Abstract
Objective:
To investigate the efficiency of endovascular smooth muscle cell (VSMC) seeding in promoting healing and stability in already-developed aneurysms obtained by matrix metalloproteases (MMPs)-driven injury.
Summary Background Data:
VSMCs are instrumental in arterial healing after injury and are in decreased number in arterial aneurysms. This cellular deficiency may account for poor healing capabilities and ongoing expansion of aneurysms.
Methods:
Aneurysmal aortic xenografts in rats displaying extracellular matrix injury by inflammation and proteolysis were seeded endoluminally with syngeneic VSMCs, with controls receiving culture medium only. Diameter, structure, and the destruction/reconstruction balance were assessed.
Results:
Eight weeks after endovascular infusion, aneurysmal diameter had increased further, from 3.0 ± 0.3 mm to 10.9 ± 6.5 mm (P = 0.009), and medial elastin content had decreased from 36.5 ± 8.5 to 5.2 ± 5.5 surface-percent (S%; P = 0.009) in controls, whereas these parameters remained stable in the seeded group (3.0 ± 0.3 to 2.7 ± 0.2 mm, P = 0.08; 36.5 ± 8.4 to 31.6 ± 9.7 S%, P = 0.22). VSMC seeding was followed by a decrease in mononuclear infiltration. MMP-1, -3, -7, -9, and -12 mRNA contents were sharply decreased in the diseased wall in response to seeding. Tissue inhibitor of metalloproteinase-1, -2, and -3 mRNAs in the intima were increased in a 2 to 10 magnitude in comparison with controls. Gelatin zymography showed the disappearance of MMP-9 activity and reverse zymography a strong increase in tissue inhibitor of metalloproteinase-3 activity in the seeded group. VSMC-seeded aneurysms were rich in collagen and lined with an endothelium instead of a thrombus in controls.
Conclusions:
VSMCs endovascular seeding restores the healing capabilities of proteolytically injured extracellular matrix in aneurysmal aortas, and stops expansion.
Expansion of already-formed arterial aneurysms once proteolytic injury has started can be interpreted as the result of a chronic healing failure. Endovascular cell therapy using vascular smooth muscle cells is shown to promote healing and stability in already-developed aortic aneurysms obtained after matrix injury by matrix metalloproteases in rats.
Changing locally the biology of arterial aneurysms to promote healing and stability could represent an alternative to prosthetic treatments. Although previous studies using genetic or pharmacological tools have been successful at preventing the degeneration of a normal artery into an aneurysm,1–4 none of these studies addressed the question of whether already-formed experimental aneurysms could be stabilized by a nonprosthetic approach.
Molecular and cellular mechanisms promoting already-formed aneurysm expansion are poorly understood. As a consequence, the structural and biologic changes required to stabilize already formed aneurysms are unknown. The degradation of arterial extracellular matrix by proteases has been shown to be instrumental in the formation of aneurysms, eg, the degeneration of a normal vessel into an aneurysm, regardless the initial mechanism triggering proteolysis, in atherosclerosis, inflammatory vascular diseases and in experimental models.4–8 Studies from human samples have shown that matrix metalloproteinases (MMPs), along with 2 other proteolytic enzyme families, are present in excess in aortic aneurysms.9,10 The role of MMPs in aneurysm formation has been validated by inhibition experiments, or gene expression invalidation, in different animal models.1–4 However, whether the interruption of extracellular matrix degradation would be sufficient to stop aortic dilatation once it has started is poorly documented.
Once extracellular matrix has been injured by proteolysis and aneurysm has formed, the vessel wall is composed of a fragmented medial elastic network, with abundant neovessels in the adventitia and infiltrating macrophages, T and B lymphocytes.9,11,12 The lumen is often separated from blood flow by a luminal thrombus. This disorganized, dilated vessel tends to dilate further,13 with no spontaneous tendency to recover a normal structure. Current treatments are based on the strengthening of the aorta, or its replacement, by means of a prosthesis.14,15
One very specific feature of aneurysmal walls is the depletion in vascular smooth muscle cells (VSMCs) in the media layer.4,8,11,12,16–20 In addition, no VSMCs are present in the luminal thrombus, which is an environment hostile to cell development. Overall, most of the wall mass of aortic aneurysms is depleted in VSMCs. In other pathologic conditions, such as atherosclerotic stenoses, restenosis, and response to injury,21–24 VSMCs appear to be the main cellular component of arterial healing. We hypothesized that adding VSMCs could restore aneurysmal healing capabilities and function, eg, the ability to support hemodynamic constraint without expanding further. The aims of this study were to set-up a model of already-formed, and expanding, aortic aneurysm and to test the impact of VSMC seeding through an endoluminal catheter on expansion, MMP-dependent proteolytic balance regulation and wall structure.
METHODS
Animals
Animals were housed and taken care of according to the European Union Standards received analgesia and were anesthetized with 5 mg/100 g b.w. of pentobarbital I.P.
Operative Procedures
Abdominal aortas from Hartley guinea pig (n = 25, Saint Antoine, France). were decellularized using sodium dodecyl sulfate as a detergent to solubilize cellular components without altering the extracellular matrix structure.7 This method produces a tube of arterial extracellular matrix, which was grafted orthotopically into male Fischer 344 rats (n = 50, Iffa Credo, Lyon, France) during a first surgical procedure.
