Abstract
Background:
Radiofrequency ablation (RFA) has become a common treatment of patients with unresectable primary and secondary hepatic malignancies. We performed this prospective analysis to determine early (within 30 days) and late (more than 30 days after) complication rates associated with hepatic tumor RFA.
Methods:
All patients treated between January 1, 1996 and June 30, 2002 with RFA for hepatic malignancies were entered into a prospective database. Patients were evaluated during RFA treatment, throughout the immediate post RFA course, and then every 3 months after RFA to assess for the development of treatment-related complications.
Results:
A total of 608 patients, 345 men (56.7%) and 263 women (43.3%), with a median age of 58 years (range 18–85 years) underwent RFA of 1225 malignant liver tumors. Open intraoperative RFA was performed in 382 patients (62.8%), while percutaneous RFA was performed in 226 (37.2%). The treatment-related mortality rate was 0.5%. Early complications developed in 43 patients (7.1%). Early complications were more likely to occur in patients treated with open RFA (33 [8.6%] of 382 patients) compared with percutaneous RFA (10 [4.4%] 226 patients, P < 0.01), and in patients with cirrhosis (25 [12.9%] complications in 194 patients) compared with noncirrhotic patients (31 [7.5%] complications in 414 patients, P < 0.05). Late complications arose in 15 patients (2.4%) with no difference in incidence between open and percutaneous RFA treatment. The combined overall early and late complication rate was 9.5%.
Conclusions:
Hepatic tumor RFA can be performed with low mortality and morbidity rates. Though relatively rare, late complications can develop and physicians performing hepatic RFA must be cognizant of these delayed treatment-related problems.
A prospective database of 608 patients who underwent radiofrequency ablation (RFA) of malignant liver tumors was queried to determine early (within 30 days of RFA) and late (more than 30 days after RFA) complication rates. Early and late complications arose in 7.1% and 2.4% of patients, respectively. The overall complication rate was 9.5%.
A burgeoning number of direct intratumoral therapies are being used to treat human solid tumors. One of the most common sites of application of these tumor-directed treatments has been the liver. The liver is second only to lymph nodes as a common site of metastasis from nonhepatic malignancies.1 Primary liver cancer, specifically hepatocellular carcinoma (HCC), is 1 of the most common human solid malignancies worldwide, with an annual incidence of over 1 million new diagnoses.2 A proportion of patients with primary or secondary hepatic malignancies with disease confined to the liver will derive long-term survival benefit from surgical resection of their disease. Unfortunately, less than 10%–30% of patients with primary or secondary hepatic malignancies are candidates for surgical resection because of the number of tumors, location of tumors that preclude a margin-negative resection, or because of coexistent chronic liver dysfunction producing an unacceptable risk of liver failure after partial hepatectomy.3–7
Patients with liver-only malignant disease who are not candidates for resection may be offered a rapidly evolving menu of direct tumor cytodestructive treatments. The in situ destruction of unresectable primary and secondary hepatic malignancies can potentially improve the median survival of patients and provide palliative relief of symptoms. The latter is particularly true in patients with pain related to tumor displacement of the hepatic capsule or in patients with symptoms related to excess hormone production from metastatic neuroendocrine tumors. Destruction of unresectable hepatic tumors has been performed by direct intratumoral injection of cytotoxic substances, including absolute ethanol, acetic acid, heated hypertonic saline, or chemotherapy agents; by intratumoral placement of cryoprobes to freeze tumors; or more recently by intratumoral placement of needles or fibers that generate heat with radiofrequency electrical current, microwaves, or laser to produce thermal tissue necrosis. An ideal direct in situ antitumor therapy would produce complete destruction of all malignant cells with no significant side effects or complications. Clearly, no such treatment exists and all in situ cytodestructive treatments must be evaluated based on improvements in patient survival rates, local tumor control rates, and complications associated with treatment.
Thermal ablation techniques, particularly radiofrequency ablation (RFA), to treat primary and secondary hepatic malignancies have gained widespread availability and use over the past 5 years.8–11 The local control and complication rates associated with microwave and laser ablation have been reported rarely; thus, it is difficult to assess the treated tumor control efficacy and risk to patients using these treatment modalities.8 Treatment-related complications are not always reported in hepatic tumor RFA studies and the complication types and incidence rates in a large, prospective series of patients have not been previously reported.10–12
MATERIALS AND METHODS
All patients treated by the hepatobiliary tumor surgery group at the University of Texas M.D. Anderson Cancer Center in Houston, Texas, U.S.A. and the G. Pascale National Cancer Institute in Naples, Italy were entered into a prospective database established in 1995. The database was queried to identify all patients who underwent RFA to treat malignant hepatic tumors. Clinical research protocols to evaluate RFA as a treatment of unresectable hepatic malignancies were approved by the Ethics and Institutional Review Boards of both institutions late in 1995. Patients were treated with RFA beginning January 1996. All patients treated with RFA as a component of therapy for malignant hepatic tumors from January 1996 through June 2002 were evaluated for this study to assess treatment-related complications that developed within 30 days (early) or more than 30 days (late) after RFA.
Specific data gathered in patients undergoing RFA include the number of tumors treated with RFA, number of additional tumors resected during the same operative procedure, the diameter of all tumors treated with RFA, the intrahepatic location (Couinaud's segment) of all tumors, proximity of tumors to major vascular structures, and the treatment approach used (percutaneous versus intraoperative). Any complication associated with RFA or the surgical procedure requiring treatment is recorded in the database, as are the date of diagnosis, management of the complication, and the ultimate patient outcome. A laboratory abnormality or radiographic finding is not scored as a complication if no treatment is required. For example, the majority of patients after open or percutaneous RFA of right hepatic lobe tumors will develop radiographic evidence of a right pleural effusion. This is not scored as a complication if it is asymptomatic and resolves without treatment. If treatment with a thoracentesis and/or diuresis is needed, the pleural effusion is scored as a treatment-related complication.
