Abstract
Objective:
Ghrelin is a novel gastric hormone recognized in 1999 as a mediator of growth hormone release. Since growth hormone is anabolic, an important function of ghrelin may be to coordinate energy needs with the growth process. Newly discovered biologic roles of ghrelin imply that it may have other important physiological functions as well. This is a review of recent clinically relevant, yet less well-known, physiologic actions of ghrelin.
Summary Background Data:
Ghrelin has profound orexigenic, adipogenic, and somatotrophic properties, increasing food intake and body weight. Secreted predominantly from the stomach, ghrelin is the natural ligand for the growth hormone secretagogue receptor in the pituitary gland, thus fulfilling criteria of a brain-gut peptide. The brain-gut axis is the effector of anabolism by regulating growth, feeding, and metabolism via vagal afferents mediating ghrelin signaling. However, the wide tissue distribution of ghrelin suggests that it may have other functions as well.
Methods:
Systematic literature review of all PubMed citations between 1999 and August 2003 focusing on clinically relevant biochemical and physiological characteristics of ghrelin.
Results:
Ghrelin is an important component of an integrated regulatory system of growth and metabolism acting via the vagus nerve, and is implicated in a variety of altered energy states such as obesity, eating disorders, neoplasia, and cachexia. It also enhances immune responses and potentially down-regulates anti-inflammatory molecules. Ghrelin's role as a brain-gut peptide emphasizes the significance of afferent vagal fibers as a major pathway to the brain, serving the purpose of maintaining physiologic homeostasis.
Conclusions:
The discovery of ghrelin has increased our understanding of feeding regulation, nutritional homeostasis, and metabolic processes. Further characterization of ghrelin's functions will likely generate new pharmacological approaches to diagnose and treat different disease entities including those related to the over-nutrition of obesity and the catabolic response to surgical trauma.
Ghrelin, a potent orexigenic, adipogenic, and somatotrophic peptide, was discovered in rat gastric mucosa in 1999. A rapidly increasing body of literature suggests that ghrelin may mediate many physiological processes relevant to surgeons. We review ghrelin's role in regulating energy balance, growth, cardiovascular dynamics, and immune function.
Anterior pituitary growth hormone (GH) secretion was initially believed to be stimulated by GH-releasing hormone (GHRH) and inhibited by the hypothalamic hormone, somatostatin. The discovery of ghrelin (from “ghre” in the Proto-Indo-European language meaning “grow,” and the suffix “relin” as in “release”), a natural ligand for the growth hormone secretagogue receptor (GHSR), established a novel independent pathway in the regulation of GH release. It was originally found to induce growth hormone release in rats through pituitary GHSR stimulation.1 However, a large body of evidence has shown other physiological functions of ghrelin, distinct from GH release and energy homeostasis. Here we provide an overview of the rapidly expanding field of ghrelin biology, and its potential for developing diagnostic and therapeutic tools.
STRUCTURAL CHARACTERISTICS OF GHRELIN
The human ghrelin gene is located on chromosome 3p26-p25, encoding a 117 amino acid peptide termed preproghrelin.2 Two isoforms of mRNA for proghrelin, ghrelin, and des-Gln14-ghrelin precursors, are produced from the gene in rat stomach by an alternative splicing mechanism.3 Ghrelin shares 36% structural resemblance with motilin,4 and is structurally identical to des-Gln14-ghrelin, except for the deletion of Gln.14 In addition, des-Gln14-ghrelin is only present in small amounts in the stomach, indicating that ghrelin is the major physiologic peptide released into the circulation. Post-translational modification of rat and human preproghrelin produces a 28 amino acid peptide, differing only in 2 residues. Esterification of the hydroxyl group of the Ser3 residue of ghrelin by n-octanoic acid increases the hydrophobicity of the ghrelin molecule, and appears to be essential for the activity of ghrelin in both rats and humans.5 A recent study suggested that the first 4 or 5 residues of ghrelin, including Ser3, are as active as the full-length molecule.6
GROWTH HORMONE SECRETAGOGUE RECEPTOR
Prior to the discovery of ghrelin, a group of peptidyl and nonpeptidyl synthetic compounds with potent GH releasing activity in vitro called growth hormone secretagogues (GHSs) had been developed.7 The receptor binding sites for the GHSs were believed to be distinct from GHRH receptor based on several studies. Radiolabelled GHSs were displaced by other GHSs, but not GHRH or somatostatin.8 GHSs and GHRH have synergistic effects on GH release, suggesting that they act, in part, via different mechanisms.9–10 Thus it was not surprising to find that GHRH increased intracellular cyclic AMP via its receptor, while GHSs increased the concentration of free intracellular calcium.5,8 The existence of an unidentified hormone that may activate GHSR was postulated. Indeed, Kojima et al reported the discovery of ghrelin, a novel natural ligand for GHSR, and demonstrated it to be one of the most potent inducers of GH release.1
The GHSR is encoded by a single gene found at chromosomal location 3q26.2.11 Alternative mRNA processing generates 2 types of GHSR proteins: GHSR1a and GHSR1b.12–13 Their amino acid sequences showed more than 50% structural homology with the neurotensin receptor and with motilin receptor type 1A.14–15 GHSR1a is a G-protein-linked receptor consisting of 366 amino acids with 7 transmembrane regions. Stimulation of GHSR1a by GHSs or ghrelin triggers the phospholipase C signaling pathway, leading to increased inositol phosphate turnover and protein kinase C activation, resulting in the release of calcium from intracellular stores.5 GHSR activation also inhibits K channels, allowing the entry of calcium through voltage-gated l- and T-type channels.7 In contrast, GHSR1b consists of 289 amino acids with only 5 transmembrane domains. It is unknown whether GHSR1b specifies a functional receptor. However, the structural differences compared with GHSR1a, and the finding that GHSs or ghrelin failed to bind GHSR1b suggested that other unknown natural ligand(s) may exist to modulate the GHSR signaling pathway.14 Recently, Hosoda et al identified multiple ghrelin-derived molecules produced by post-translational processing. Future characterizations of these molecules may detect functional differences with respect to the ghrelin peptide.16
LOCALIZATION OF INTRACELLULAR GHRELIN PROTEINS
Ghrelin is found throughout the gastrointestinal tract with the greatest concentration in the fundus of the stomach. In situ hybridization and immunohistochemical analyses indicated that ghrelin-containing cells, known as X/A-like cells, are a distinct endocrine cell type found within the acid-producing oxyntic glands in humans and rats.17 The X/A-like cells synthesize round, electron-dense granules containing ghrelin proteins. The mechanisms governing the biosynthesis and secretion of this peptide are unknown. Normal adult plasma samples contain 100–120 fmol ghrelin per ml, indicating that it is not secreted in the gastrointestinal tract, but into the systemic circulation, exhibiting endocrine, paracrine, and possibly autocrine effects.17
Although most of circulating ghrelin is believed to arise from the stomach,17 it is found in substantially lower amounts in many other tissues, including pituitary, lung, pancreas, gall bladder, esophagus, colon, liver, spleen, breast, thyroid, and heart.18–19 The relative contribution of ghrelin derived from nongastric sources in the circulation and its physiological function are unknown. It is interesting to note that GHSR1a is detected most abundantly in the pituitary gland, with lesser distribution in the thyroid, pancreas, spleen, heart, and adrenal glands.18–19 In contrast, GHSR1b is found most commonly in the skin, myocardium, and pituitary gland, and its tissue distribution is comparable to ghrelin, but much broader than GHSR1a.18–19 Since ghrelin is widely distributed in peripheral tissues, it is likely that ghrelin may interact with other unidentified GHSR subtype(s). In addition, the extensive tissue distribution of GHSR1b and the lack of natural endogenous ligand(s) reflect the complexity of ghrelin and GHSR interactions. Future investigations are needed to identify GHSR subtypes to further define the physiological significance of the ghrelin protein.
