Abstract
Objective:
To describe clinical characteristics and outcomes of a large cohort of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas affecting the main pancreatic duct.
Summary Background Data:
IPMNs are being diagnosed with increasing frequency. Preoperative determination of malignancy remains problematic, and reported results of long-term survival following resection are conflicting.
Methods:
The combined databases from the Massachusetts General Hospital and the Pancreatic Unit of the University of Verona were analyzed. To avoid confusing overlap with mucinous cystic neoplasms, only patients with tumors of the main pancreatic duct (with or without side branch involvement) were included. A total of 140 tumors consecutively resected between 1990 and 2002 were classified as either benign (adenoma and borderline tumors) or malignant (carcinoma in situ or invasive cancer) to compare their characteristics and survival.
Results:
Men and women were equally affected (mean age 65 years). Seven patients (12%) had adenomas, 40 (28%) borderline tumors, 25 (18%) carcinoma in situ, and 58 (42%) invasive carcinoma. The median age of patients with benign IPMN was 6.4 years younger than those with malignant tumors (P = 0.04). The principal symptoms were abdominal pain (65%), weight loss (44%), acute pancreatitis (23%), jaundice (17%), and onset or worsening of diabetes (12%); 27% of patients were asymptomatic. Jaundice and diabetes were significantly associated with malignant tumors. Five- and 10-year cancer-specific survival for patients with noninvasive tumors was 100%, and comparable survival of the 58 patients with invasive carcinoma was 60% and 50%.
Conclusions:
Cancer is found in 60% of patients with main-duct IPMNs. Patients with malignant tumors are 6 years older than their benign counterparts and have a higher likelihood of presenting with jaundice or new onset diabetes. No patients with benign tumors or carcinoma in situ died of their disease following resection, and those with invasive cancer had a markedly better survival (60% at 5 years) than pancreatic ductal adenocarcinoma. These findings support both the concept of progression of benign IPMNs to invasive cancer and an aggressive policy of resection at diagnosis.
Sixty percent of resected main-duct intraductal papillary mucinous neoplasms of the pancreas harbor either carcinoma in situ or invasive cancer. Compared with patients with benign tumors, these patients are older and have a higher likelihood of presenting with jaundice or diabetes, but 29% of them are asymptomatic. Resection can be done safely and long-term survival is excellent, with a low likelihood of recurrence in the remaining pancreas.
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are being diagnosed with increasing frequency. While the majority of these patients have symptoms, others are being identified by radiologic studies done for unrelated problems. These “incidentally discovered” lesions may account for as many as 27% of IPMNs1 and can pose management challenges, since many of these patients are elderly and the only curative treatment is surgical resection.
IPMNs comprise a histologic spectrum that ranges from adenoma to invasive carcinoma with different degrees of aggressiveness.2 Not infrequently, varying degrees of cytoarchitectural atypia are seen in the same tumor, and current thinking is that all IPMNs with invasive carcinoma progressed from an adenoma that underwent transformation, perhaps reflecting stepwise molecular genetic changes. It is not known whether all IPMNs have this malignant potential or what the time course of this progression may be.
Several recent series have divided IPMNs into 2 “geographic” groups: those with involvement of the main pancreatic duct and those with predominant involvement of the side branches of the ductal system. It has been suggested that “branch-duct” IPMNs may be more indolent than the main-duct type, have a lesser frequency of in situ and invasive cancer, and therefore be potentially amenable to nonsurgical observation or a more limited resection.3–5 However, the criteria to differentiate these 2 anatomic varieties of tumor have not been clearly defined, and main-duct and branch-duct IPMNs frequently are not distinguished or separated in reported series. Also, in all likelihood, there has been confusion between mucinous cystic neoplasms and branch-duct IPMNs, especially in older series.
The purpose of this study is twofold. By using a large database of patients with main-duct IPMNs, we first sought to determine whether preoperative clinical characteristics reliably correlate with malignancy in an IPMN, or conversely, whether absence of symptoms exonerates cancer. The second objective was to analyze long-term survival in patients who underwent resection for these tumors.
PATIENTS AND METHODS
Prospective databases of patients with IPMNs and other cystic neoplasms are kept in the Pancreatic–Endocrine Unit of the Department of Surgical and Gastroenterological Sciences of the University of Verona (UV) and the Department of Surgery of the Massachusetts General Hospital (MGH). From January 1988 to June 2002, 352 IPMNs and mucinous cystic neoplasms (MCN) were resected at both institutions. Unresectable mucinous cancers were not tabulated. An initial comparative review of these cases showed that the histologic criteria for MCNs differed between the 2 institutions. Whereas at the UV the diagnosis of MCN required the identification of an ovarian-like stromal layer in the tumor (resulting in an almost exclusive restriction of prevalence to the distal pancreas of females), at the MGH such a stromal layer was not required for diagnosis, just a lack of demonstrable communication with the pancreatic ductal system; consequently, there was inclusion of many lesions in the head and neck of the pancreas, some of which were in males, which potentially could be IPMNs of the branch-duct variety rather than MCNs.