Patients in this series were treated using the RF 2000 or RF 3000 generator system produced by Boston Scientific Corporation/RadioTherapeutics (Natick, MA). The RF 2000 system consists of a generator which supplies up to 100W of power, while the RF 3000 system provides up to 200W of power. Indifferent dispersive electrode pads applied to the patient's legs are attached to the generator and the generator is connected to a LeVeen monopolar multiple array RF needle electrode. The technique, treatment planning, and algorithms for hepatic tumor RFA using this equipment has been previously reported.10–12 Any evidence of excessive bleeding from the needle insertion site upon withdrawal of the RFA needle electrode was measured and all organs and structures adjacent to the liver were carefully inspected for any evidence of thermal injury. In the intraoperative treatment group, some patients underwent resection of dominant or large tumors in 1 lobe of the liver and RFA of smaller tumors in the contralateral lobe during a single operative procedure.
All patients were followed during their inpatient hospitalization for any evidence of acute complications. Patients were evaluated with physical examination and serum laboratory tests in the outpatient clinic within 2 weeks after RFA treatment, and were then seen at 3-month intervals with a chest radiograph, abdominal computed tomography (CT) or magnetic resonance imaging (MRI) scan, serum liver function tests, and serum tumor markers as appropriate. Chest radiographs and abdominal CT or MRI scans were used to detect evidence of recurrent tumor in RFA-treated lesions, to monitor for the development of new hepatic or extrahepatic metastatic disease, and to detect any late sequelae or complications related to RFA treatment.
A general linear model univariate and multivariate analysis of variables to detect associations with the development of early or late complications after hepatic RFA was performed. Variables analyzed included patient age, sex, tumor histology, number of tumors treated with RFA, size of tumors treated with RFA, proximity of treated tumors to major vascular structures, percutaneous versus intraoperative treatment, partial hepatectomy performed in association with RFA of additional malignant tumors, hepatic segmental location of tumors treated with RFA (central: segments 1, 4, 5, and 8 versus peripheral: segments 2, 3, 6, and 7), presence of cirrhosis, and any prior systemic or regional chemotherapy. Analysis of statistical significance of differences between groups was performed using the χ2 test with Yate's correction, univariate and multivariate regression analysis, or ANOVA (analysis of variance) using SPSS statistics software (SPSS, Inc., Chicago, Illinois).
RESULTS
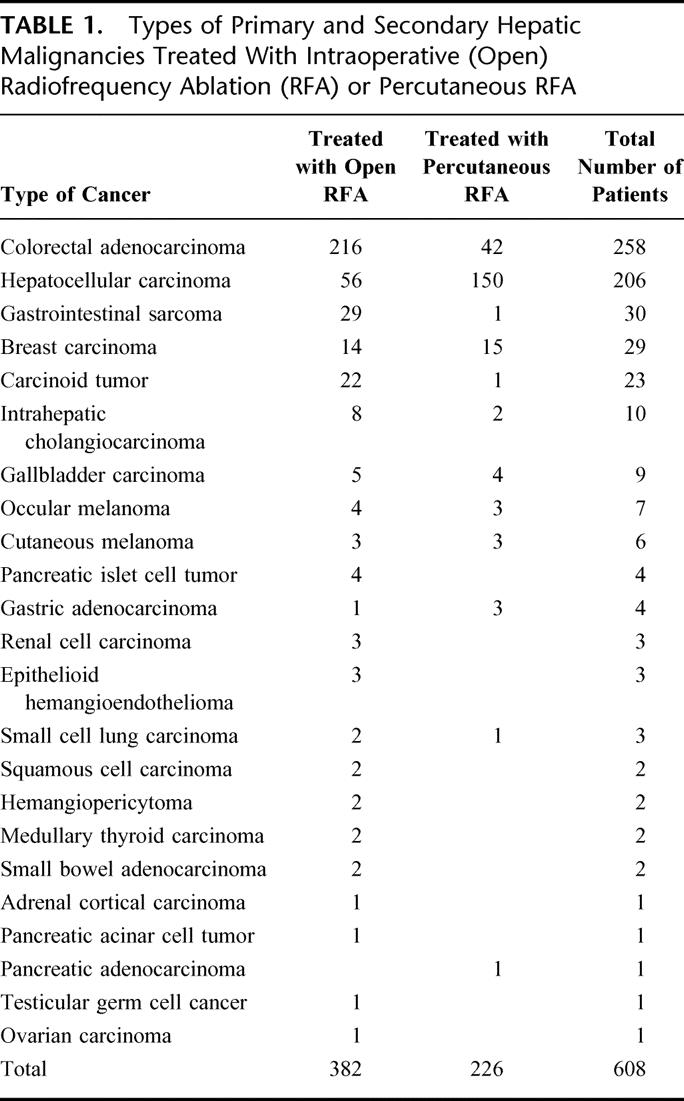
Six hundred and eight consecutive patients who underwent RFA of malignant hepatic tumors were identified from our prospective hepatobiliary tumor surgery database. None of these 608 patients were lost to follow-up during the study period. Of these, 345 (56.7%) were men and 263 (43.3%) were women. The age of the patients treated with RFA ranged from 18–85 years (median 58 years). The types of primary and secondary hepatic malignancies treated are listed in Table 1. Open intraoperative RFA was performed in 382 patients (62.8%) and ultrasound-guided percutaneous RFA was performed in 226 (37.2%). A total of 1225 tumors were treated with RFA, an average of 2.0 tumors per patient with a range of 1–12 tumors treated per patient. A total of 626 RFA procedures were performed in the 608 patients because 18 patients developed new hepatic tumors detected during follow-up that were treated with a second course of thermal ablation. The size of tumors treated with open RFA ranged from 0.4–12.0 cm in diameter (2.7 cm ± 1.7 cm), compared with a range of 1.0–10.0 cm in diameter (2.6 cm ± 1.1 cm) for tumors treated percutaneously. The average number of tumors treated percutaneously was 1.4 per patient (316 tumors in 226 patients), which was significantly less than the average of 2.4 tumors per patient (909 tumors in 382 patients) treated during an open surgical procedure (P < 0.01). Of the 382 patients treated with open RFA, 184 (48.1%) underwent segmental, lobar, or nonanatomic resection of large or dominant tumors, followed immediately by RFA of the remaining tumors during the same surgical procedure.