EFFECTS OF GHRELIN ON SOMATOTROPHS
The stimulatory effect of ghrelin on GH secretion in somatotrophs is specific, potent, dose-dependent, and synergistic with GHRH.20–21 Intravenous injection of ghrelin in rats induced a strong GH release, peaked at about 5–15 minutes and returned to basal level 1 hour later.17 Single intracerebroventricular (icv) administration of ghrelin in rats also increased plasma GH concentration in a dose-dependent manner.22 In humans, ghrelin induced a significant and long-lasting increase in circulating GH levels, an effect more potent than GHRH itself.20,23
Ghrelin stimulated GH release in isolated pituitary cells in vitro,1 but also caused a significant rise in ACTH, cortisol, aldosterone, prolactin, and epinephrine, without affecting gonadotrophin or TSH release in vivo.24 These neuroendocrine effects are transitory during prolonged treatment, and observed only at supraphysiological concentrations of ghrelin.25–26 The GH-independent actions of ghrelin may be explained from an evolutionary perspective as a physiological adaptive response serving survival. During periods of food shortage and stress, activation of the hypothalamo-pituitary-adrenal axis, eg, with ghrelin release, may activate counter-regulatory hormones for the mobilization of substrate to defend the host against perceived stress or noxious stimuli. The ensuing energy-depleted state, together with the physiological action of ghrelin in fasting-induced feeding, implies an essential role for ghrelin in restoring energy balance of the host.
Interestingly, ghrelin has been implicated in the induction of anxiogenic activities and memory retention in rats.27–28 Hunger and food-seeking behavior are not only associated with anxiogenesis, but also the ability to process and memorize information about prior experiences with food consumption. Consistent with these observations, specific ghrelin binding sites have been detected in the hippocampus,29 indicating that the mechanisms of learning, memory, and anxiogenesis may be linked to the roles of ghrelin in neuroendocrine responses to stress and feeding behavior.
ENGERGY HOMEOSTASIS: OREXIGENIC AND ADIPOGENIC EFFECTS OF GHRELIN
Ghrelin has profound orexigenic and adipogenic properties, generating signals to the hypothalamus when an increase in metabolic substrates is needed. The presence of ghrelin and GHSR in the arcuate nucleus of the hypothalamus suggested that ghrelin acts centrally to regulate food intake.18–19 Intraventricular ghrelin injection potently stimulated food intake,30–31 while antighrelin antibody reduced food intake in rats.30 Furthermore, food intake and body weight increased dose-dependently following ghrelin administration.31–33 Subcutaneous ghrelin caused weight gain, with an increase in fat mass over a 2-week period.32 This induction of positive energy balance was independent of GH effects. In addition to increasing food intake, exogenous ghrelin decreased basal metabolic rate16 and caused obesity32 in experimental animals.
Intravenous ghrelin stimulated hunger and food intake in healthy volunteers,23,34 while dramatic spontaneous preprandial rises and postprandial falls in plasma ghrelin support the notion that ghrelin is a physiological meal initiator in humans.35 Although acute administration of ghrelin reduced insulin secretion and caused hyperglycemia,36 plasma ghrelin levels were not affected by glucose or insulin levels in healthy subjects.37
GHRELIN AND GASTRIC BYPASS SURGERY
The Roux-en-Y gastric bypass (RYGB) is one of the most effective treatments for morbid obesity,38 yet the mechanisms leading to sustained weight loss after RYGB are controversial. Cummings et al reported that 24-hour plasma ghrelin levels were increased in response to diet-induced weight loss, while levels were lower in matched, weight-stable, obese subjects.39 In addition, they showed that plasma ghrelin levels after weight loss from gastric bypass remained significantly lower than in other subjects, irrespective of body weight. The authors suggested that gastric bypass-induced weight loss may partly stem from suppression of ghrelin production by “override inhibition.”39 The uninterrupted diversion of food from contact with the distal stomach and duodenum initially produces stimulatory signals to release gastric ghrelin, and later paradoxically suppresses its release.
Other investigators have similarly documented ghrelin suppression following RYGB, despite discrepant results in the magnitude of postoperative ghrelin reduction.40–41 However, recent reports have generated a growing debate over whether gastric bypass surgery causes stable weight reduction via ghrelin suppression.42–43 Holdstock et al prospectively examined 66 morbidly obese patients before RYGB and at 12 months after surgery.42 At 12 months following RYGB the average body mass index decreased by 30% and average ghrelin levels increased by 62%. Although these authors argued that RYGB did not directly affect circulating ghrelin levels, Faraj et al suggested that postoperative serum ghrelin may be dependent on the dynamic status of weight loss.43 Indeed, weight loss usually continues for 22–24 months after diversionary operations.38 During the weight loss phase, patients are in a state of negative energy balance, and may continuously secrete ghrelin into the circulation from nongastric sources, thus demonstrating higher serum ghrelin levels than controls. In contrast, weight-stable patients are in energy balance, and therefore do not exhibit higher serum ghrelin levels despite massive weight loss.43 Consequently, it appears that energy balance, but not body weight per se, may be a critical determinant of circulating ghrelin levels following RYGB.
The disparate results in the literature imply that the mechanisms by which RYGB induces weight loss engage complex interactions between the mechanical effects of the surgery and neuroendocrine responses involved in energy homeostasis. Future studies may elucidate the neurohumoral pathways mediating weight loss following RYGB, and perhaps define the potential role of pharmacologic ghrelin blockade as a novel approach to treating obesity.
