Abstract
Objective:
To update the authors’ experience with intraductal papillary mucinous neoplasms (IPMNs) of the pancreas.
Background Data:
IPMNs are intraductal mucin-producing cystic neoplasms of the pancreas with clear malignant potential. Since the authors’ 2001 report, the number of IPMNs resected at our institution has more than doubled, providing an opportunity to define the clinical features of this distinct neoplasm.
Methods:
All patients undergoing pancreatic resection for an IPMN at the Johns Hopkins Hospital between January 1987 and March 2003 were evaluated. Noninvasive IPMNs were classified as “adenoma,” “borderline,” or “carcinoma-in situ” (CIS) depending on the degree of dysplasia within the specimen. Invasive cancers were classified as tubular, colloid, mixed, or anaplastic types. Pathology was retrospectively reviewed to identify main-duct or branch-duct origin of the tumors. Long-term overall survival for patients having IPMNs with invasive cancer was compared with those patients having IPMNs without an invasive component.
Results:
Between January 1987 and March 2003, inclusive, 136 pancreatic resections were performed for patients with IPMNs, with 78 resections performed since January 2001. The mean age of the patients was 66.8 ± 1.1 years, with 57% being male and 89% white. Pancreaticoduodenectomy was performed in 71% of patients, total pancreatectomy in 15%, distal pancreatectomy in 12%, and central pancreatic resection in 2%. IPMNs without evidence of invasive cancer were identified in 62% (n = 84) of patients (17% adenoma, 28% borderline, or 55% CIS). The remaining 38% (n = 52) of patients had IPMNs with associated invasive cancer (60% tubular, 27% colloid, 7% mixed, and 6% anaplastic). The mean age of patients with IPMN adenoma was 63.2 years, 66.7 years for those with borderline/CIS IPMNs, and 68.1 years for those with invasive cancer (P = 0.08, adenomas vs. invasive cancer). In those patients with invasive cancers, 15% had invasive cancer at the final surgical margin, 23% had IPMN without invasive cancer at the margin, and 54% had lymph node metastases. Residual IPMN was identified at the neck or uncinate margin in 24% of patients with noninvasive IPMNs. The overall 5-year survival for patients having IPMNs without invasive cancer was 77% (several deaths secondary to metachronous invasive cancer), compared with 43% in those patients with an invasive component (P < 0.0001). There were no differences in survival when comparing adenomas, borderline neoplasms, and CIS. Similarly, there were no statistically significant differences in survival when comparing branch-duct, main-duct, and combined variants; however, the branch-duct variants were more often noninvasive. For those patients with invasive IPMNs, 2-year survival was 40% when margins were positive for invasive cancer or for IPMN without invasive cancer, and 60% when margins were tumor-free (P = 0.15). Those patients with colloid carcinomas (n = 14) had improved survival compared with those with tubular carcinomas (n = 31), with 5-year survival rates of 83% and 24%, respectively. IPMN recurrences and deaths from cancer occurred in patients with both invasive and noninvasive IPMNs at initial resection.
Conclusions:
IPMNs continue to be recognized with increasing frequency. Five-year survival for those patients following resection of IPMNs with invasive cancer (43%) is improved compared with those patients with resected pancreatic ductal adenocarcinoma in the absence of IPMN (averages 15%–25%). Survival following resection of IPMNs without invasive cancer (regardless of degree of dyplasia) is good, but recurrent disease in the residual pancreas suggests that long-term surveillance is critical. Based on the age at resection data, there appears to be a 5-year lag time from IPMN adenoma (63.2 years) to invasive cancer (68.1 years).
A single institution, retrospective review of 136 patients with resected intraductal papillary mucinous neoplasms (IPMNs) of the pancreas was performed. Patients with noninvasive IPMNs (adenoma, borderline, or carcinoma in situ) had a 5-year overall survival rate of 77%, compared with 43% in those patients with IPMNs with invasive carcinoma (P < 0.0001).
Intraductal papillary mucinous neoplasms (IPMNs) are a well-characterized group of intraductal mucin-producing cystic neoplasms of the pancreas with clear malignant potential. They have been reported with increasing frequency over the last decade.1–8 In 1996, the World Health Organization (WHO) established criteria to classify IPMNs and to distinguish them from other mucin-producing cystic neoplasms of the pancreas such as mucinous cystadenomas and cystadenocarcinomas.9 The WHO defines IPMNs as intraductal mucin-producing neoplasms with tall, columnar, mucin-containing epithelium with or without papillary projections. These neoplasms extensively involve the main pancreatic ducts and/or major side branches. In addition, IPMNs lack the ovarian stroma characteristic of mucinous cystic neoplasms.
Prior to their unification under the heading of IPMN, many neoplasms were reported under various names, reflecting the spectrum of pathologic appearances now attributed to IPMNs. A portion of tumors previously termed papillary carcinoma, ductectatic mucinous cystadenoma, villous adenoma, and mucin-producing tumors of the pancreas are now classified as IPMNs, partially attributing to the observed increased incidence.10 It has also been suggested that some of the observed increase in IPMNs may be attributed to improved diagnostic imaging.11 In a previous study of patients with IPMNs at the Johns Hopkins Hospital,7 the number of patients with IPMNs sharply increased from the late 1980s through 2000. During that same time period, the number of surgically resected mucinous cystic neoplasms remained constant, suggesting that reclassification alone did not account for the increased number of IPMNs seen.
Similar to the well-defined adenoma-carcinoma sequence in colorectal cancer12 and pancreatic ductal adenocarcinoma (pancreatic intraepithelial neoplasia [PanIN] to invasive ductal carcinoma),13 IPMNs seem to follow a similar pattern progressing from IPMN adenoma, to borderline IPMN with dysplasia, to IPMN with carcinoma in situ (CIS), and eventually to invasive carcinoma. The genetic changes associated with this progression have not been entirely established but are thought to be distinct from those associated with the development of pancreatic ductal adenocarcinoma. Labeling of IPMNs for Dpc4 protein expression supported the idea that IPMNs are genetically distinct from Pan-INs and pancreatic ductal adenocarcinoma, with 84% of invasive IPMNs but less than half of pancreatic ductal adenocarcinomas expressing Dpc4.7 The types of mucin expressed also differ. Most Pan-INs and invasive ductal adenocarcinomas express MUC1, while most IPMNs express MUC2, but not MUC1.10 The length of time required for progression from IPMN adenoma to IPMN with invasive carcinoma is not known at this time.
As noted earlier, IPMNs can be distinguished from mucinous cystic neoplasms by the presence of ovarian stroma and the absence of large duct involvement in the latter. Completely resected mucinous cystic neoplasms without evidence of invasive cancer (CIS, borderline, and cystadenomas) do not recur or metastasize.14,15 In contrast to patients with benign mucinous cystic neoplasms, patients with both invasive and noninvasive IPMNs have been reported to recur after partial pancreatectomy.2,7,16
Several controversies remain over the treatment of IPMNs. Follow-up has been limited in many series. In addition, methods of evaluating survival were not standardized, with some reports grouping patients with an IPMN with CIS together with those having an IPMN with an associated invasive cancer for survival analysis, while others included IPMN with CIS together with the other noninvasive IPMNs. Controversy still remains over prognostic indicators, including branch-duct versus main-duct variants3,6,8 and mucus production.17 The invasive carcinomas associated with IPMNs can be categorized as tubular, colloid, mixed, or anaplastic, and these various histologic types may serve as prognostic indicators.