TABLE 1. Types of Primary and Secondary Hepatic Malignancies Treated With Intraoperative (Open) Radiofrequency Ablation (RFA) or Percutaneous RFA
Early complications occurred in 43 patients (7.1%). As seen in Table 2, patients undergoing open RFA had a significantly higher early complication rate (33 [8.6%] complications in 382 patients), compared with patients treated percutaneously (10 [4.4%] complications in 226 patients, P < 0.01). In the 43 patients who developed an early complication, 19 had HCC; 15 had colorectal cancer metastases; 2 each had gastrointestinal sarcoma, gallbladder, or breast cancer metastases; and 1 each had cholangiocarcinoma, carcinoid tumor, or pancreatic islet cell tumor metastases. The RFA-related mortality rate was 0.5% (3 of 608 patients). One patient with bilobar colorectal liver metastases and significant fatty infiltration of the liver underwent right hepatic lobectomy and RFA of 2 left lobe tumors; this patient died 10 days after operation because of progressive liver failure. Another patient with breast cancer liver metastases and a history of chronic obstructive pulmonary disease died 2 weeks following percutaneous RFA after developing a bacterial pneumonia on post-treatment day 6; the patient progressed to respiratory failure, sepsis, and multisystem organ failure. The third death after RFA occurred 3 days after open surgical treatment of unresectable colorectal liver metastasis in a patient with no history of cardiac problems; however, bleeding into a tumor treated with RFA caused hypotension leading to a fatal myocardial infarction.
TABLE 2. Complications Developing Within First 30 Days After Radiofrequency Ablation (RFA) of Primary or Secondary Hepatic Malignancies
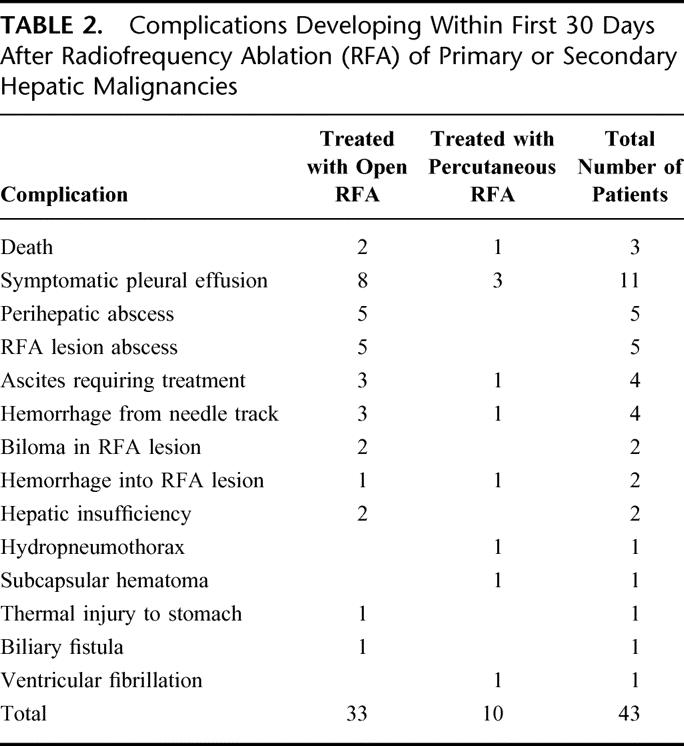
Symptomatic pleural effusions and the single case of a hydropneumothorax were treated with thoracentesis and/or diuresis, with all cases resolving within 3 weeks of initiating treatment.
All 5 cases of perihepatic abscess occurred in patients who underwent resection combined with RFA, and each case was resolved with percutaneous CT-guided drainage and intravenous antibiotics. Similarly, the 5 cases of abscess arising within a RFA lesion resolved with percutaneous CT-guided drainage and intravenous antibiotics to treat the gram negative microbes isolated from the infected cavities. One of the 5 patients who developed an early abscess after RFA had this complication arise following the second RFA procedure for recurrent pancreatic islet cell metastases. This was the solitary complication arising after a second RFA procedure in the 18 patients treated on 2 separate occasions with RFA (5.5%). The 4 patients who developed symptomatic ascites were all cirrhotic HCC patients. Ascites was successfully managed with diuretic therapy in all 4 patients. Hemorrhage from the RFA needle track did not exceed 100 mL in the 4 patients noted, and resolved with direct intraoperative pressure to the liver in the 3 open cases and to the skin overlying the liver in the 1 percutaneous case. A subcapsular hematoma causing pain 2 days after percutaneous RFA was managed expectantly with oral analgesics and serial CT scans; no other intervention was required.