CENTRAL REGULATION OF FEEDING
Appetite and feeding behavior are regulated by a complex balance of stimulatory and inhibitory signals in the central nervous system, particularly in the hypothalamus.44 Essential elements of this control system are ghrelin and leptin, both of which signal nutritional status and energy storage levels to the hypothalamic feeding centers (Fig. 1). Ghrelin is orexigenic when administered both centrally and peripherally, and it is one of several appetite-regulating humoral signals to the central nervous system. The critical role of the afferent vagus nerve as a mediator of feeding behavior is consistent with the findings of early satiety, lack of hunger, and stable weight reduction in obese patients following truncal vagotomy.45–46 Weight reduction may be, at least in part, due to alterations in vagally mediated central and peripheral mechanisms for appetite regulation.45 In contrast, leptin is an anorexigenic hormone secreted by adipose tissue involved in thermogenesis and the control of several crucial neuroendocrine functions in the hypothalamic-pituitary-adrenal axis.47–50
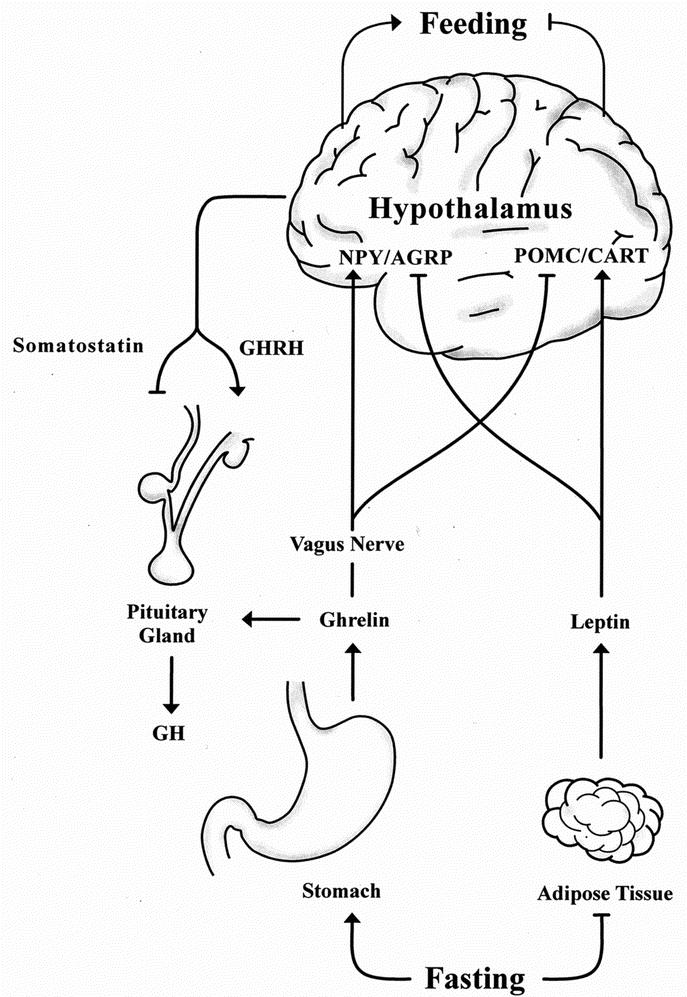
FIGURE 1. Neuroendocrine regulation of central feeding and growth hormone release. NPY, neuropeptide Y; AGRP, agouti-related peptide; POMC, proopiomelanocortin; CART, cocaine and amphetamine-regulated transcript; GHRH, growth hormone releasing hormone; GH, growth hormone.
The reciprocal actions of leptin and ghrelin are mediated by hypothalamic neurons in the arcuate nucleus containing neuropeptide Y (NPY) and agouti-related peptide (AGRP), which induce feeding, and neurons containing proopiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART), which inhibit feeding.44 Leptin inhibits NPY neurons and stimulates POMC neurons to suppress feeding, whereas ghrelin activates NPY neurons and inhibits POMC neurons to promote food intake. These appetite-regulating neurons thus provide a common route to integrate leptin- and ghrelin-mediated feeding regulation with other homeostatic mechanisms such as the recently demonstrated melanocortin 4 receptor (MC4R) effects on binge-eating.51
GHRELIN IN OBESITY
The discovery of ghrelin and its influence on appetite, fuel utilization, and body weight added complexity to centrally regulated energy balance. In general, human plasma ghrelin levels are inversely correlated with positive energy balance, body mass index, body fat mass, adipocyte size, and leptin levels,52–54 while they are lower in obese subjects than in controls.54 Pima Indians, known for their propensity to develop type II diabetes and obesity, also have lower circulating ghrelin levels, independent of body mass index, compared with matched controls.54 Patients with anorexia nervosa exhibit high plasma ghrelin levels when compared with age- and sex-matched controls, and weight gain decreases their elevated ghrelin concentrations.55 Thus, fluctuations of plasma ghrelin levels may reflect physiological adaptation to long-term alterations in energy balance.
Plasma leptin correlates with body fat content despite its anorectic effects, suggesting that human obesity is associated with a state of leptin resistance.56–57 However, 5%–10% of obese people have relatively low levels of leptin, indicative of a reduced rate of leptin production.56–57 While a negative correlation has been reported between basal leptin and ghrelin concentrations in humans in one report,54 other investigators have documented conflicting results.58 A recent study by Barazzoni et al reported that hyperleptinemia blunted the increase of serum ghrelin during caloric restriction, suggesting that the anorectic effects of leptin act both via direct central actions, and via peripheral inhibition of orexigenic effects of ghrelin.59
Given the orexigenic and adipogenic properties of ghrelin, treatment with an antagonist would seem logical in obesity. In animal studies, the biologic actions of the ghrelin antagonist ([D-Lys-3]-GHRP-6) appear to be mediated through GHSR antagonism.60–61 This antagonist has been shown to inhibit ghrelin-induced GH release59 and to reduce food intake and body weight gain in mice.61 However, several concerns should be addressed prior to using a ghrelin antagonist in clinical trials. First, it is uncertain whether suppressing ghrelin would add any benefits to the already depressed ghrelin levels in obese individuals. Furthermore, Palmiter et al reported that NPY knockout mice eat and grow normally with all endocrine responses to fasting intact, implying that compensatory and redundant mechanisms are activated to protect nutritional integrity by driving the animal to feed.62–63 In addition, the antiobesity effects of a ghrelin antagonist may cause functional GH deficiency. Since the time course of the observed changes in body mass and composition induced by ghrelin is unknown, future studies will have to show which effects of ghrelin antagonism, antiadipogenic or antisomatotrophic, dominate during long-term blockade of ghrelin bioactivity. As yet there are no reports of a GHSR1a antagonist, although an inverse agonist of the ghrelin receptor was just described.64
Recent exciting findings linked obesity to genetic variations of the ghrelin gene. Ravussin et al reported that a decrease in ghrelin concentration in response to overfeeding and baseline ghrelin levels were more alike within pairs of monozygotic twins than in heterozygous pairs, indicating a probable genetic effect underlying the variability in plasma ghrelin levels.52 Korbonits et al further substantiated this observation noting that obese children carrying the single nucleotide polymorphism (a nonconservative amino acid change in the protein sequence in the C-terminal tail of the preproghrelin protein) have higher body mass index, and reduced insulin secretion.65 Consistent with these findings, Ukkola et al identified a mutation at amino acid position 51 (Arg51Gln) of the preproghrelin sequence in obese subjects, and found that a mutation at codon 72 of the preproghrelin gene (Leu72Met) was associated with lower age of onset of obesity.66 Genetic modifications at the ghrelin locus may play a role in the etiology of obesity. Future studies are warranted to define the relationships between genetic alterations of the ghrelin gene and its associated phenotypic expression in the context of body weight regulation.