The previous report from this institution evaluated 60 patients with IPMNs and compared their survival to patients with invasive ductal adenocarcinoma of the pancreas.7 The current report evaluates 136 patients. This larger number of patients allows further subgroup analysis of noninvasive IPMNs (adenoma, borderline, CIS), of branch-duct and main-duct variants, and of different pathologic tumor types in invasive cancers (tubular, colloid, mixed, anaplastic). By analyzing subgroups of patients undergoing resection of IPMNs with progressive degrees of dysplasia, we attempt to estimate the time of progression from IPMN adenoma to IPMN with invasive carcinoma.
PATIENTS AND METHODS
Between January 1987 and March 2003, 136 patients underwent surgical resection for an IPMN of the pancreas at the Johns Hopkins Hospital. Seventy-eight patients were resected after December 2000. A retrospective review of this prospectively collected database was performed. The demographics, presenting symptoms, operative management, pathology, postoperative course, and long-term survival were evaluated.
Preoperative imaging was not standardized but was performed as per the preference of the attending surgeon. Diagnostic and staging modalities performed included spiral or multidetector 3-dimensional CT scan, MRI, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiography/percutaneous biliary drainage.
Patients with an IPMN in the head, neck, or uncinate process of the pancreas underwent pancreaticoduodenectomy, while those with a neoplasm in the body and tail underwent distal pancreatectomy. Total pancreatectomy was performed for neoplasms diffusely involving the gland or those involving the head and extending into the body of the gland. Central pancreatectomy was performed in a small number of patients with IPMNs localized in the neck of the gland. The majority of pancreaticoduodenal resections were pylorus-preserving, reserving distal gastrectomy for neoplasms involving the distal stomach or proximal duodenum. As part of a prospective, randomized trial, retroperitoneal lymphadenectomy and distal gastrectomy were used in some patients with an infiltrating carcinoma from 1996 through 2001.18 All distal pancreatic resections included splenectomy, and most extended proximally to the level of the pancreatic neck. The estimated blood loss, transfusion requirement, and operative time were noted for all cases.
All pathologic specimens were reviewed by 2 pathologists (R.H.H. and N.F.) to confirm the diagnosis of IPMN. The tumors were classified according to established criteria by the World Health Organization.9 IPMNs were defined as neoplasms with tall, columnar, mucin-containing epithelium with or without papillary proliferations and extensively involving the main pancreatic ducts or major side branches. By definition, IPMNs lacked the “ovarian”-type stroma seen in mucinous cystic neoplasms. Size was the major criterion used to distinguish IPMNs from PanINs. To be classified as an IPMN, the lesion had to measure ≥1 cm or be grossly and/or radiographically visible. If they were not, they were classified as a PanIN. The IPMNs were also classified into main-duct and branch-duct variants by retrospective review of the gross and microscopic pathology. Main duct IPMNs were defined as IPMNs grossly or microscopically involving the main pancreatic duct. Branch duct IPMNs were defined as IPMNs that did not grossly or microscopically involve the main pancreatic duct. Combined duct variants were defined as IPMNs that microscopically or grossly involved both the main pancreatic duct and its main side branches.
Patients with IPMNs were divided into noninvasive (IPMN without associated infiltrating carcinoma) and invasive (IPMN with associated infiltrating carcinoma) groups based on pathologic examination (Fig. 1). Those patients with noninvasive IPMNs were further classified into 1 of 3 groups based on the degree of cytologic and architectural dysplasia: 1) adenoma, 2) borderline, or 3) CIS (Fig. 1). Among patients with IPMNs with associated invasive carcinoma, 4 different tumor types were identified: 1) tubular, 2) colloid, 3) mixed, and 4) anaplastic.
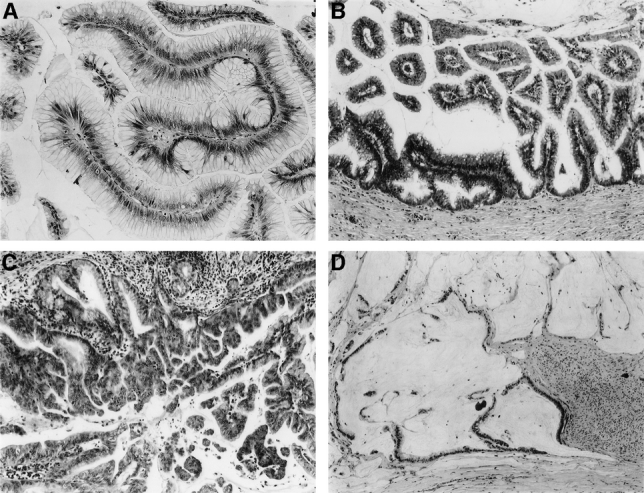
FIGURE 1. A, IPMN adenoma of the pancreas. B, Borderline IPMN of the pancreas. C, IPMN with CIS. D, IPMN with associated infiltrating colloid carcinoma.
The demographics, presenting symptoms, operative management, pathology, postoperative course, and long-term survival were evaluated for patients with IPMN adenoma, borderline IPMNs, IPMNs with CIS, and IPMN with invasive carcinoma. Survival analyses were performed comparing noninvasive and invasive IPMNs as well as among the noninvasive subgroups.
Perioperative mortality was defined as in-hospital death or death within 30 days of surgery. The overall incidence of postoperative complications was evaluated using previously defined criteria.19
Overall survival information was available on all patients. Follow-up information was obtained by contacting the U.S. Social Security Administration and through direct patient contact, hospital charts, and surgeons’ records. The cause of death was not available in all cases; thus, disease-specific survival could not accurately be reported. Hence, the survival statistics presented represent overall, not disease-specific survival.
All continuous data are presented at mean ± standard error (SE) of the mean. Differences between categorical variables were evaluated by χ2 analysis, while Student's t test was used for all comparisons among continuous variables. Survival analysis was performed using the method of Kaplan and Meier.20 Differences in survival were compared using a log-rank test. Significance was accepted at the 5% level.
RESULTS
Between January 1987 and March 2003, inclusive, 136 pancreatic resections were performed for patients with an IPMN, with 78 resections performed since January 2001. The number of IPMNs resected per year is shown in Figure 2.