Inasmuch as there is no consensus among pathologists as to the requirement for a stromal layer to make the diagnosis of an MCN, and to avoid possible contamination of our observations by inadvertently including neoplasms of nonuniform types, pathogenesis, and natural history, we elected to include in the study only those patients with IPMN that had involvement of the main pancreatic duct, either as the principal component of the tumor or secondarily as an extension from a branch-duct (or peripheral) IPMN. Thus, both MCN and isolated side-branch IPMN have been excluded. This resulted in the identification of 140 patients (60 from UV and 80 from the MGH) that are the focus of this study.
The tumors were classified, in accordance with World Health Organization criteria,6 as intraductal papillary mucinous adenomas, intraductal papillary mucinous tumor with moderate dysplasia or borderline tumor, in situ or noninfiltrating intraductal papillary mucinous carcinoma, or infiltrating intraductal papillary mucinous carcinoma.
The pancreatic transection margins were analyzed intraoperatively by frozen section and reported as negative (normal ductal epithelium, simple mucinous metaplasia), positive (presence of definite dysplastic changes, varying from moderate to high grade), or equivocal (hyperplasia or low-grade dysplasia, or difficult to interpret). These results were correlated with the subsequent definitive permanent histologic interpretations.
The overall clinical characteristics, including the presence or absence and type of symptoms, were compared between patients with benign (intraductal papillary mucinous adenomas and intraductal papillary mucinous tumor with moderate dysplasia or borderline tumor) and malignant tumors (intraductal papillary mucinous carcinoma and infiltrating intraductal papillary mucinous carcinoma). The type of surgical procedure, operative morbidity (pancreatic and biliary fistulae, delayed gastric emptying, intra-abdominal collections), length of postoperative hospital stay, and mortality were recorded. Patients or their referring physicians were contacted at 6-month intervals to determine the presence or absence of recurrence and the disease-specific and overall survival.
Statistical Analysis
For a description of the continuous variables, we used the median, first quartile and third quartile (Q1 and Q3), respectively, as measures of position and dispersion. In-between group comparisons were done with Student t test or Mann-Whitney rank sum test, depending on the distribution of the data (normal or skewed). Categorical variables were compared using the χ2 test. Survival analysis was done using the Kaplan-Meier function, comparing the groups with the log-rank test and the Breslow test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Clinical Characteristics
Of the 140 patients with resected IPMNs involving the main pancreatic duct, 17 (12%) had adenomas, 40 (28.4%) had borderline tumors, 25 (18%) carcinoma in situ, and 58 (41.6%) invasive carcinoma.
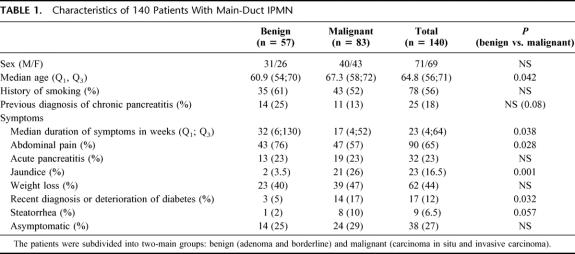
The clinical characteristics of the entire cohort, and the subgroups with benign (adenoma or borderline) or malignant (carcinoma in situ or invasive cancer) tumors are shown in Table 1. Significant differences included older age (6.4 years), a higher incidence of jaundice, and recent onset or deterioration of diabetes in patients with malignant tumors, versus a longer duration of symptoms and more abdominal pain in patients with benign IPMNs. Although not quite statistically significant because of the relatively small numbers of patients, there was a fivefold increase in the prevalence of steatorrhea in patients with malignant tumors. In 38 patients (27%), the tumors were incidentally discovered during workup of an unrelated problem, and there were no symptoms related to IPMN. There was no difference in the frequency of asymptomatic patients between benign and malignant tumors.
TABLE 1. Characteristics of 140 Patients With Main-Duct IPMN
Tumor Location and Surgical Procedures
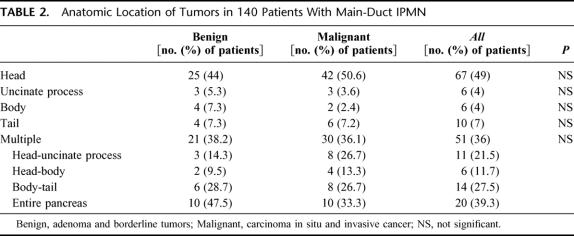
The location of the tumors is described in Table 2. There was no significant difference when comparing the location of benign and malignant tumors. In all, the head and/or uncinate process of the pancreas was involved in 99 patients (70.7%), and in keeping with this the most common surgical procedure performed was a pancreatoduodenectomy (88 patients, 63%). In 13 of these patients, the resection was extended to the left of the mesenteric vein. A distal pancreatectomy was done in 24 cases (17%), a total pancreatectomy in 26 (18.5%), and a middle pancreatectomy in the remaining 2 patients. It is noteworthy that in this series of main-duct IPMNs we did not observe any instances of discontinuous involvement or skip lesions (ie, head and tail with a normal duct in the pancreatic body).
