Abstract
Objective:
To compare the outcomes of mitral repair and replacement in revascularized patients with ischemic mitral regurgitation.
Summary Background Data:
Combined coronary bypass (CABG) and mitral procedures have been associated with the highest mortality (>10%) in cardiac surgery. Recent studies have suggested that mitral valve replacement (MVR) with sparing of the subvalvular apparatus had comparable results to mitral repair when associated with CABG.
Methods:
Over the past 7 years, 54 patients had CABG/mitral repair versus 56 who had CABG/MVR with preservation of the subvalvular apparatus. The groups were similar in age at 69.2 years in the replacement group versus 67.0 in the repair group. We compared these 2 groups based on hospital mortality, incidence of complications including nosocomial infection, neurologic decompensation (stroke), pulmonary complication (pneumonia, atelectasis, and prolonged ventilation), and renal complications (acute renal failure or insufficiency).
Results:
The mitral repair group had a hospital mortality of 1.9% versus 10.7% in the replacement group (P = 0.05). Infection occurred in 9% of repairs compared with 13% of replacements (P = 0.59). The incidence of stroke was no different between groups (2 of 54 repairs vs. 2 of 56 replacements, P = 1.00). Pulmonary complication rate was 39% in repairs versus 32% in replacements (P = 0.59). Worsening renal function occurred in 15% of repairs versus 18% of replacements (P = 0.67).
Conclusions:
Mitral repair is superior to mitral replacement when associated with coronary artery disease in terms of perioperative morbidity and hospital mortality. Although preservation of the subvalvular apparatus with MVR has a theoretical advantage in terms of ventricular function, mitral repair clearly adds a survival benefit in patients with concomitant ischemic cardiac disease.
Ischemic mitral regurgitation remains difficult to manage for cardiac surgeons. Because of poor survival, recent literature suggests that these patients may not benefit from mitral repair compared with replacement. This study evaluates our experience with ischemic mitral regurgitation, which supports an early benefit for patients undergoing mitral valve repair.
Ischemic mitral regurgitation remains one of the most difficult entities to manage for cardiac surgeons. The operative risk of patients with ischemic mitral regurgitation is significantly greater than one would expect from combined risks of either mitral valve or ischemic pathology. Historically, mortality in these patients is reported to approach 20% and higher with surgical therapy in the perioperative period alone.1–4 Revascularization of the heart without intervening on the valve may be sufficient in patients with documented mitral insufficiency due to cardiac ischemia.5,6 However, only patients with well-defined pathophysiology and minimal regurgitation benefit from the revascularization strategy without addressing the valve. The exact mechanism of the insufficiency can be extremely difficult to characterize intraoperatively, which further limits the ability to determine who benefits from revascularization without direct repair of the mitral valve. Consideration of mitral valve repair is warranted partly by the incidence of persistent mitral regurgitation if coronary bypass grafting alone is performed.
Recent literature demonstrates the improvement in the technique of mitral valve replacement (MVR).7–10 With preservation of the mitral subvalvular apparatus, the ventricles appear to function bet ter with improved survival compared with replacement with sacrifice of the ventriculo-annular relationship. However, the outcome of mitral repair continues to be superior to MVR in patients with mitral pathology independent of ischemia.
Several institutions have examined the surgical outcomes of the ischemic mitral regurgitation subset. The Cleveland Clinic recently published a study with a homogeneous group of patients with what appears to be pure ischemic mitral regurgitation.11 They concluded that these patients had better outcomes with mitral repair than with mitral replacement except in the sickest patients. Results in the latter group were indistinguishable because of their poor performance with either procedure. In contrast, Thourani et al (from Emory) could not identify any benefit for repair over replacement in patients with combined revascularization and mitral valve repair compared with replacement.12 The optimal procedure for patients with mitral regurgitation and coronary ischemia remains controversial in even the most recent literature.
The hypothesis to be tested in this study is that, in patients with ischemic disease, mitral repair is superior to MVR.