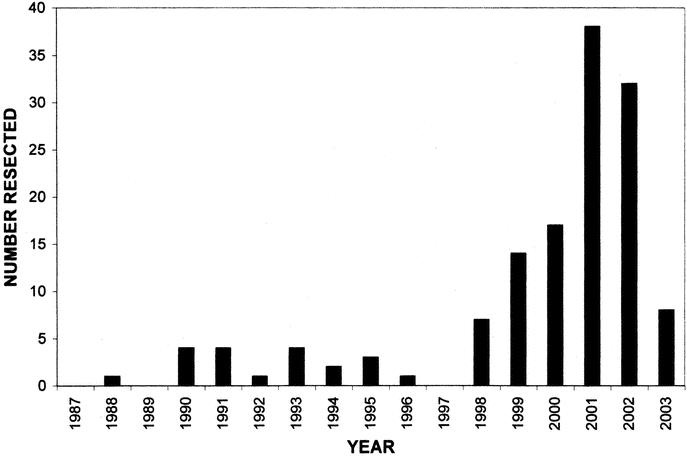
FIGURE 2. Histogram representing the number of resections per year for IPMNs. Note the sharp increase in numbers during the late 1990s. The data for 2003 only include the first 3 months of the year.
Pathologic Features and Classification of Resected IPMNs
Of the 136 IPMNs resected, 84 patients (62%) had an IPMN without evidence of invasive cancer (Table 1). In these cases, the entire surgically resected specimen was submitted for histologic examination. The remaining 52 patients (38%) had an IPMN with an associated invasive carcinoma. In the 84 patients with noninvasive IPMNs, 14 (17%) had an IPMN adenoma, 24 (28%) had a borderline IPMN, and 46 (55%) had an IPMN with CIS. This distribution is depicted graphically in Figure 3. Of the 52 IPMNs with an associated invasive cancer, 31 of those invasive carcinomas were tubular carcinoma (60%), 14 were colloid carcinoma (27%), 4 were mixed carcinoma (tubular and colloid, 7%), and 3 were anaplastic carcinoma (6%). Fifty-four percent of patients with invasive IPMNs had positive lymph nodes in the resection specimen.
TABLE 1. Pathologic Features and Classification of Resected Intraductal Papillary Mucinous Neoplasms (IPMNs)
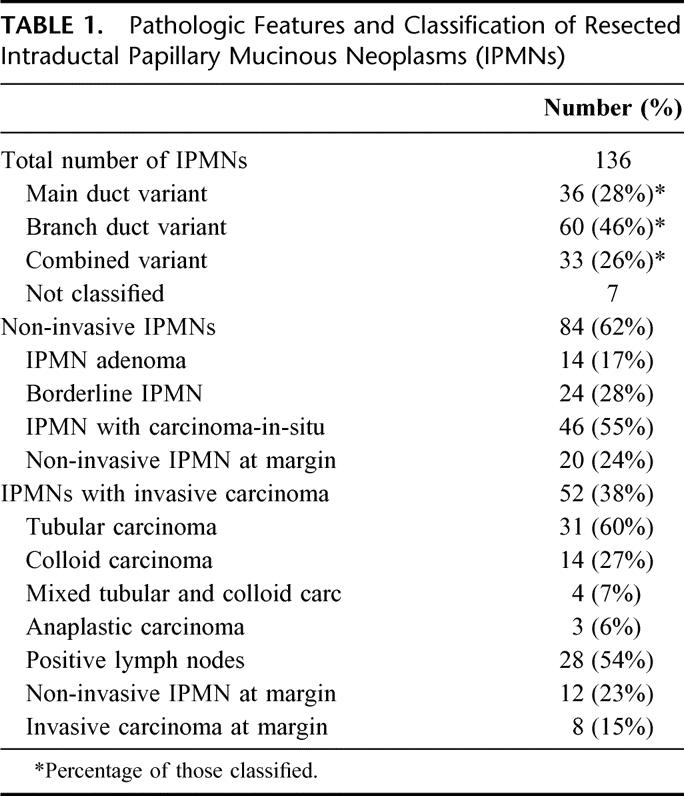
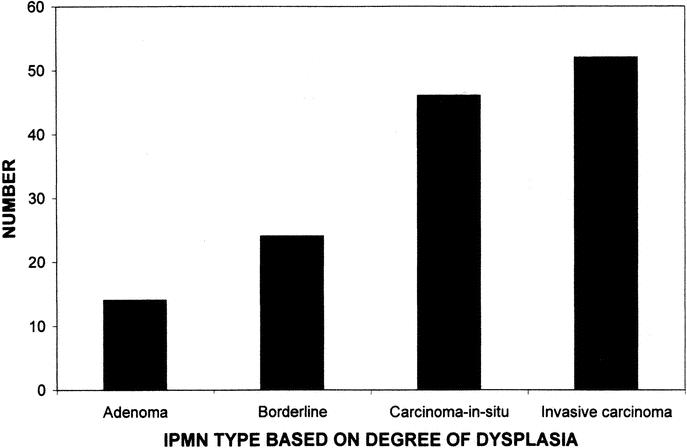
FIGURE 3. Histogram showing the distribution of IPMNs based on the degree of dysplasia. IPMN adenomas were the least common (n = 14), followed by increasing numbers for each degree of dysplasia; borderline IPMNs (n = 24), IPMN with CIS (n = 46), and IPMN with invasive carcinoma (n = 52).
On retrospective review of the gross and microscopic pathology, 129 of the 136 IPMNs were able to be classified as main-duct, branch-duct, or combined variants. Thirty-six IPMNs (28%) were main-duct variants, 60 (46%) IPMNs were branch-duct variants, and 33 (26%) were combined variants. The branch-duct variants were more often noninvasive. Only 50% of main-duct IPMN variants were noninvasive (adenoma, borderline, CIS), whereas 70% of branch-duct variant IPMNs and 60% of combined IPMN variants were noninvasive. Moreover, noninvasive IPMNs were comprised of 53% branch-duct variants, 22% main-duct variants, and 25% combined variants. The distribution for invasive IPMNs was 25% branch-duct, 35% main-duct, and 25% combined variants.
The mean tumor diameter was 3.2 ± 1.0 cm for IPMN adenoma, 3.5 ± 0.7 cm for borderline IPMNs, and 3.5 ± 0.3 cm for IPMNs with CIS. The mean tumor diameter of the invasive component was 4.0 ± 0.6 cm for IPMNs with invasive carcinoma. This demonstrates a trend toward increasing tumor size, which correlates with increasing grades of dysplasia, although this difference is only significant between IPMN with CIS and IPMN with invasive carcinoma (P = 0.03).
Residual noninvasive IPMN was identified at the neck or uncinate margin of resection in 24% of the 84 patients with noninvasive IPMNs. Similarly, residual noninvasive IPMN was seen at the final surgical margin in 23% of the 52 patients with invasive IPMNs. Fifteen percent of patients (n = 8) having IPMNs with invasive carcinoma had final surgical margins positive for invasive cancer. Four of these final positive margins occurred at the uncinate process: 2 at the uncinate and neck, 1 at the neck, and 1 at the bile duct and neck margins.
Demographics and Presentation
The mean age of all patients with IPMNs was 66.8 ± 1.1 years. Fifty-seven percent of patients were male and 88% were white. The most common presenting signs and symptoms were abdominal pain in 52% of patients, followed by weight loss in 29%, jaundice in 17%, nausea and vomiting in 14%, and acute pancreatitis in 13% of patients.
