Abstract
Objective:
The objective of the study was to compare the results of open versus laparoscopic gastric bypass in the treatment of morbid obesity.
Summary Background Data:
Gastric bypass is one of the most commonly acknowledged surgical techniques for the management of morbid obesity. It is usually performed as an open surgery procedure, although now some groups perform it via the laparoscopic approach.
Patients and Methods:
Between June 1999 and January 2002 we conducted a randomized prospective study in 104 patients diagnosed with morbid obesity. The patients were divided into 2 groups: 1 group with gastric bypass via the open approach (OGBP) comprising 51 patients, and 1 group with gastric bypass via the laparoscopic approach (LGBP) comprising 53 patients. The parameters compared were as follows: operating time, intraoperative complications, early (<30 days) and late (>30 days) postoperative complications, hospital stay, and short-term evolution of body mass index.
Results:
Mean operating time was 186.4 minutes (125–290) in the LGBP group and 201.7 minutes (129–310) in the OGBP group (P < 0.05). Conversion to laparotomy was necessary in 8% of the LGBP patients. Early postoperative complications (<30 days) occurred in 22.6% of the LGBP group compared with 29.4% of the OGBP group, with no significant differences. Late complications (>30 days) occurred in 11% of the LGBP group compared with 24% of the OGBP group (P < 0.05). The differences observed between the 2 groups are the result of a high incidence of abdominal wall hernias in the OGBP group. Mean hospital stay was 5.2 days (1–13) in the LGBP group and 7.9 days (2–28) in the OGBP group (P < 0.05). Evolution of body mass index during a mean follow-up of 23 months was similar in both groups.
Conclusions:
LGBP is a good surgical technique for the management of morbid obesity and has clear advantages over OGBP, such as a reduction in abdominal wall complications and a shorter hospital stay. The midterm weight loss is similar with both techniques. One inconvenience is that LGBP has a more complex learning curve than other advanced laparoscopic techniques, which may be associated with an increase in postoperative complications.
Laparoscopic gastric bypass has clear advantages over open gastric bypass, such as a reduction in abdominal wall complications and a shorter hospital stay. The midterm weight loss is similar with both techniques.
Morbid obesity is a serious health problem that occurs more and more frequently and at younger ages, usually associated with a series of comorbidities that justify its treatment. According to the National Institutes of Health in 1991,1 surgery is the only effective treatment of morbid obesity, indicated by a body mass index (BMI) of >40 or >35 with associated comorbidities. The most commonly used surgical techniques are vertical banded gastroplasty and gastric bypass, the latter regarded by some as the gold standard for the surgical treatment of obesity.2–4 The laparoscopic approach for treating morbid obesity has increased considerably in recent years because of the use of simple techniques involving low morbidity and mortality rates, such as gastric banding.5,6 As the surgeon's experience and ability progress, other more complex techniques, such as gastric bypass or biliopancreatic diversions, are performed via laparoscopic surgery.7–9 The aim of this randomized prospective study is to compare open versus laparoscopic gastric bypass in a work group with previous experience in the surgical treatment of obesity and in advanced laparoscopic surgery.
PATIENTS AND METHODS
Between June 1999 and January 2002, we conducted a randomized prospective study in 104 patients diagnosed with morbid obesity and with selection criteria of BMI >40 kg/m2 without coexisting pathologic disorders or BMI >35 kg/m2 with coexisting pathologic disorders. The patients were evaluated by the Psychiatry, Endocrinology, Anesthesia and Surgery units to rule out significant contraindications for surgery. They all gave their written consent for each of the operations. They were divided into 2 groups: 1 group with gastric bypass via the open approach (OGBP) comprising 51 patients, and 1 group with gastric bypass via the laparoscopic approach (LGBP) comprising 53 patients. Both were similar with regard to age, gender, preoperative weight, and BMI (Table 1), with no significant between-group differences. The parameters compared were as follows: operating time; intraoperative complications; early (<30 days) and late (>30 days) postoperative complications; hospital stay; and short-term evolution of BMI.