TABLE 2. Anatomic Location of Tumors in 140 Patients With Main-Duct IPMN
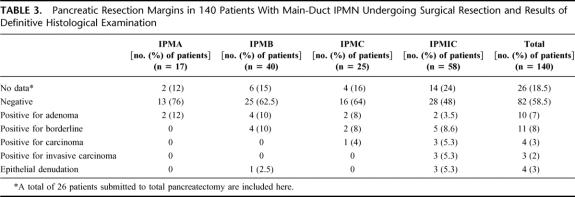
The results of the pathologic examination of the pancreatic transection margins are shown in Table 3. In all cases, definitive examination of the margins confirmed the diagnosis obtained on the cryostatically examined material. In 29 patients, the results of the intraoperative frozen section analysis altered the surgical plan. In 82 of the 114 patients (72%) who had a pancreatic transection margin (26 patients had a total pancreatectomy), this was unequivocally negative for tumor (mucinous metaplasia and hyperplasia were considered as a negative margin). Four of the 32 patients with a positive or indeterminate margin had a late recurrence in the remaining pancreas.
TABLE 3. Pancreatic Resection Margins in 140 Patients With Main-Duct IPMN Undergoing Surgical Resection and Results of Definitive Histological Examination
Operative Outcomes
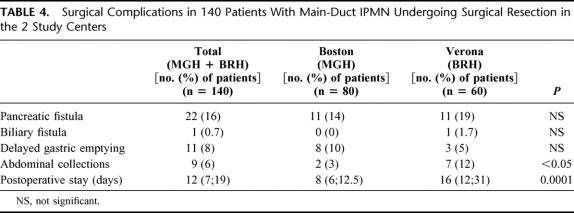
There was no operative mortality in the 140 patients, and the median number of blood transfusion units was 0. Postoperative complications for the entire group as well as for those patients operated at MGH and at the UV are shown in Table 4. There were no differences in the complication rates between the 2 institutions with the exception of a higher incidence of intra-abdominal collections at the UV (12% vs. 2.5%). The postoperative hospital stay at the UV was twice that at the MGH (median of 16 vs. 8 days), likely reflecting differences in medical culture.
TABLE 4. Surgical Complications in 140 Patients With Main-Duct IPMN Undergoing Surgical Resection in the 2 Study Centers
Long-term Follow-up, Recurrence, and Survival
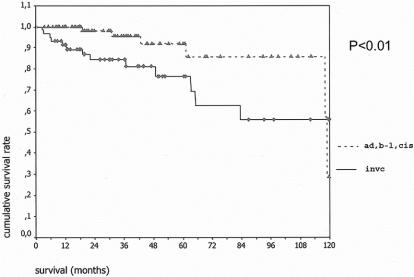
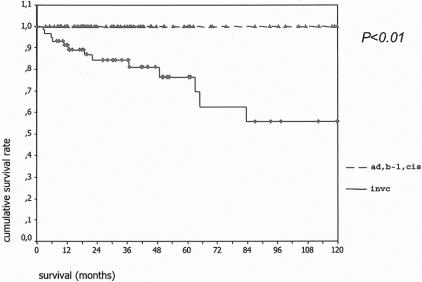
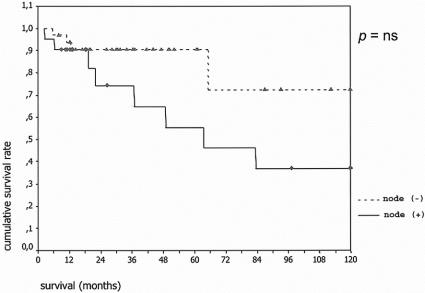
Complete follow-up information was available in 137 (98%) of patients. Of the 3 patients lost to follow-up, 2 had benign tumors and 1 invasive carcinoma. Mean and median follow-up for the remaining patients was 40 and 31 months, respectively. The overall actuarial survival curves for both groups are shown in Figure 1. Five- and 10-year disease-specific survival for the 80 patients with adenoma, borderline tumors, or carcinoma in situ was 100%, and the comparable statistics for the 57 patients with invasive carcinoma were 60% and 50% (Fig. 2); 24 of 58 (41%) patients with IPMN and invasive carcinoma had positive lymph nodes. Their survival was worse than that of patients with invasive cancer and negative lymph nodes, although the difference did not quite reach statistical significance (Fig. 3).
FIGURE 1. Overall actuarial survival of 80 patients with noninvasive IPMNs (adenoma, borderline or in situ carcinoma) (top curve) versus 57 patients with IPMN and invasive carcinoma (bottom curve). The curves are significantly different (P < 0.01).
FIGURE 2. Disease-specific actuarial survival of 80 patients with noninvasive IPMNs (adenoma, borderline or in situ carcinoma) (top curve) versus 57 patients with IPMN and invasive carcinoma (bottom curve). The curves are significantly different (P < 0.01).
