Abstract
Objective:
Determine the effect of insulin on the systemic inflammatory response, pro- and anti-inflammatory cytokines and hepatic acute-phase-response in severely burned pediatric patients.
Summary Background Data:
The systemic inflammatory and hepatic acute-phase-response contribute to hypermetabolism, multi-organ failure, and mortality. Insulin has been recently shown to decrease mortality and to prevent the incidence of multi-organ failure in critically ill patients; however, the underlying mechanisms have not been defined.
Methods:
Thirteen thermally injured children received insulin to maintain blood glucose at a range from 120 to 180 mg/dl, 15 children received no insulin with blood glucose levels also at range from 120 to 180 mg/dl and served as controls. Our outcome measures encompassed the effect of insulin on pro-inflammatory mediators, the hepatic acute-phase-response, fat, and the IGF-I system.
Results:
Insulin administration decreased pro-inflammatory cytokines and proteins, while increasing constitutive-hepatic proteins (P < 0.05). Burned children receiving insulin required significantly less albumin substitution to maintain normal levels compared with control (P < 0.05). Insulin decreased free fatty acids and serum triglycerides when compared with controls (P < 0.05). Serum IGF-I and IGFBP-3 significantly increased with insulin administration (P < 0.05).
Conclusion:
Insulin attenuates the inflammatory response by decreasing the pro-inflammatory and increasing the anti-inflammatory cascade, thus restoring systemic homeostasis, which has been shown critical for organ function and survival in critically ill patients.
The systemic inflammatory and hepatic acute-phase-response contribute to hypermetabolism, multi-organ failure, and mortality. In the present study we show that insulin attenuates the inflammatory response by decreasing the pro-inflammatory and increasing the anti-inflammatory cascade, thus restoring systemic homeostasis, which has been shown critical for organ function and survival in critically ill patients.
The reaction of trauma, sepsis, or major operations is characterized by hypermetabolism and catabolism, leading to peripheral protein waste, compromise of the immune system and the skin, and multi-organ dysfunction.1,2 During the aftermath of these multiple reactions, the liver has been shown to play a crucial role. Under physiologic conditions the liver synthesizes mainly constitutive-hepatic proteins, such as albumin, prealbumin, or transferrin. After trauma the synthesis shifts from constitutive-hepatic proteins to acute-phase proteins, such as haptoglobin, α2-macroglobulin, α1-acid glycoprotein, and C-reactive protein (CRP).3 This reaction of the liver is called the hepatic acute-phase-response. The goal of the hepatic acute-phase-response is to restore homeostasis; however, a prolonged and exaggerated response leads to the enhancement of hypermetabolism and catabolism, thus to increased morbidity and mortality.4–8 Mediators of the acute-phase-response are pro-inflammatory cytokines, such as interleukin-1 (IL-1 β), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor-necrosis factor (TNF), or the anti-inflammatory cytokine interleukin-10 (IL-10).3 Several groups showed that increased pro-inflammatory cytokine synthesis also contributes to hypermetabolism and catabolism.9 Therefore, a therapeutic agent attenuating the hepatic acute-phase-response and cytokine release may improve morbidity and mortality after trauma, sepsis, or major operation.
Recently, intensive insulin therapy was shown to decrease mortality in critically ill patients.10 Insulin given at doses to maintain blood glucose below 110 mg/dl prevented the incidence of multi-organ failure and thus improved clinical outcome and rehabilitation.10 There is now evidence that insulin improves hypermetabolism by affecting pro-inflammatory cytokine production and hepatic signal transcription factor expression.11 However, it remains unclear whether insulin affects the systemic inflammatory response and the hepatic acute-phase response in humans and whether insulin directly exerts its effects or through glucose metabolism.
We hypothesized that insulin exerts an anti-inflammatory effect on cellular mediators and the hepatic acute-phase-response after a major trauma. To test our hypothesis we determined the effect of insulin on the systemic inflammatory response, pro- and anti-inflammatory cytokines, and hepatic acute-phase-response in severely burned pediatric patients that received insulin but had glucose levels in the normal range, and compared the findings to patients who had similar glucose levels but did not receive insulin.
MATERIALS AND METHODS
In the present study we retrospectively analyzed 2 different patient cohorts. One study cohort was severely thermally injured children who required insulin substitution to maintain normal blood glucose levels of a range 120 to 180 mg/dl. The other study cohort was severely thermally injured children who did not require insulin substitution and their blood glucose levels without insulin were in the normal range of 120 to 180 mg/dl. Insulin was administered either as a continuous drip or as single dosages according to the blood glucose concentration.