TABLE 1. Patient Characteristics

Surgical Technique
All the patients in the pre- and postoperative periods were given an antibiotic prophylaxis with clavulanic amoxicillin (2 g iv/8 hours/48 hours) and an antithrombotic prophylaxis with low-molecular-weight heparin (40 mg/12 hours/45 days) and lower extremities compression. The laparoscopic gastric bypass was performed according to the technique previously described by us,10 with creation of a small pouch between the first and second coronary vessels and the angle of His after inserting the head of the circular stapler (Ethicon™) through the gastrotomy. The intestine is dissected 60–80 cm from the angle of Treitz. A side-to-side jejuno-jejunal anastomosis is performed 150–200 cm from the dissection using a linear stapler, and the opening for the insertion of the stapler is closed with running suture. The bowel loop for the gastroanastomosis is drawn up transmesocolic and antegastric, the anastomosis is done with a 21-mm circular stapler, and the anterior face is reinforced with loose sutures to relieve tension. The defect in the transverse mesocolon is closed with several interrupted sutures.
The open gastric bypass was performed via a bilateral subcostal incision to create the gastric pouch with stapling of the bariatric TEA 90 from the lesser curvature without division of the stomach, 4 cm from the esophagogastric junction, as far as the angle of His. A gastrotomy is then created in the pouch, through which the head of the circular stapler is inserted and then secured with a purse string. Division of the small intestine (biliopancreatic loop) is performed 60–80 cm from the angle of Treitz, and from here the jejunoileal anastomosis is created at 150–200 cm depending on the patient's degree of obesity (alimentary loop). An antegastric transmesocolic gastrojejunal anastomosis is created with the circular stapler with reinforcement of the anterior face of the anastomosis to relieve the tension. A Penrose-type drain is placed in all the patients, which is then removed on the third or fourth day.
All the patients received a control intestinal transit on the second or third postoperative day with gastrograffin, and if it was normal they began taking fluids. The patients were seen in follow-up postoperatively at 15 days, and 3, 6, 12, 18, 24 months and annually thereafter. There was a 100% follow-up.
Randomization was performed before assessment by computer generated numbers, details of which were concealed in sequentially numbered, sealed opaque envelopes. The mean, standard deviation, medians and range were calculated for all data. The comparisons between the 2 groups were performed with the Student t test for quantitative variables and with the χ2 Pearson test for qualitative variables.
RESULTS
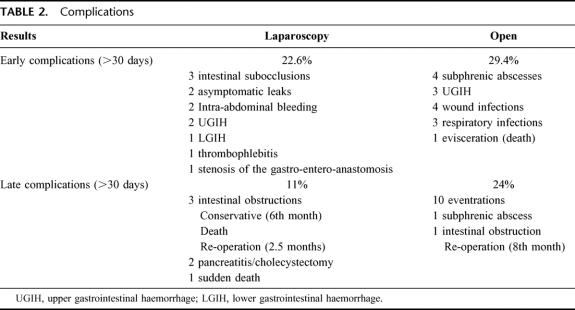
The mean operating time was 186.4 minutes (125–290) in the LGBP group and 201.7 minutes (129–310) in the OGBP group (P < 0.05). In the LGBP group conversion to laparotomy was necessary in 4 patients (8%) due to extreme hepatomegaly, portal hypertension secondary to hepatic cirrhosis discovered in the course of the operation, anesthetic problems (hypercapnia), and splenic lesion during dissection of the angle of His. All the conversions occurred in the first 20 patients. In the OGBP group there were intraoperative complications in 4 patients (8%): 3 splenectomies and 1 splenic vein tear requiring suture. Three patients died in the postoperative period, 2 in the LGBP group, 1 case not related with the surgical procedure and 1 in the OGBP group. Complications in the early postoperative period (<30 days) occurred in 12 patients (22.6%) of the LGBP group versus 15 patients (29.4%) of the OGBP group, with no significant differences (Table 2). Of note in the LGBP group were 2 asymptomatic leaks of the gastro-entero-anastomosis, diagnosed during a control intestinal transit performed in the immediate postoperative period. A further transit was performed at 6 days, with no evidence of leaks, and a liquid diet was started, with good patient progress. Two patients developed intra-abdominal bleeding revealed by blood in the drains, without hemodynamic repercussions or need for transfusion, which ceased spontaneously, probably because of bleeding of the gastric dissection line. Two patients presented with an upper gastrointestinal hemorrhage, 1 requiring blood transfusion, with bleeding at the gastrojejunal anastomosis, which sclerosed with upper gastrointestinal endoscopy. Another patient presented with a lower gastrointestinal hemorrhage with no hemodynamic repercussions or need for transfusion, probably due to bleeding of the entero-anastomosis. Another patient presented with a stenosis of the gastro-entero-anastomosis, which required endoscopic dilatation. Four patients in the OGBP group presented with a subphrenic abscess, which was drained by radiologic puncture. The origin of these abscesses is unclear, as all the patients presented with a normal gastrointestinal transit; it cannot be ruled out that the origin was due to leaks occurring in the late postoperative period. Three had an upper gastrointestinal hemorrhage, which evolved favorably with medical treatment without the need for transfusion, and 1 patient died of broncho-aspiration in the reoperation for evisceration secondary to coughing on the 2nd postoperative day.