A symptomatic biloma (bile filling and expanding the necrotic RFA lesion) was diagnosed 15 and 23 days, respectively, after RFA when patients returned with worsening upper abdominal pain. CT scans revealed the large fluid-filled cavities stretching the hepatic capsule. Both patients were treated with percutaneous CT-guided drainage of the bile collection, followed by endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy to exclude a proximal biliary stricture or increased intrabiliary pressure as a contributing cause for the biloma. The percutaneous drainage catheters were removed within 4 weeks in both patients with no recurrence of the bilomas. The single case of an early biliary fistula was evident from bilious drainage from a subcostal incision 10 days after RFA. The treated tumor involved segment 5 of the liver and cholecystectomy was performed during the operation to prevent thermal injury to the gallbladder. CT-guided percutaneous drainage of the subhepatic space combined with ERCP sphincterotomy led to resolution of the fistula within 3 weeks.
The 2 episodes of bleeding into the RFA lesion presented as acute onset of pain within 7 days of the RFA procedure and both were successfully managed with transfemoral hepatic arterial branch embolization and blood transfusion. Hepatic insufficiency was diagnosed in 2 cirrhotic HCC patients who developed jaundice; both episodes resolved within 30 days with medical management. The solitary thermal injury to an adjacent organ or structure in our 608 patients was a 3 cm zone of thermal necrosis noted on the anterior lesser curvature of the stomach after open RFA of a left lobe colorectal cancer metastasis. The damaged area of the stomach wall was excised full thickness with a 2-layer closure of the gastrotomy. The patient suffered no detectable ill effects as this problem was identified intraoperatively during a routine survey of all organs and structures near the treated tumors. The single episode of ventricular fibrillation occurred at the conclusion of RFA of a right lobe liver tumor and resolved with a single electrocardioversion. The patient showed no evidence of myocardial damage on serial electrocardiograms, serum myocardial enzymes, and an echocardiogram.
Late complications after the RFA procedure were diagnosed in 15 patients (2.4%). There was no significant difference in the incidence of late complications comparing patients treated with open RFA (10 [2.6%] complications in 382 patients) and those treated with percutaneous RFA (5 [2.2%] complications in 226 patients, Table 3). Thirteen of the 15 complications arose in patients with the 2 most common histologic tumor types treated in our series, colorectal adenocarcinoma metastases and HCC, with 1 patient each with breast or carcinoid metastases developing a late complication. Two cases of late biliary fistulae presented between 45 and 90 days after RFA with new onset of jaundice and abdominal pain. CT scans revealed subhepatic fluid collections and dilated intrahepatic bile ducts. Percutaneous drainage of the subhepatic fluid collections produced bile. Subsequent fistulagrams through the subhepatic drainage catheters and ERCP cholangiograms revealed stricture of the proper hepatic duct. Both of the RFA-treated tumors involved segments 4B and 5, and both were managed successfully with endostents placed across the proximal bile duct stricture. Stenting across the stricture led to resolution of the fistula with resultant removal of the percutaneous drainage catheters.
TABLE 3. Complications Developing More Than 30 Days After Radiofrequency Ablation (RFA) of Primary or Secondary Hepatic Malignancies
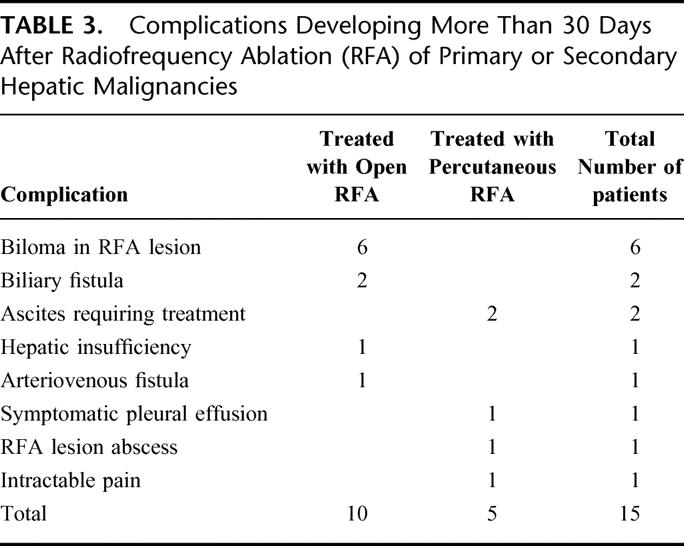
All 6 of the patients who developed a late symptomatic biloma were treated with open RFA of central tumors involving segments 4B, 5, and/or 8. All of these tumors were between 4.0 and 7.0 cm in maximum diameter. These late bilomas occurred in 4 patients with colorectal metastases and 2 patients with HCC. These 6 late bilomas presented as onset of progressively worsening right upper quadrant abdominal and flank pain in 5 patients and as a gastric outlet obstruction from duodenal compression in 1 patient. These symptoms leading to the diagnosis of late biloma developed from 65–137 days after RFA treatment. In 4 of the cases of late biloma, it was possible to treat the intrahepatic bile collection with percutaneous drainage catheters and ERCP sphincterotomy (Fig. 1). The percutaneous drainage catheters were subsequently removed in 2 patients, but 2 patients reaccumulated bile in the cavitary lesions with clamping of the percutaneous drainage catheters necessitating maintenance and periodic exchange of the percutaneous drains more than 9 and 15 months after RFA, respectively. The final 2 patients who developed late bilomas also required percutaneous drainage, but it was possible to internalize the drains through dilated proximal bile ducts and then down the common bile duct into the duodenum (Fig. 2). While symptoms related to the biloma resolved completely in both of these patients, it was necessary to maintain the external biliary drainage catheters in both patients up to the time of their death from recurrent metastatic malignant disease 14 months and 24 months after RFA, respectively.