FIGURE 3. Actuarial survival according to lymph node status. Top curve shows patients with invasive carcinoma and negative lymph nodes (n = 34), and bottom curve patients with positive lymph nodes (n = 24). P = not significant.
Subsequent recurrence in the remnant pancreas occurred in 8 patients (7%). Only 1 of these did not have invasive cancer in the initial pathology. He underwent a Whipple procedure for an adenoma and had a pancreatic transection that was negative. Five years later he developed a recurrence detected by CT and underwent a completion pancreatectomy for a carcinoma in situ in the distal pancreas. The other 7 recurrences occurred in patients who had presented with invasive cancer in the primary lesion. Two of them recurred within 6 months but also had distant metastases and died shortly thereafter. The other 5 recurred in the remnant between 28 and 67 months after the initial operation. Three underwent further resection but subsequently developed metastases and died, but the other 2 are currently free of disease following reresection. None of the transection margins of these 5 patients showed invasive cancer, but 2 had borderline tumor, 2 were de-epithelialized (and therefore indeterminate), and only 1 was read as negative.
DISCUSSION
What we now call IPMN of the pancreas was first described only 20 years ago. In that report, Ohashi et al described 4 cases of what they called “mucin-producing cancer” that affected the main pancreatic duct and produced excessive quantities of mucus, which filled and distended the ductal system.7 They remarked on the surprisingly good survival of these patients when compared with the usual ductal pancreatic adenocarcinoma. Since then many other reports, initially only from Japan and later on from the rest of the world, confirmed the existence of this “new” tumor,8 frequently called mucinous ductal ectasia in the early 1990s9 but later termed IPMN by the World Health Organization and the Armed Forces Institute of Pathology in 1996 and 1997.6,10 Subsequent reports have shown that IPMN can occur without hypersecretion of mucus, does not always affect the main pancreatic duct, and can exhibit a spectrum of histopathological abnormalities ranging from adenoma to borderline tumors, carcinoma in situ, and invasive cancer, and that not infrequently more than 1 of these features can be found within the same tumor. Later series have also highlighted potential incorrect overlap in reporting of IPMNs and the mucinous cystic neoplasm of the pancreas,11 inclusion of side-branch IPMNs in reports of mucinous cystadenomas, and vice versa.8 This confusion is likely to continue until firm criteria for the definition of each entity is agreed upon.
The present combined series from UV and MGH is the largest cohort of IPMNs reported in the literature to this time. By including only patients whose tumors affected the main pancreatic duct, we have scrupulously avoided any potential overlap with mucinous cystic neoplasms. While our case-selection methodology has also almost certainly excluded pure side-branch IPMNs, we propose that the resulting experience more accurately represents and characterizes the disease originally described by Ohashi et al7 and avoids the skewing of its observations and conclusions, which might have been affected by including a related but possibly different tumor with a lower incidence of malignancy and better prognosis.3–5
Our results confirm that IPMNs comprise a wide range of histopathological atypia, and carcinoma, either in situ or invasive, was present in 60% of the resected specimens. In other large series, this proportion has ranged from 52% to 88%,3,12–15 although distinction between branch-duct and main-duct types is not always made. We found that IPMNs can present with a wide variety of symptoms or no symptoms at all, as was the case in 27% of our cases. One of the goals of this study was to determine if any particular symptoms were associated with a higher likelihood of malignancy. We found that jaundice was present in 17% of patients with IPMNs, and recent onset or worsening of diabetes in 12%, and that both symptoms had a significantly higher prevalence in malignant tumors (eightfold and threefold increase, respectively). A fivefold increase in the likelihood of steatorrhea was also found in patients with malignant IPMNs, but the low prevalence of the symptom (6.5%) did not make the finding statistically significant. Interestingly, patients with benign tumors had a higher frequency of abdominal pain (76% vs. 57%) and a longer duration of symptoms (32 vs. 17 weeks). This apparent paradox could be used as an argument indicating that benign and malignant IPMNs are 2 different disease processes (rather than a single entity where tumors progress from benign to malignant); however, the subjective and heterogeneous nature of abdominal pain is difficult to interpret, and there is likely recall bias in patients with more severe symptoms (ie, jaundice), where the onset of disease tends to be linked to the appearance of the most severe symptom. The fact that as many as 29% of patients with either carcinoma in situ or invasive cancer were asymptomatic underscores that reliance on symptoms cannot be used to exclude malignancy in this neoplasm.