TABLE 2. Complications
There were late complications (>30 days) in 11% of the LGBP group compared with 24% of the OGBP group, with statistically significant differences (P < 0.05) (Table 2). Of note in the LGBP group was a sudden death on postoperative day 32 due to a possible pulmonary thrombo-embolism (no autopsy) and 3 intestinal obstructions: 1 resolved with medical treatment and 2 requiring surgery: 1 was caused by a hernia through the mesocolon. Another patient presented with an internal hernia between the mesocolon and the mesentery of the small intestine (Peterson's space), which caused an obstruction of the alimentary loop leading to perforation of the gastrojejunal anastomosis with acute peritonitis. Undergoing surgery, the patient presented with multiorgan failure in the postoperative period and died at 48 hours. In the OGBP group there was a subphrenic abscess, which was drained by radiologic puncture, and an intestinal obstruction due to adhesions, which was operated on 8 months after surgery. The differences observed between the 2 groups are due to the presence of 10 abdominal wall hernias (Table 2) in the OGBP group, 3 receiving surgery and 7 awaiting operation.
Mean hospital stay was 5.2 days (1–13) in the LGBP group and 7.9 days (2–28) in the OGBP group (P < 0.05). Evolution of BMI in both groups during a follow-up averaging 23 months is shown in Figure 1, with no significant between-group differences.

FIGURE 1. Evolution of the percentage of excess weight loss (%EWL) and BMI.
DISCUSSION
Since the introduction of laparoscopic surgery, a wide variety of techniques have been performed via this approach, some widely accepted such as cholecystectomy or treatment of gastroesophageal reflux disease, and others the subject of heated debates such as the treatment of colorectal cancer. This relentless advance of laparoscopic surgery has been due to the advantages it offers, ie, less pain in the postoperative period, short hospital stay, early return to normal physical activity, better esthetic results and a decrease in incisional hernias. It also causes less alteration in systemic and immunologic stress. As laparoscopic surgery is a minimally invasive technique it would benefit patients with morbid obesity since they present with a series of associated comorbidities that make them more susceptible to complications in the postoperative period. Therefore, surgeons began using the different techniques that exist for treating obesity via the laparoscopic approach. Gastric banding was the first laparoscopic technique described, which became extremely popular due to its relatively low technical difficulty and low morbidity and mortality rates, but the long-term results regarding weight loss are still unknown.5,6 Another of the procedures performed via laparoscopy is vertical banded gastroplasty. This technique is not difficult to perform by laparoscopy and has low morbidity and mortality rates, but the long-term results are worse than with gastric bypass.11,12 This technique is used by few bariatric surgeons due to the discomfort it causes the patient and the high incidence of staple-line disruption.13 Gastric bypass is the operation recommended by the National Institutes of Health for treatment of morbid obesity due to its low morbidity and mortality rates and excellent long-term results regarding weight loss. Witgrove was the first to perform it via the laparoscopic approach in 1994.7 Since then, others have performed this technique with different technical variations such as creation of a gastro-entero-anastomosis, Roux-en-Y positioning of the loop in relation to the colon and stomach, the length of this loop, and use of an entero-enterostomy, the great majority reporting good results.8,14–16 Others, however, have not seen their perspectives on LGBP maintained and attribute this mainly to the learning curve, which is more difficult than for other laparoscopic procedures.17 For example, anastomotic leak is the most feared complication when performing gastric bypass, with an incidence of 1–2%,18 which increases with laparoscopic surgery but gradually decreases as the surgeon's experience grows.16 In our study there were 2 patients in the LGBP group who were totally asymptomatic and diagnosed during the control transit in the postoperative period. These leaks occurred in the first 20 patients and in our experience are directly related to the learning curve.