Syngeneic male Fischer 344 rat VSMCs were isolated from thoracic aortas25 and were grown in RPMI 1640 and Medium 199, with l-glutamine and 10% fetal calf serum (Gibco-BRL). More than 98% of isolated cells grown to 80% confluence were stained positively with an anti-alpha actin antibody (Dakopatts, Denmark).
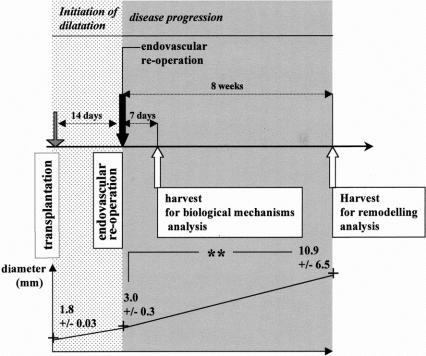
A second surgical procedure was performed 14 days after the first one, a delay at which the xenograft diameter had increased to more than 50%, to comply with aneurysm definition.26 The dilated xenograft was isolated from blood flow by clamps. Its lumen was gently rinsed with culture medium through a PE10 catheter introduced by an aortectomy performed downstream in the native aorta. Passage 5 to 8 VSMCs were resuspended in culture medium with 5% fetal calf serum, injected into the dilated xenograft lumen (107 cells per xenograft), and allowed to attach for 8 minutes. Cell passage in culture was chosen to allow the production of a sufficient number of cells. To assess their presence and location in vivo, in some experiments VSMCs were stained before seeding with the fluorescent dye PKH26 (Sigma).27 As controls, dilated xenografts were infused with culture medium with 5% fetal calf serum without cell. Graft diameter was measured immediately after transplantation at reoperation and before euthanasia, at day 7 or at week 8 after seeding (Fig. 1).
FIGURE 1. Experimental design: 14 days after transplantation, aortic xenografts were dilated to more than 50% of their initial diameter and reoperated by the endovascular route. Graft diameter was measured immediately after transplantation, at reoperation, and before euthanasia. Endovascular infusion of culture medium (control group) was followed 8 weeks later by a statistically significant increase in diameter of the already dilated aorta (curve below, **P < 0.01). Analysis of the inflammatory infiltration, MMP and TIMP expression in vivo, was performed on dilated aortas harvested 7 days after the reoperation, with the assumption that biologic changes at an intermediate delay provide mechanistic explanations for the impact of cell therapy on the remodeling observed 8 weeks after treatment.
Histology and Immunohistochemistry
Cryostat cross sections (5 μm) were made from the center of the xenografts. Computerized quantification of elastin in the media was performed after orcein staining (software from Clara Vision, France). Primary antibodies were mouse antirat monoclonals: ED1 clone for monocytes and macrophages, RLN-9D3 for B cells, R73 for TCR α/β receptor of T lymphocytes, RECA for endothelial cells (Medgene Science, Pantin, France), and 1A4 for alpha-actin (Dakopatts). An alkaline phosphatase-antialkaline phosphatase technique was used (Dakopatts). Control sections were generated by omission of the primary antibody and with a nonrelevant primary antibody. Labeled cells were counted with a grid in the microscope eyepiece. To assess the dynamic of intimal growth after endovascular VSMC seeding, the thickness of the intima was measured with a grid eyepiece on cross sections of the aneurysmal vessels after anti-alpha actin immunostaining. Measurements were made in 4 directions with 90° rotations and averaged for each graft. Comparisons were made between 1- and 8-week delays in the seeding group.
Analysis of MMP, Tissue Inhibitor of Metalloproteinase (TIMP), and Collagen mRNA Contents
MMPs, TIMPs, and collagen I and III mRNA levels were analyzed using reverse transcription polymerase chain reaction (RT-PCR), comparative to the domestic gene 18s (QuantumRNA™ 18s Internal Standards kit, Ambion, Montrouge, France). Intima, on the one hand, and media plus adventitia, on the other hand, were separated by microdissection and pooled by layers and groups. Total RNA was extracted with TRIzol™ (Life Technologies, USA) and treated with grade I DNAse (Roche Molecular Biomedicals). Reverse transcription was done with random primers, and Superscript II (Life Technologies), dNTP, dithiothreitol, and ribonuclease inhibitor (Roche Molecular Biochemicals). PCR was performed in a PCR Express thermo cycler (Hybaid, UK), in the same tube for both the gene of interest (primers are listed in Table 1; Genset Oligos SA, France) and 18s. Final PCR conditions were chosen to avoid interference between the 2 sets of primers. The PCR mix contained Taq Polymerase (EurobioTaq, Eurobio) in buffer, dNTP, and MgCl2. Negative controls were done without Superscript II. Ten microliters of the PCR products was run in a 2% agarose gel with 5 μg/mL ethidium bromide, visualized under UV light by a video camera. Bands of amplified sequences corresponding to the gene of interest and to 18s were quantified with Gel Analyst™ (Iconix, France). Results were expressed as a ratio between signals corresponding to the gene of interest and 18s.
