy. Two patients had carcinoma in situ at the margin. One underwent a total pancreatectomy and the other was extended from a distal pancreatectomy to a subtotal pancreatectomy, but stopped here despite a persistently positive margin.
There were 40 complications in 31 patients yielding a 50% morbidity rate. The majority of complications were gastrointestinal or anastomotic (40%) and infectious (18%). Urinary, cardiovascular, and hemorrhagic complications accounted for 13%, 10%, and 10% of all complications, respectively. There were 2 in-hospital deaths (3%) and 2 other (3%) out-of-hospital deaths within 30 days, yielding a total operative mortality of 6%. The out-of-hospital deaths were secondary to a myocardial infarction in 1 case and a massive gastrointestinal bleed in the other. Data on long-term morbidity were available for the remaining 59 patients. New onset malabsorption requiring oral pancreatic enzyme supplementation occurred in 21 patients (33%) whereas new-onset or worsening of diabetes from baseline occurred in 11 patients (17%). Two patients (3%) developed a biliary stricture and 1 patient (2%) developed a persistent pseudocyst.
Pathology
The pathologic findings are detailed in Table 2. Forty-six (74%) of the tumors were located in the head, and 15 (24%) were located in the body or tail. There was 1 case of multifocal tumor, with 1 in the head and 1 in the tail. The median size of the primary tumor was 3.4 cm with a range of 0.8 to 14 cm. In tumors with an invasive component (n = 30) the median size of the invasive component was 1.5 cm (range, 0.1–4.8 cm) and the median percentage of the overall tumor estimated to be invasive was 40% (range, 1–80%). Of the 10 cases with involved lymph nodes, 7 involved a single node, 1 involved 3, and 1 involved 6 nodes.
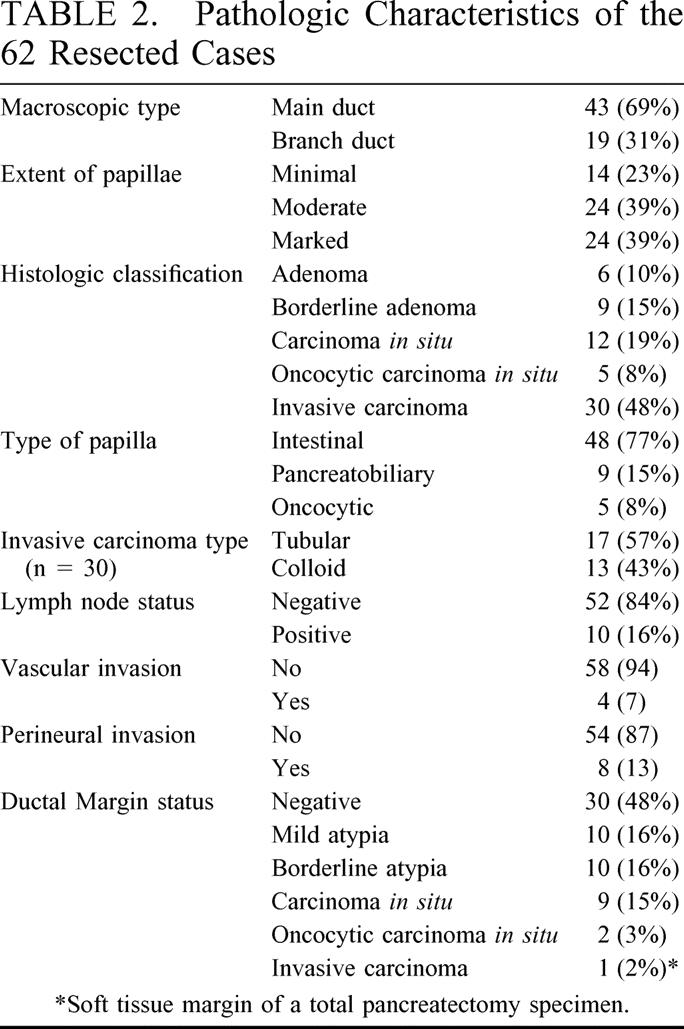
TABLE 2. Pathologic Characteristics of the 62 Resected Cases
Outcome
Of the 63 cases taken to the operating room with curative intent, 1 (2%) was found to be locally unresectable. The diagnosis of IPMN was made by intraoperative biopsy. This patient died of disease 21 months after surgery of local tumor progression and hepatic failure. The median follow up for all 62 resected patients was 32 months (range, 0.1–177), and the median follow up for survivors was 38 months (range, 6–177). Figure 1 illustrates the disease specific survival for the 62 completely resected patients. The actuarial 5- and 10-year survival rates were 75% and 60%, respectively.