Patients with malignant tumors were found to be 6.4 years older than those with adenomas or borderline neoplasms. This is the first time a significant difference in age has been described, and although not useful for diagnosis or exclusion of malignancy, given the large overlap between groups, it does give insight into the time required for progression into malignant transformation. Interestingly, a similar age differential has been described in mucinous cystic neoplasms, which share many phenotypical and genetic alterations with IPMNs. In 1 study, a significant age difference of 9.5 years was found between patients with cystadenocarcinoma (invasive or in situ) and those with benign mucinous cystadenomas or borderline tumors,16 and in another there was a stepwise age increase between patients with mucinous cystadenomas, borderline and in situ tumors, and those with invasive carcinoma (48, 53, and 64 years, respectively).17
One of the important findings of this study is the excellent survival of IPMNs following resection. None of the 80 patients with adenomas, borderline tumors, or carcinoma in situ died as a consequence of the disease (although about 10% of them died of other causes during follow-up), and the survival of the 58 patients with invasive cancer was a remarkable 60% at 5 years and 50% at 10 years. Like our experience, a recent series from Oita Medical University in Japan cites a 100% and 70% 5-year survival, respectively, for 25 benign and 18 malignant tumors.5 Results from other large series are conflicting. A report from the Virginia Mason Medical Center describes an 83% 5-year survival for 30 patients with benign IPMNs and 44% for 33 malignant tumors.12 In the Mayo Clinic experience with 113 patients (of which 40 had invasive carcinoma), the 5-year survival was 36% for patients with invasive tumors and 88% for those with noninvasive IPMN.14 Inexplicably, in the Johns Hopkins series, the 4-year survival of 22 patients with IPMN and infiltrating carcinoma was no different than that of 38 patients with noninvasive tumors (62% and 64%, respectively).18 Perhaps some of these differences may be accounted for by inclusion of both main-duct and branch-duct IPMNs, or by differences in the pathologic interpretation. For example, tumor producing an overabundance of mucus can erroneously be diagnosed as invasive cancer when mucus is seen in lakes extruded outside of the ductal system into the parenchyma; conversely, the more aggressive mucinous adenocarcinoma can be included with the infiltrating intraductal papillary mucinous carcinoma group.
Twenty-four of the 58 patients (41%) with IPMN and invasive cancer in the present series had lymph node metastases, in keeping with the 29% to 46% incidence from other reports.14,18,19 The fact that survival of these patients was 45% at 5 years further underscores that carcinoma arising in IPMNs is a different disease, more amenable to cure than ductal adenocarcinoma of the pancreas, wherein long-term survival of resected patients with positive lymph nodes is a rare occurrence.20,21 This behavioral difference may provide clues to a unique opportunity to discover genetic factors that lead to aggressive growth and metastases in pancreatic ductal adenocarcinoma.
The issue of recurrence in the pancreatic remnant following resection for IPMN remains unresolved. In the present series, tumor recurred in 8 patients (7%). However, 2 of them did so in a very short period of time after resection and had distant metastases as well. Whether or not the pancreatic remnant was the origin of the recurrence is indeterminate. The remaining 6 patients (5%) presented with recurrent IPMN in the pancreatic remnant at a median of 50 months after their initial surgery, and all underwent re-resection. Although 3 of these patients eventually died of metastatic disease, 2 remain disease free. Chari et al14 have described a disturbingly high incidence of recurrence (67%) following partial pancreatectomy for invasive IPMN. The bulk of those recurrences occurred as or in conjunction with distant metastases; only 6 of 27 (22%) of patients with invasive IPMN and 4 of 60 (7%) of noninvasive IPMN recurred locally in the pancreas. Six of the recurrences described by them were also re-resected. Sohn et al18 also described recurrence amenable to further pancreatic resection in 3 of 60 patients, 2 of them 10 years after the initial operation. Because of the long interval between initial resection and recurrence described in these and other experiences, it is quite possible that the 5% recurrence in the remnant that we are reporting at 31 months will increase as our cohort matures. Given that the chance of recurrence in the remnant is acceptably low and that many of the recurrences have been successfully resected, we prefer a segmental resection to total pancreatectomy when the extent of the disease permits. The risk of recurrence and of death from that recurrence seems preferable to the morbidity and mortality of total pancreatectomy. It is our current practice to follow these patients with CT scans at least on a yearly basis. Perhaps with more knowledge of the natural history of the disease or novel genetic markers, we will eventually be able to predict which patients are at a higher risk of recurrence. Inasmuch as some patients have recurred with well-documented negative transection margins, this criterion alone is a reasonable guide to the extent of resection but does not guarantee that IPMN will not develop in the remnant. Metachronous tumors elsewhere in the pancreas may reflect either multifocality or a “field defect,” as has been suggested by others.18
CONCLUSION
This combined experience from 2 large centers of expertise shows that 60% of resected IPMNs affecting the main pancreatic duct harbor cancer. Although jaundice and diabetes are more common in malignant tumors, 29% of patients who have either carcinoma in situ or invasive cancer have no symptoms at all. The 6.4-year difference in age between patients with benign versus malignant tumors supports the concept of oncogenic transformation from benign IPMNs to invasive carcinoma. Surgical resection of these tumors can be accomplished with minimal morbidity and mortality in experienced centers and produces excellent long-term survival for both noninvasive and invasive tumors.