Inclusion criteria for the study were: 1–18 years of age, admission to our institute within 3 days after injury, and burns covering more than 40% total body surface area (TBSA) with a 3rd–degree component of >10%, which required a minimum harvesting of 1 donor site for skin grafting. Patient demographics (age, date of burn and admission, sex, burn size, and depth of burn) and concomitant injuries, such as inhalation injury, sepsis, morbidity and mortality were obtained from records. Sepsis was defined as the systemic inflammatory response syndrome (SIRS) associated with an identifiable focus of infection.
Nutritional Markers and Albumin Supplementation Requirement
Amount and distribution of caloric intake was determined for each patient. The body weight was determined every week. The change of the body weight was determined at the end of the study period. The feeding protocol at our institute is calculated using the Curreri formula, which calls for 25 kcal/kg/d plus 40 kcal/% TBSA burned per day. This formula provides for maintenance needs, plus the additional caloric needs of the burn wounds. Based on the findings that TPN was associated with higher mortality, patients at our institute are fed by enteral feeding.
Serum albumin was measured daily at 4:30 am. If serum albumin concentrations were less than 2.0 g/dl, albumin was supplemented based upon age and body weight to maintain albumin at 2.0 g/dl. Children < 2 years of age and < 20 kg body weight received 6.25 g/d exogenous albumin over 6 hours, children 2–9 years old weighing > 20 kg but < 40 kg received 12.5 g/d over 6 hours, and children 10–18 years old weighing > 40 kg received 25 g/d. Total albumin infused, albumin infused per day, and grams of albumin infused per meter square burn were compared for patients receiving insulin and control patients.
Serum Calcium, Phosphate, Constitutive Hepatic Proteins, Acute Phase Proteins, and Serum Fat
Serum calcium; phosphate; constitutive hepatic proteins, such as transferrin, prealbumin, and retinol binding protein; serum acute phase proteins, such as α1-acid glycoprotein, C-reactive protein, α2-macroglobulin, α1-antitrypsin, haptoglobin, and free fatty acids; and triglycerides were measured using a Behring nephelometer (Behring, Dearfield, IL). Acute-phase-protein serum amyloid A (SAA) was determined by enzyme linked immuno assay (ELISA), (Biosource Int., Camarillo, CA).
Serum Cytokines, IGF-I, IGFBP-1, and IGFBP-3
Serum TNF, IL-1β, IL-6, IL-8, and IL-10 were determined by a human ELISA (Endogen, Woburn, MA. or Biosource Int., Camarillo, CA) on days 0 (admit), 10, 20, and 40 days after burn. Serum IGF-I, IGFBP-1, and IGFBP-3 were determined by RIA (Nichols Inst. Diagnostics, San Juan Capistrano, CA).
Ethics and Statistics
The study was reviewed and approved by the Institutional Review Board of the University of Texas Medical Branch, Galveston, Texas. Prior to the study, each subject, parent, or child's legal guardian had to sign a written informed consent form.
Analysis of variance (ANOVA) with post hoc Bonferroni's correction, paired and unpaired Student t test, χ2 analysis, and Mann-Whitney tests were used where appropriate. Data are expressed as percentages, means ± SD or means ± SEM, where appropriate. Significance was accepted at P < 0.05.
RESULTS
Twenty-eight severely burned children were included into the study, 13 children into the insulin group and 15 into the control group. The average insulin amount administered was 348 ± 170 IU over an average time period of 44 ± 22 days. Insulin was administered either by continuous drip or by single dose. With the administration of insulin a target blood glucose concentration of 120 to 180 mg/dl could be achieved. The control group demonstrated also a blood glucose range of 120 to 180 mg/dl. There was no statistical significant difference between the 2 groups at each time point.