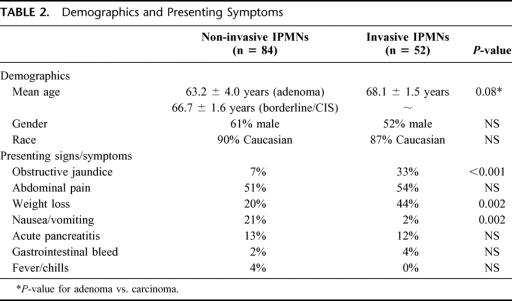
The demographic characteristics and presenting symptoms of those with noninvasive IPMNs are compared with those with invasive IPMNs in Table 2. Noninvasive IPMNs include IPMN adenomas, borderline IPMNs, and IPMNs with CIS. The mean age of patients with IPMNs increased with the degree of dysplasia. For those with IPMN adenoma, the mean age was 63.2 ± 4.0 years. This increased to 66.7 ± 1.6 years for those with borderline IPMNs and IPMNs with CIS, and to 68.1 ± 1.5 years for those with invasive cancers (P = 0.08, adenoma vs. invasive carcinoma, Fig. 4). The gender and race distributions were similar for those with noninvasive and invasive IPMNs. Patients with IPMNs with invasive carcinoma were more likely to present with obstructive jaundice (33% vs. 7%, P < 0.001) and weight loss (44% vs. 20%, P = 0.002), and less likely to present with nausea and vomiting (2% vs. 21%, P = 0.002), as compared with those patients with noninvasive IPMNs.
TABLE 2. Demographics and Presenting Symptoms
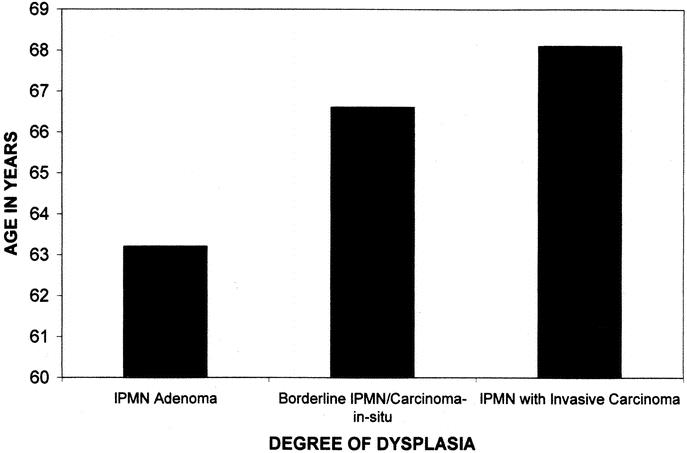
FIGURE 4. Histogram demonstrating the increase in age of patients with increasing degrees of dysplasia within their tumors. Those with IPMN adenomas (n = 14) had a mean age of 63.2 years, those with IPMN borderline/CIS (n = 70) had an average age of 66.7 years, and those with IPMNs with invasive cancer (n = 52) had an average age of 68.1 years.
Intraoperative Course
Ninety-six patients (71%) underwent pancreaticoduodenectomy for tumors involving the head, neck, or uncinate process of the pancreas, while 16 patients (12%) underwent distal pancreatectomy for neoplasms isolated to the body and tail of the gland. Twenty-one patients (15%) had neoplasms diffusely involving the pancreas and required total pancreatectomy. Three patients (2%) had noninvasive IPMNs in the neck of the gland and underwent central pancreatic resection.
Pancreaticoduodenectomy was performed with a mean operative time of 6.6 ± 0.2 hours, a median estimated blood loss of 700 mL, and median transfusion requirement of zero units of packed red blood cells. Pylorus preservation was performed in 86% and classic resection in 14% of patients undergoing pancreaticoduodenectomy.
The mean operative time, median estimated blood loss, and median transfusion requirements for patients undergoing total pancreatectomy were 8.0 ± 0.6 hours, 1350 mL, and 1.5 units packed red blood cells, respectively. For those undergoing distal pancreatectomy, the values were 3.3 ± 0.3 hours, 475 mL, and zero units packed red blood cells, respectively. Averages for central pancreatectomy were not calculated given the small number of patients.
Postoperative Course
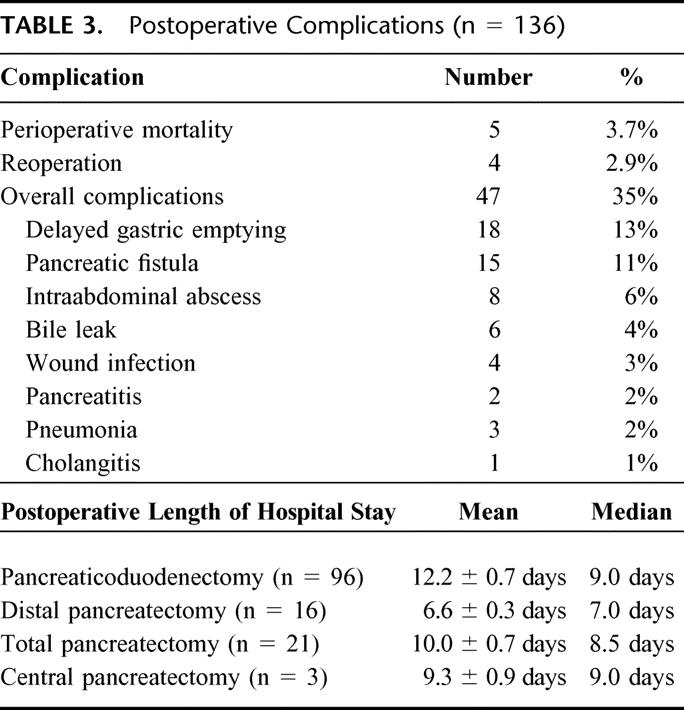
There were 5 postoperative deaths among the 136 IPMN patients, for an overall perioperative mortality rate of 3.7%. Three deaths were in the noninvasive IPMN group and 2 were in the group of IPMNs with invasive cancer (Table 3). Three patients died of intraabdominal sepsis and related multisystem organ failure, 1 died of ischemic bowel, and 1 died of a massive postoperative cerebrovascular accident with associated brain stem infarct and brain death. The overall complication rate was 35%. Four patients required reoperation (2.9%), 2 of which were in the perioperative mortality group. Two reoperations were for anastomotic dehiscences: 1 at the pancreaticojejunostomy and 1 at the hepaticojejunostomy. One patient was reexplored for bleeding from a gastroduodenal artery pseudoaneurysm, and 1 was reexplored for ischemic bowel. The specific complications and postoperative length of hospital stay are displayed in Table 3.
TABLE 3. Postoperative Complications (n = 136)
Long-term Survival and Recurrence
The mean live patient follow-up was 24 months for all patients with IPMNs. Seventy-two of 84 patients (86%) with noninvasive IPMNs remained alive at the time of follow-up, while only 29 of 52 patients (56%) having IPMNs with invasive carcinoma were alive. The 5 patients who died in the immediate postoperative period were not included in the long-term survival analysis.