TABLE 1. Primers for PCR (Forward/Reverse)
Analysis of MMP and TIMP Activities
Intima on the one hand, and media plus adventitia on the other hand, from 6 grafts were separated and pooled by groups. After extraction with a guanidine buffer,2 MMP-2 and MMP-9 activities were analyzed on 1% gelatin zymograms. MMP-2– and MMP-9–related bands were quantified with the ImageMaster™ software (Pharmacia Biotech, Uppsala, Sweden). Results were plotted against a standard curve generated on the same gel with purified human recombinant MMP-2 or MMP-9 (Medgene Science, France). To quantify MMP-9 complexed with TIMP molecules, in some experiments, TIMPs were inactivated by reduction-alkylation.2 TIMP activities were detected with a Reverse Zymography Kit2 (University Technologies Intl. Inc., Calgary, Alberta, Canada).
Data Analysis
Results were expressed as mean ± SD. Comparisons between 2 groups were conducted using the nonparametric Mann–Whitney U test (Statview, version 4.5).
RESULTS
A Model of Aortic Extracellular Matrix Injury for Testing Endovascular Cell Therapy in Dilated Aortas
As a first step, we characterized an experimental situation that could be used to evaluate endovascular cell therapy for already dilated arteries. The diameter of all xenografts at day 14 had increased of more than 50% in comparison to value at implantation. Control dilated xenografts at 14 days were infused endoluminally with culture medium plus 5% calf serum during a second procedure and were shown to retain a potential of disease progression (Fig. 1, Fig. 2A). Xenograft diameter increased from 3.2 ± 0.3 mm to 10.9 ± 6.5 mm, P < 0.01 (delta = 7.7 ± 6.4 mm) during the 8 weeks after reoperation, n = 5 in each group (Fig. 2A). Elastin content decreased from 36.5 ± 8.4 surface-percent (S%; value from 5 xenografts after 14 days implantation, used as a reference value for the 2 groups) to 17.2 ± 8.2 S% at 7 days and to 5.2 ± 5.5 S% at 8 weeks, after the second procedure, P = 0.014.
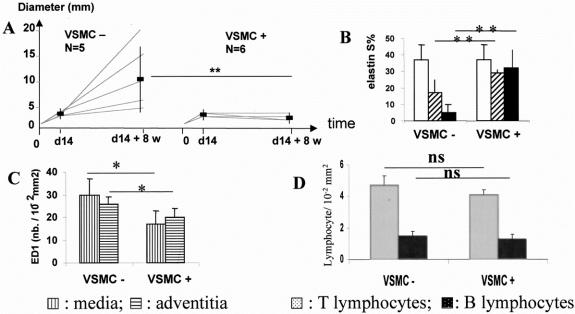
FIGURE 2. Endovascular cell therapy stops aortic disease progression. External diameter (A) increased in the control group infused endoluminally with culture medium with no cell (VSMC−) and was stabilized after endoluminal seeding of VSMCs (VSMC+). Medial elastin content (B) remained constant in dilated aortas stabilized by cell therapy (VSMC+). □, At endovascular reoperation; ⧄: 7 days, and ▪: 8 weeks after endovascular reoperation. S%: percentage of surface of the media occupied by orcein staining. Seven days after endovascular reoperation, monocyte–macrophage infiltration in the media and in the adventitia of xenografts had decreased in the treated group (VSMC+) in comparison with the control group (VSMC−; C), whereas T and B lymphocyte infiltration was not affected by seeding (D). ED1 is a monoclonal antibody specific for rat monocytes and macrophages. *P < 0.01; **P < 0.01; ns: non significant.
Endovascular Cell Therapy Stops the Progression of Aortic Dilation
At reoperation, the diameter of all xenografts had increased more than 50% with no statistical difference between the 2 groups. Eight rats (16,7%) died within 24 hours after endovascular reoperation (3 in the endovascular seeding group, 5 in the control group). At autopsy, no occlusive thrombus was visible in the lumen of the xenograft.
Seeding Efficiency
One week after endovascular seeding, syngeneic VSMCs labeled with the fluorescent dye PKH26 were observed on the endoluminal aspect of dilated aortas, forming a multilayered intima. This result demonstrates that VSMCs seeded into the lumen of the aneurysmal vessels can attach to the thrombus and survive. No PKH-26 positive cells were observed in the adventitia or in the diseased wall itself.
Prevention of Further Dilation
All xenografts of were patent at harvest. In sharp contrast with the control group, the endovascular seeding of VSMCs prevented further diameter increase of dilated aortas (diameter in the seeding group: 3.1 ± 0.3 mm at reoperation versus 2.7 ± 0.17 mm 8 weeks later, P = 0.076; delta: −0.3 ± 0.1 mm). The stabilizing effect was observed for all seeded dilated aortas. The difference in diameter between the 2 groups at 8 weeks after the endovascular reoperation was significant (P < 0.01). The percentage of diameter variation was + 240.5 ± 210.4% in controls and −10.6 ± 9.3% in seeded vessels (P < 0.01; Fig. 2A).
Prevention of Elastin Loss
In contrast with culture medium infusion in controls, the endoluminal seeding of VSMCs prevented further medial elastin degradation at one and 8 weeks (32.1 ± 4.3 S% at one week, and 31.6 ± 9.7 S% at 8 weeks, NS; Fig. 2A). The difference between seeded and nonseeded aortas at 8 weeks was significant (P = 0.01).