The hypothesis that ghrelin hypersecretion may contribute to genetic obesity is equally intriguing. Prader-Willi syndrome (PWS), due to a lack of expression of paternal genes on chromosome 15 (15q11-q13), is one of the most common forms of genetic obesity.67 It is characterized by hypothalamic dysfunction leading to GH deficiency, insatiable hunger, morbid obesity, hypogonadotropic hypogonadism, short stature, mental retardation, aberrant body temperature control, and sleep disturbances.67 Recent evidence indicated that elevated serum ghrelin may be responsible, at least in part, for the hyperphagia observed in patients with PWS.68–70 The mechanism underlying elevated ghrelin levels in PWS is unknown, but is not likely to reflect a mutation of the genes encoding ghrelin or GHRS, but rather genetic alteration(s) on chromosome 15 indirectly affecting ghrelin expression. High ghrelin levels in PWS are also unlikely to arise from a decreased negative feedback from GH; Janssen et al have shown that ghrelin concentrations are not elevated in adults with GH deficiency, and GH therapy in these same individuals does not alter their ghrelin levels.71 Similar findings were also observed in obese subjects and children with PWS.72–73 Regardless of the etiology of elevated ghrelin in PWS, additional studies will be required to validate the functional activity of ghrelin by demonstrating whether ghrelin antagonists effectively reduce food intake in PWS.
GHRELIN AND GASTROINTESTINAL FUNCTIONS
Gastric
Recently, Masuda et al demonstrated that intravenous administration of ghrelin in rats not only increased gastric acid secretion, but also accelerated gastric motility.74 Similarly, intravenous administration of ghrelin has been shown to induce gastrin release in rats,75 and circulating ghrelin levels accelerated gastric emptying rate in humans.53 These effects of ghrelin were abolished in rats by pretreatment with either atropine or bilateral cervical vagotomy, but not a histamine H2-receptor antagonist, suggesting that ghrelin may play a role in vagal control of gastric function.74 Likewise, intracerebro-ventricular administration of ghrelin also increased gastric acid output in a dose-dependent manner, a phenomenon abolished by vagotomy or atropine injection.76 In a series of elegant experiments, Date et al showed that blockade of the gastric vagal afferent abolished ghrelin-induced feeding, GH secretion, and activation of NPY-producing and GHRH-producing neurons.77 Taken together, these findings indicated that ghrelin conveys signals for GH secretion and feeding to the central nervous system, and stimulates the vagal efferent system to increase gastric acid secretion.
Pancreatic
Immunohistochemical analyses have shown colocalization of ghrelin proteins with glucagon in the α islet cells of the pancreas.78 In addition, ghrelin was found to increase cytosolic-free calcium concentration in β cells and stimulate insulin secretion in isolated rat pancreatic islets.78 In contrast, Broglio et al showed that ghrelin induced hyperglycemia and reduced insulin secretion in humans, suggesting that endogenous factor(s) act in concert with ghrelin to regulate insulin release in vivo.36 Furthermore, Zhang et al reported that ghrelin infusion at 12 nmol/kg/h dependently suppressed cholecystokinin (CCK)-induced and central vagal stimulant-induced pancreatic exocrine secretion in vivo.79 Similarly, ghrelin abolished exocrine secretion caused by CCK in rats with subdiaphragmatic vagotomy. In vitro, ghrelin produced no change in basal amylase release from purified acinar cells, and coincubation of ghrelin with CCK demonstrated no inhibition of CCK-stimulated amylase release.79 These data suggest that the suppressive actions of ghrelin on pancreatic exocrine secretion, analogous to its role in insulin regulation, are indirect in nature, requiring cooperative, not yet identified factor(s) in the physiologic microenvironment. Thus, ghrelin functions as a gastro-entero-pancreatic hormone, participating in the regulation of glucose metabolism, insulin release, and pancreatic exocrine secretion to integrate the hormonal and metabolic responses to fasting.
NOVEL ACTIONS AND CLINICAL IMPLICATIONS OF GHRELIN
Cardiovascular Effects of Ghrelin
Increasing evidence supports a functional role of ghrelin in myocardial growth associated with improved cardiac function. Both ghrelin and GHSR have been detected in the aorta and myocardium, indicating that ghrelin may modulate cardiovascular parameters through GH-independent mechanisms.80 Recent evidence indicates that ghrelin inhibits apoptosis in cardiomyocytes and endothelial cells through activation of extracellular signal-regulated kinase (ERK) 1/2 and Akt serine kinases.81 It is interesting to note that although cardiomyocytes bind ghrelin with high affinity, they do not express GHSR1a. Taken together, these findings imply the existence of other unknown GHSR subtype(s) distinct from the classic GHSR1a in the cardiovascular system.
Cardiac cachexia is a catabolic state characterized by weight loss and muscle wasting. It occurs in some patients with end-stage heart failure and is a strong independent risk factor for mortality in patients with chronic heart failure.82 Nagaya et al reported that ghrelin improved left ventricular dysfunction and attenuated the development of cardiac cachexia in rats.83 In addition, they showed that ghrelin reduced cardiac afterload and increased cardiac output without an increase in heart rate in healthy humans.84 Expectedly, plasma ghrelin was elevated in cachetic patients with congestive heart failure (CHF), and was associated with high levels of GH and tumor necrosis factor (TNFα) and decreased body weight.85 Collectively, these data indicated that an increase in plasma ghrelin might mediate compensatory mechanisms during catabolic-anabolic imbalance in cachectic patients with CHF.
Ghrelin has been shown to possess direct vasorelaxant properties in endothelium-denuded human internal mammary arteries and to potently reverse endothelin-1-induced vasoconstriction.86 In addition, recombinant human GH (rhGH) was shown to be beneficial in idiopathic dilated cardiomyopathy.87 Similar findings were observed in a later randomized, double-blind, placebo-controlled trial of rhGH in patients with chronic heart failure.88 These interesting data indicate that ghrelin administration may represent a new therapeutic approach in the management of chronic heart failure. Thus, the cardiovascular activities of ghrelin suggest potential clinical uses of ghrelin. Theoretically, GHSs analogs might be used to protect from coronary ischemia and prevent the progression of dilated cardiomyopathy.
Critical Illness and Immune Function
Ghrelin and the GHSR signaling system were detected in human T cells, B cells, and neutrophils, regardless of the maturity of the cell types.89 Ghrelin and GHSR were expressed not only in B cells, but also in T cells and neutrophils that did not express substantial GH transcripts, suggesting that ghrelin has GH-independent functions in the immune system. Koo et al reported that ghrelin induced significant increases in peripheral blood lymphocytes, and thymic cellularity and differentiation in young and old mice, respectively.90 In addition, they showed that ghrelin-treated mice exhibited significant increases in cytotoxic lymphocytes, cycling of lymphoid cells in the spleen, and reduction in tumor initiation and subsequent metastases. These observations indicated an immune enhancing property of ghrelin, with clinical implications for the treatment of immunocompromised states, eg, in aging, transplantation, and acquired immune deficiency syndrome.