Patients with noninvasive IPMNs had 1-, 2-, and 5-year actuarial overall survival rates of 97%, 94%, and 77%, respectively. In those patients with IPMNs with an associated infiltrating cancer, the 1-, 2-, and 5-year actuarial overall survival rates were 72%, 58%, and 43%, respectively (Fig. 5, P < 0.0001). There were 9 deaths in the noninvasive group, not including the 3 postoperative mortalities. These 9 deaths were identical to those reported in our previous publication.7 Four patients died of unknown causes. Of the 5 with known causes of death, 1 died of complications of diabetes and 4 died of disseminated adenocarcinoma (3 of whom had negative surgical margins). There were no further deaths from cancer in the noninvasive IPMN group. Of the 21 long-term deaths in the invasive IPMN group (not including 2 postoperative deaths), all patients died of disseminated adenocarcinoma.
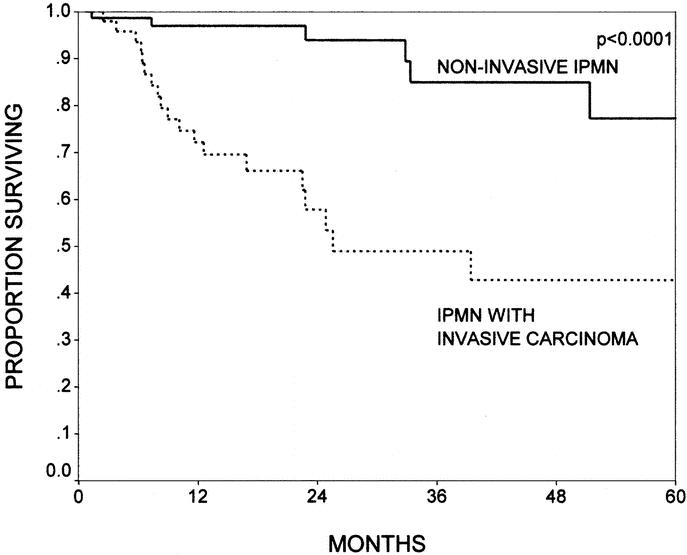
FIGURE 5. The Kaplan-Meier actuarial survival curves comparing patients with noninvasive intraductal papillary mucinous neoplasms (IPMNs, n = 84) and patients with IPMNs with invasive carcinoma (n = 52, P < 0.0001). Patients with noninvasive IPMNs had 1-, 2-, and 5-year survival rates of 97%, 94%, and 77%, respectively; patients with IPMNs with an associated invasive carcinoma had survival rates of 72%, 58%, and 43%, respectively.
In addition to the 4 patients with noninvasive IPMNs who succumbed to invasive adenocarcinoma, there was 1 additional patient with an initial noninvasive IPMN who developed an invasive adenocarcinoma in the tail of his gland 5 years after margin-negative pancreaticoduodenectomy. He underwent completion pancreatectomy in November 2000 and remains alive without evidence of disease. Two more patients have developed recurrent noninvasive IPMN in the tail of the gland. The first patient had a noninvasive borderline, branch-duct variant IPMN with a positive neck margin at the time of pancreaticoduodenectomy. He recurred in the tail 1 year after his initial surgery and underwent completion pancreatectomy. Final pathology showed a borderline IPMN in the residual gland. The second patient had a margin negative pancreaticoduodenectomy for a noninvasive IPMN with CIS. She recurred 11 years after initial surgery and underwent completion pancreatectomy for a noninvasive IPMN with CIS. Both patients are alive without evidence of disease. Two patients with invasive IPMNs were discovered to have infiltrating adenocarcinoma in the pancreatic remnant approximately 10 years after margin-negative pancreaticoduodenectomy. Both underwent completion pancreatectomy. One subsequently died of disseminated disease; the other remains alive without evidence of disease.
There were no differences in survival seen for patients with noninvasive IPMNs, when comparing groups with varying degrees of dysplasia. The 5-year actuarial survival rates were 80%, 72%, and 79% for adenoma, borderline, and CIS, respectively (Fig. 6, P = NS). Likewise, when broken down by branch versus main-duct variants, there were no differences between branch-duct, main-duct, or combined variants with overall 5-year actuarial survival rates (including invasive and noninvasive IPMNs) of 69%, 56%, and 62%, respectively (Fig. 7, P = not significant).
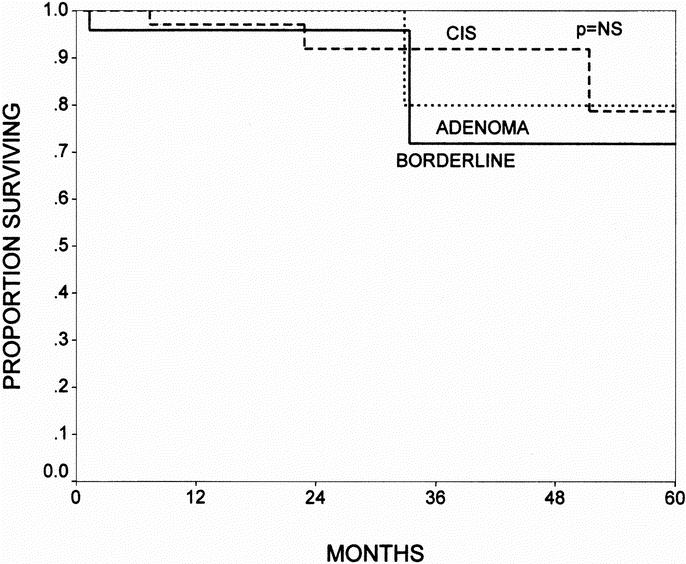
FIGURE 6. The Kaplan-Meier actuarial survival curves comparing patients with IPMN adenomas (n = 14), borderline IPMNs (n = 24), and IPMNs with CIS (n = 46). There were no differences in survival among the 3 noninvasive groups. The 5-year survival rates were 80%, 72%, and 78% for adenoma, borderline, and CIS, respectively (P = NS).
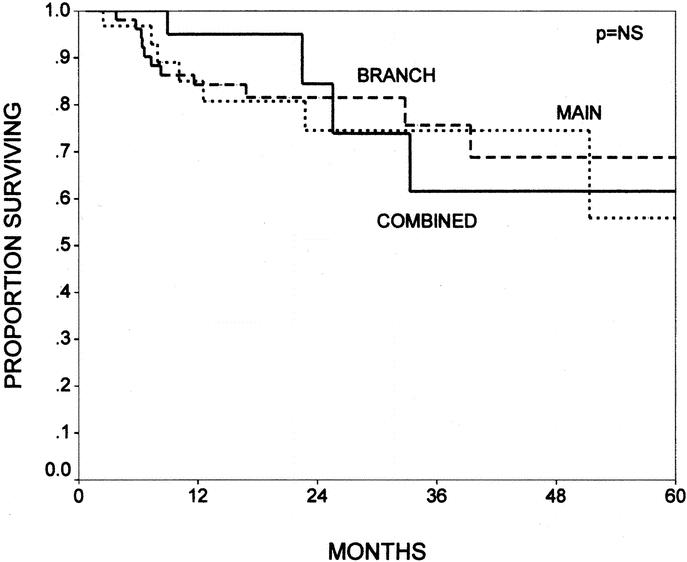
FIGURE 7. The Kaplan-Meier actuarial survival curves comparing patients with main-duct (n = 36), branch-duct (n = 60), and combined variants (n = 33) of IPMN (P = not significant). When broken down by tumor variant, there were no differences between main-duct, branch-duct, or combined variants with overall 5-year survival rates (including invasive and noninvasive IPMNs) of 69%, 56%, and 62%, respectively.