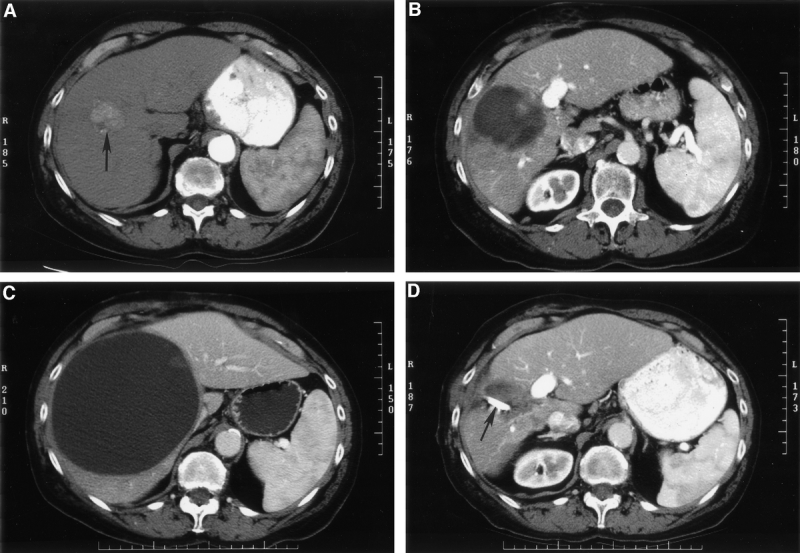
FIGURE 1. (A) An arterial phase computed tomography (CT) shows a right lobe hepatocellular cancer (arrow) in a patient with hepatitis C virus-induced cirrhosis. This lesion was treated with intraoperative ultrasound-guided radiofrequency ablation (RFA). (B) CT obtained 3 months after RFA of the hepatocellular cancer shows a necrotic cavity larger than the original tumor. This is typical because RFA treatment is planned to destroy the tumor and a rim of surrounding hepatic parenchyma when possible. (C) CT almost 5 months after RFA shows a very large intrahepatic biloma producing upper abdominal and flank pain. (D) CT 1 month after percutaneous CT-guided drainage of the biloma. The percutaneous drain (arrow) was removed 6 weeks after it was placed (and after endoscopically-performed sphincterotomy) with no recurrence of the biloma.
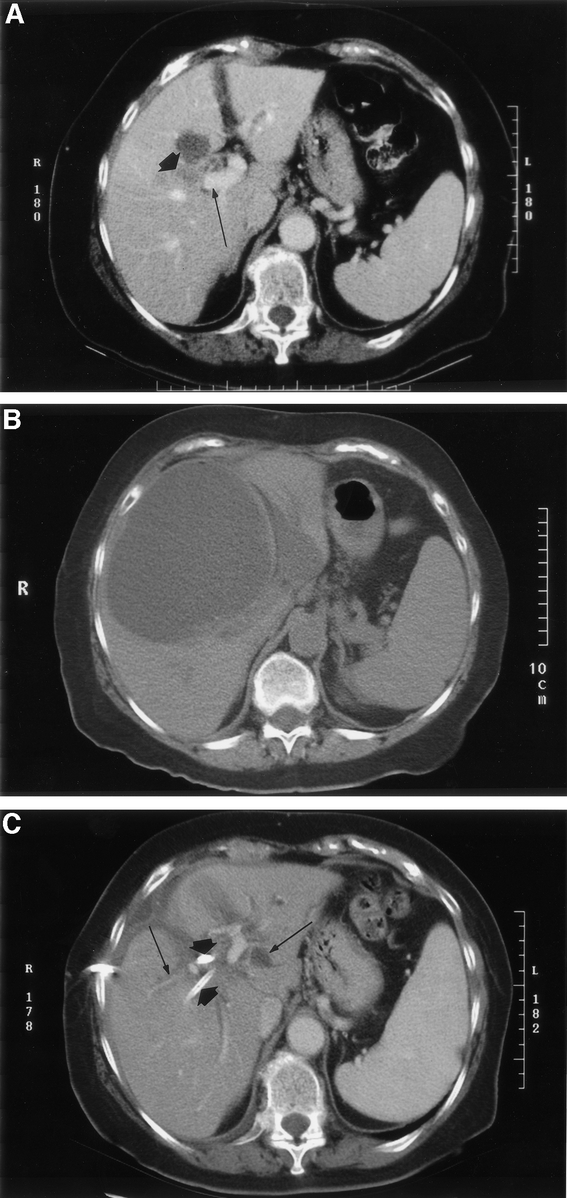
FIGURE 2. (A) Computed tomography (CT) of a colorectal cancer liver metastasis (short arrow) involving the central aspect of the right and left liver lobes near the portal vein (long arrow). This lesion and 2 other liver metastases were treated with intraoperative ultrasound-guided radiofrequency ablation (RFA). (B) CT 10 weeks after RFA shows a large intrahepatic biloma that presented as gastric outlet obstruction caused by duodenal compression. (C) CT shows 2 percutaneous drainage catheters (short arrows) placed into the biloma and directed into 2 bile duct branches draining into the cavity. Both drains were advanced down the common bile duct into the duodenum. The biloma is completely resolved but bilobar dilated intrahepatic bile ducts (long arrows) remain. The external biliary drainage catheters remained in placed for the 14-month duration of this patient's life.