The catabolic state of globally impaired anabolism in critical illness activates both sympathetic and parasympathetic nervous systems to control a wide range of cellular and systemic functions.91 Sympathetically mediated processes such as mobilization of stored energy to deliver energy substrates to vital organs such as the brain and heart, and inhibition of subsequent energy storage and gluconeogenesis are initially observed. This is ultimately reflected at the target tissue level as a reduction of metabolic activity, translating into a catabolic state of globally impaired anabolism with impaired secretion of GH and insulin-like growth factor I (IGF-1).91 On the other hand, a role for ghrelin in neuroimmunological control is implicated in the adaptive response directed at balancing the pro-inflammatory and anti-inflammatory pathways. Wang et al have shown that direct vagal stimulation, acting via nicotinic acetylcholine receptors on macrophages, suppressed the release of TNFα and interleukin 1 and 6 induced by endotoxin lipopolysaccharide.92–93 In addition, the vagus nerve has been shown to convey the immunologic state of the gastrointestinal tract to the hypothalamus.94 Thus, the vagus nerve provides the endogenous mechanism to regulate the magnitude of innate immune responses and attenuate proinflammation. Although this physiological mechanism has major implications in the prevention of overt life-threatening infections, the potential target molecule(s) for the development of possible therapeutic agents are not known. Ghrelin might be the mediator of this vagal signaling to the immune system, and future investigations are warranted to define its functional role in the parasympathetic anti-inflammatory pathway.
Neoplasia
An autocrine or paracrine role of ghrelin in neoplasia has been implied in a variety of tumors (Table 1). Ghrelin mRNA has been found in normal human pituitary tissue and pituitary tumors.95 Nonfunctioning adenomas expressed the highest level of ghrelin mRNA, followed by GH- and gonadotrophin-producing adenomas, while prolactinomas appeared to express the lowest level.96 There was an inverse correlation between the level of mRNA expression and size in GH-producing adenomas. In addition, ghrelin has also been linked to several cancer cell lines and neuroendocrine tumors. Specific binding sites for GHSs are present in neoplastic human thyroid and lung tissues.97–98 Ghrelin was found to stimulate cell proliferation in vitro in prostate cancer cell lines, implying a potential tumor-promoting role for ghrelin in prostate cancer.99 Murata et al reported that ghrelin modulated insulin signaling downstream and activated cell proliferation in hepatoma cells.100 Further studies suggested that ghrelin is produced substantially in human medullary thyroid carcinoma and endocrine tumors of the stomach and intestine.101–102 In addition, some pancreatic endocrine tumors expressed ghrelin and GHSR, regardless of the type of pancreatic hormone produced.103
TABLE 1. Localization of Ghrelin in Tumor Cells
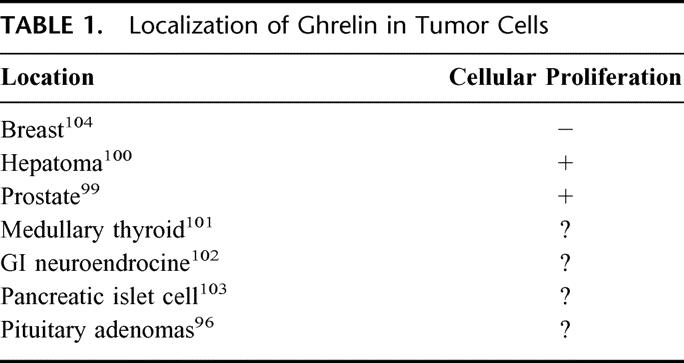
Conversely, Cassoni et al documented an antiproliferative effect of ghrelin in breast cancer cells.104 In their study, specific binding for ghrelin and GHSs in human breast carcinomas and cell lines was observed. This entity of binding was independent of histologic type, stage, ER status, proliferative index of the tumor, or pre- or postmenopausal status of the patients, but it was directly related to grade of tumor differentiation. Ghrelin and GHSs (peptidyl and nonpeptidyl) inhibited cellular proliferation in vitro, independent of GH releasing activity. Taken together, these observations suggest that the antiproliferative actions of ghrelin appear to function in providing a homeostatic growth balance in concert with existing proliferative signals in the mammary gland. However, as breast tumors acquire resistance to ghrelin, its inhibiting effects in mammary tissue may be suppressed. This suppression of ghrelin-responsiveness may be due to a lack of ghrelin/GHSR expression or receptor down-regulation. Wisse et al reported that plasma ghrelin levels are increased in their cancer cachexia model,105 suggesting that pathways associated with GHSR modulation (decreased synthesis or down-regulation) may exist by which malignant breast cancer cells acquire resistance to ghrelin. Future studies involving the expression of ghrelin or GHSR in mammary tumors are warranted to establish the role of ghrelin, if any, in breast cancer progression.
Cachexia
Ghrelin secretion has been proposed to counter further energy deficit and to defend against starvation in cachexia,24 indicating its role in an adaptive feeding response.106 Because weight loss is a potent stimulus for food intake in humans and animals, the persistence of anorexia indicates a failure of this adaptive feeding response, reflected in an insignificant rise in plasma ghrelin levels.107–108 The role of ghrelin in cancer anorexia is not well known, although as a mediator of hunger it is a key candidate for an important role. The expression of ghrelin mRNA in the stomach is decreased by IL-1β,24 a cytokine known to be elevated in malignancy.109 Conversely, peripherally administered ghrelin was able to reverse, at least in part, the IL-1β-induced loss of appetite and body weight.24 It is interesting to note that GHSs have been shown to reverse diet-induced catabolism and improve the alterations in the somatotrophic axis and protein catabolism in patients with prolonged, severe illness.110–111 Therefore, diminished ghrelin signaling may be linked to the etiology of cancer cachexia.
Wisse et al suggested that melanocortin, but not ghrelin pathways are involved in cancer anorexia.105 In their study, prostate cancer was induced by subcutaneous implantation of prostate adenocarcinoma cells in Lobound-Wistar rats. Intracerebroventricular injection of a synthetic antagonist of melanocortin (SHU9119) reversed cancer anorexia in rats; the increase in food intake was seen in anorexic animals treated with SHU9119, but not with neuropeptide Y or ghrelin. These authors further showed changes in the serum concentrations of ghrelin, leptin, and other serum markers in 4 groups of rats: ad libitum-fed control, pair-fed control, tumor-bearing, and SHU9119-treated. Ghrelin levels were increased to the same degree in both anorexic tumor-bearing and pair-fed control rats, and both were significantly higher than levels in ad libitum-fed controls. Treatment of tumor-bearing rats with SHU9119 reduced serum levels of ghrelin to the levels of controls. A reciprocal pattern was seen with serum leptin and insulin concentrations in all 4 groups.
The role of melanocortin antagonists in ameliorating cancer anorexia deserves further clinical evaluation. However, it is also evident from these experiments that the increased plasma ghrelin levels in anorexic animals compared with controls, suggest that ghrelin is ineffective in cancer anorexia. Interestingly, although centrally administered ghrelin failed to reverse anorexia in tumor-bearing rats,105 intramuscular injection of GHRH-expressing plasmid in tumor-bearing dogs raised the serum concentrations of insulin-like growth factor I (IGF-1), a measure of GHRH activity, and reversed cancer cachexia.112 Since an anabolic agent that stimulates appetite may be of great value in the treatment of cancer cachexia, it remains to be seen how useful ghrelin or its analogues could be in other tumors that cause cachexia.