Endovascular Cell Therapy Modulates Inflammation
Infiltration by rat monocyte-macrophages identified with the ED1 monoclonal antibody was significantly reduced 1 week after endovascular seeding of VSMCs as compared with nonseeded aortas (Fig. 2B; media: 28 ± 7 vs. 16 ± 6 10−2 ED1+ cells/mm2, P = 0.025; adventitia: 25 ± 3 vs. 19 ± 4 10-2 ED1+ cells/mm2, P = 0.025, n = 5 in the control group, n = 3 in the seeded group). In contrast, neither adventitial infiltration by T lymphocytes (control: 4.7 ± 0.6, seeded: 4.1 ± 0.3 10−2 R73 + cells/mm2, P = 0.29) and B lymphocytes (control: 1.5 ± 0.3, seeded: 1.3 ± 0.3 10−2 RLN-9D3 + cells/mm2, P = 0.29; Fig. 2D), nor the number of adventitial endothelial cells (control: 2.2 ± 0.3, seeded: 2.5 ± 0.1 10−2 RECA + cells / mm2) were modified by seeding.
Endovascular Cell Therapy Decreases MMP Expression
Levels of MMP mRNA
Decellularized xenografts at 2 weeks contained high levels of MMP-1, -2, -3, -7, -9, and -12 mRNA (data not shown). VSMCs in culture before seeding constitutively expressed MMP-2 (Fig. 3A). Xenograft layers microdissection was used to study separately the biologic activity of the seeded cells and their impact on the surrounding dilated aortic wall. Concordant with the VSMC culture data, MMP-2 mRNA contents were significantly increased by seeding in the intima. The expression of the other MMP mRNAs in the intima was not modified by VSMC seeding. In contrast, endovascular seeding of VSMCs decreased mRNA contents by a 3 to 80 magnitude, for all studied MMPs, except MMP-2 (Fig. 3A) in the medial and adventitial layers, ie, in the diseased wall itself. This result was consistently reproduced on different batches of pools of 3 grafts.
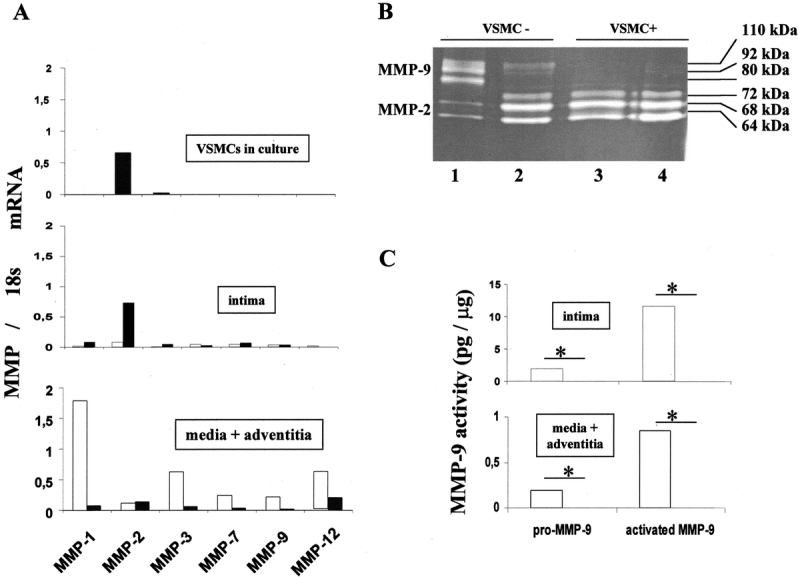
FIGURE 3. Endovascular cell therapy decreases MMP expression in dilated aortas. RT-PCR comparative to the domestic gene 18s for interstitial collagenase (MMP-1), gelatinases (MMP-2 and -9), and elastases (MMP-3, -7, and -12) in VSMCs in culture before seeding and in vivo (intima, media + adventitia) 7 days after the endovascular reoperation (A). After cell therapy (VSMC+), MMP expression had decreased, except constitutive MMP-2, in the media and adventitia corresponding to the diseased aorta itself. RT-PCR data were generated from pools of 3 grafts in each group for each layer. Gelatin zymography (B) shows decreased MMP-9 gelatinolytic activity (Mr: 110 to 80 kd) in the treated vessels (VSMC+). The increase of constitutive MMP-2 expression in VSMC+ vessels reflects increase of cell content in the intima after seeding. Gel zymography quantification of MMP-9 activity (Mr 110 to 80 kd; C). Active forms of MMP-9 are 92- and 80-kd bands. □, Dilated aortas infused with culture medium as a control (VSMC−); ▪, dilated aortas seeded with VSMCs (VSMC +); *P < 0.05.
Gelatinase Activity
On gelatin gel zymography, MMP-9 activity was detected mainly in the thrombus of control xenografts. VSMC seeding suppressed MMP-9 activity (total MMP-9: 13.5 ± 1.62 to 0 equivalent ng rMMP-9/ μg, P < 0.05; active [80 kd] MMP-9: 11.56 ± 1.51 to 0, P < 0.5). Conversely, seeding increased MMP-2 activity in the intima. MMP-9 activity was also detected in the media plus adventitia of control xenografts. Similarly, MMP-9 activity was suppressed in the arterial wall by VSMC intimal seeding (total MMP-9: 1.04 ± 0.12 to 0 equivalent ng rMMP-9/ μg, P < 0.05; active (80 kd) MMP-9: 0.85 ± 0.08 to 0, P < 0.5; Fig. 3B and C).