Two patients who developed late symptomatic ascites and the 1 patient who developed late hepatic insufficiency were cirrhotic HCC patients. These complications developed between 30 and 60 days after RFA and were managed successfully with medical treatment. One patient presented with a symptomatic, nonmalignant pleural effusion 10 weeks after percutaneous RFA of a solitary right lobe breast cancer liver metastasis. The patient ultimately responded to diuretic therapy, but required 3 therapeutic thoracenteses. A single arteriovenous (AV) fistula from a hepatic arterial to portal venous branch within the ablation cavity was noted on a scan 6 weeks after RFA, and a 2.5 cm pseudoaneurysm developing at the site was noted on a CT scan 3 months after RFA. The AV fistula was successfully treated with a transfemoral approach arterial coil embolization to prevent bleeding into the RFA cavity. A single RFA lesion abscess presenting as fever 48 days after treatment was treated successfully with percutaneous drainage and oral antibiotics to treat the Enterococcus that was isolated from cultures of the purulent drainage. The single patient with intractable pain had percutaneous RFA of a colorectal cancer metastasis in segment 7 and developed a radicular pain syndrome slightly more than 2 months after RFA. While oral analgesics partially relieved the pain in this patient, resolution did not occur until percutaneous subcostal nerve block in the affected region was performed.
We have not observed needle track tumor seeding or grounding pad burns in any of our 608 patients. We have observed an additional late CT finding in less than 2% of our patients that has not been scored as a complication because the patients are asymptomatic with normal serum bilirubin and liver function tests. These patients developed sectoral or segmental intrahepatic biliary ductal dilation radiating peripherally from a deep or central RFA site.13 This CT finding has developed between 6 and 12 months after hepatic RFA, and has not been a harbinger of local tumor recurrence or progression to symptomatic bilomas.
Univariate analysis of factors predictive of a higher risk to develop complications after hepatic RFA indicated that patient age, sex, hepatic tumor histology, number of tumors treated with RFA, size of tumors treated with RFA, proximity of tumors to major intrahepatic blood vessels, and performing resection in combination with RFA were not factors that could be used to predict an increased risk for complications. Similarly, central location of tumors treated with RFA compared with peripheral location was not a factor predictive of increased risk for early or late complications. A total of 337 (55.4%) of the 608 patients had received prior systemic or regional chemotherapy to treat hepatic malignancies. There was no higher incidence of RFA-related complications in this group compared with the 271 patients (45.6%) who had not received previous chemotherapy. The only 2 factors on univariate analysis predictive of an increased risk for complications after hepatic tumor RFA were open surgical RF treatment of tumors (43 [11.2%] complications in 382 patients) versus a percutaneous RFA approach (15 [6.6%]rsqb) complications in 226 patients, P < 0.01), and the presence of cirrhosis was also a predictor of higher risk for complications with 25 complications (12.9%) arising in 194 cirrhotic patients, compared with 31 complications (7.5%, P < 0.05) occurring in 414 noncirrhotic patients. However, on multivariate analysis, none of these factors achieved statistical significance as an independent variable predictive of an increased risk to develop complications after RFA.
DISCUSSION
Reported complication rates after RFA of malignant liver tumors in series as small as 6 patients and as large as 2320 patients range from 0%–27%.9,11,14–31 RFA treatment-related mortality is nonexistent or rare in most reports. Reported RFA treatment-related complications include pneumothorax; symptomatic pleural effusion; bleeding from the needle track or into the treated tumor; biliary fistula; biliary stricture; biloma; abscess in the treated tumor; skin burn; cholecystitis; thermal injury to adjacent structures including the diaphragm, stomach, duodendum, and transverse colon; liver failure; segmental hepatic infarction; paralysis of the hemidiaphragm; arterial-portal venous fistula; systemic hemolysis; tumor lysis syndrome; myoglobinemia or myoglobinuria; transient acute renal failure; and prolonged post treatment pain for lesions near the hepatic capsule.
Complications reported following percutaneous RFA of malignant liver tumors in 2320 patients treated at 41 different hospitals in Italy indicates that the mortality rate was 0.3% with an overall complication rate of 7.1%.31 This complication rate is similar to our overall rate of 9.5%, and is identical to our 7.1% rate of early complications. The multicenter Italian study did not report on the incidence of late complications following percutaneous RFA of 1610 HCC patients, 693 patients with liver metastases, and 17 patients with intrahepatic cholangiocarcinoma. The authors did distinguish between major complications (2.4% incidence) including death, hemorrhage, RFA needle track seeding, RFA lesion abscess, perforation of a gastrointestinal viscus, liver failure, biloma, biliary stricture, portal vein thrombosis, and hemothorax or pneumothorax requiring drainage and minor complications (4.7% incidence) that included pain, fever, and asymptomatic pleural effusion.