MATERIALS AND METHODS
The charts of patients undergoing coronary artery bypass grafting (CABG) and mitral valve repair or MVR were retrospectively reviewed over the past 7 years (1995–2002). Excluded were patients in shock and those with ruptured papillary muscles requiring emergent MVR. A total of 110 patients were identified who fit these criteria. Fifty-four patients underwent combined CABG and mitral valve repair, while 56 patients underwent CABG with MVR. All patients were completely revascularized, and all had an internal mammary artery graft unless it had been used previously. Mitral annuloplasty was preformed using a rigid complete ring. An undersized ring was used (in most a 28 mm in males and a 26 mm in females) to correct the annular dilatation. Finally, a few patients with posterior papillary muscle tethering also received a posterior papillary stitch, as previously described, to restore the relationship between the papillary muscle and annulus.13
MVR was performed as described by Miller and others preserving the subvalvular apparatus with sparing of the papillary muscles.14
The charts were reviewed for preoperative characteristics, including age at operation and gender. Additionally, we compared comorbidities, which included cardiac disease that could be further broken down into a history of any arrhythmia, congestive heart failure requiring previous admission for congestive heart failure, history of rheumatic disease, and finally severity of mitral regurgitation based on preoperative echocardiography. Comorbidities included any cerebral vascular disease (including cerebral vascular accident, transient ischemic attack, or reversible ischemic neurologic defect), peripheral vascular disease (including claudication, previous vascular surgery, or surveillance of significant vascular disease), significant pulmonary disease (chronic obstructive pulmonary disease; current or previous treatment of emphysema), chronic renal insufficiency (creatinine > 1.5 mg/dL or need for dialysis), and diabetes (requiring medical treatment). Left ventricular ejection fraction (normal > 50%, mild 40%–49%, moderate 30%–39%, and severe < 30%) and mitral regurgitation severity by preoperative echocardiography (mild, moderate, severe) were graded by a cardiologist based on echocardiographic or cardiac catheterization.
Operative characteristics were also evaluated, including number of vessels revascularized, time on pump, cross-clamp time, and transfusion requirements.
Comparisons of perioperative outcome were based on hospital mortality, nosocomial infection, stroke, pulmonary decompensation (pneumonia, significant atelectasis, or prolonged ventilation), and renal complications (creatinine rising above 1.5 mg/dL or new need for hemodialysis). Finally, length of hospital stay was examined.
An independent statistician performed statistical analysis. Depending on the variable, a Student's t test, χ2 analysis, or Fisher exact test was used for comparison among groups. All results are reported as mitral repair first unless otherwise stated.
RESULTS
Preoperative Comparison
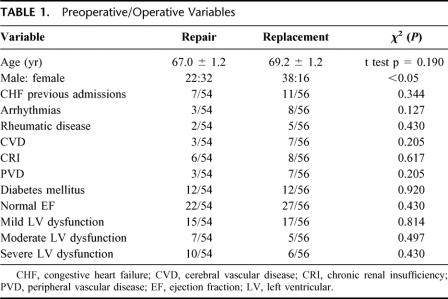
Preoperative comparisons are summarized in Table 1. The mean age in the mitral valve repair group was 67 ± 1.2 years (range, 42–84 years) compared with mitral replacement group mean age of 69.2 ± 1.2 years (range, 42–84 years) (t test, P = 0.190). There was a lower male:female ratio in the repair group (22:32) compared with the replacement group (38:18, χ2, P < 0.05).