Discussions
Dr. B. Mark Evers (Galveston, Texas): The paper represents another outstanding addition from Dr. Warshaw and his colleagues in further delineating the management of a recently recognized entity of the pancreas, namely, intraductal papillary mucinous neoplasms (IPMNs).
Within the last decade this entity has been increasingly reported in the pancreas literature. In fact, IPMN accounts for up to 20% of all pancreatic resections at some institutions. Confusion further exists due to the multiple nomenclature of this entity which has been variously referred to as mucin producing tumor, intraductal mucin hypersecreting neoplasm, mucin hypersecreting tumor, intraductal mucin producing tumor and on and on just to name a few.
As the authors have correctly noted in this presentation, IPMNs are unpredictable and the possibility of field defects, multi-focality and unpredictability combined with the difficulty of the clinical follow-up has led authors to increasingly take a more aggressive approach in the management of these cases which appears prudent given the progressive nature of pancreatic cancer invasion and metastasis and the outstanding long-term results reported by these authors.
I have 3 questions for Dr. Warshaw regarding this presentation.
The first question has to do with the incidental findings. I am confused by what you are calling incidental. You note that incidental IPMNs were noted in 18% of your cases. Yet by definition, IPMNs are mass forming neoplasias, the size is usually about 4 centimeters; they manifest as either papillary or cystic lesions. It is sometimes difficult to distinguish between another entity called pancreatic intraepithelial neoplasm, commonly referred to as PanIn, which represents microscopic changes in the ducts, and they truly are detected incidentally and have a different clinical course. Therefore, many authors use clinical detectability or a size larger than 1 centimeter as the criteria to distinguish between these 2 entities which have distinct clinical behaviors and aggressive patterns. With this high incidence of incidental findings, have you ensured that these lesions truly are as you state or could they represent pancreatic intraepithelial neoplasias?
The second question has to do with molecular characteristics of your IPMNs and whether you have evaluated these characteristics in this particular study. Various authors are reporting the fact that these lesions appear more genetically stable than conventional ductal adenocarcinomas and the fact that mutations of the K-ras, p16, or the tumor suppressor gene DPC4, are significantly less common in these lesions than conventional ductal adenocarcinomas. In fact, the IPMNs frequently express the muc2 gene, which has tumor suppressor activity, and some authors are reporting increased p53 mutations in these lesions. Therefore, it would be of interest to know the molecular profile of the IPMNs in your series.
Finally, this entity is more frequently recognized, and I was wondering if you could speculate regarding why, over the last decade, we have seen such a dramatic increase in the reporting of this entity. Some of this obviously can be explained quite easily due to better diagnostic technology and recognition of this as a distinct clinical entity; however, this cannot explain entirely why IPMNs were not seen as commonly in autopsy series and suggest that part of this increase may be due to an increasingly aging population of which this entity is most prevalent.
Dr. Charles J. Yeo (Baltimore, Maryland): I too rise to congratulate to Dr. Warshaw on several accounts: For his clear, concise presentation; for a top-notch manuscript and his analysis; for pursuing a collaboration with the fine surgical unit at the University of Verona. And lastly, for being such a fine member of the Southern Surgical Association, even though I think he is really from central Boston. This material adds notably to our knowledge about IPMNs.
In analyzing these 140 patients without mortality and focusing on only main pancreatic duct involvement, they report that jaundice and diabetes are associated with malignant disease, that 1 out of 4 patients were asymptomatic and had their IPMN discovered as a “cystic incidentaloma,” that there is a 6-year difference between patients with adenoma and borderline tumors as compared to those with CIS or invasive tumors, and lastly, that with mean follow-up of about 3 years there were no tumor related deaths in the group of patients with resected noninvasive tumors. My suspicion is that this 100% disease specific survival will not stand as patients are further followed; however, I suspect that the vast majority of the data presented today will remain robust for years to come.
I have a few questions for the MGH/Verona team.
First, did any of the main pancreatic duct IPMNs that you discussed today have underlying ovarian stroma? Because this would be an issue as regards conflict with mucinous cystic neoplasia.
Second, I am curious why in your manuscript you have lumped IPMN carcinoma in situ as a malignant tumor initially and then analyzed it subsequently as a noninvasive tumor. I would suggest that CIS should be classified as a noninvasive lesion and not as a malignant lesion.
Third, we in Baltimore struggle to obtain negative margins, and from your data fully 28% of patients had some issues as regards margin positivity. How do you deal with a neck or body margin that is positive for noninvasive disease in a patient that you have just done either a right- or a left-sided resection? How many additional “slices” of the gland are you willing to take? And does patient age factor into your decision whether or not to complete the pancreatectomy?
Lastly, your current report doesn't tell us the answer to an important question: When do we offer resection to patients with imaging studies suspicious for IPMN? Does your group find EUS with FNA or aspiration helpful, as you have previously reported? Does that help prompt resection? Or does imaging alone, in the proper clinical setting, prompt resection?