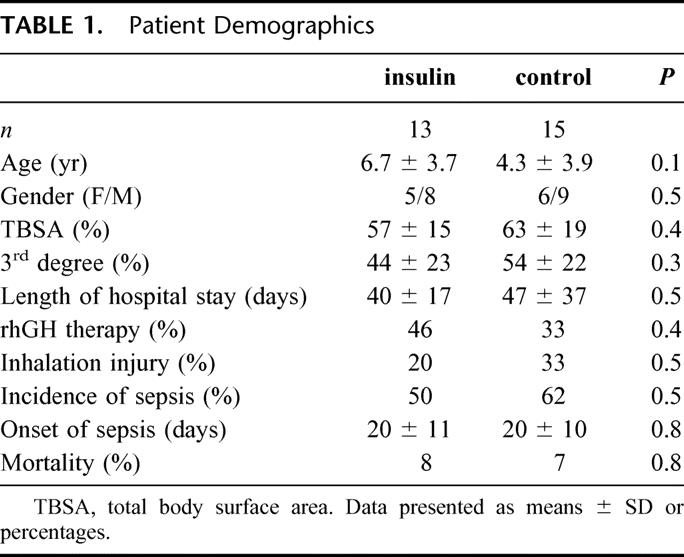
There were no significant differences between children receiving insulin or control in age, sex, size, and depth of burns. Furthermore, there were no differences between these 2 groups in rhGH treatment, incidence of sepsis, incidence of inhalation injury, or mortality rates. By looking at infection rate, it was mostly burn wound infection that resulted in a SIRS. The incidence of sepsis was around 20 days in both groups, with a range from 10 to 35 days in the insulin group and from 11 to 39 days in the control group (Table 1). No significant differences in length of hospital stay between insulin and control treated children could be shown (Table 1).
TABLE 1. Patient Demographics
Nutritional Markers and Albumin Substitution Requirement
Both groups had an adequate and equal amount of caloric intake (1500 kcal/m2 body surface + 1500 kcal/m2 area burn), as well as equal distribution of the calories. Dietary delivery was also the same in both groups, being fed by enteral nutrition. The change of body weight was recorded from the enrollment of the study until the study end. The control group had a body weight loss of −0.3 ± 1.3% from the admission body weight. Children receiving insulin had a body weight gain of 3.9 ± 1.2% from the original body weight (P < 0.05).
The amount of albumin required for substitution, expressed as total albumin amount, albumin amount per day, or as albumin per square meter burned surface area, was significantly reduced in patients receiving insulin compared with controls (P < 0.05) (Table 2).
TABLE 2. Nutritional Intake and Albumin Substitution Requirements

Serum Calcium, Phosphate Constitutive Hepatic Proteins, Acute Phase Proteins, and Fat
The range of serum calcium was from 2.6 to 5.7 mg/dl in the insulin group, while the range was from 2.7 to 6.1 mg/dl in the control group. Two patients required calcium substitution in the insulin group and the control group, respectively. The range of serum phosphate was similar in both groups. In the insulin group, the range was 1.0 to 7.0 mg/dl, in the control group from 1.4 to 7.1 mg/dl (normal 2.5 mg/dl to 5.5 mg/dl). Phosphate substation was required in 1 patient in the insulin group and 1 patient in the control group, which was not statistically significant different.
All constitutive-hepatic-proteins dropped below normal levels within 1 day after burn. Over time constitutive-hepatic-proteins increased but still remained below normal levels. Insulin significantly increased serum prealbumin at days 30 and 40 post trauma when compared with controls (P < 0.05) (Fig. 1a). Increased prealbumin levels would give an explanation why albumin substitution requirements were significantly reduced in the insulin group. Furthermore, insulin increased serum retinol binding protein concentrations at 20, 30, and 40 days after trauma compared with controls (P < 0.05) (Fig. 1b). There was no significant difference in serum transferrin between insulin and control.