TABLE 1. Preoperative/Operative Variables
Comorbidities between the groups were similar. Groups had similar rates of congestive heart failure (8 of 54 in repair vs. 11 of 56 in replacement, χ2, P = 0.34), preoperative arrhythmias (3 of 54 repair vs. 8 of 56 replacement, χ2, P = 0.15), and rheumatic disease (2 of 54 repair vs. 6 of 56 replacement, Fisher exact test, P = 0.27). Noncardiac comorbidities (repair vs. replacement, χ2) were also similar in terms of cerebral vascular disease (3 of 54 vs. 7 of 56, P = 0.21), chronic renal insufficiency (6 of 54 vs. 8 of 56, P = 0.67), peripheral vascular disease (3 of 54 vs. 7 of 56, P = 0.21), chronic obstructive pulmonary disease (9 of 54 vs. 9 of 56, P = 0.93), and diabetes (12 of 54 vs. 12 of 56, P = 0.92). When broken down by severity of left ventricular dysfunction, functional classification were similar (normal 22 of 54 vs. 28 of 56, P = 0.43; mild 15 of 54 vs. 17 of 56, P = 0.814; moderate 5 of 56 vs. 7 of 54, P = 0.497; severe 10 of 54 vs. 6 of 56, P = 0.246) with similar severities of left ventricular dysfunction in the replacement group (mean left ventricular ejection fraction of 43.9 ± 1.2 in repair in vs. 40.0 ± 1.7% in replacement, P = 0.09).
Overall, there were minor differences between the groups that should not limit conclusions made from comparisons in the study.
Operative Comparisons
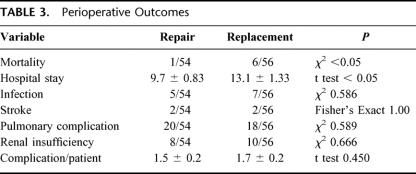
Operative statistics are summarized in Table 2. The mean number of revascularized grafts was similar, with patients undergoing repair receiving an average of 2.3 ± 0.15 grafts compared with the replacement group who received 2.5 ± 0.15 grafts (P = 0.17). The average time on pump and cross-clamp time was significantly shorter in length in the repair group (pump time, 152 minutes vs. 171 minutes, P < 0.05; cross-clamp time, 112 minutes vs. 133 minutes, P < 0.05). The utilization of red blood cells was similar between groups as well (1.5 units vs. 1.1 units, P = 0.39).
TABLE 2. Operative Statistics
Perioperative Outcomes
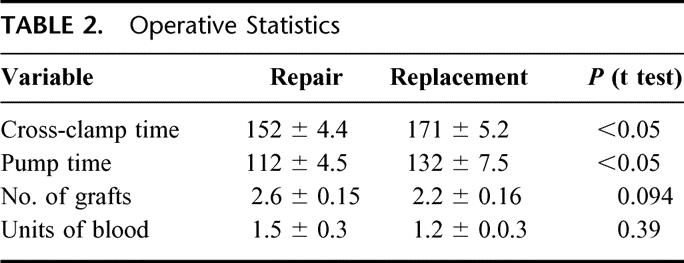
Perioperative comparisons are summarized in Table 3. The mitral repair group had a hospital mortality of 1.9% (1 of 54) versus 10.7% (6 of 56) in the replacement group (P = 0.05). The mean hospital stay was significantly shorter in the mitral repair group compared with the mitral replacement group (9.7 ± 0.83 days vs. 13.1 ± 1.33 days, t test, P < 0.05). Infection occurred in 13% (5 of 54) of replacement patients compared with 9% (7 of 56) of repair patients (P = 0.59). The incidence of stroke (2 of 54 vs. 2 of 56, P = 1.00) was no different. Pulmonary complication rate was 39% (21 of 54) in repairs versus 32% (18 of 56) in replacements (P = 0.59). However, there were trends toward increases likelihood of prolonged ventilation in repair patients (20 of 54 vs. 12 of 56, P = 0.07) and toward increased likelihood of pneumonia in replacement patients (0 of 54 vs. 5 of 56, P = 0.06). Worsening renal function occurred in 18% (10 of 56) of replacement patients versus 15% (8 of 54) of repair patients (P = 0.67). Additionally, no differences were found in postoperative need for dialysis (0 of 54 vs. 1 of 56, P = 1.00)
TABLE 3. Perioperative Outcomes
DISCUSSION
Ischemic mitral regurgitation remains a distinctly different pathology in cardiac surgery from both coronary disease and other forms of mitral regurgitation. The mortality from the Society of Thoracic Surgeons Database on MVR plus CABG has been more than 10% for each of the last 10 years. This procedure consistently carries the highest mortality of any procedure published in the database. The combined pathology, which includes the ventricle, the papillary muscles, the annulus, and the valve leaflets, amplifies the risk of complications and reduces their survival with or without surgical correction. The progression of disease is relentless enough that some authors question whether the choice between mitral valve repair and MVR makes any difference in outcome.12
Tissue hypoxia, which is the fundamental mechanism of ischemic mitral regurgitation, leads to acute and chronic structural and/or functional changes in the ventriculo-annular apparatus. Two specific types regurgitation can be addressed by mitral valve intervention and revascularization. First, the injured ventricle and/or mitral annulus can dilate leading to Carpentier Type I regurgitation. This is defined as valvular leaflets unable to coapt secondary to the increased annular size creating a central jet. Second, with Carpentier Type IIIb regurgitation, the ischemia can lead to restriction of the distance between the leaflet edge and the ventricular wall. The shortened papillary or hypokinetic ventricular wall tethers the leaflets into the ventricle, not allowing the valve to coapt completely. The ventricular annular relationship appears to be very important in preserving ventricular function. Preservation of this ventricular relationship appears to play a role in ventricular function, particularly with the systolic performance. Thus, preservation of the ventriculo-annular relationship preserves long-term ventricular function with annuloplasty, and to a lesser extent, in MVR with preservation of the subvalvular apparatus.