Another recent review revealed a complication rate of 8.9% following percutaneous, laparoscopic, or open RFA of hepatic tumors in 3670 patients.32 The mortality rate after RFA of hepatic malignancies was 0.5%. Complications directly related to the liver included bleeding (1.6%), intrahepatic abscess (1.1%), biliary or hepatic vascular injury (1.7%), and liver failure (0.8%). Complications that arose in less than 1% of hepatic tumor RFA patients included pulmonary problems (pneumothorax, hydrothorax, pleural effusion), grounding pad skin burn, myoglobinemia or myoglobinuria, renal failure, coagulopathy, tumor seeding of the needle track, excessive hormone release from treated neuroendocrine tumors, cardiac problems (myocardial infarction, dysrhythmias), and injury to the diaphragm or adjacent viscera.32 The complication rates for percutaneous, laparoscopic, and open RFA were 7.2%, 9.5%, and 9.9%, respectively. However, the complication rate associated with open RFA rose to 31.8% in patients who underwent a combination of resection and RFA of hepatic malignancies, which is consistent with the higher complication rates associated with major hepatic resections.5–7
Our overall mortality and complication rates in this large series of 608 patients treated with hepatic tumor RFA are nearly identical to the rates determined in recent reviews.31,32 Unlike previous studies, this is the first report, prospective or retrospective, to distinguish early and late complications following RFA of malignant hepatic tumors. While the incidence of complications that arose more than 30 days after RFA was only 2.3%, clinicians utilizing RFA to treat liver tumors must be aware that treatment-related problems can develop months after the initial treatment. None of the patients on our series developed complications that have not previously been reported. However, the late development of symptomatic bilomas and the management of this complication have not been previously reported. Similarly, this is the first report with adequate statistical power to determine that tumor size, the number of tumors treated with RFA, or previous treatment with cytotoxic chemotherapy are not factors that increase the risk of a patient to develop complications after RFA. While central location of tumors was not associated with an overall increased risk to develop complications after RFA, it should be noted that all 11 cases of symptomatic bilomas or biliary fistulae developed in patients who underwent RFA of central hepatic lesions. Based on our preclinical experimental studies with hepatic RFA, it is known that large primary and secondary intrahepatic bile ducts are damaged or destroyed following RFA.33 Thus, we avoid or employ extreme caution in selection of patients to undergo RFA of tumors near the hilum of the liver. Clearly, we were not cautious enough in the 11 patients who developed biliary tract complications following RFA of a central liver tumor. We have also not attempted to mitigate injury to large central bile ducts by irrigation of the biliary tree with cooled fluid during intraoperative RFA.34 It is not known if techniques that cool or otherwise protect the bile ducts during RFA will prevent late injury such as stricture or biloma.
The higher complication rate associated with open RFA compared with percutaneous RFA in our series may be related to several factors. First, our preferred approach to treat centrally located tumors is an open laparotomy to optimize accuracy of RFA needle electrode placement with intraoperative ultrasonography. Secondly, almost half of the patients treated with open RFA underwent concomitant liver resection of additional malignant tumors. Seven of the 8 patients who developed an early symptomatic pleural effusion and all 5 of the patients who developed a perihepatic abscess underwent liver resection of dominant or large tumors prior to RFA of smaller lesions; these complications are likely related to the hepatic mobilization and liver resection. Similarly, the patients who developed hepatic insufficiency underwent resection combined with RFA to treat multifocal liver tumors. Combining resection of dominant liver tumors with RFA of the remaining lesions can be expected to increase the complication rate.32 Finally, patients treated with a percutaneous approach underwent RFA of fewer lesions than patients undergoing open RFA, and the tumors treated percutaneously were carefully selected for peripheral location in the liver away from major blood vessels or bile ducts and adjacent organs and structures.
Predictably, cirrhotic patients undergoing RFA, generally for HCC, had a slightly higher complication rate than noncirrhotic patients. Nonetheless, although almost two-thirds of our cirrhotic patients were Child's class B or C, the overall complication rate in 194 cirrhotic patients was only 12.9%. There were no treatment-related deaths in the cirrhotic patients, and the complication rate is almost identical to the 12.7% rate we reported in our initial experience with RFA of HCC in 110 cirrhotic patients.12 The low complication rate associated with intraoperative and percutaneous RFA in cirrhotic HCC patients has been confirmed by 2 other studies describing 47 and 62 patients, respectively, with treatment-related complication rates of less than 10%.35,36 In contrast, most patients with Child's class B or C cirrhosis are not candidates for major surgical resection, and the complication rate after resection of HCC in Child's class A cirrhotic patients ranges from 30%–60%, with a mortality rate of up to 5%.8 A possible explanation for the relatively low complication rate after hepatic tumor RFA is the recently reported finding that there is no systemic increase in inflammatory cytokines or cytokine receptors following RFA.37
High resolution CT or MRI are both useful in diagnosing early and late complications after RFA of hepatic tumors. Abdominal helical CT can be used to diagnose RFA treatment-related abscesses, biliary fistulae, bilomas, arteriovenous fistulae, ascites, and hemorrhage into a treated lesion.13 High resolution CT scans can demonstrate asymptomatic changes associated with RFA including segmental or subsegmental biliary duct dilatation, atrophy, and enhancing inflammatory changes around the RFA site, and are used to exclude local recurrence as a cause of contrast enhancement or biliary dilatation.13 High resolution CT or MRI has also been useful in detecting needle track seeding after percutaneous RFA of HCC.38 We have not had any cases of needle track recurrence utilizing a system that produces intratumoral temperatures in excess of 100°C with out-gassing and release of steam along the needle track.
RFA intended to produce complete thermal necrosis of tumor is a recent addition to the modalities available to clinicians treating patients with unresectable primary or secondary hepatic malignancies. The success of this treatment must be judged based on local recurrence (incomplete treatment), survival, and complication rates. Proper patient selection combined with meticulous surgical planning and performance of RFA should result in local recurrence rates below 5%. This study indicates that RFA of hepatic tumors can and should be performed with low morbidity rates and with mortality rates below 1%. Clinicians performing hepatic tumor RFA must recognize early and late complications associated with RFA and then intervene with appropriate treatment. Long-term disease-free and overall survival rates following RFA of primary and secondary hepatic malignancies are not yet established, but survival data will be forthcoming over the next several years as adequate follow-up of patients becomes available.
Footnotes
Steven A. Curley, MD, and Francesco Izzo, MD, receive financial support for research nurses and database management from Boston Scientific Corporation.
Reprints: Steven A. Curley, MD, FACS, Department of Surgical Oncology, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 444, Houston, Texas 77030–4095. Email: scurley@mdanderson.org.