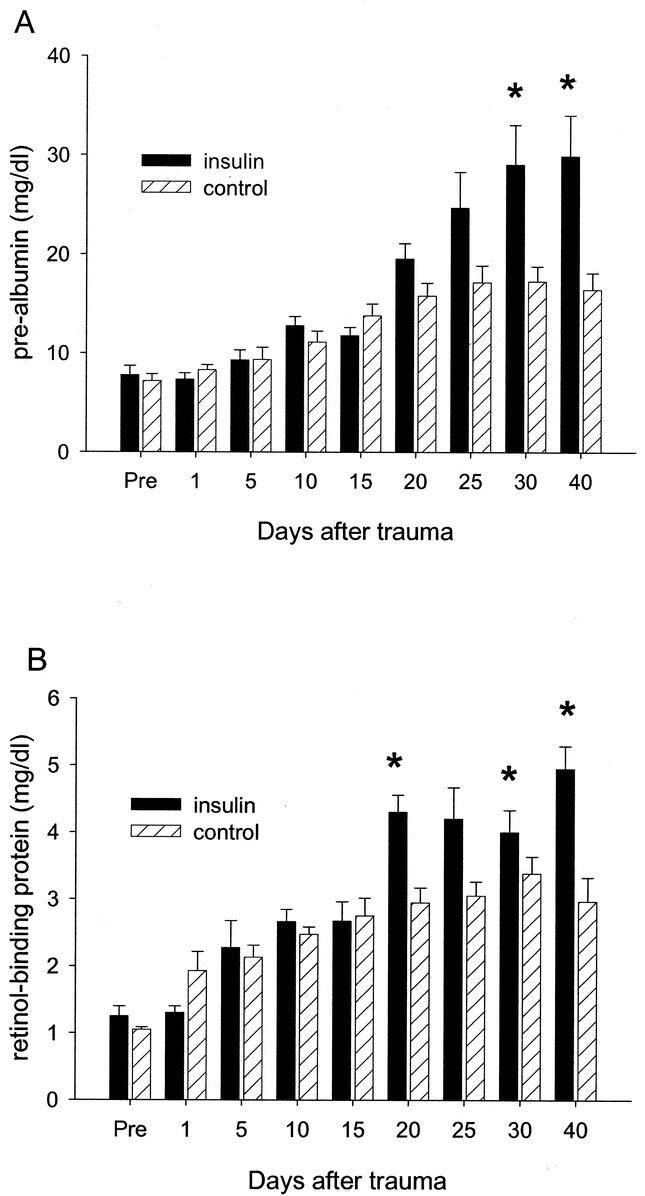
FIGURE 1. Serum prealbumin and retinol-binding protein after trauma. (A) Serum prealbumin decreased 3- to 4-fold in the insulin and control groups immediately after the injury compared with normal levels. Insulin increased serum prealbumin 30 and 40 days after trauma when compared with control (normal prealbumin, 25–45 mg/dl). (B) Serum retinol-biding protein dropped also by 3- to 4-fold compared with normal levels after the burn. Insulin significantly increased retinol-binding protein levels 20, 30, and 40 days after the insult when compared with controls. The insulin administration levels reached normal range while levels remained decreased in the control group (normal retinol-binding protein, 4–6 mg/dl). *Significant difference between insulin and control, P < 0.05. Data presented as means ± SEM.
All acute phase proteins determined in this study increased above normal levels within 5 to 10 days after the thermal injury and remained elevated throughout the entire study period. Insulin was found to decrease α2-macroglobulin at 30 and 40 days after trauma when compared with controls (P < 0.05) (Fig. 2a). More importantly, insulin was found to decrease serum CRP levels. CRP increased immediately after trauma and approached normal levels in the insulin group, not in the control group. Insulin further significantly decreased CRP at days 5, 10, 30, and 40 after trauma when compared with controls (P < 0.05) (Fig. 2b). There were no significant changes in the concentration of serum α1-acid glycoprotein, haptoglobin, or α1-anti trypsin.

FIGURE 2. Serum acute-phase-proteins α2-macroglobulin and C-reactive protein. (A) Serum α2-macroglobulin increased after burn. In the control group, α2-macroglobulin increased over the study period, while insulin decreased α2-macroglobulin at 30 and 40 days after trauma (normal α2-macroglobulin, <110 mg/dl). (B) Serum C-reactive protein increased 2-fold immediately after trauma in both groups. In the control group, CRP levels remained elevated throughout the study period, while serum CRP was significantly decreased and approached normal range in the insulin group (normal CRP, <5 mg/dl). *Significant difference between insulin and control, P < 0.05. Data presented as means ± SEM.
Insulin had a positive effect on serum free fatty acids and serum triglycerides. In contrast to other growth factors, insulin decreased serum triglycerides on days 25, 30, and 40 when compared with controls (P < 0.05) (Fig. 3a). Furthermore, insulin prevented an increase of free fatty acids immediately after burn and decreased free fatty acids at 15 and 20 days after burn when compared with controls (P < 0.05) (Fig. 3b).