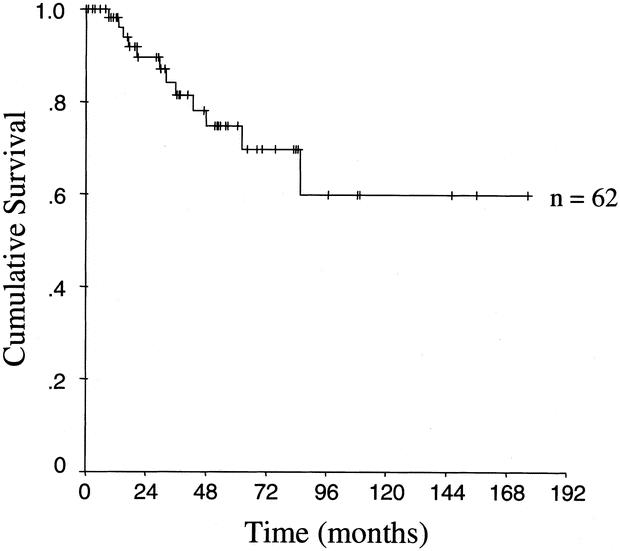
FIGURE 1. Kaplan–Meier actuarial disease-specific survival curve of 62 patients who had complete resection.
Table 3 demonstrates the clinical and pathologic factors associated with outcome in the 62 completely resected patients. Of the clinical factors analyzed, a serum bilirubin elevated above normal (>1 mg/dL) was the only factor significantly associated with a poor outcome. Elevated serum alkaline phosphatase predicted outcome in a similar way (data not shown).
TABLE 3. Log Rank Univariate Comparisons of Clinical and Pathologic Factors Associated With Outcome
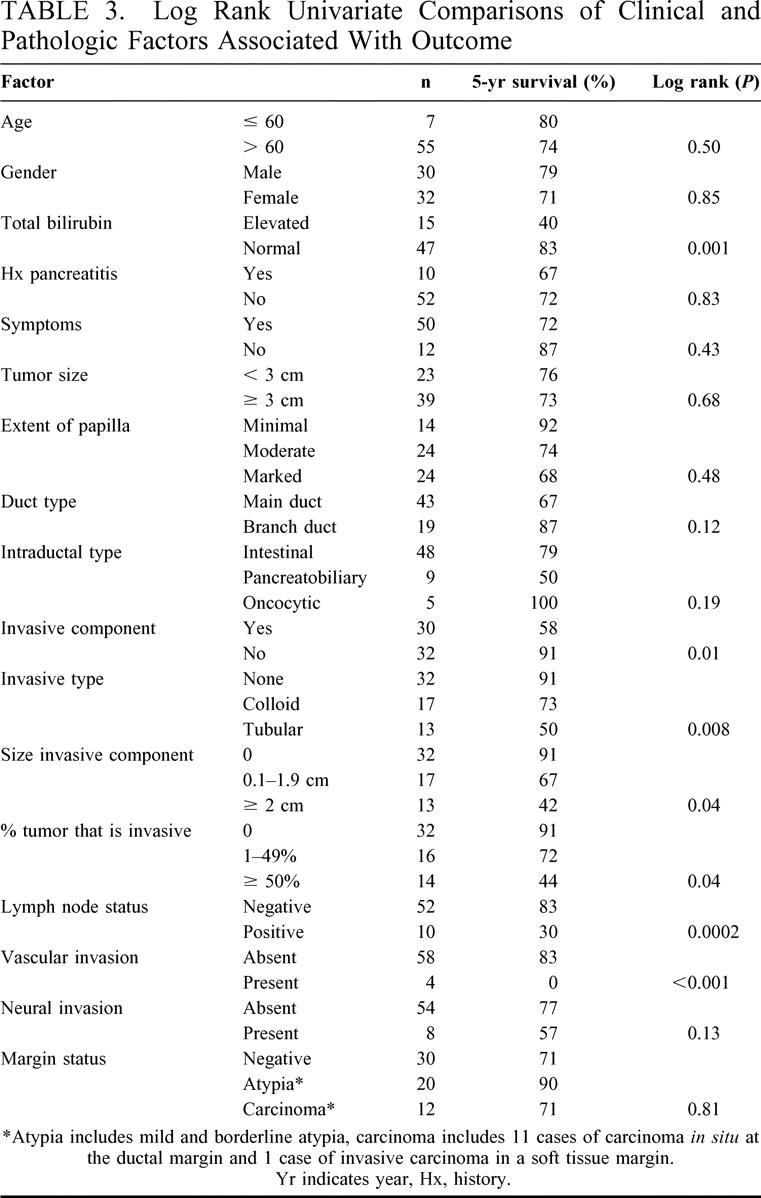
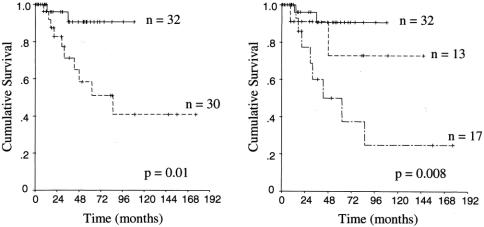
A number of pathologic factors were significantly associated with outcome. The presence of an invasive component, found in 48% of cases, was a strong predictor of poor outcome (Fig. 2A). Of the 32 cases in which no invasive component was found in the specimen, 3 cases had a poor outcome. One patient with IOPC had IOPC at the resection margin and recurred in the pancreatic stump. This patient's tumor was re-resected at 39 months, and she is currently alive without evidence at disease at 47 months from the original resection. The second patient had IPMC with a negative margin and recurred in the pancreatic stump at 8 months postoperatively. This patient did not undergo repeat surgery and died of disease 36 months later. The third patient had a tumor with borderline atypia and a negative margin. This patient died of disease 13 months postoperatively of diffuse intraabdominal recurrence. The histologic type of invasive carcinoma (tubular or colloid) also had a strong association with outcome (Fig. 2B). The extent of the invasive component appeared to have an effect on survival. As seen in Table 3, increasing size of the invasive component (not including the intraductal component of the tumor) and percent of the tumor containing an invasive component portended a worse prognosis.
FIGURE 2. Kaplan–Meier actuarial disease-specific survival curves of (A) noninvasive (——) versus invasive (- - - -) disease and (B) noninvasive (——) versus invasive colloid carcinoma (- - - -) versus invasive tubular carcinoma (— - —).
Lymph node positivity was associated with poor outcome (Fig. 3). The median survival of the 10 patients with involved lymph nodes was 29 months. Vascular invasion, although an uncommon event, was a significant predictor of poor outcome as well. All 4 patients with vascular invasion were dead of disease within 4 years. The 8 patients with perineural invasion also had a worse prognosis (Table 3), but the difference only approached statistical significance (P = 0.13). Patients with a tumor originating from a main duct appeared to have a worse prognosis than those with a branch duct origin (Table 3), but this did not reach statistical significance (P = 0.12).