THE BRAIN-GUT AXIS
The localization and secretion of ghrelin in the stomach and gastrointestinal tract, the presence of ghrelin receptor synthesis in the vagus nerve, and the numerous gastrointestinal effects of the hormone imply an important role in the brain-gut axis. Ghrelin's primacy as a “hunger hormone” with orexigenic effects mediated by the hypothalamic peptides, agouti-related peptide (AGRP), and neuropeptide Y (NPY), and the fact that it is the most potent peripheral signal of diminishing energy stores, implies that ghrelin release might be the most important of the many redundant mechanisms ensuring human survival in times of famine. This developmentally successful capacity has become detrimental in the industrial era when labor-saving devices, increased production and distribution of food, and environmental stressors result in the global epidemic of obesity.
In this context it is interesting to note that ghrelin signaling initiates phospholipase C and augments protein kinase C activation, which in itself increases both intracellular calcium and diacylglycerol.5,113 This sequence contributes to the development of insulin resistance,114–115 a key component in the pathogenesis of metabolic obesity with its multiple comorbidities.116–117 The newly discovered intimate relationship between ghrelin and the vagus nerve, recognized in the 19th century to affect feeding behavior, finally provides the missing hormonal link to the “hunger nerve.” This might justify further exploration of vagotomy as a means of attenuating the effects of a hyperactive brain-gut axis in the treatment of obesity.
SUMMARY AND CONCLUSIONS
Since the discovery of ghrelin in 1999, the volume of literature on ghrelin biology is impressive. Ghrelin's clinical potential both as a diagnostic and treatment modality has been recognized. In addition to affecting energy homeostasis, ghrelin may have cardioprotective effects, serve as a diagnostic or therapeutic tool in GH deficiency, and function as a prognosticator for neuroendocrine tumors. Theoretically, ghrelin is a promising candidate for treating catabolic states and enhancing immune function in cachexia or acquired immunodeficiency syndrome, as well as for treating eating disorders such as obesity and anorexia nervosa.
Many unanswered questions remain. Identification of the stimuli and pathways regulating ghrelin synthesis and release is fundamental to discovering clinical uses for ghrelin. Since acyl modification is essential for the activity of ghrelin, isolation of enzyme(s) that catalyze acylation should be important. The normal physiology of ghrelin during steady-state is largely unknown; most experimental models have focused on short-term pharmacological effects. Analysis of the functional significance of ghrelin in various physiological processes will require studies of both pulsatile and continuous infusion of physiologic and pharmacologic doses of ghrelin at equilibrium. In addition, biologic actions of ghrelin other than its GH-releasing activity should be clarified by identifying ghrelin isoforms or receptor subtypes.
The discovery of ghrelin is a major step forward in understanding networks regulating energy homeostasis, a critical biologic function. It offers the possibility of developing new approaches to diseases of impaired growth and development and energy imbalance. As future research efforts unravel the puzzle of ghrelin physiology, there will be many more surprises and exciting opportunities for research into its diverse physiological functions.
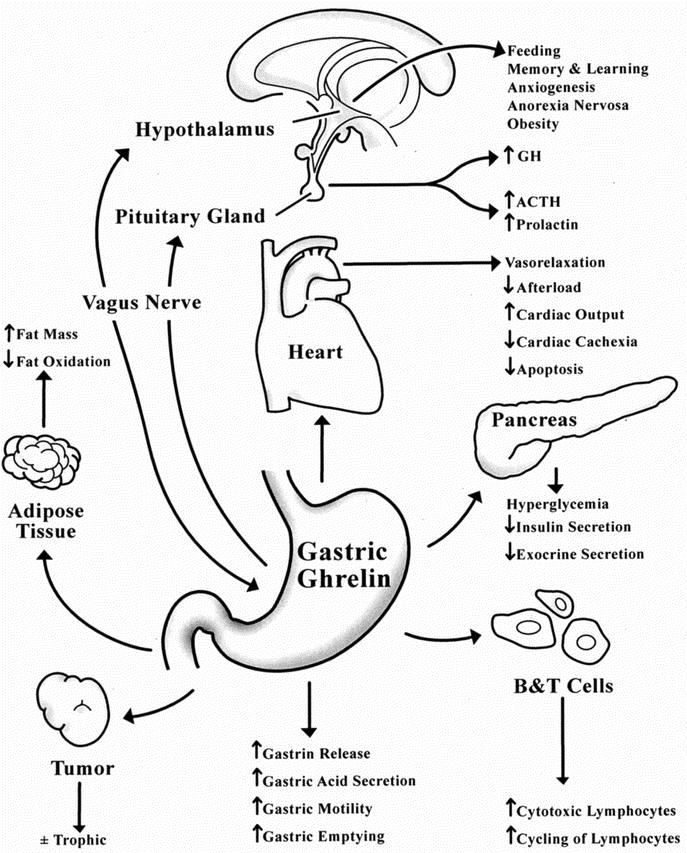
FIGURE 2. Physiological actions of ghrelin.
Footnotes
Reprints: James T. Wu, MD, Department of Surgery, SUNY Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203. E-mail: jwu@downstate.edu.
REFERENCES
- 1.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 2.Wajnrajch MP, Ten IS, Gertner JM, et al. Genomic organization of the Human GHRELIN Gene. J Endocr Genet. 2000;1:231–233. [Google Scholar]
- 3.Hosoda H, Kojima M, Matsuo H, et al. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem. 2000;275:21995–22000. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, Hosoda H, Matusuo H, et al. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–121. [DOI] [PubMed] [Google Scholar]
- 6.Bednarek MA, Feighner SD, Pong SS, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–4376. [DOI] [PubMed] [Google Scholar]
- 7.Casanueva FF, Dieguez C. Growth hormone secretagogues: physiological role and clinical utility. Trends Endocrinol Metab. 1999;10:30–38. [DOI] [PubMed] [Google Scholar]
- 8.Smith RG, Van der Ploeg LH, Howard AD, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. [DOI] [PubMed] [Google Scholar]
- 9.Bluet-Pajot MT, Tolle V, Zizzari P, et al. Growth hormone secretagogues and hypothalamic networks. Endocrine. 2001;14:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum GS, Bowers CY. Interactions of growth hormone secretagogues and growth hormone-releasing hormone/somatostatin. Endocrine. 2001;14:21–27. [DOI] [PubMed] [Google Scholar]
- 11.McKee KK, Palyha OC, Feighner SD, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol. 1997;11:415–423. [DOI] [PubMed] [Google Scholar]
- 12.Petersenn S, Rasch AC, Penshorn M, et al. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology. 2001;142:2649–2659. [DOI] [PubMed] [Google Scholar]
- 13.Smith RG, Palyha OC, Feighner SD, et al. Growth hormone releasing substances: types and their receptors. Horm Res. 1999;51:1–8. [DOI] [PubMed] [Google Scholar]
- 14.Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. [DOI] [PubMed] [Google Scholar]
- 15.Feighner SD, Tan CP, McKee KK, et al. Receptor for motilin identified in the human gastrointestinal system. Science. 1999;284:2184–2188. [DOI] [PubMed] [Google Scholar]
- 16.Hosoda H, Kojima M, Mizushima T, et al. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem. 2003;278:64–70. [DOI] [PubMed] [Google Scholar]
- 17.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. [DOI] [PubMed] [Google Scholar]
- 18.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988–2991. [DOI] [PubMed] [Google Scholar]
- 19.Papotti M, Ghe C, Cassoni P, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–3807. [DOI] [PubMed] [Google Scholar]
- 20.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–4911. [DOI] [PubMed] [Google Scholar]
- 21.Hataya Y, Akamizu T, Takaya K, et al. A low dose of ghrelin stimulates growth hormone (gh) release synergistically with gh-releasing hormone in humans. J Clin Endocrinol Metab. 2001;86:4552–4555. [DOI] [PubMed] [Google Scholar]
- 22.Date Y, Murakami N, Kojima M, et al. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem Biophys Res Commun. 2000;275:477–480. [DOI] [PubMed] [Google Scholar]