For those patients with invasive IPMNs, lymph node status was predictive of survival in a univariate model. The 1-, 2-, and 5-year actuarial survival rates were 45%, 24%, and 0% for those patients resected with positive lymph nodes, compared with 95%, 95%, and 85%, respectively (P < 0.0001), for those patients resected with negative lymph nodes. Patients with surgical margins negative for invasive cancer showed a trend toward improved survival with 1-, 2-, and 5- year actuarial survival rates of 78%, 62%, and 46% compared with 40%, 0%, and 0%, respectively, for those with positive resection margins (P = 0.08). In those patients with invasive IPMNs, survival was no worse for those with noninvasive IPMN at the surgical margin, tumors ≥ 3 cm, or poorly differentiated tumors. Patients with colloid carcinomas (n = 14) had better survival than those with tubular carcinomas (n = 30, 1 postoperative death), with 1-, 2-, and 5-year actuarial survival rates of 83%, 83%, and 83%, compared with 62%, 24%, and 24%, respectively (P = 0.01).
For those patients with noninvasive IPMN, residual IPMN at the surgical margin was not predictive of survival. It was also not predictive of recurrence, as many who recurred had negative margins and many who had positive margins did not recur in the follow-up period.
DISCUSSION
In this cohort of 136 patients with IPMNs, the 5-year overall survival was 77% for patients with noninvasive IPMNs and 43% for those patients with IPMNs with associated invasive cancer. Patients with IPMNs with CIS were included in the noninvasive group. While the overall 5-year survival in patients with noninvasive IPMNs is good, 4 patients died of disseminated adenocarcinoma, 1 developed an invasive cancer in the pancreatic remnant that was surgically resected, and 2 developed noninvasive IPMNs in the pancreatic remnant, also surgically resected. Only 2 of these patients had noninvasive IPMN at the surgical margin at their initial resection. Similarly, Chari et al reported a series of 113 patients with IPMNs; 5 of 73 patients with noninvasive IPMNs developed local recurrences 3 to 6 years after surgery.2 Azar et al21 and Nagai et al22 also demonstrated recurrence in 2 of 15 patients with noninvasive IPMNs. These data suggest that some IPMNs are multifocal; therefore, patients with an IPMN have a small, but definite, risk of developing invasive or noninvasive IPMN in the remaining pancreas after apparent complete resection of noninvasive tumors. These data illustrate a point critical to managing patients with IPMNs: the role of postoperative surveillance.
The development of noninvasive or invasive IPMNs in the pancreatic remnant of patients with completely resected noninvasive IPMNs may have occurred as a result of residual dysplastic tissue at the surgical margins or a missed invasive cancer within the resected specimen. However, some recurrences occurred in patients with negative margins, and in this and other series2 the pathology was carefully reviewed (all noninvasive IPMNs were examined in their entirety histologically), making a missed invasive cancer unlikely. Furthermore, in the series reported from the Mayo Clinic,2 no recurrences were observed following total pancreatectomy for a noninvasive IPMN. It is therefore more likely that the recurrences observed were due to multifocal disease, with a synchronous IPMN present within the remaining gland or the development of a second metachronous IPMN as a result of a widespread neoplastic field defect in the pancreatic duct epithelium. In either case, careful surveillance of the pancreatic remnant appears warranted.
The 5-year survival rate of 43% for those with resected invasive adenocarcinoma is similar to that seen in other series of IPMNs2,8,17 and is better than the 15% to 25% 5-year survival typically reported for resected invasive ductal adenocarcinoma of the pancreas.7,19 The presence of invasive carcinoma is the single strongest adverse predictor of survival and it appears that patients with advanced stage disease (node positive, margin positive) do poorly, similar to their counterparts with invasive ductal adenocarcinoma. The differences in survival observed between invasive IPMN cancers associated with an IPMN and invasive pancreatic ductal cancers may be a function of earlier presentation of these lesions. However, stage for stage, the outcomes may be similar once invasive cancer has developed. Noninvasive IPMNs are often radiographically identifiable and symptomatic years before an invasive cancer develops. As a result, invasive cancers are often picked up when they are only a microscopic focus associated with a large noninvasive IPMN. This is evidenced by the lower rate of nodal and margin positivity of IPMN associated adenocarcinomas seen in this series, as compared with the rates seen with resected ductal adenocarcinoma.7
The finite but definite risk of recurrence or development of noninvasive or invasive IPMN in the pancreatic remnant, even in patients with initial noninvasive disease, and the high risk of recurrence with invasive cancer have led some to suggest total pancreatectomy for this disease. Those with recurrent disease following intended curative resection of invasive IPMNs recur primarily in distant sites such as the liver and lymph nodes. Therefore, total pancreatectomy is not likely to improve their survival. While demonstrating no recurrences in patients with noninvasive IPMNs undergoing total pancreatectomy, Chari et al2 suggest that the physiologic consequences of total pancreatectomy are so grave that the consequences may outweigh the small risk of recurrence. In agreement with their conclusion, at this time we think the goal should be complete resection of the IPMN with negative surgical margins, but not prophylactic total pancreatectomy, followed by mandatory remnant surveillance. We currently favor either multidetector 3-dimensional CT or magnetic resonance cholangiopancreatography as the imaging study of choice for surveillance, performed at yearly intervals. As additional data are accumulated, we may evolve to the use of other modalities for surveillance such as endoscopic ultrasonography with or without intraductal sonography.