Given the fact that ischemia leads to the dysfunction in ischemic mitral regurgitation, logical thinking would conclude that revascularization should prevent or correct the dysfunction. Several studies have suggested that revascularization with CABG alone does not adversely affect survival or functional status in patients who are left with mild to moderate mitral regurgitation postoperatively.15–17 However, Aklog et al demonstrated that CABG alone for patients with moderate regurgitation can result in significant residual mitral regurgitation.5 This moderate amount of regurgitation was significant enough to increase late symptoms and decrease late survival. Furthermore, in a later study, Adams and colleagues reinforce the idea that intraoperative transesophageal echocardiography can underestimate the severity of the mitral regurgitation, making postoperative evaluation of the regurgitation tenuous.18 They concluded that anyone with more than mild mitral regurgitation would probably benefit from valvular intervention.
The revascularization alone remains ineffective because of irreversible anatomic distortions from ventricular scarring, ventricular dilatation, or annular dilatation. In regard to the added risk of valvular correction, other authors would argue that any further risk of the additional procedure could be offset by the benefits of complete repair.12 Previously, we examined mitral valve repair in patients with ischemic cardiomyopathy undergoing CABG.19 We found that the combined procedure resulted in symptomatic improvement with no added surgical risk. In this high-risk population, valvular intervention does not make the procedure more risky for the patient. Our overall perioperative mortality of 6% (7 of 110) is lower than other studies of ischemic mitral regurgitation with good symptomatic improvement in the short term. However, we do feel that CABG alone can reverse ischemic mitral regurgitation in patients with small atria, active ischemia, and in the absence of chronic structural changes.
As Miller reiterates, ischemic mitral regurgitation is a dysfunction of the entire ventricle.14 The corrective procedure must be based on the structural pathology on preoperative echocardiography, as the intraoperative echo can be misleading with underestimation of the severity of the regurgitation. Mitral valve annuloplasty directly corrects annular dilatation (Carpentier Type I) and in many cases via restoration of the ventriculo-annular relationship distorted by other mechanism of mitral regurgitation. However, the results of annuloplasty in patients with ischemic mitral regurgitation have been reported to be inconsistent. This is due to the inability to deal with the tethering mechanism with the ring alone. At our institution, we have had excellent short-term results in patients with Carpentier Type IIIb pathology using an annular ring in addition to a stitch between the posterior annuls and the posterior papillary muscle to pull ventricular wall into a better relationship with the annulus. Ten patients in this study underwent this procedure with improvement in all from 3 to 4+ mitral regurgitation to 0 to 1+.13 In cases of anatomic deviation that would not be restored with CABG alone, mitral valve annuloplasty can directly address the pathology with correction in a majority of patients with good long-term results.