- 23.Arvat E, Di Viteo L, Broglio F, et al. Preliminary evidence that ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–495. [DOI] [PubMed] [Google Scholar]
- 24.Muccioli G, Tschop M, Papotti M, et al. Neuroendocrine and peripheral activites of ghrelin: implications in metabolism and obesity. Eur J Pharmacol. 2002;440:235–254. [DOI] [PubMed] [Google Scholar]
- 25.Arvat E, Maccario M, Di Vito L, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–1174. [DOI] [PubMed] [Google Scholar]
- 26.Bowers CY. Unnatural growth hormone-releasing peptide begets natural ghrelin. J Clin Endocrinol Metab. 2001;86:1464–1469. [DOI] [PubMed] [Google Scholar]
- 27.Asakawa A, Inui A, Kaga T, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74:143–147. [DOI] [PubMed] [Google Scholar]
- 28.Carlini VP, Monzon ME, Varas MM, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299:739–743. [DOI] [PubMed] [Google Scholar]
- 29.Muccioli G, Ghe C, Ghigo MC, et al. Specific receptors for synthetic GH secretagogues in the human brain and pituitary gland. J Endocrinol. 1998;157:99–106. [DOI] [PubMed] [Google Scholar]
- 30.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. [DOI] [PubMed] [Google Scholar]
- 31.Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. [DOI] [PubMed] [Google Scholar]
- 32.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. [DOI] [PubMed] [Google Scholar]
- 33.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. [DOI] [PubMed] [Google Scholar]
- 34.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. [DOI] [PubMed] [Google Scholar]
- 35.Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in human. Diabetes. 2001;50:1714–1719. [DOI] [PubMed] [Google Scholar]
- 36.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. [DOI] [PubMed] [Google Scholar]
- 37.Schaller G, Schmidt A, Pleiner J, et al. Plasma ghrelin concentrations are not regulated by glucose or insulin: a double-blind, placebo-controlled crossover clamp study. Diabetes. 2003;52:16–20. [DOI] [PubMed] [Google Scholar]
- 38.Kral JG. Surgical treatment of obesity. In: Bray GA, Bouchard C, James WPT, eds. Handbook of obesity. New York: Marcel Dekker; 1989:977–994. [Google Scholar]
- 39.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. [DOI] [PubMed] [Google Scholar]
- 40.Geloneze B, Tambascia MA, Pilla VF, et al. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13:17–22. [DOI] [PubMed] [Google Scholar]
- 41.Tritos NA, Mun E, Bertkau A, et al. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. [DOI] [PubMed] [Google Scholar]
- 42.Faraj M, Havel PJ, Phelis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. [DOI] [PubMed] [Google Scholar]
- 43.Holdstock C, Engstrom BE, Ohrvall M, et al. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz MW, Woods SC, Porte D Jr, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 45.Kral JG. Vagotomy for treatment of severe obesity. Lancet. 1978;1:307–308. [DOI] [PubMed] [Google Scholar]
- 46.Kral JG. Behavioral effects of vagotomy in humans. J Auton Nerv Syst. 1983;9:273–281. [DOI] [PubMed] [Google Scholar]
- 47.Elmquist JK, Maratos-Flier E, Saper CB, et al. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. [DOI] [PubMed] [Google Scholar]
- 48.Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:575–579. [DOI] [PubMed] [Google Scholar]
- 49.Bornstein SR, Uhlmann K, Haidan A, et al. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes. 1997;46:1235–1238. [DOI] [PubMed] [Google Scholar]
- 50.Heiman ML, Ahima RS, Craft LS, et al. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–3863.9275075 [Google Scholar]
- 51.Branson R, Potoczna N, Kral JG, et al. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med. 2003;348:1096–1103. [DOI] [PubMed] [Google Scholar]
- 52.Ravussin E, Tschop M, Morales S, et al. Plasma ghrelin concentration and energy balance: overfeeding and negative energy balance studies in twins. J Clin Endocrinol Metab. 2001;86:4547–4551. [DOI] [PubMed] [Google Scholar]
- 53.Tschop M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21. [DOI] [PubMed] [Google Scholar]
- 54.Tschop M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. [DOI] [PubMed] [Google Scholar]
- 55.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673. [PubMed] [Google Scholar]
- 56.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. [DOI] [PubMed] [Google Scholar]
- 57.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. [DOI] [PubMed] [Google Scholar]
- 58.Ikezaki A, Hosoda H, Ito K, et al. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes. 2002;51:3408–3411. [DOI] [PubMed] [Google Scholar]
- 59.Barazzoni R, Zanetti M, Stebel M, et al. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124:1188–1192. [DOI] [PubMed] [Google Scholar]
- 60.Pinilla L, Barreiro ML, Tena-Sempere M, et al. Role of ghrelin in the control of growth hormone secretion in prepubertal rats: interactions with excitatory amino acids. Neuroendocrinology. 2003;77:83–90. [DOI] [PubMed] [Google Scholar]
- 61.Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmiter RD, Erickson JC, Hollopeter G, et al. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- 63.Marsh DJ, Hollopeter G, Kafer KE, et al. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;6:718–721. [DOI] [PubMed] [Google Scholar]
- 64.Holst B, Cygankiewicz A, Halkjar J, et al. High constitutive signaling of the ghrelin receptor-identification of a potent inverse agonist. Mol Endocrinol. 2003;Aug 7 epub. [DOI] [PubMed]
- 65.Korbonits M, Gueorguiev M, O'Grady E, et al. A variation in the ghrelin gene increases weight and decreases insulin secretion in tall, obese children. J Clin Endocrinol Metab. 2002;87:4005–4008. [DOI] [PubMed] [Google Scholar]
- 66.Ukkola O, Ravussin E, Jacobson P, et al. Role of ghrelin polymorphisms in obesity based on three different studies. Obesity Res. 2002;10:782–791. [DOI] [PubMed] [Google Scholar]
- 67.Burman P, Ritzen EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev. 2001;22:787–799. [DOI] [PubMed] [Google Scholar]
- 68.DelParigi A, Tschop M, Heiman ML, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2002;87:5461–5464. [DOI] [PubMed] [Google Scholar]
- 69.Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–644. [DOI] [PubMed] [Google Scholar]
- 70.Haqq AM, Farooqi IS, O'Rahilly S, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:174–178. [DOI] [PubMed] [Google Scholar]
- 71.Janssen JA, van der Toorn FM, Hofland LJ, et al. Systemic ghrelin levels in subjects with growth hormone deficiency are not modified by one year of growth hormone replacement therapy. Eur J Endocrinol. 2001;145:711–716. [DOI] [PubMed] [Google Scholar]
- 72.Murdolo G, Lucidi P, Di Loreto C, et al. Circulating ghrelin levels of visceral obese men are not modified by a short-term treatment with very low doses of GH replacement. J Endocrinol Invest. 2003;26:244–249. [DOI] [PubMed] [Google Scholar]