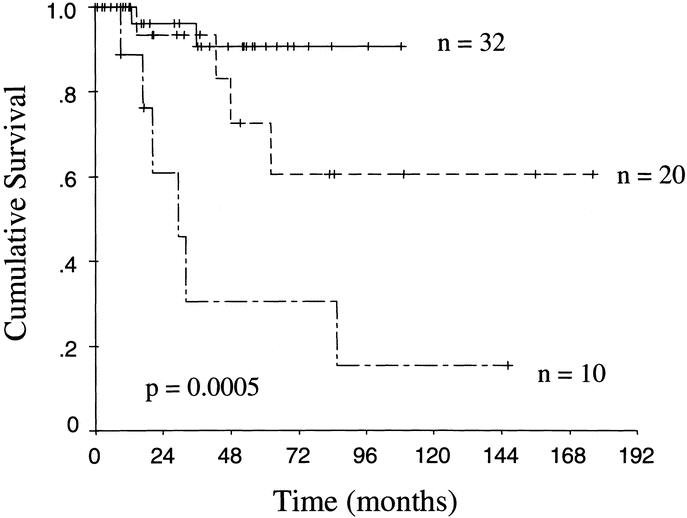
FIGURE 3. Kaplan–Meier actuarial disease-specific survival curves of patients with noninvasive disease and uninvolved lymph nodes (——), invasive disease with uninvolved lymph nodes (- - - -), and invasive disease with involved lymph nodes (— - —).
The presence of atypia (mild or borderline) or carcinoma in situ at the resection margin had no association with outcome (Table 3). Carcinoma in situ was demonstrated at the ductal margin in 11 patients and in 1 case, invasive carcinoma in the soft tissue margin of a total pancreatectomy specimen was noted. Of these 12 cases, there were 3 cases of recurrent disease with a median follow-up 19 months. Two of these cases were in tumors containing tubular invasive carcinoma with involved lymph nodes. The third case was a case of IOPC that had a recurrence in the pancreatic stump and has subsequently been resected. The patient with invasive carcinoma at the soft tissue margin of a total pancreatectomy specimen died in the hospital from multiple postoperative complications. There were 10 patients with borderline atypia at the resection margin, 3 of whom had recurrence of disease with a median follow-up of 33 months. All 3 of the recurrences occurred in patients with tumors containing lymph node positive tubular invasive carcinoma. Ten patients had mild atypia at the resection margin and at a median follow-up of 30 months there has been no recurrence of disease. Only 2 of these 10 cases had invasive disease, but they were both colloid carcinomas without lymph node metastases.
Inter-relationships of Predictive Factors
A multivariate analysis of factors predictive of outcome was not statistically feasible because of the small sample size. It is therefore important to analyze how the significant prognostic factors are related to each other. Of the clinical factors analyzed, serum total bilirubin was the only significant factor found. Using χ2 comparisons, total bilirubin was significantly associated with the presence of an invasive component (P = 0.03), increasing size (P = 0.05) and percent (P = 0.02) of the invasive component, involved lymph nodes (<0.0001), and tubular type carcinoma (P = 0.03) but was not significantly associated with the presence of vascular invasion (P = 0.24).
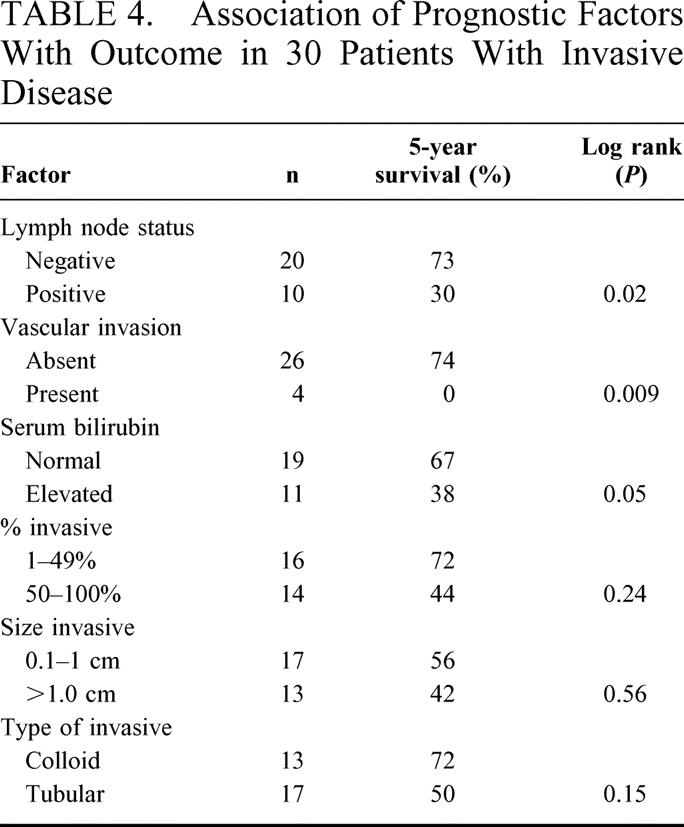
All patients with involved lymph nodes and vascular invasion had invasive disease in the primary tumor. As the size and percent of the invasive component increased there was a statistically significant increase in lymph node metastases (P < 0.0001), and a statistical association approaching significance with vascular invasion (P = 0.1 and 0.06). Additionally, tubular carcinoma (as compared with colloid) was significantly associated with lymph node metastases (P < 0.0001) and closely associated with vascular invasion (P = 0.06). Lastly, among the subset of patients with invasive disease (n = 30), serum total bilirubin, lymph node metastases and vascular invasion continued to predict outcome implying an association somewhat independent of simply having invasive disease. There were trends toward a worse survival in patients with an increasing extent of invasive carcinoma and in those with tubular carcinoma, but these did not reach statistical significance in this subset (Table 4).
TABLE 4. Association of Prognostic Factors With Outcome in 30 Patients With Invasive Disease
Recurrence Patterns
Of the 62 completely resected patients, there were 14 disease recurrences (23%), 12 of whom are dead of disease. The median time to recurrence was 20 months (range, 8–70). Half of the recurrences occurred in the first 2 years after surgery, and all except 1 occurred within 4 years.