Effect of Endovascular Cell Therapy on TIMP Expression
VSMCs in culture before seeding expressed high levels of TIMP-1, -2, and -3 (Fig. 4A). Endovascular cell therapy strongly increased TIMP-1 and TIMP-3 mRNA contents 1 week after treatment (6- to 10-fold) (Fig. 4A) in the intima without changing TIMP mRNAs in the media plus adventitia. Reverse zymography evidenced a strong increase in TIMP-3 activity in the media and adventitia of seeded dilated aortas (Fig. 4B).
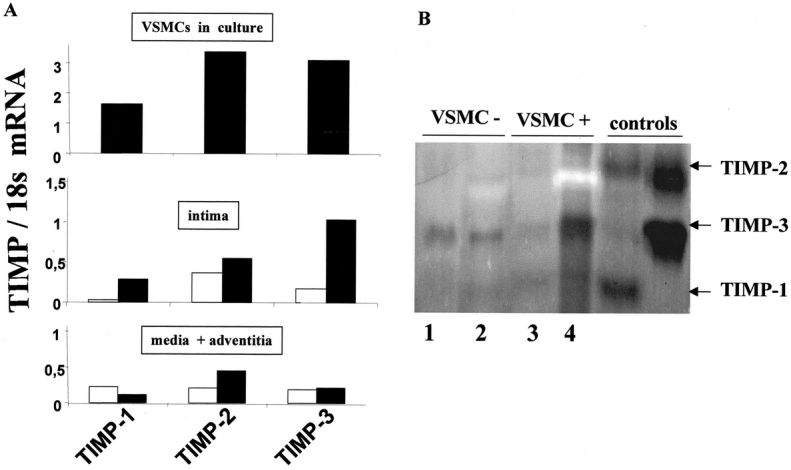
FIGURE 4. Endovascular cell therapy increases TIMP expression. RT-PCR comparative to the domestic gene 18s for TIMP-1, -2, and -3 in VSMCs in culture, before seeding, and in vivo (intima, and media plus adventitia) one week after the endovascular treatment (A). The expression of TIMP-1 and -3 increased in the intima of dilated aortas after treatment (VSMC+), demonstrating that the seeded VSMCs produce high amounts of MMP inhibitors. Reverse zymography (B) shows TIMP activity as dark bands. TIMP-3 was detected in the media and adventitia. □, Dilated aortas infused with culture medium as a control (VSMC−); ▪, dilated aortas seeded with VSMCs (VSMC+). Lanes 1 and 3: intima or thrombus; lanes 2 and 4: media plus adventitia. Reverse zymography was performed with extracts from 3 grafts, pooled by group. RT-PCR data were generated from pools of 3 grafts in each group for each layer.
Endovascular Cell Therapy Increases Fibrillar Collagen Expression
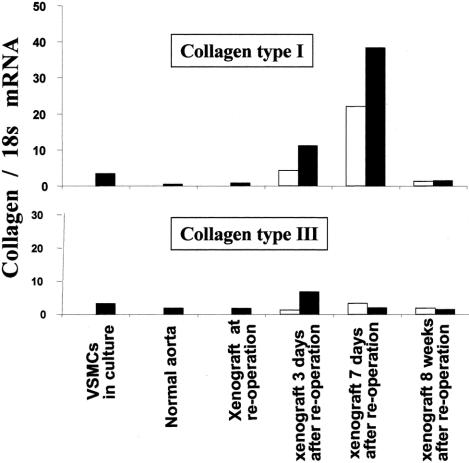
Before seeding, VSMCs in culture expressed high levels of type I and III collagen mRNAs (Fig. 5). Dilated xenografts just before seeding exhibited a massive loss in fibrillar collagen, as shown by Sirius red staining. VSMC seeding increased type I and III collagens mRNA contents in the media and adventitia of dilated xenografts very early on in comparison with untreated dilated aortas and normal rat aortas. Eight weeks after treatment, mRNAs encoding for collagens I and III had decreased to levels similar to those measured in a normal aorta.
FIGURE 5. Endovascular cell therapy induces fibrillar collagen genes expression. The endovascular seeding of VSMCs was followed by an early increase in mRNAs encoding for fibrillar collagens I and III, as shown by RT-PCR analysis from the all wall thickness. The increase is transient. mRNA levels in dilated aortas 8 weeks after the endovascular treatment decrease to those of a normal artery. □, Dilated aortas infused with culture medium, as controls (VSMC−); ▪, dilated aortas seeded with VSMCs (VSMC+). RT-PCR data were generated from pools of 3 grafts in each group for each layer.
Morphology of the Dilated Aortas Stabilized by Endovascular Cell Therapy
Dilated xenografts before endovascular reoperation and 8 weeks after reoperation in the control group contained few alpha-actin positive cells (Fig. 6). A loose collagen network had replaced the destroyed media and adventitia. In sharp contrast, in seeded xenografts 8 weeks after stabilization by cell therapy, VSMCs had accumulated on the luminal aspect, not in the aneurysmal wall itself, and had formed a multicellular intimal layer rich in collagen. The thickness of the intima after endovascular VSMC seeding decreased between 1 and 8 weeks, from 1.35 ± 0.144 mm to 0.65 ± 0.15 mm, respectively (P = 0.034, Mann–Whitney U test). The intima resulting from VSMC accumulation after seeding never resulted in lumen occlusion. In contrast with control vessels, the luminal aspect of xenografts 8 weeks after VSMC seeding was lined by a continuous endothelium (Fig. 6).