- 73.Haqq AM, Stadler DD, Jackson RH, et al. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:2206–2212. [DOI] [PubMed] [Google Scholar]
- 74.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophy Res Commun. 2000;276:905–908. [DOI] [PubMed] [Google Scholar]
- 75.Lee HM, Wang G, Englander EW, et al. Ghrelin: a new gastrointestinal endocrine peptide that stimulates insulin secretion - enteric distribution, ontogeny, influence of endocrine and dietary manipulations. Endocrinology. 2002;143:185–190. [DOI] [PubMed] [Google Scholar]
- 76.Date Y, Nakazato M, Murakami N, et al. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. [DOI] [PubMed] [Google Scholar]
- 77.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. [DOI] [PubMed] [Google Scholar]
- 78.Date Y, Nakazato M, Hashiguchi S, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–129. [DOI] [PubMed] [Google Scholar]
- 79.Zhang W, Chen M, Chen X, et al. Inhibition of pancreatic protein secretion by ghrelin in the rat. J Physiol. 2001;537:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katugampola S, Davenport A. Emerging roles for orphan G-protein-coupled receptors in the cardiovascular system. Trends Pharmacol Sci. 2003;24:30–35. [DOI] [PubMed] [Google Scholar]
- 81.Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anker SD, Coats AJS. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–847. [DOI] [PubMed] [Google Scholar]
- 83.Nagaya N, Uematsu M, Kojima M, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. [DOI] [PubMed] [Google Scholar]
- 84.Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol (Regul Integr Comp Physiol). 2001;280:R1483–R1487. [DOI] [PubMed] [Google Scholar]
- 85.Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic-catabolic factors. Circulation. 2001;104:2034–2038. [DOI] [PubMed] [Google Scholar]
- 86.Wiley KE, Davenport AP. Comparison of vasodilators in human mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol. 2002;136:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fazio S, Sabatini D, Capaldo B, et al. A preliminary study of growth hormone in the treatment of dilated cardiomyopathy. N Engl J Med. 1996;334:809–814. [DOI] [PubMed] [Google Scholar]
- 88.Osterziel KJ, Strohm O, Schuler J, et al. Randomized, double-blind, placebo-controlled trial of human recombinant growth hormone in patients with chronic heart failure due to dilated cardiomyopathy. Lancet. 1998;351:1233–1237. [DOI] [PubMed] [Google Scholar]
- 89.Hattori N, Saito T, Yagyu T, et al. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4248–4291. [DOI] [PubMed] [Google Scholar]
- 90.Koo GC, Huang C, Camacho R, et al. Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166:4195–4201. [DOI] [PubMed] [Google Scholar]
- 91.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. [DOI] [PubMed] [Google Scholar]
- 92.Borovikova S, Ivanova M, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Wang BR, Zhang XJ, et al. Evidence for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8:540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korbonits M, Jacobs RA, Aylwin SJ, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:881–887. [DOI] [PubMed] [Google Scholar]
- 96.Kim K, Arai K, Sanno N, et al. Ghrelin and growth hormone (GH) secretagogue receptor (GHSR) mRNA expression in human pituitary adenomas. Clin Endocrinol. 2001;54:759–768. [DOI] [PubMed] [Google Scholar]
- 97.Cassoni P, Papotti M, Catapano F, et al. Specific binding sites for synthetic growth hormone secretagogues in non-tumoral and neoplastic human thyroid tissue. Endocrinol. 2000;165:139–146. [DOI] [PubMed] [Google Scholar]
- 98.Ghe C, Cassoni P, Catapano F, et al. The antiproliferative effect of synthetic peptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology. 2002;143:484–491. [DOI] [PubMed] [Google Scholar]
- 99.Jeffery PL, Herington AC, Chopin LK. Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J Endocrinol. 2002;172:R7–R11. [DOI] [PubMed] [Google Scholar]
- 100.Murata M, Okimura Y, Iida K, et al. Ghrelin modulates the downstream of insulin signaling in hepatoma cells. J Biol Chem. 2002;277:5667–5674. [DOI] [PubMed] [Google Scholar]
- 101.Kanamoto N, Akamizu T, Hosoda H, et al. Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J Clin Endocrinol Metab. 2001;86:4984–4990. [DOI] [PubMed] [Google Scholar]
- 102.Papotti M, Cassoni P, Volante M, et al. Ghrelin-producing endocrine tumors of the stomach and intestine. J Clin Endocrinol Metab. 2001;86:5052–5059. [DOI] [PubMed] [Google Scholar]
- 103.Volante M, Allia E, Gugliotta P, et al. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab. 2002;87:1300–1308. [DOI] [PubMed] [Google Scholar]
- 104.Cassoni P, Papotti M, Ghe C, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab. 2001;86:1738–1745. [DOI] [PubMed] [Google Scholar]
- 105.Wisse BE, Frayo RS, Schwartz MW, et al. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 2000;16:866–873. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz MW, Dallman MF, Woods SC. Hypothalamic response to starvation: implications for the study of wasting disorders. Am J Physiol. 1995;269:R949–R957. [DOI] [PubMed] [Google Scholar]
- 108.Inui A. Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59:4493–4501. [PubMed] [Google Scholar]
- 109.Plata-Salaman CR. Anorexia during acute and chronic disease. Nutrition. 1996;12:69–78. [DOI] [PubMed] [Google Scholar]
- 110.Murphy MG, Plunkett LM, Gertz BJ, et al. MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol Metab. 1998;83:320–325. [DOI] [PubMed] [Google Scholar]
- 111.Van den Berghe G. Neuroendocrine pathobiology of chronic critical illness. Crit Care Clin. 2002;18:509–528. [DOI] [PubMed] [Google Scholar]
- 112.Draghia-Akli R, Hahn KA, King GK, et al. Effects of plasmid-mediated growth hormone-releasing hormone in severely debilitated dogs with cancer. Mol Ther. 2002;6:830–836. [DOI] [PubMed] [Google Scholar]
- 113.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. [DOI] [PubMed] [Google Scholar]
- 114.Karasik A, Rothenberg PL, Yamada K, et al. Increased protein kinase C activity is linked to reduced insulin receptor autophosphorylation in liver of starved rats. J Biol Chem. 1990;265:10226–10231. [PubMed] [Google Scholar]
- 115.Pillay TS, Whittaker J, Siddle K, et al. Phorbol ester-induced downregulation of protein kinase C potentiates insulin receptor tyrosine autophosphorylation: evidence for a major constitutive role in insulin receptor regulation. Biochem Soc Trans. 1990;18:494–495. [DOI] [PubMed] [Google Scholar]
- 116.Kral JG. Morbidity of severe obesity. Surg Clin North Am. 2001;81:1039–1061. [DOI] [PubMed] [Google Scholar]
- 117.Marceau P, Biron S, Hould FS, et al. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84:1513–1517. [DOI] [PubMed] [Google Scholar]