In 12 of the 14 cases of recurrent disease, data on the recurrence pattern was obtained. There were 3 cases of distant recurrence alone (25%). In 4 cases (42%), diffuse intraabdominal recurrence occurred, 1 of which was in association with distant metastases. There were 4 pancreatic stump recurrences (33%) and 2 of these were in association with distant metastases. There was one case of locoregional nodal recurrence in conjunction with distant metastases. Overall, distant sites were involved in 7 of the recurrences (58%).
Elevated serum total bilirubin, presence, extent and type (tubular > colloid) of invasive component, lymph node metastases, vascular invasion and perineural invasion were all significantly associated with the recurrence of tumor. Margin status was not associated with disease recurrence (data not shown) and of note, of the 4 documented stump recurrences only 1 had an involved ductal margin.
Recurrence patterns were documented in 9 patients with an invasive primary tumor and all were multifocal or involved distant sites. There were 3 recurrences in patients with a noninvasive primary tumor. Two were isolated stump recurrences and 1 was a diffuse intra-abdominal recurrence.
Predicting the Presence of Invasive Disease
An analysis of factors significantly associated with the presence of invasive disease was carried out. The presence of any symptom, elevated serum bilirubin, jaundice and weight loss were significantly associated with the presence of invasive disease. All other preoperative factors including age, gender, tumor location, history of pancreatitis and all other specific symptoms had no significant association with the presence of invasive disease. Pathologic factors that may have implications for preoperative radiologic studies were also studied. Tumors greater than 3 cm and tumors with a main duct origin were significantly associated with the presence of an invasive component. Cyst type (multicystic versus single cyst) had no association with the presence of invasive disease.
DISCUSSION
IPMN of the pancreas is now being identified with increasing frequency.10,12,13 To date, small numbers of cases and minimal follow-up have limited studies on this entity.10–14 Therefore a number of clinical and pathologic controversies about this neoplasm persist.
In our series, the presentation of the patients was similar to previous studies. The median age was higher than most other studies (70) and although many studies have suggested a male preponderance,2–6,8,11 our study and most recent larger series have found an equal sex preponderance.10–13 IPMN presents with symptoms and, like other studies, 80% of our patients presented with some symptom, most commonly pain (50% abdominal 10% back). Unlike adenocarcinoma of the pancreas, jaundice was an uncommon presentation.13
In common with others,6,9–13 we have shown that 40–50% of resected IPMN specimens contain invasive carcinoma (48% in our series), with the other tumors containing varying degrees of borderline atypia or carcinoma in situ. The tumors with invasive components all occurred within areas of carcinoma in situ, and the percent of the overall tumor that was invasive was variable. This may suggest that this tumor progresses slowly along a spectrum of neoplastic transformation as more than half of the specimens had not yet progressed to invasive carcinoma. This is in stark contrast to ductal pancreatic adenocarcinoma, which commonly presents in an advanced state.