REFERENCES
- 1.Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM, Rustgi VK, Hoofnagle JH, et al. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390–401. [DOI] [PubMed] [Google Scholar]
- 3.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–20; discussion 520–522. [DOI] [PMC free article] [PubMed]
- 4.Lau WY, Leung TW, Lai BS, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. 2001;233:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilimoria MM, Lauwers GY, Doherty DA, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. [DOI] [PubMed] [Google Scholar]
- 6.Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North Am. 2002;82:1075–1090. [DOI] [PubMed] [Google Scholar]
- 7.Topham C, Adam R. Oncosurgery: a new reality in metastatic colorectal carcinoma. Semin Oncol. 2002;29(5 Suppl 15):3–10. [DOI] [PubMed] [Google Scholar]
- 8.Curley SA, Cusack JC Jr, Tanabe KK, et al. Advances in the treatment of liver tumors. Curr Probl Surg. 2002;39:449–571. [DOI] [PubMed] [Google Scholar]
- 9.Seidenfeld J, Korn A, Aronson N. Radiofrequency ablation of unresectable liver metastases. J Am Coll Surg. 2002;195:378–386. [DOI] [PubMed] [Google Scholar]
- 10.Barnett CC, Curley SA. Ablation techniques, ethanol injection, cryoablation, and radiofrequency ablation. Op Tech Gen Surg. 2002;4:65–75. [Google Scholar]
- 11.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi H, Loyer EM, Dubrow RA, et al. Radiofrequency ablation (RFA) of liver tumors: Assessment of therapeutic response and complications. Radiographics. 2001;21:S41–S54. [DOI] [PubMed] [Google Scholar]
- 14.Siperstein AE, Rogers SJ, Hansen PD, et al. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery. 1997;122:1147–1154; discussion 1154–1155. [DOI] [PubMed]
- 15.Podnos YD, Henry G, Ortiz JA, et al. Laparoscopic ultrasound with radiofrequency ablation in cirrhotic patients with hepatocellular carcinoma: technique and technical considerations. Am Surg. 2001;67:1181–1184. [PubMed] [Google Scholar]
- 16.Cuschieri A, Bracken J, Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy. 1999;31:318–321. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda M, Okada S, Ueno H, et al. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: a comparative study. Jpn J Clin Oncol. 2001;31:322–326. [DOI] [PubMed] [Google Scholar]
- 18.Chung MH, Wood TF, Tsioulias GJ, et al. Laparoscopic radiofrequency ablation of unresectable hepatic malignancies. A phase 2 trial. Surg Endosc. 2001;15:1020–1026. [DOI] [PubMed] [Google Scholar]
- 19.Montorsi M, Santambrogio R, Bianchi P, et al. Radiofrequency interstitial thermal ablation of hepatocellular carcinoma in liver cirrhosis. Role of the laparoscopic approach. Surg Endosc. 2001;15:141–145. [DOI] [PubMed] [Google Scholar]
- 20.Choy PY, Koea J, McCall J, et al. The role of radiofrequency ablation in the treatment of primary and metastatic tumours of the liver: initial lessons learned. N Z Med J. 2002;115:U128. [PubMed] [Google Scholar]
- 21.Jiang HC, Liu LX, Piao DX, et al. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong SL, Edwards MJ, Chao C, et al. Radiofrequency ablation for unresectable hepatic tumors. Am J Surg. 2001;182:552–557. [DOI] [PubMed] [Google Scholar]
- 23.Kosari K, Gomes M, Hunter D, et al. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg. 2002;6:255–263. [DOI] [PubMed] [Google Scholar]
- 24.de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–1625. [DOI] [PubMed] [Google Scholar]
- 25.Bowles BJ, Machi J, Limm WM, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–869. [DOI] [PubMed] [Google Scholar]
- 26.Wood TF, Rose DM, Chung M, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. [DOI] [PubMed] [Google Scholar]
- 27.Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. [DOI] [PubMed] [Google Scholar]
- 28.Solbiati L, Ierace T, Tonolini M, et al. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound. 2001;13:149–158. [DOI] [PubMed] [Google Scholar]
- 29.Ma K, Min C, Ian HX, et al. Prevention and cure of complications from multiple-electrode radiofrequency treatment of liver tumors. Dig Dis. 2001;19:364–366. [DOI] [PubMed] [Google Scholar]
- 30.Iannitti DA, Dupuy DE, Mayo-Smith WW, et al. Hepatic radiofrequency ablation. Arch Surg 2002;137:422–426; discussion, 427. [DOI] [PubMed]
- 31.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. [DOI] [PubMed] [Google Scholar]
- 32.Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. [DOI] [PubMed] [Google Scholar]
- 33.LeVeen RF. Laser hyperthermia and radiofrequency ablation of hepatic lesions. Sem Interven Radiol. 1997;14:313–324. [Google Scholar]
- 34.Dominique E, El Otmany A, Goharin A, et al. Intraductal cooling of the main bile ducts during intraoperative radiofrequency ablation. J Surg Oncol. 2001;76:297–300. [DOI] [PubMed] [Google Scholar]
- 35.Nicoli N, Casaril A, Marchiori L, et al. Intraoperative and percutaneous radiofrequency thermal ablation in the treatment of hepatocellular carcinoma. Chir Ital. 2000;52:29–40. [PubMed] [Google Scholar]
- 36.Buscarini L, Buscarini E. Therapy of HCC-radiofrequency ablation. Hepatogastroenterology. 2001;48:15–19. [PubMed] [Google Scholar]
- 37.Schell SR, Wessels FJ, Abouhamze A, et al. Pro- and antiinflammatory cytokine production after radiofrequency ablation of unresectable tumors. J Am Coll Surg. 2002;195:774–781. [DOI] [PubMed] [Google Scholar]
- 38.Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. [DOI] [PubMed] [Google Scholar]