Our data suggest a lag-time of approximately 5 years from the time of development of an IPMN adenoma to the progression to IPMN with an associated invasive carcinoma. When evaluating the groups of IPMNs with progressive degrees of dysplasia, the average age of the patients increased with the degree of dysplasia. Patients with IPMN adenomas were 63.2 years old, those with borderline/CIS IPMNs were 66.7 years old, and those with IPMNs with invasive cancer were 68.2 years old. These data are similar to the data in the report by Chari et al,2 wherein patients with IPMN adenoma were 64 years old on average and those with invasive cancers were 67 years old. Only 14 patients in our series had IPMN adenomas, 24 had borderline IPMNs, 46 had IPMNs with CIS, and 52 had IPMNs with invasive cancer, suggesting that we are identifying most IPMNs late in their progression. Although we have not followed untreated patients to define the natural history of the disease, our data suggest that progression to invasive carcinoma occurs relatively quickly once dysplasia (CIS) is found in the tumor. The molecular steps of such progression have not been established in the same detail as that for pancreatic adenocarcinoma, but it has been shown that sequential acquisition of hypermethylation at multiple gene promoter sites is associated with tumor progression in IPMNs. Indeed, IPMNs associated with invasive cancers demonstrated higher rates of aberrant tumor suppressor gene methylation than their noninvasive counterparts.23 In addition, labeling of IPMNs for Dpc4 protein expression supports the idea that IPMNs are genetically distinct from Pan-INs and invasive pancreatic ductal adenocarcinoma, with only 16% of invasive IPMNs showing loss of Dpc4, compared with more than half of invasive pancreatic ductal adenocarcinomas.7
Controversy still exists over the prognosis of main-duct versus branch-duct variants of IPMNs. The reader should be cautioned that our classification of IPMNs in this series into main-duct, branch-duct, or combined variants was done retrospectively, making it perhaps less accurate than if it were done at the time of initial procurement of the specimen. Our findings are consistent with those of Doi et al4 and Bernard et al,3 showing that a greater proportion of branch-duct variants were noninvasive IPMNs when compared with main-duct IPMNs. However, there was no survival difference in the overall cohort or in the invasive group alone, when the different ductal variants were compared.
Unlike the microscopic PanIN lesions that develop into invasive pancreatic ductal adenocarcinoma over time, IPMNs are a grossly and radiographically identifiable premalignant lesion. IPMN adenomas can be symptomatic (most commonly abdominal pain and nausea/vomiting) and they may produce characteristic CT findings including a distinct cystic mass or markedly dilated pancreatic duct. Based on our data, we estimate the lag-time between IPMN adenoma and IPMN with invasive cancer to be approximately 5 years. The combination of a recognizable precursor to invasive cancer and the relatively lengthy time course for this progression to invasive cancer offers a unique opportunity for early diagnosis and aggressive management. Patients with IPMNs should undergo surgical resection yielding negative margins for all invasive and noninvasive disease. Unlike those patients with completely resected noninvasive mucinous cystic neoplasms of the pancreas (who are routinely cured), patients with completely resected noninvasive IPMNs should undergo careful follow-up and surveillance for the development of recurrent disease. Furthermore, patients with resected invasive IPMNs should also undergo careful follow-up and surveillance as they, too, remain at risk for the development of recurrent disease. In some cases, disease recurrence may be isolated to the remnant pancreas, allowing for reoperation and reresection with the potential for long-term survival.
Discussions
Dr. Andrew L. Warshaw (Boston, Massachusetts): I would like to congratulate Dr. Yeo and Dr. Sohn and their colleagues for another fine study on pancreatic neoplasms. Hopkins frequently leads the way and continues to do so.
Needless to say, it is comforting to note how much they and we agree upon. So it all must be true. We agree that we are seeing increasing numbers of new cases of IPMN. We agree on the histological and spatial presentations of the IPMN. We agree on their treatment. And I think we even agree on our recommendations. So I have several questions for my own edification.
First, you have included branch-duct IPMNs in your study. You asked me previously, and I turn the question back, do you rely on the ovarian-like stroma to distinguish IPMN clearly and completely from MCNs?
Second, some have asserted that the branch-duct IPMNs are less likely to become invasive malignancies. You seem to agree with that. And yet you saw no significant difference in outcomes between branch-duct and main-duct tumors. How do these observations affect the relative aggression of your surgical approach versus observation? Is there a size threshold for nonoperative observation for either a branch-duct or main-duct IPMN, and are the thresholds different?
Third, how much should we rely on frozen sections to ensure adequate resection margins? We do rely on them, but I think we both have found that we are occasionally on thin ice. Both of us have had a few treatment failures through recurrence related to margins that either were positive on permanent section or misleading because of epithelial denervation. How do we correct for this except by total pancreatectomy on every patient (which we are not prepared to do?)
Fourth, when comparing your experience and ours, there is a disturbing difference in the survival curves in the apparently benign group, which includes adenoma, borderline, and CIS, as you have suggested. We have no disease-specific deaths in these patients. I am not sure whether your curves are for disease-specific survival or whether they include deaths from causes other than pancreatic cancer. Perhaps you can clarify that point. Since our follow-up periods are similar, I am puzzled by the disparity in survival.
Finally, can you clarify for me the relationship of PanINs to IPMNs? I believe that Ralph Hruban's work at Hopkins has primarily related the progression of PanINs to ductal adenocarcinoma. Is there any evidence that PanINs are similarly precursors for IPMNs? If they are not, why should we even be considering them in this discussion? Aren't there molecular markers that distinguish them?
Dr. David B. Adams (Charleston, South Carolina): At discussions of IPMN, I always like to remember an early report from Japan in which they reported 4 patients with IPMN and classified the disease into 4 types. So I think our reports today from Hopkins and MGH have added great clarity to this fascinating disease. The questions I have for Dr. Sohn relate to their expertise in the technique of this operation as well as their extensive experience with chronic pancreatitis.
My first question relates to the patients who have chronic pancreatitis in their presentation with IPMN and present chiefly with pain. My experience is that their outcome in terms of pain relief is poor. Could the authors please comment on their experience in pain relief in the management of patients with IPMN?
The second question relates to the issue of total pancreatectomy, which I fear to do simply because of the issue of the brittle diabetes. Many say it is not a problem. My experience is that the diabetes is an issue not because of the hyperglycemia but because of hypoglycemia that presents with no premonitions and presents simply with unconsciousness. Many patients that I followed have had terrible outcomes in terms of the management of diabetes.
The third question relates to the issue of the Hopkins experience with pancreaticogastrostomy. Has this been utilized in patients with IPMN and is it something we should all think of in patients who undergo resections of the head and require surveillance with subsequent ERCP? Would pancreaticogastrostomy be something we all should be doing in order to follow up the patients with endoscopic surveillance who have pancreatic remnant?
The other issue relates to that of resection margins. Have you found intraoperative pancreatoscopy to be valuable? My experience is that it has been falsely positive when not accompanied with histologic evidence. Is this something that we should be looking at further?
The final question relates to long-term follow-up and surveillance in patients with both invasive and noninvasive disease. Do you prefer CT or MRI?
Dr. William H. Nealon (Galveston, Texas): I rise first to congratulate Dr. Sohn, who, I think if you look back, has contributed massively to our literature in pancreatic disease, and she hasn't yet finished her residency.
I mostly rise to mention some of the first reports that appeared in the literature on these entities, all of which were small and several of which followed patients over a period of time before instituting resections. Interestingly, each of these reports strongly suggested that there was a progression to malignancy, but many also mentioned the question of multifocality and some recommended total pancreatectomy.
I think every one of us in the room who does pancreatic surgery will agree that total pancreatectomy leaves us with what I believe is a significant medical problem over the remaining lifetime of those patients. And for that reason alone, it is something that I don't welcome.