Long-term survival for completely resected IPMN of the pancreas is favorable and the tumor exhibits an indolent course in most cases. Five-year actuarial survival was 75%, but 2 deaths from disease occurred after 5 years resulting in an actuarial 10 year survival of 60%. There were 16 actual 5-year survivors. This is consistent with other recently published series.10–13 Despite the overall favorable outcome, there are clearly cases with poor prognosis.
The presence of an invasive component in the tumor was a significant predictor of poor outcome. It appears uncommon that adverse oncologic events occur in patients with noninvasive IPMN. Three of 32 patients with noninvasive disease in this series had recurrence of tumor. Two of them were pancreatic stump recurrences in patients with carcinoma in situ (1 of which had a positive margin). The third case was a case of borderline atypia with a normal margin that rapidly died of intra-abdominal recurrence. It is possible that an invasive component was not appreciated in this patient due pathologic sampling error, or a separate unresected focus of invasive cancer was present, as it is difficult to ascribe any biologic mechanism to his rapid demise. A recent series from Johns Hopkins reported similar survival in patients with and without an invasive component, but the cause of death in the patients with noninvasive disease was not known in more than half of the cases.13
Our study demonstrated that other pathologic factors beyond the presence of an invasive component were associated with poor outcome. An increasing extent (size and percent of tumor) of invasive component and the type of invasive component (tubular worse than colloid) were significant predictors of outcome. The presence of vascular and neural invasion both were associated with a poor outcome as well, although only vascular invasion reached statistical significance. Analysis of these factors has not been reported in other series. Lymph node involvement, while not common, was also associated with a poor outcome. This was also demonstrated in the recent study from Johns Hopkins.13 The only clinical factor that was associated with a poor outcome was an elevated serum bilirubin. Additionally, the presence of an elevated serum bilirubin was associated with nearly all the adverse pathologic factors described above. While we do not believe that the bilirubin level is causally related to outcome, it appears to be a significant clinical marker of adverse pathology and outcome.
The extent of resection required for adequate treatment of IPMN of the pancreas is controversial and much of this controversy lies in the status of the margin. The majority of published series have reported a much lower rate of margin positivity, although they usually did not perform specific pathologic re-review of the margins.9,10,13 In a recent series from Italy where margins were specifically analyzed, the rate of margin involvement was approximately 50% and mirrors our experience.12 We recorded even small duct involvement with minimal atypia, a lesion otherwise designated PanIn 1, and regarded to be very common and of minimal clinical significance, as positive. Others may have called this a negative margin.
Our analysis demonstrates that the pathologic characteristics of the primary tumor and not the margin status determine early oncologic outcome. Overall, the rate of disease specific outcomes (recurrence or death) was no different among those with and without involved margins. Of the 4 patients who developed pancreatic stump recurrences, only 1 had an involved margin. The Hopkins group has also reported that the status of the margin had no association with outcome or tumor recurrence.13 The effect of an involved margin, however, must be viewed with caution as IPMN is an indolent neoplasm and follow-up in this series may not be of sufficient length to capture all recurrences. The use of intraoperative frozen sections of the transection margin is a difficult and controversial issue because the true long-term implication of transection margins containing noninvasive disease is not completely understood. When contemplating extended resections or total pancreatectomy one must weigh the long-term morbidity of the procedure against the fact that no benefit has ever been documented. We believe that routine total pancreatectomy is not indicated and should only be performed for obvious extensive, but resectable disease involving the whole gland.