Despite these possible advantages with mitral repair, some recent studies argue that the choice of valvular procedure makes no difference in outcomes. Cohn et al reviewed 150 patients with ischemic mitral regurgitation.20 They found that the choice repair or replacement of the ischemic mitral valve was less important than the underlying pathophysiology of the mitral regurgitation and the patient's functional status.20 Reviewing the patients at Emory undergoing mitral procedures, Thourani et al found that patients with concomitant coronary artery bypass grafting did not realize a survival advantage at the 10-year follow-up because of disease progression.6 In contrast, although Gillinov et al found no difference between repair and replacement in the highest-risk patients with ischemic mitral regurgitation, they did conclude that the majority of patients (70%) have a long-term survival advantage with mitral valve repair.11 Other studies have shown that mitral repair may be superior to replacement. Grossi et al found that patients undergoing repair had lower short-term morbidity and death rates among patients undergoing replacement.21 A more recent study from Gillinov et al22 reviewed patients undergoing mitral procedures and CABG. They concluded that 89% of patients would benefit from repair over replacement and that this advantage would be evident within 2 years of surgery.22 Although the literature remains controversial on the subject, there is significant evidence for the preferential treatment of ischemic mitral regurgitation with restoration of the anatomic relationships of the ventricle by mitral valve repair.
Our perioperative mortality rate of 2% with annuloplasty and 11% in replacement of the ischemic mitral valve is lower than other studies of ischemic MR. At the same time, we have demonstrated a perioperative survival advantage for the patients undergoing mitral valve repair. Our patients with mitral regurgitation and ischemic cardiac disease have acted more like nonischemic mitral regurgitation. Patients undergoing mitral valve repair also spend less time in the hospital, adding to the benefit of repair over replacement.
Although we feel that mitral valve repair is superior to replacement, MVR is still appropriate in some patients with ischemic mitral regurgitation. Despite increased risk with mitral replacement in this cohort, the risk of operation does not compare with the natural progression of this disease without intervention. Survival and hospital stay are improved in these patients with mitral replacement; however, if possible, the results in these patients will be better with repair.
This study does have several limitations. First, the study is retrospective and not randomized. We are limited by the choices made by 5 individual surgeons on what they feel is better for their patients. Second, we report only the perioperative outcomes in this cohort. The long-term outcomes have been difficult to compile with sufficient completeness to make observations. Third, the cohort is relatively small, but despite this limitation, we still identified an advantage for the patients undergoing repair, which suggests a significant benefit for this procedure.
CONCLUSION
Most patients with combined mitral regurgitation and ischemic cardiac disease will have superior perioperative outcomes with revascularization and mitral valve repair. Ischemic mitral regurgitation patients will benefit from repair just as patients with other mechanisms of mitral pathology benefit.
Discussions
Dr. William A. Baumgartner (Baltimore, Maryland): I would like to congratulate Dr. Reece and his associates on a fine study and an excellent presentation. The issue of mitral regurgitation in patients with coronary artery disease has a number of controversial facets.
For starters, as Dr. Reece pointed out, this is one of the highest risk groups of patients that we face. There is a question of whether to only perform coronary bypass grafting without intervening on the mitral valve. The other controversial aspect is whether to replace or repair the mitral valve, which is the subject of Dr. Reece's retrospective analysis.
The authors demonstrated that superior outcomes, including hospital mortality and length of stay, were seen in the cohort of patients who underwent mitral valve repair rather than replacement for ischemic heart disease. Their outcomes and mortality are particularly commendable in this high risk group of patients.
The authors have acknowledged in their manuscript the limitations of this study but do provide evidence of the superiority of repair versus replacement for this high risk group of patients. I have a few questions.
Are you able to tell us what you used to determine whether patients underwent repair or replacement over this 7-year period of study?
What would you now consider as an indication for mitral valve replacement rather than repair, since repair seems to be the procedure of choice?
What criteria do you and your group use to consider doing anything to the mitral valve rather than just performing coronary artery grafting alone?
Do you use any provocative methods in the operating room to better determine the degree of mitral regurgitation? This is a real issue since once a patient is put under anesthesia, mitral regurgitation often is diminished. Performing a preoperative transesophageal echo, as has been done in this group of patients, is very important. But if you don't have that information, do you do anything in the operating room to try and induce mitral gurgitation?