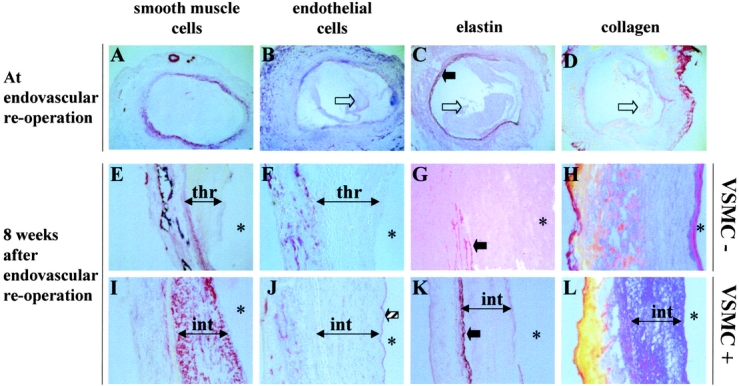
FIGURE 6. Microscopic appearance of dilated aortas. Just before endovascular reoperation (A–D), few smooth muscle cells (alpha actin positive) were located in the media and adventitia (A), neovessels were present in the adventitia, but no endothelial cells were detected in the thrombus (empty arrow; B). The elastin network in the media (plain arrow) was disorganized, often missing (C). Collagen was largely deleted (D). Eight weeks after endovascular reoperation in the control group (E–H), as diameter of dilated aortas had increased further, the endoluminal surface was covered by a thrombus (thr). Elastin in the media had nearly disappeared (G), and little collagen had accumulated (H). Eight weeks after endoluminal cell therapy (I–L), a dense and thick intima (int) covered the luminal aspect where no thrombus could be visualized. This intima was rich in alpha actin-positive cells (I), covered by a continuous endothelium (striped arrow; J). Elastin in the media of the dilated aortas had not undergone further degradation (K), and a dense collagen network occupied the intima (l). The structure of the aneurysm treated by endovascular cell therapy was shifted towards the structure of a normal artery wall. The renewed artery had healed and recovered the ability to contain hemodynamic stain without dilating. *lumen. A, E, I: immunostaining with an anti-alpha actin antibody; B, F, J: immunostaining with anti-rat endothelial cells (RECA clone); C, G, K: orcein staining; D, H, L: Sirius red staining. Nuclei were counterstained with hematoxylin. Original magnifications A–D: ×4; E–L: ×20.
DISCUSSION
Using a model of already developed and expanding aneurysm, this study provides evidence that endovascular VSMC seeding stabilizes aneurysm diameter, blocks the initiated extracellular matrix degradation, and regenerates the diseased wall.
In the xenograft model, the xenogeneic aorta is depleted from cells by a detergent treatment before implantation, so that the graft is composed of an acellular matrix tube harboring no VSMCs.7 With time, as wall infiltration by inflammatory cells proceeds, fibroblasts, endothelial cells and few alpha-actin–positive cells colonize the xenograft. When a significant amount of extracellular matrix has been digested by MMPs,1–3 the aneurysm is formed, and its general structure and cellular content resemble that of a human aneurysm, with a luminal thrombus, a deteriorated medial elastic network and a thickened adventitia with abundant neovessels. Experimental aneurysms in the control group of our experiments reproducibly display a constant expansion rate, reproducing a key feature of human atherosclerotic aneurysms.
Endovascular Cell Therapy Suspends Ongoing Aortic Wall Destruction
The elastin network in the media of human and experimental aneurysms is damaged.6–8,26 We and others have shown that prevention of aortic dilation correlates with medial elastin preservation.1–3 Endovascular VSMC seeding resulted in both diameter and medial elastin content stabilization in already dilated, but stabilized aortas.
Inflammation in the adventitia, and more specifically in the media, is observed in both human and experimental aneurysms9,11,12,26,28 and is thought to be a prominent source of proteases. Endovascular VSMC seeding significantly decreased monocyte-macrophage infiltration in already formed aneurysms. However, the most dramatic effect was a decrease in MMP expression, to levels barely detectable by RT-PCR and zymography, 2 very sensitive methods. Experimental and human atherosclerotic aneurysms exhibit increased levels of MMP.2,4,9,10,29 Concordant experimental data suggest that MMP activities are instrumental in extracellular matrix degradation and aortic dilation.2,3,29 However, no previous experiments had been aimed at decreasing MMP activity in experimental aneurysms already subjected to inflammation and proteolysis, which may be closer to an attempt-to-treat situation in a clinical setting. In previous studies, we have shown that in intact decellularized xenografts, the seeding of syngeneic VSMCs prevents aneurysmal degeneration, suggesting that VSMCs may control the initial steps of extracellular matrix injury.2,30 In the present work, wall layer microdissection demonstrated that MMP expression at mRNA level was decreased in the aneurysmal wall itself, in an area distant from the seeded cells. Additionally, TIMP-3 produced by the intimal cells diffused to the injured wall. Our hypothesis is that endoluminally seeded VSMCs produce paracrine factors, including TIMP-3, which diffuse to the aortic injured wall and down-regulate MMP activity in a centrifugal manner. These data demonstrate that cell therapy can stop the evolution of the aortic destructive process after its onset in the xenograft model.