Other series4,12,13 and cases in our series have shown that patients can recur in the pancreatic stump regardless of margin status and that these patients can be re-resected for cure. In contrast, Sho et al21 reported on 4 patients that were re-explored for stump recurrences and all 4 were unresectable because of distant metastases or massive local invasion. There were 2 cases of isolated stump recurrences in our series, both potentially resectable, but only 1 came to operation. Neither of these cases involved invasive disease. The recurrences in patients with invasive disease in our series almost always involved distant sites and were usually multifocal.
In summary, IPMN of the pancreas is a unique tumor that is generally indolent, but has significant malignant potential warranting surgical treatment. We have shown that outcome is dictated by the pathologic characteristics of the tumor. The presence of noninvasive disease at transection margins has little effect on early disease recurrence and disease specific death. Diligent follow-up in patients who are candidates for further surgery is warranted, as there is the potential for curative re-resections of recurrent pancreatic tumor. The extent of resection and intraoperative management of the pancreatic margin remain controversial issues that will only be answered by ongoing long-term follow-up.
Footnotes
Current address of Kevin Conlon: Department of Surgery, Adelaide and Meath Hospital, Dublin, Ireland.
Reprints: Murray F. Brennan, MD, FACS, Chairman, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: brennanm@mskcc.org.
REFERENCES
- 1.Ohashi K, Murakami Y, Muruayama M, et al. Four cases of “mucin-producing” cancer of the pancreas on specific findings of the papilla of vater. Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 2.Nagai E, Ueki T, Chijiwa K, et al. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called “mucinous ductal ectasia”. Am J Surg Pathol. 1995;19:576–589. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima N, Mukai K, Kanai Y, et al. Intraductal papillary tumors and mucinous cystic tumors of the pancreas: clinicopathologic study of 38 cases. Human Pathol. 1997;28:1010–1017. [DOI] [PubMed] [Google Scholar]
- 4.Madura JA, Wiebke EA, Howard TJ, et al. Mucin-hypersecreting intraductal neoplasms of the pancreas: a precursor to cystic pancreatic malignancies. Surgery. 1997;122:786–792. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Ogawa Y, Chijiiwa K, et al. Mucin-hypersecreting tumors of the pancreas: assessing the grade of malignancy preoperatively. Am J Surg. 1996;171:427–431. [DOI] [PubMed] [Google Scholar]
- 6.Azar C, VandeStadt J, Rickaert F, et al. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milchgrub S, Campuzano M, Casillas J, et al. Intraductal Carcinoma of the Pancreas. Cancer. 1992;69:651–656. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV, Olivares Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroenterology. 1996;110:1909–1918. [DOI] [PubMed] [Google Scholar]
- 9.Rivera JA, Fernandez del Castillo C, Pins M, et al. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms—a single malignant clinicopathologic entity. Ann Surg. 1997;225:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traverso LW, Peralta EA, Ryan JA, et al. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas—imaging studies and treatment strategies. Ann Surg. 1998;228:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falconi M, Salvia R, Bassi C, et al. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg. 2001;88:376–381. [DOI] [PubMed] [Google Scholar]
- 13.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siech M, Tripp K, Schmidt-Rohlfing B, et al. Intraductal papillary mucinous tumor of the pancreas. Am J Surg. 1999;177:117–120. [DOI] [PubMed] [Google Scholar]
- 15.Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–994. [DOI] [PubMed] [Google Scholar]
- 16.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas–an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. [DOI] [PubMed] [Google Scholar]
- 17.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary mucinous neoplasms. Semin Diagn Pathol. 2000;17:16–30. [PubMed] [Google Scholar]
- 18.Solcia E, Capella C, Kloppel G. Tumors of the Pancreas. Atlas of Tumor Pathology. 3rd series, fascicle 20. Washington, DC: Armed Forces Institute of Pathology; 1997. [Google Scholar]
- 19.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. [DOI] [PubMed] [Google Scholar]
- 20.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. [DOI] [PubMed] [Google Scholar]
- 21.Sho M, Nakajima Y, Kanehiro H, et al. Pattern of recurrence after resection for intraductal papillary mucinous tumors of the pancreas. World J Surg. 1998;22:874–878. [DOI] [PubMed] [Google Scholar]