Finally, do you have any long-term follow-up on these patients that you presented, or on patients who had mitral regurgitation but did not undergo mitral valve repair or replacement?
I enjoyed your presentation very much.
Dr. Fred A. Crawford, Jr. (Charleston, South Carolina): I also enjoyed this very nice presentation by Dr. Reece and his colleagues from the University of Virginia. The authors should first be congratulated because the overall outcome when both groups, that is both repair and replacement groups, are combined, is significantly superior to that reported in other studies as well as the data from the STS database.
Mitral regurgitation in the setting of surgery for ischemic heart disease continues to be a problem. One must first decide if anything needs to be done, and if so what procedure should be performed. Carpentier and others have taught us that in most instances mitral valve repair is superior to mitral valve replacement. It is tempting to extend this conclusion to mitral regurgitation associated with ischemic heart disease, but the data are mixed. And this presentation attempts to clarify the situation.
As noted, this study demonstrated a significant difference in outcomes between the 2 groups insofar as survival and hospital stay is concerned. The major problems with the study, which again are acknowledged by authors in the manuscript, are that it is retrospective and involves a relatively small group of patients.
The groups are similar statistically, but the replacement patients tended to be older and have worst ventricular function and heart failure, more arrhythmias, and more peripheral vascular disease. It may be that these patients were felt to be higher risk and therefore were selected for valve replacement. If so, it is not too surprising that the replacement patients had a higher mortality.
I have several questions for the authors that may help clarify these issues. First, was the degree of mitral regurgitation comparable in the 2 groups? Next, were the repair and replacement patients evenly spaced throughout the study, or did more patients have replacement in the early years and more have repair in the later years? Were the chondal-sparing techniques consistent among all surgeons and did it always involve both papillary muscles? Finally, mitral valve replacement abolishes mitral regurgitation. Did any patient in the repair group have persistent or recurring mitral regurgitation and did any require reoperation?
This is a very difficult group of patients that we all struggle with, and I congratulate the authors for their outstanding results and Dr. Reece for this very nice presentation.
Dr. Lynn H. Harrison, Jr. (New Orleans, Louisiana): This very nice presentation by one of our former residents who is now in Dr. Kron's stable manifests the same smooth persuasiveness which is characteristic of so many of Dr. Kron's papers, and is in concert with reports from other institutions which point to the advantages both immediate and long term of mitral repair as compared with replacement.
However, the differences shown by the Virginia group are so dramatic, it leads me to wonder if in fact there are some important differences between those 2 cohorts that were not brought out in the presentation. The questions that I would ask, which are along the lines of those asked by Dr. Crawford, are: Do you have information on the mean greatest left atrial dimension from your echo studies on these patients? A surrogate would be, was there a difference in the chronicity of the mitral insufficiency between the groups? Because if there was, I would predict that those patients with more long-standing mitral insufficiency with larger left atria are those who fell into the repair group and those with a more acute process, perhaps a shorter interval between a myocardial infarction and operation, fell into the replacement group. It is much easier for most surgeons to repair a valve in a capacious left atrial than it is in the acute circumstances when the left atrium dimension is 4 centimeters or less. And although with manipulation you can see everything you need to see in order to do a valve replacement, in my own hands, at least, if I can't see pretty much the entire circumference of the mitral annulus without distracting the leaflets, then my results with mitral repair are not as good.
Dr. Irving L. Kron (Charlottesville, Virginia): All the questions asked are very good questions and this paper clearly has the issue of not being a randomized study. I can tell you having spent the last 10 years trying to get something like that together, it is just so difficult to find enough patients like this even in multiple centers to come up with an answer. This is the best we could do.
Dr. Baumgartner asked the question: Why repair versus replacement? This was a surgical preference. We have a group of senior surgeons at UVA, so there is not a bunch of young guys doing the replacements versus repairs done by the old guys. Truthfully, if you look STS database results for our surgeons, they match up for any subset.