FIGURE 3. Serum triglycerides and free fatty acid concentrations in burned children. (A) Serum triglycerides concentration increased above normal 10 days after burn in both groups. Insulin significantly decreased serum triglycerides 25, 30, and 40 days after the insult where levels were in the normal range. In the control group, serum triglyceride levels remained significantly elevated up to 40 days after burn (normal triglycerides, 35–150 mg/dl). (B) Serum free fatty acids increased immediately after burn and remained slightly elevated in the control group during the entire study period. Insulin decreased serum free fatty acids on days 15 and 20 after injury when compared with controls (serum FFA, 0.1–0.4 meq/L). *Significant difference between insulin and control, P < 0.05. Data presented as means ± SEM.
Serum Cytokines
Serum IL-1β levels increased immediately after trauma in both groups and were still increased 15 days after trauma. IL-1β demonstrated a slight increase 30 days after burn in the control group. In contrast, children receiving insulin had a significant decrease in IL-1β levels (P < 0.05) (Fig. 4a). Serum TNF was elevated in both groups immediately after burn. Insulin decreased the elevation observed for the TNF-α concentrations 30 days after injury compared with controls (P < 0.05) (Fig. 4b).

FIGURE 4. Serum pro- and antiinflammatory cytokines. (A) Serum IL-1β increased immediately after the severe insult and elevated 30 days after trauma. Insulin decreased IL-1β 30 days after burn when compared with controls (normal IL-1β, 0–2pg/ml). (B) Serum TNF was significantly decreased in the insulin 30 days after burn when compared with controls (normal TNF-α, 0–2 pg/ml). (C) Serum IL-10 increased 10-fold immediately after burn, decreased, and approached normal levels over the study period. Insulin increased IL-10 15 days after burn compared with controls (normal IL-10, <15 pg/ml). *Significant difference between insulin and control, P < 0.05. Data presented as means ± SEM.
Serum IL-10 also increased immediately after the thermal trauma. In both, group IL-10 decreased over the study period. However, insulin increased IL-10 15 days after trauma when compared with controls (P < 0.05) (Fig. 4c). There were no significant differences found 30 days after trauma.
No significant differences between insulin and control could be shown for serum IL-6 and IL-8.
Serum IGF-I, IGFBP-1, and IGFBP-3
As previously shown, serum IGF-I concentration dropped 3–4 fold after the severe burn injury. In the control group, levels remained low throughout the study period. Children treated with insulin demonstrated 3-fold increase in serum IGF-I starting 10 days after trauma. Serum IGF-I significantly increased with insulin administration 20 and 30 days after trauma (P < 0.05) (Fig. 5a).

FIGURE 5. Serum IGF-I and IGFBP-3 after the severe insult. (A) Serum IGF-I decreased 3- to 4-fold in both groups after burn. Insulin increased IGF-I 20 and 30 days after burn compared with controls (normal IGF-I, 365 ± 15 μg/ml). (B) IGFBP-3 levels dropped 5- to 6-fold after injury. Insulin significantly increased IGFBP-3 20 and 30 days after burn compared with controls (normal IGFBP-3, 2.8 ± 0.9 μg/ml). *Significant difference between insulin and control, P < 0.05. Data presented as means ± SEM.
Serum IGFBP-3 was also drastically decreased after injury. Insulin administration significantly increased serum IGFBP-3 20 and 30 days after trauma when compared with controls (P < 0.05) (Fig. 5b).
There were no significant differences found for serum IGFBP-1 between the 2 groups.
DISCUSSION
The systemic inflammatory response after trauma leads to hypermetabolism and thus protein degradation and catabolism. As a consequence, the structure and function of essential organs, such as the muscle, skin, heart, immune system, and liver are compromised and contribute to multi-organ failure and mortality.1,2 Pro-inflammatory mediators such as pro-inflammatory cytokines and acute-phase-proteins were thought to trigger and enhance this response and to mediate the catabolic effects, eg, by the inhibition of the growth hormone-insulin-like growth factor-I (IGF-I)-insulin axis.12–14 After the clinical failure of anti-inflammatory agents or antibodies against pro-inflammatory cytokines such as tumor necrosis (TNF), interleukin-1β (IL-1β), or their receptors, different approaches were taken to attenuate hypermetabolism. Insulin at a dose that kept blood glucose below 110 mg/dl decreased mortality and prevented the incidence of multi-organ failure in critically ill patients.10 In an animal model, insulin had anti-inflammatory effects by decreasing pro-inflammatory signal transcription factors and pro-inflammatory cytokines, while increasing anti-inflammatory cytokines.11 However, it is still unknown whether insulin exerts its effects directly through modulating pro-inflammatory mediators or indirectly through modulating glucose concentration. Several studies suggested that even mild hyperglycemia is detrimental and it aggravates endotoxemic shock and hypermetabolism.15,16 Hyperglycemia may further contribute to morbidity and mortality after burns or surgery.17,18 The possible detrimental mechanisms of hyperglycemia are increased production of superoxide and increased stress.19
In the present study we investigated the effect of insulin on the systemic inflammatory response in severely burned pediatric patients. One patient group received no insulin with blood glucose levels at a normal range, while the other group received insulin with the same range of blood glucose as the control group. Our results showed that insulin decreased pro-inflammatory cytokine and hepatic acute-phase-protein concentrations. At the same time, insulin significantly increased the anti-inflammatory cytokine IL-10. Given the fact that the insulin treated group and the control group had normal blood glucose levels, these data suggest that insulin acts as an anti-inflammatory molecule through direct cellular effects rather then through indirect effects, which would be by modulating glucose concentration.