Endovascular Cell Therapy Promotes Wall Reconstruction
In addition to stopping the initiated aortic wall destruction, VSMC seeding triggered 2 events that promoted endovascular vessel reconstruction. One event was the increase in collagen type I and III mRNA contents and the increase in collagen accumulation at histology, in particular in the thick neointima that had formed, presumably derived from the seeded VSMCs. Aneurysm formation primarily results from the destruction of the fibrillar connective tissue. In vitro, excessive destruction of collagen causes arterial rupture.31 Likewise, the strengthening of already-formed aneurysms might depend on new collagen accumulation. The second event was the accumulation of endothelial cells on the lumen of seeded vessels, whereas no endothelial cell was detected on the thrombus of control vessels, as observed in human aortic aneurysms. This result suggests that endovascular VSMC seeding changes the biology at the interface between blood and aneurysmal vessel, allowing endothelial cell repopulation and endoluminal healing. Of note, although no attempt was made to controlling growth of the intima after endovascular seeding, accumulation of VSMCs never induced vessel occlusion, and tended to stabilize over time.
Study Limitations
The pathophysiology of the xenograft aneurysmal degeneration and that of human atherosclerotic aneurysms are clearly different, whatever role autoimmunity may play in human aortic aneurysms32. However, terminal cellular and enzymatic effectors of extracellular matrix degradation appear to share similarities in the 2 settings.2,9,11,12,30
In the xenograft model, VSMC endovascular seeding may prevent extracellular matrix injury by inducing local changes in the inflammatory infiltration, a consequence of the immune response towards the xenogeneic proteins of the matrix. However B and T lymphocyte infiltration was not significantly modified by VSMC seeding. In contrast, MMP expression was abolished, and TIMPs were strongly induced. Therefore, direct regulation of the MMP-dependent proteolytic balance was the main mechanism by which extracellular matrix degradation was prevented, rather than changes in the immune response. Concordant with the idea that syngeneic cells do not profoundly affect the immune response, the endoluminal seeding of VSMCs syngeneic to the recipient did not modify adventitial inflammation in a rat model of aortic allograft rejection.27
The luminal aspect of aneurysmal xenografts is lined by a thrombus. However, the thrombus in human aneurysms is thicker, multilayered, and has developed during a longer period of time.33 It probably represents an environment hostile to VSMC development. This spontaneous hostility to VSMC growth is also a characteristic of the thrombus in aneurysmal xenografts, as suggested by the absence of VSMCs at 8 weeks in controls. The large number of VSMCs used for seeding may have overwhelmed factors preventing spontaneous cellular repopulation in the thrombus in our experiments.
CLINICAL RELEVANCE AND CONCLUSION
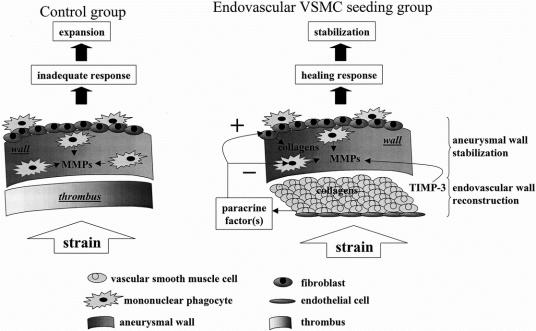
The external diameter is the most powerful parameter for the prediction of aortic rupture.26 Therefore diameter stabilization may represent a relevant clinical endpoint. The diseased aorta in the present study was used as a scaffold to form a new stable vessel incorporating the seeded cells, while VSMC therapy restored a healing potential in spontaneously nonhealing vessels (Fig. 7).
FIGURE 7. Schematic representation of cell therapy and its impact on aneurysmal wall summarizing our hypothesis. The endovascular addition of VSMCs allows the diseased wall to respond to radial hemodynamic stress in an appropriate manner, resulting in diameter stabilization.
Whether VSMC seeding could represent an approach to the stabilization of arterial aneurysms in humans, regardless of their etiology and location, needs further investigations. The viability of VSMCs in contact with the thrombi of atherosclerotic aneurysms is under investigation, as well as the possibility of modifying gene expression in human aneurysmal tissues by VSMC seeding in vitro.
ACKNOWLEDGMENTS
The authors thank Jean-Baptiste Michel, MD, PhD, INSERM 460 for constructive criticism of the manuscript. The authors thank P. Druelle, J. Magnard, and P. Mario for animal care and A. Maurette and N. Sauvant for administrative and secretary assistance.
Footnotes
Supported by the CNRS, La Fondation de l'Avenir pour la Recherche Médicale Appliqué (grant ET7-210), and by a grant PROGRES from INSERM. Mrs B. Muscatelli-Groux was supported by a grant from Laboratoire Louis Lafon, Maisons-Alfort, France.
Reprints: Eric Allaire, Centre de Recherches Chirurgicales, 8, rue du Général Sarrail, 94010 Créteil, France. E-mail: allaire@club-internet.fr.