The question was asked about my indications for mitral valve replacement. I am aggressive about repairs personally, and I would say that this is a disease not of the leaflets but of the muscle. The only time I would replace a valve would be a failed repair by another surgeon, or myself for that matter, or a situation if there was so much calcium that I couldn't put a ring around the valve.
Now, the real question is repair versus isolated CABG for mitral regurgitation. This is a question that we as cardiac surgeons argue ad nauseam. We think this is really an important issue, and it bores the rest of you. The bottom line is that in cases of acute ischemia, a coronary bypass is an excellent operation. When there is evidence of chronic ventricular dysfunction, evidence of a big left atrium, a big left ventricle, a repair or replacement must be done. That decision is not to be made in the operating room; it is to be made preoperatively. When we try to make this decision in the operating room it is to the lack of benefit to our patients. We tend not to use provocative methods to induce mitral regurgitation intraoperatively.
What Bill says is correct if you go to the operating room and say, “I really don't want to do a repair here, I want to get out of there.” If you get the pressure down to 80, they are not going to have regurgitation. So you look real good for about 15 minutes or so.
Well, short-term follow-up for most heart surgeons is about 15 minutes. We have had situations where we go to the operating room planning to repair, there is virtually no mitral regurgitation. You give them a little bit of phenylephrine and suddenly you have wide open MR. So you have got to make the decision ahead of time.
Long-term follow-up is a very good question, and we are troubled with this. We are a victim, as Paper 11 of this program stated, of HIPAA regulations. We have a database of our own, of course. But many patients are long-distance referrals. For us to call their referring physician about their outcomes 3 or 4 years hence is very difficult. We have not gotten around that yet, but we will figure out a methodology.
Dr. Crawford asked the question: Was the degree of MR comparable? Yes, I believe so. Sparing of the subvalvular apparatus was pretty consistent. Was the cordal sparing consistent? Yes. In terms of spacing of procedures, I don't think we suddenly got the message about repair in the last 2 years or anything of that sort.
Finally, the late failure of repair, to my knowledge, has been one of my own patients that had a repair that required replacement. That is the only one that required reoperation to my knowledge.
Dr. Harrison asked several very good questions. The sum of his questions are: Did the real sick acutely ill patients with papillary muscle rupture get mitral replacement and the others get repair? The answer is no. In this manuscript we excluded specifically patients who had papillary muscle rupture because that would bias the mitral valve replacement group. These were basically chronic patients; we excluded patients in shock.
The major question is the ease of repair, is a big atrium easier than a little atrium? That is true. But those of us who were brought up in the days of Wolf Parkinson White surgery, an operation that is now obsolete, unfortunately, we learned to operate through very small left atria. The truth is you can repair any one of these valves. I think even in a small atrium, a repair is actually easier than a replacement. You have just got to work at it a little bit, and there are ways of detecting with it.
I think the final message I would like to have you take home is if you compare the results of our repair with isolated coronary bypass, they are equivalent. A repair basically is as safe with coronary bypass as coronary bypass alone. So you ought not think that any repair will add to the mortality of your patients; conversely, if you leave someone with mitral regurgitation they will do poorly. I can tell you that it is not easy to do a re-do mitral valve operation in a patient with live grafts.
Footnotes
Reprints: T. Brett Reece, MD, HSC Box 801359, MR4 Building, Room 3116, Charlottesville, VA 22908. E-mail: tbr5q@virginia.edu.