Dr. James A. O'Neill, Jr. (Nashville, Tennessee): I rise to ask a question regarding the change from benign to malignant form. This question is based on a current follow-up study a Japanese colleague and I are doing on patients with choledochal cysts. There is a subset of that group of patients who have a pattern of mucus plug obstruction, induction of chronic pancreatitis over time, and the induction of biliary malignancy anywhere in the biliary tree. So the pattern that you present, which is a period of time over which malignancy appears to develop, the continuous nature of it, et cetera, is at least suggestive of chronic inflammation as an inducer of DNA change, and it is of interest that some of the same genes that Mark Evers brought up are involved. In this case it is DPC4 and Smad4 alterations that are involved in this oncogenic change. So the question then comes up, how many of the specimens that you found had evidence of chronic pancreatitis, which very often has little or no symptomatology?
Dr. Reid Adams (Charlottesville, Virginia): I would like to congratulate Dr. Warshaw on a very nice paper and for progressing our knowledge of this disease. I have some specific questions about how you manage your follow-up in these patients, which you described as having very low recurrence.
My first question is, do you think that your time to recurrence is longer than your follow-up period considering the length for development of this disease?
Secondly, how do you follow these patients? Do you scan them? If so, how frequently? What are your criteria for determining recurrence? And then, how do you decide who you are going to take back to the operating room for recurrent disease?
Dr. Martin J. Heslin (Birmingham, Alabama): Dr. Warshaw and colleagues, an excellent presentation. My question would be the extent of operation. Because total pancreatectomy, as we all know, is not a trivial operation, with many patients becoming brittle diabetics. Your data suggested that in the absence of invasive cancer there was zero mortality due to the disease. If that is the case, my question would be, how do you judge the extent of operation at the time of operation in the absence of an invasive cancer?
Dr. Andrew L. Warshaw (Boston, Massachusetts): Thank you very much for your interest and for those excellent questions. They are questions that certainly have plagued us and that we continue to wrestle with.
First, Dr. Evers, what we meant by incidental tumors was incidentally found, as in an asymptomatic patient who is sent for renal ultrasound, and something anticipated is seen in the pancreas. That is not the circumstance of the PanIn lesions, the pancreatic or intraepithelial neoplasms, which are microscopic in size and invisible to imaging until they progress into either a macroscopic mass lesion such as adenocarcinoma. There has been genetic analysis which suggests that PanIns are not precursors of IPMNs. In any case these were lesions that were fully developed cystic tumors which are completely different from the PanIns.
You are correct that the molecular characteristics of the cystic neoplasms are somewhat different from pancreatic adenocarcinoma. We have previously presented our work with K-ras and p53 suggesting that those molecular changes occur along the oncogenic spectrum, that they are not found in the adenomas, and that they develop progressively as the tumor evolves through increasing dysplasia to invasive cancer. The Hopkins group has worked in particular with DPC4, and perhaps you will hear more about that in the next paper.
Why an increasing number? Is this a new disease that truly has occurred or are we simply seeing it with better imaging? Or are we picking it out of what had previously been diagnosed as chronic pancreatitis? I think the latter is true in many cases.
I recently operated on a patient who had been treated nonsurgically for chronic pancreatitis for 21 years. By the time I saw him he had a huge pancreatic duct with obvious tumor in the head of the pancreas. He has now had a Whipple operation with negative margins, but he was thought to have recurrent acute and chronic pancreatitis until now.
Supporting that hypothesis is a study done by the Mayo Clinic group, Mike Sarr's group, in which they asked, “Okay, this disease was described in 1982, but why didn't we see it before?” They went back to their own pathology archives and compared the 20 years before 1982 with the 20 years after and found the same number of these IPMNs buried in amongst their pancreatic specimens. They just hadn't been called IPMN. We are seeing more of them, I conclude, because of imaging and recognition.
Dr. Yeo, I am sure you are correct that ours is a relatively short follow-up. It is quite similar to your follow-up in your study. There may well be some fall-off in survival with the further passage of time. That is to be expected. We did eliminate patients who had ovarian stroma since those would be classified as MCNs rather than IPMNs according to the WHO.
You ask why we changed our assigned location of carcinoma in situ, including it with the benign lesions in the first part of our analysis and with cancer subsequently? We did so for logical reasons. Since carcinoma in situ can become cancer, we would choose to resect it. But when you really look further, none of the patients with carcinoma in situ died after resection. So including cancer in situ with invasive cancer would misleadingly make our results look better than they should be. We think the clarity of the respective outcomes is enhanced by demonstrating the different post-resection natural history of benign and in situ tumors versus invasive cancers.
How much further to chip away at a positive margin before taking the rest of the pancreas? That is a very difficult question. If we see mucinous metaplasia, we do not pursue it. If we see adenoma or carcinoma in situ, we do take more pancreas and repeat the frozen section. We will usually try about 2 more times to get a negative margin, but then you get to a point where there is not enough remaining pancreas to be worth preserving.