Another aim of the study was to define the effect of insulin on the hepatic acute-phase-response. While a thermal injury in our pediatric population increased pro-inflammatory responses as indicated by a rise in both serum TNF and IL-1β, these values were significantly lower in pediatric burned patients receiving insulin. Decreases in serum TNF and IL-1β were associated with decreased acute-phase-protein concentrations of α2-macroglobulin and C-reactive protein. Insulin did not affect serum IL-6 or serum IL-8 concentration or their dependent acute-phase-proteins haptoglobin and α1-antitrypsin. Decreased acute-phase-protein expression was associated with increased synthesis of constitutive-hepatic-proteins, such as prealbumin and retinol binding protein. As a clinical relevant consequence, albumin substitution requirement to maintain normal serum albumin levels was significantly decreased in the insulin group when compared with the control group. Improved constitutive-hepatic-protein synthesis may result from either direct cellular signaling effects of insulin on CCAAT/enhancer-binding-proteins (C/EBPs) or signal transducer and activator of transcription (STATs) or indirect from a better liver synthesis capacity. We have recently shown that insulin alters the intracellular signal cascade in the liver. Insulin decreased the pro-inflammatory signal transcription factors STAT-5 and C/EBP-β.11 An up-regulation of both transcription factors lead to impaired organ function and protein synthesis, such as albumin.20,21 Therefore, it appears that insulin improves organ function and protein synthesis during the hypermetabolic response through these signal transcription factors. In addition to pro-inflammatory transcription factors, we determined signal transcription factors that were identified to either suppress cytokine signaling (SOCS) or regulate the T-cell function (RANTES) and showed increased hepatic SOCS-3 and RANTES mRNA expression.11 Thus insulin may act as an antiinflammatory molecule by 2 different pathways by decreasing pro-inflammatory mediators and by increasing antiinflammatory mediators.
Insulin is known to have vasodilatory properties and to increase glucose uptake in ischemic tissues for anaerobic ATP production after augmented glycolysis.22,23 Insulin may also stimulate pyruvate dehydrogenase during ischemia and thereby exerts a beneficial effect associated with an improved energy state and less lactate production.22,23 Furthermore it was speculated that insulin may inhibit the synthesis and release of free fatty acids.22 Increased levels of free fatty acids lead to increased hepatic triglyceride accumulation, and thus to fatty liver infiltration and consecutively to hepatic failure, which is associated with increased incidence of sepsis and mortality.24,25 The mechanisms have been discussed in clinical studies in which the authors speculated that burn increased peripheral lipolysis and due to a lack of transporter proteins (LDL, HDL) triglycerides accumulate in the liver.25,26 In the present study, we have shown that insulin administration decreases free fatty acids and serum triglycerides after thermal injury. This metabolic action of insulin represents an advantage, as liver failure and death due to increased fat accumulation could be prevented.