REFERENCES
- 1.Czer LS, Maurer G, Trento A, et al. Comparative efficacy of ring and suture annuloplasty for ischemic mitral regurgitation. Circulation. 1992;86(suppl 5):46–52. [PubMed] [Google Scholar]
- 2.Cohn LH, Allred EN, Cohn LA, et al. Early and late risk of mitral valve replacement: a 12 year concomitant comparison of the porcine bioprosthetic and prosthetic disc mitral valves. J Thorac Cardiovasc Surg. 1985;90:872–881. [PubMed] [Google Scholar]
- 3.Hickey MS, Smith LR, Muhlbaier LH, et al. Current prognosis of ischemic mitral regurgitation: implications for future management. Circulation. 1988;78(3 Pt 2):51–59. [PubMed] [Google Scholar]
- 4.Flameng W, Szecsi J, Sergeant P, et al. Combined valve and coronary artery bypass surgery: early and late results. Eur J Cardiothorac Surg. 1994;8:410–419; discussion 419. [DOI] [PubMed]
- 5.Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation. 2001;104(12 suppl 1):68–75. [DOI] [PubMed] [Google Scholar]
- 6.Thourani VH, Weintraub WS, Guyton RA, et al. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting [comment]. Circulation. 2003;108:298–304. [DOI] [PubMed] [Google Scholar]
- 7.David TE. Papillary muscle-annular continuity: is it important? J Cardiac Surg. 1994;9(suppl 2):252–254. [DOI] [PubMed] [Google Scholar]
- 8.Rose EA, Oz MC. Preservation of anterior leaflet chordae tendineae during mitral valve replacement [comment]. Ann Thorac Surg. 1994;57:768–769. [DOI] [PubMed] [Google Scholar]
- 9.David TE. Mitral valve replacement with preservation of chordae tendinae: rationale and technical considerations. Ann Thorac Surg. 1986;41:680–682. [DOI] [PubMed] [Google Scholar]
- 10.Chitwood WR Jr. Mitral valve repair: an odyssey to save the valves! J Heart Valve Dis. 1998;7:255–261. [PubMed] [Google Scholar]
- 11.Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–1141. [DOI] [PubMed] [Google Scholar]
- 12.Thourani VH, Weintraub WS, Craver JM, et al. Influence of concomitant CABG and urgent/emergent status on mitral valve replacement surgery. Ann Thorac Surg. 2000;70:778–783; discussion 783–784. [DOI] [PubMed]
- 13.Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74:600–601. [DOI] [PubMed] [Google Scholar]
- 14.Miller DC. Ischemic mitral regurgitation redux: to repair or to replace? J Thorac Cardiovasc Surg. 2001;122:1059–1062. [DOI] [PubMed] [Google Scholar]
- 15.Duarte IG, Shen Y, MacDonald MJ, et al. Treatment of moderate mitral regurgitation and coronary disease by coronary bypass alone: late results. Ann Thorac Surg. 1999;68:426–430. [DOI] [PubMed] [Google Scholar]
- 16.Connolly MW, Gelbfish JS, Rose DM, et al. Early coronary artery bypass grafting for complicated acute myocardial infarction. J Cardiovasc Surg. 1988;29:375–382. [PubMed] [Google Scholar]
- 17.Arcidi JM Jr, Hebeler RF, Craver JM, et al. Treatment of moderate mitral regurgitation and coronary disease by coronary bypass alone. J Thoracic Cardiovasc Surg. 1988;95:951–959. [PubMed] [Google Scholar]
- 18.Adams DH, Filsoufi F, Aklog L. Surgical treatment of the ischemic mitral valve. J Heart Valve Dis. 2002;11(suppl 1):21–25. [PubMed] [Google Scholar]
- 19.Gangemi JJ, Tribble CG, Ross SD, et al. Does the additive risk of mitral valve repair in patients with ischemic cardiomyopathy prohibit surgical intervention? Ann Surg. 2000;231:710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohn LH, Rizzo RJ, Adams DH, et al. The effect of pathophysiology on the surgical treatment of ischemic mitral regurgitation: operative and late risks of repair versus replacement. Eur J Cardiothorac Surg. 1995;9:568–574. [DOI] [PubMed] [Google Scholar]
- 21.Grossi EA, Goldberg JD, LaPietra A, et al. Ischemic mitral valve reconstruction and replacement: comparison of long-term survival and complications [comment]. J Thorac Cardiovasc Surg. 2001;122:1107–1124. [DOI] [PubMed] [Google Scholar]
- 22.Gillinov AM, Faber C, Houghtaling PL, et al. Repair versus replacement for degenerative mitral valve disease with coexisting ischemic heart disease. J Thorac Cardiovasc Surg. 2003;125:1350–1362. [DOI] [PubMed] [Google Scholar]