We are not afraid of total pancreatectomy. It has the bad rap of producing very brittle diabetes, but in fact we have had no late deaths from total pancreatectomy. In my opinion, the safe approach is not to try to control the blood sugar too tightly, but rather to accept blood sugars in the 150 rage or even higher. That way you don't see the deaths from insulin overdose that otherwise can occur.
We certainly look at our patients with an eye to their overall physical condition, age among the considerations that have a part in our decisions.
We do use endoscopic ultrasound and fine needle aspiration, mainly to determine whether or not there is mucin and an elevated CEA. If it is a mucin-producing tumor, we worry about it since most mucinous tumors have malignant potential. If the tumor is not mucinous we are happier to sit and watch.
Size does make a difference. For a 1-cm cystic lesion in the head of the pancreas in an 80-year-old, it is pushing it to do a Whipple operation. We will follow that patient, usually with CT at 6–12 months, unless and until there is significant change.
Dr. O'Neill, you make the comparison between what might be a scar carcinoma in choledochal cysts and the possibility that chronic inflammation in the pancreas with IPMN might induce cancer, as suggested by Mark Evers in his paper presented at this meeting. We don't have an answer to that, but it must be a relatively small factor at best because the incidence of cancer is only 1–4% after 20 years of chronic pancreatitis. Also, inflammation has not been prominent in our resected specimens even though many have been followed with a presumptive diagnosis of chronic pancreatitis.
What is our protocol for follow-up after partial resection for IPMN? We will generally obtain a CT scan on a yearly basis. Patients are more comfortable with surveillance than they are with benign neglect. We will reoperate for recurrence. Fortunately this has been quite rare.
Finally, what part of the pancreas do we take out? Is it necessary to take out the entire pancreas for fear of recurrence in the remnant? We look at it like a golf game. You play it where it lies. We take out what is relevant to elimination of known tumor, and we are willing to take the 5% risk of a recurrence in what we leave behind. We are not afraid of doing a total pancreatectomy, but especially in an older patient we prefer to be more conservative.
Footnotes
Reprints: Carlos Fernández-del Castillo, MD, Massachusetts General Hospital, ACC/336, Boston, MA 02114. E-mail: cfernandez@partners.org.
Dr. Salvia was supported in part by the Azienda Ospedaliera di Verona, COFIN 2003063754, and the Fundazione Giorgio Zanotto.
REFERENCES
- 1.Fernández-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinico-pathologic characteristics and comparison to symptomatic patients. Arch Surg. 2003;138:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adsay NV. The “new kid on the block”: intraductal papillary mucinous neoplasms of the pancreas. Current concepts and controversies. Surgery. 2003;133:459–463. [DOI] [PubMed] [Google Scholar]
- 3.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. [DOI] [PubMed] [Google Scholar]
- 4.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–265. [DOI] [PubMed] [Google Scholar]
- 6.Kloppel G, Solcia E, Longnecker DS, et al. Histological Typing of Tumors of the Exocrine Pancreas: International Histological Classification of Tumors. Berlin: Springer, 1996. [Google Scholar]
- 7.Ohashi K, Murakami Y, Maruyama M. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater [in Japanese]. Prog Dig Endoscopy. 1982;20:348–351. [Google Scholar]
- 8.Yamaguchi K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas: a historical review of the nomenclature and recent controversy. Pancreas. 2001;23:12–19. [DOI] [PubMed] [Google Scholar]
- 9.Bastid C, Bernard JP, Sarles H, et al. Mucinous ductal ectasia of the pancreas: a premalignant disease and a cause of obstructive pancreatitis. Pancreas. 1991;6:15–22. [DOI] [PubMed] [Google Scholar]
- 10.Solcia E, Capella C, Kloppel G. Tumors of the exocrine pancreas. In: Solcia E, Capella C, Kloppel G, eds. Tumors of the Pancreas. Washington: Armed Forces Institute of Pathology, 1997:145–210. [Google Scholar]
- 11.Yanigasawa A, Ohashi K, Hori M, et al. Ductectatic-type mucinous cystadenoma and cystadenocarcinoma of the human pancreas: a novel clinicopathological entity. Jpn J Cancer Res. 1993;84:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–19. [DOI] [PubMed] [Google Scholar]
- 13.Doi R, Fujimoto K, Wada M, et al. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002;132:80–85. [DOI] [PubMed] [Google Scholar]
- 14.Chari ST, Yadav D, Smyrk T, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. [DOI] [PubMed] [Google Scholar]
- 15.Nakagohri T, Kenmochi T, Kainuma O, et al. Intraductal papillary mucinous tumors of the pancreas. Am J Surg. 1999;178:344–347. [DOI] [PubMed] [Google Scholar]
- 16.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. [DOI] [PubMed] [Google Scholar]
- 17.Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas. Ann Surg. 2000;231:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adsay NV, Conlon K, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. [DOI] [PubMed] [Google Scholar]
- 20.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2. Ann Surg. 2002;236:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delcore R, Rodriguez FJ, Forster J, et al. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. Am J Surg. 1996;172:463–469. [DOI] [PubMed] [Google Scholar]