Intestinal obstruction in the immediate postoperative period is a complication associated with LGBP (1%-5%).8,16 These obstructions are due to strictures of the enteroenterostomy, since the defect is closed with an endoscopic stapler, which may result in a stenosis or to internal hernias through the mesocolon or between the jejunal mesentery and mesocolon (Peterson's space). Stenosis of the entero-enterostomy can be avoided if closure of the defect is done with manual suture; as for internal hernias, is easy to avoid those across the orifice of mesocolon closing the defect. However the orifice between the jejunal mesentery and the mesocolon to avoid Peterson's hernia is difficult to close via laparoscopy. There were no obstructions in our OGBP group, as all the possible defects were closed, but there were 3 obstructions in the LGBP group: 1 due to a hernia through the mesocolon, since at the beginning of the series this defect was not closed, and another with fatal consequences due to a Peterson hernia. This defect is habitually not closed when an OGBP is performed, because the incidence of herniation through it is low due to adhesion formation in the postoperative period. Conversely, when a LGBP is performed there is less formation of adhesions and the small intestine can insert easily through this defect, so it is preferable to close it. This maneuver is quite difficult via the laparoscopic approach. This is why we currently draw up the bowel loop antecolic and antegastric in Roux-en-Y to avoid this potential complication.
Stenosis of the gastrojejunostomy is a common complication following gastric bypass (3–12%), via both the open and laparoscopic approaches.19 The cause of this stenosis is unclear.20 Our incidence was 2% in the LGBP group, which is relatively low, possibly because we establish the anastomosis on the anterior face of the gastric pouch, 7–10 mm from the transsection line, leaving a well-vascularized anastomosis as shown by a 4% incidence of bleeding with a gastrojejunal anastomosis.
The incidence of intra-abdominal bleeding was greater in the LGBP group, since to create the gastric pouch the stomach is dissected with the stapler, leaving a suture line which often presents bleeding despite using a 3.5-mm stapler (blue load) and performing careful hemostasis of all the dissection lines with electrocautery if the hemorrhage is of little significance and occasionally with running and interrupted manual suture if it is a gushing hemorrhage. Conversely, dissection of the gastric pouch instead of stapling avoids the sometimes high incidence of staple-line disruption.14
Pulmonary thromboembolism is a cause of morbidity and mortality in surgery for morbid obesity. After gastric bypass via both the open and laparoscopic approaches the incidence is 0%-3%.21 This incidence is no greater after laparoscopic surgery, providing there is adequate prophylaxis.
One of the disadvantages of laparoscopic surgery is that the costs in the operating theater are higher because of the use of nonreusable instruments and longer operating times.8,17 This increase in intraoperative costs may be compensated by a shorter intensive care stay during the first hours or days of the postoperative period, a shorter hospital stay and a lower incidence of incisional hernias that require subsequent reoperations, as has been shown in this study. Operating time decreases considerably as the surgeon's experience progresses. In our experience, operating time is longer in OGBP, probably because of the time spent during opening and closure of the laparotomy and because to create the gastric pouch we perform dissection, probably excessive, of the angle of His and the greater curvature of the stomach.