Pro-inflammatory mediators enhance catabolism and hypermetabolism by the inhibition of the growth hormone–insulin-like growth factor-I (IGF-I)–insulin axis.12,13,14 After major trauma, burns or in critically ill patients anabolic growth factor levels decrease 4- to 10-fold and remain mostly decreased over a long period of time.27 It is well documented that the administration of growth hormone after trauma increases IGF-I levels and rhGH may thus act through increased IGF-I levels.27,28 IGF-I itself was shown to attenuate hypermetabolism and the hepatic acute-phase-response and therefore may act as an antiinflammatory agent.29 In the present study, we found that insulin increased serum IGF-I and IGFBP-3 levels. Insulin increased IGF-I 2- to 3-fold when compared with controls. Twenty days after burn, serum IGF-I levels were in the range of normal levels in the insulin group, while it was still decreased in the control group. Similarly, insulin increased serum IGFBP-3 and brought levels up to normal levels 20 days after burn. The question that may arise is whether insulin or IGF-I exerts the effects in the present study can be answered by a recent animal study in which the authors showed that insulin exerts stronger anabolic effect than IGF-I in the state of septicemia, suggesting that insulin is the dominant factor with antiinflammatory properties.30
There are 2 limitations of the present study, 1 of which being that patients who received insulin may have had a different metabolic profile than the untreated controls. In addition it appears that the insulin group was older and bigger then the control group. Although this was not statistically significant, the number of patients in the present study was too small to detect differences. But the authors suggest that the differences observed in pro-inflammatory and antiinflammatory mediators are not due to age and body size differences, rather then effects of insulin. The second limitation being that in the insulin group 46% of the patients received rhGH, compared to 33% in the control group. Therefore, some of the effects observed, such as increases in IGF-I or IGFBP-3 could be due to the greater number of patients receiving rhGH in the insulin group and to a lesser extend due to insulin treatment.
In the present study, we have shown that insulin acts as an antiinflammatory mediator by decreasing pro-inflammatory cytokines and pro-inflammatory hepatic acute-phase-proteins. By decreasing pro-inflammatory mediators, constitutive proteins were increased, improving liver synthesis after severe burn. Insulin further decreased serum triglycerides and free fatty acids which could attenuate fatty liver infiltration and thus liver function and liver homeostasis. Insulin increased the anabolic growth factor IGF-I and its major binding protein IGFBP-3. As blood glucose was kept in the same range in both groups, these data suggest that insulin acts through direct cellular mechanisms rather then indirect mechanisms, which would be through modulation of glucose concentrations. In conclusion, we have shown that insulin attenuates hypermetabolism after severe trauma, and we suggest that insulin may decrease morbidity and mortality in a variety of critical conditions.
ACKNOWLEDGMENTS
The authors like to thank Wade Berlin, Marsha Hildreth, and Deb Benjamin for their technical assistance.
Footnotes
Reprints: Priv. Doz. Dr. med Marc G Jeschke, Klinik und Poliklinik für Chirurgie, Abteilung für Plastische Chirurgie und Handchirurgie, Universität Erlangen-Nürnberg, Krankenhausstr. 12, 91054 Erlangen, Germany. Email: Mcjeschke@hotmail.com.
REFERENCES
- 1.Rennie MJ. Muscle protein turnover and wasting due to injury and disease. Br Med Bull. 1985;41:257–264. [DOI] [PubMed] [Google Scholar]
- 2.Takala LE, et al. Increase mortality associated with growth hormone treatment in critically ill patients. N Engl J Med. 1999;341:785–792. [DOI] [PubMed] [Google Scholar]
- 3.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. [DOI] [PubMed] [Google Scholar]
- 4.Livingston DH, Mosenthal AC, Deitch EA. Sepsis and multiple organ dysfunction syndrome: A clinical-mechanistic overview. New Horizons. 1995;3:276–287. [PubMed] [Google Scholar]
- 5.Selzman CH, Shames BD, Miller SA, et al. Therapeutic implications of interleukin-10 in surgical disease. Shock. 1998;10:309–318. [DOI] [PubMed] [Google Scholar]
- 6.De Maio A, de Mooney ML, Matesic LE, et al. Genetic component in the inflammatory response induced by bacterial lipopolysaccharide. Shock. 1998;10:319–323. [DOI] [PubMed] [Google Scholar]