REFERENCES
- 1.Petrinec D, Liao S, Holmes DR, et al. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg. 1996;23:336–346. [DOI] [PubMed] [Google Scholar]
- 2.Allaire E, Forough R, Clowes MM, et al. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaire E, Hasenstab D, Kenagy RD, et al. Prevention of aneurysm development and rupture by local overexpression of plasminogen activator inhibitor-1 [see comments]. Circulation. 1998;98:249–255. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Moons L, Lijnen R, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–444. [DOI] [PubMed] [Google Scholar]
- 5.MacSweeney ST, Powell JT, Greenhalgh RM. Pathogenesis of abdominal aortic aneurysm. Br J Surg. 1994;81:935–941. [DOI] [PubMed] [Google Scholar]
- 6.Anidjar S, Salzmann JL, Gentric D, et al. Elastase-induced experimental aneurysm in rat. Circulation. 1990;82:973–981. [DOI] [PubMed] [Google Scholar]
- 7.Allaire E, Guettier C, Bruneval P, et al. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994;19:446–456. [DOI] [PubMed] [Google Scholar]
- 8.Osborne-Pellegrin M, Coutard M, Poitevin P, et al. Induction of aneurysms in the rat by stenosing cotton ligature around the inter-renal aorta. Int J Exp Path. 1994;75:179–190. [PMC free article] [PubMed] [Google Scholar]
- 9.Freestone T, Turner RJ, Coady A, et al. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscl Thromb Vasc Biol. 1995;15:1145–1151. [DOI] [PubMed] [Google Scholar]
- 10.Newman KM, Jean C, Li H, et al. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J Vasc Surg. 1994;20:814–820. [DOI] [PubMed] [Google Scholar]
- 11.Allaire E, Bruneval P, Mandet C, et al. The immunogenicity of the arterial extracellular matrix in arterial xenografts. Surgery. 1997;122:73–81. [DOI] [PubMed] [Google Scholar]
- 12.Allaire E, Mandet C, Bruneval P, et al. Cell and extracellular matrix rejection in arterial concordant and discordant xenografts in rat. Transplantation. 1996;62:794–803. [DOI] [PubMed] [Google Scholar]
- 13.Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 14.Dubost C, Allary M, Deconomos N. Resection of an aneurysm of the abdominal aorta. Re-establishment of the continuity by a preserved human arterial graft, with a result after five months. Arch Surg. 1952;64:405–407. [PubMed] [Google Scholar]
- 15.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. [DOI] [PubMed] [Google Scholar]
- 16.Henderson EL, Geng YJ, Sukhova GK, et al. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Candales A, Holmes DR, Liao S, et al. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 18.Powell J. Models of arterial aneurysm: for the investigation of pathogenesis and pharmacotherapy–a review. Atherosclerosis. 1991;87:93–102. [DOI] [PubMed] [Google Scholar]
- 19.Anidjar S, Salzmann JL, Gentric D, et al. Elastase-induced experimental aneurysm in rat. Circulation. 1990;82:973–981. [DOI] [PubMed] [Google Scholar]
- 20.Brophy CM, Tilson JE, Braverman IM, et al. Age of onset, pattern of distribution, and histology of aneurysm development in a genetically predisposed mouse model. J Vasc Surg. 1988;8:45–48. [PubMed] [Google Scholar]
- 21.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 22.Kraiss LW, Kirkman TR, Kohler TR, et al. Shear stress regulates smooth muscle proliferation and neointimal thickening in porous polytetrafluoroethylene grafts. Arterioscler Thromb. 1991;11:1844–1852. [DOI] [PubMed] [Google Scholar]
- 23.Mattsson EJ, Kohler TR, Vergel SM, et al. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245–2249. [DOI] [PubMed] [Google Scholar]
- 24.Clowes AW, Gown AM, Hanson SR, et al. Mechanisms of arterial graft failure. I. Role of cellular proliferation in early healing of PTFE protheses. Am J Pathol. 1985;118:43–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Battle T, Arnal JF, Challah M, et al. Selective isolation of rat aortic wall layers and their cell types in culture. Application to converting enzyme activity measurement. Tissue Cell. 1994;26:943–955. [DOI] [PubMed] [Google Scholar]
- 26.Ernst CB. Abdominal aortic aneurysm. N Engl J Med. 1993;328:1167–1172. [DOI] [PubMed] [Google Scholar]
- 27.Gomes D, Louedec L, Plissonnier D, et al. Endoluminal smooth muscle cell seeding limits intimal hyperplasia. J Vasc Surg. 2001;34:707–715. [DOI] [PubMed] [Google Scholar]
- 28.Anidjar S, Dobrin PB, Eichorst M, et al. Correlation of inflammation infiltrate with the enlargement of experimental aneurysm in rats. J Vasc Surg. 1992;16:139–147. [DOI] [PubMed] [Google Scholar]
- 29.Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allaire E, Hasenstab D, Kenagy R, et al. Overexpression of plasminogen activator-inhibitor prevents arterial extracellular matrix degradation by blocking matrix metalloproteinase activity. Circulation. 1998;98:249–255. [DOI] [PubMed] [Google Scholar]
- 31.Dobrin PB, Baker WH, Gley WC. Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch Surg. 1984;119:405–409. [DOI] [PubMed] [Google Scholar]
- 32.Pearce WH, Koch AE. Cellular components and features of immune response in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:175–185. [DOI] [PubMed] [Google Scholar]
- 33.Adolph R, Vorp DA, Steed DL, et al. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25:916–926. [DOI] [PubMed] [Google Scholar]