Weight loss was similar in both groups, although a longer follow-up is necessary. However, it is expected that if the only thing that changes is the method of approach and the surgical technique is similar, the long-term results should not be different.
In conclusion, we think that LGBP is superior to OGBP for treating morbid obesity. In this type of patient it highlights certain advantages of laparoscopic surgery, such as the incidence of abdominal wall complications, and the midterm results for weight loss are similar to OGBP. As a disadvantage LGBP involves a more complex learning curve than other advanced laparoscopic techniques, which may be associated with an increase in postoperative complications.
Footnotes
Reprints: Juan A. Luján, MD, PhD, Servicio de Cirugía General, Hospital Universitario “Virgen de la Arrixaca,” 30120 El Palmar. Murcia, Spain. E-mail: jlujanm@teleline.es.
REFERENCES
- 1.NIH Conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 2.Reinhold RB. Late results of gastric bypass surgery for morbid obesity. J Am Coll Nutr. 1994;13:326–331. [DOI] [PubMed] [Google Scholar]
- 3.Fobi MA, Lee H, Holness R, et al. Gastric bypass operation for obesity. World J Surg. 1998;22:925–935. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman HJ, Kellum JM, Engle KM, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr. 1992;55(suppl 2):560s–566s. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Abeid S, Szold A. Results and complications of laparoscopic adjustable gastric banding an early an intermediate experience. Obes Surg. 1999;9:188–190. [DOI] [PubMed] [Google Scholar]
- 6.Berrevoet F, Pattyn P, Cardon A, et al. Retrospective analysis of laparoscopic gastric banding technique: short-term and midterm follow-up. Obes Surg. 1999;9:272–275. [DOI] [PubMed] [Google Scholar]
- 7.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopy gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4:353–7. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10:514–523. [DOI] [PubMed] [Google Scholar]
- 10.Luján JA, Hernandez Q, Frutos MD, et al. Laparoscopic gastric bypass in the treatment of morbid obesity. Preliminary results of a new technique. Surg Endosc. 2002;16:1658–1662. [DOI] [PubMed] [Google Scholar]
- 11.Howard L, Malone M, Michalek A, et al. Gastric bypass and vertical banded gastroplasty. Obes Surg. 1995;5:55–60. [DOI] [PubMed] [Google Scholar]
- 12.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus nonsweets eaters. Ann Surg. 1987;205:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLean LK, Rhode BM, Forse RA. Late results of vertical banded gastroplasty for morbid obesity and super obesity. Surgery. 1990;107:20–25. [PubMed] [Google Scholar]
- 14.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass:1040 patients—what have we learned? Obesity Surg. 2000;10:509–513. [DOI] [PubMed] [Google Scholar]
- 15.De la Torre RA, Scott JS. Laparoscopic Roux-en-Y gastric bypass: a totally intra-abdominal approach—technique and preliminary report. Obes Surg. 1999;9:492–498. [DOI] [PubMed] [Google Scholar]
- 16.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westling A, Gustavsson S. Laparoscopic vs open Roux-en-Y gastric bypass: a prospective, randomized trial. Obes Surg. 2001;11:284–292. [DOI] [PubMed] [Google Scholar]
- 18.Buckwalter JA, Herbst CA. Leaks occurring after gastric bariatric operations. Surgery. 1988;103:156–160. [PubMed] [Google Scholar]
- 19.Sanyal AJ, Sugerman HJ, Kellum JM, et al. Stomal complications of gastric bypass: incidence and outcome of therapy. Am J Gastroenterol. 1992;87:1165–1169. [PubMed] [Google Scholar]
- 20.Pope GD, Goodney PP, Burchard KW, et al. Peptic ulcer/stricture after gastric bypass: a comparison of technique and acid suppression variables. Obes Surg. 2002;12:30–33. [DOI] [PubMed] [Google Scholar]
- 21.Hall JC, Watts JM, O′Brien PE, et al. Gastric surgery for morbid obesity. The Adelaide Study. Ann Surg. 1990;211:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]