- 7.Pruitt JH, Copeland EM, Moldawer LL. Interleukin-1 and interleukin-1 antagonism in sepsis systemic inflammatory response syndrome and septic shock. Shock. 1995;3:235–251. [DOI] [PubMed] [Google Scholar]
- 8.Williams G, Giroir B. Regulation of cytokine gene expression: tumor-necrosis factor, interleukin-1, and the emerging biology of cytokine receptors. New Horizons. 1995;2:276–287. [PubMed] [Google Scholar]
- 9.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. [DOI] [PubMed] [Google Scholar]
- 10.Van den Berghe G, et al. Intensive insulin therapy in critically ill patients. New Eng J Med. 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 11.Jeschke MG, Einspanier R, Klein D, et al. Insulin attenuates the systemic inflammatory response to thermal trauma. Mol Med. 2002;8:443–450. [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berghe G, de Zegher F, Veldhuis JD. The somatotropic axis in critical illness: effect of continuous growth hormone (GH)-releasing hormone and GH-releasing peptide-2 infusion. J Clin Endocrin Metab. 1997;82:590–599. [DOI] [PubMed] [Google Scholar]
- 13.Frost RA, Lang CH, Gelato MC. Transient exposure of human myoblasts to tumor necrosis factor-α inhibits serum and insulin-like growth factor-I stimulated protein synthesis. Endocrinology. 1997;138:4153–4159. [DOI] [PubMed] [Google Scholar]
- 14.Timmins AC, Cotterill AM, Hughes SC. Critical illness is associated with low circulating concentrations of insulin-like growth factors-I and -II, alterations in insulin like growth factors binding proteins, and induction of an insulin-like growth factor binding protein 3 protease. Crit Care Med. 1996;24:1460–1466. [DOI] [PubMed] [Google Scholar]
- 15.Losser MR, Bernard C, Beaudeux JL, et al. Glucose modulates hemodynamic, metabolic, and inflammatory respnses to lipopolysaccharide in rabbits. J Appl Physiol. 1997;83:1566–1574. [DOI] [PubMed] [Google Scholar]
- 16.Ling PR, Lydon E, Frederich RC, et al. Metabolic effects of insulin and insulin-like growth factor-1 in endotoxemic rats during total parenteral nutrition feeding. Metaboism. 2000;49:611–615. [DOI] [PubMed] [Google Scholar]
- 17.Gore DC, Chinkes D, Heggers J, et al. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. [DOI] [PubMed] [Google Scholar]
- 18.Ljungqvist O, Nygren J, Thorell A. Insulin resistance and elective surgery. Surgery. 2000;128:757–760. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. [DOI] [PubMed] [Google Scholar]
- 20.Trautwein C, et al. C/EBP-beta/LAP controls down-regulation of albumin gene transcription during liver regeneration. J Biol Chem. 1996;271:22262–22270. [DOI] [PubMed] [Google Scholar]
- 21.Niehof M, et al. Interleukin-6-induced tethering of STAT3 to the LAP/C/EBPbeta promoter suggests a new mechanism of transcriptional regulation by STAT3. J Biol Chem. 2001;276:9016–9027. [DOI] [PubMed] [Google Scholar]
- 22.Groeneveld ABJ, Beishuizen A, Visser FC. Insulin: a wonder drug in the critically ill? Crit Care. 2002;6:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao V, Merante F, Weisel RD, et al. Insulin stimulates pyruvate dehydrogenase and protects human ventricular cardiomyocytes from stimulated ischemia. J Thorac Cardiovasc Surg. 1998;116:485–494. [DOI] [PubMed] [Google Scholar]
- 24.Aarsland A, Chinkes D, Wolfe RR. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J Clin Invest. 1996;98:2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in paediatric burn patients: Autopsy findings and clinical implications. J Trauma. 2001;51:736–739. [DOI] [PubMed] [Google Scholar]
- 26.Aarsland A, Chinkes D, Wolfe RR. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretions remains unchanged. Ann Surg. 1996;223:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeschke MG, Barrow RE, Herndon DN. Recombinant human Growth Hormone (rhGH) treatment in pediatric burn patients and its role during the acute phase response. Crit Care Med. 2000;28:1578–1584. [DOI] [PubMed] [Google Scholar]
- 28.Bichell DP, Kikuchi K, Rotwein P, et al. Growth hormone rapidly activates insulin-like growth factor 1 gene transcription in-vivo. Mol Endocrinol. 1992;6:1899–1908. [DOI] [PubMed] [Google Scholar]
- 29.Jeschke MG, Barrow RE, Herndon DN. IGF-I/IGFBP-3 attenuates the pro-inflammatory acute phase response in severely burned children. Ann Surg. 2000;231:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vary TC, Jefferson LS, Kimball SR. Insulin fails to stimulate muscle protein synthesis in sepsis despite unimpaired signalling to 4E-BP1 and S6K1. Am J Physiol. 2001;281:E1045–E1053. [DOI] [PubMed] [Google Scholar]