But I wonder if the authors have considered the fact that what they are terming metachronous lesions might possibly represent multifocality, and even the fact that they have patients with benign lesions who then developed malignancy, whether those patients might have had multifocal disease from the outset. And does that raise the issue that Dr. Adams just mentioned of whether to be a little more precise? Are you, for example, at all finding usefulness in measuring CA 19–9 just to get some kind of a biochemical representation of recurrence of disease and would endoscopic ultrasound, which should give us a little more detail, possibly help in trying to detect and answer this question of multifocality, particularly over long-term follow-up?
Dr. Edward M. Copeland, III (Gainesville, Florida): I am thinking of these neoplasms relative to diseases that I take care of, such as colorectal and breast cancer where the progression from premalignant to malignant disease is reasonably well established. I make the assumption that you find adenomas, in situ disease, and invasive disease all in the same specimen, and I assume that there is an established pathologic progression within these pathologic entities from benign to malignant, but I did not pick up this point in your presentation. Also, how do you know that it takes 5 years to complete this process?
Dr. Taylor A. Sohn (Baltimore, Maryland): Our surgical specimens have varying degrees of differentiation ranging from adenoma to invasive carcinoma. Dr. Warshaw noted the same thing about the specimens in his series. When we classify an IPMN as adenoma, borderline, carcinoma in situ, or invasive cancer, we are referring to the greatest degree of dysplasia observed in the specimen, as this gives us the best indication of long-term prognosis.
Our information on the time for progression from adenoma to invasive carcinoma is skewed because we are seeing patients at the time they present for resection. Many of these patients may have adenomas for years before they are clinically apparent. Our data show an age difference of 5 years (range 63–68 years) between patients with adenomas and patients with invasive carcinomas. As many patients may be asymptomatic for months to years before their tumors are clinically evident, this progression is probably longer than we are estimating.
None of our IPMNs had ovarian stroma. Mucinous cystic neoplasms of the pancreas are defined as having ovarian stroma and no connection to the main pancreatic duct, distinguishing them from IPMNs. Therefore, any tumor with ovarian stroma was classified as a mucinous cystic neoplasm and not as an IPMN.
Dr. Warshaw asked about branch-duct variants in our series, which were not included in their study. Patients with branch-duct variants more often had noninvasive tumors. Of the noninvasive IPMNs, 53% were branch-duct, 22% were main-duct, and 25% were combined variants, whereas in the invasive IPMNs only 25% were branch-duct, 35% were main-duct, and 25% were combined variants. Despite this difference, we did not show a survival difference between the main-duct, branch-duct, and combined variants. We should also state that IPMNs were classified retrospectively into main-duct or branch-duct variants.
There are differences in survival observed between our data and the Massachusetts General Hospital data. For those patients with invasive cancer, our patients presented at a more advanced stage. Dr. Warshaw states a 41% incidence of positive lymph nodes, while we had a 54% incidence of positive lymph nodes, which we demonstrated to be predictive of survival. With regard to benign disease, we do not present disease-specific curves because we have 4 patients in whom we do not know the cause of death. Given the small numbers of deaths in this group, we thought it would not be accurate to exclude them. However, Dr. Warshaw's group presents disease-specific survival, and this, too, could account for the observed differences.
PanINs are distinct from IPMNs. They do not progress to IPMNs but rather to invasive pancreatic adenocarcinomas, which are genetically distinct. I think the only reason to include them in the discussion is to distinguish them from IPMNs. IPMNs present with a clinically or radiographically identifiable mass, whereas PanINs do not. PanINs are all microscopic lesions and tend to be found incidentally.
Dr. Adams asked about improvement in pain in these patients. The majority of these patients have some improvement in their pain, although we really did not look at this specifically.
The issue of prophylactic total pancreatectomy ties into Dr. Nealon's question about multifocality and field defects in patients with IPMNs. We have identified several patients with benign disease at initial resection who have recurred or developed tumor in the pancreatic remnant or died of metastatic disease. It is possible that such patients had an unidentified tumor in the initial resection specimen; however, this is unlikely, as all specimens were carefully reviewed. It is also possible that patients developed a synchronous or metachronous tumor in the remnant gland, most likely as a result of a field defect within the pancreatic ducts predisposing them to neoplasm formation.
At this time, we do not recommend prophylactic total pancreatectomy in patients with disease localized to the head or tail of their gland. The majority of patients with invasive cancer who recurred, recurred distant from the pancreas, with liver and peritoneal metastases. As Dr. Warshaw and Dr. Yeo pointed out, there are very few patients who present with isolated disease in the pancreatic remnant that can be reresected. We feel that the physiologic consequences of prophylactic pancreatectomy probably far outweigh the small risk of developing cancer in the remnant. Therefore, we recommend partial pancreatectomy and careful surveillance of the remaining pancreas with annual multidetector 3-dimensional CT or magnetic resonance cholangiopancreatography. However, we do not hesitate to perform total pancreatectomy for patients with IPMN adenoma or invasive carcinoma involving the entire gland. We did have 1 death in our series from complications of diabetes, but this death occurred in a patient who underwent partial pancreatectomy. With the newer, long-acting insulins and insulin pumps, diabetes is much easier to control.
In our series, we did not do a pancreaticogastrostomy in any of the pancreaticoduodenectomy patients. We did perform pancreaticogastrostomy in the 3 patients who underwent central pancreatic resections, as this was technically easier and did not require a Roux-en-Y reconstruction.
Lastly, Dr. Warshaw raised a question regarding resection margins and the utility and reliability of intraoperative frozen sections. The majority of our positive margins are in the uncinate process and cannot be further resected. And while not perfect, our pathologists are fairly accurate in reading out pancreatic neck margins and guiding our intraoperative management.
I'd like to thank the Southern Surgical Association for the privilege of closing this discussion.
Footnotes
Correspondence: Charles J. Yeo, MD, Departments of Surgery and Oncology, Johns Hopkins Medical Institutions, Blalock 606, 600 N. Wolfe Street, Baltimore, MD 21287-4606. E-mail: cyeo@jhmi.edu.
REFERENCES
- 1.Weisenauer CA, Schmidt CM, Cummings OW, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138:610–617. [DOI] [PubMed] [Google Scholar]
- 2.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2002;123:1500–1507. [DOI] [PubMed] [Google Scholar]
- 3.Bernard P, Scoazec JY, Joubert M, et al. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–1278. [DOI] [PubMed] [Google Scholar]
- 4.Doi R, Fujimoto K, Wada M, et al. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002;132:80–85. [DOI] [PubMed] [Google Scholar]
- 5.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection: comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of branch duct type of intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–265. [DOI] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traverso LW, Peralta EA, Ryan JA, et al. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. [DOI] [PubMed] [Google Scholar]
- 9.Kloppel G, Solcia E, Longnecker DS, et al. Histological typing of tumours of the exocrine pancreas. In: World Health Organization International Classification of Tumors, 2nd ed. Berlin: Springer, 1996:11–20. [Google Scholar]
- 10.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol. 2000;17:16–30. [PubMed] [Google Scholar]
- 11.Longnecker DS. Observations on the etiology and pathogenesis of intraductal papillary-mucinous neoplasms of the pancreas. Hepatogastroenterology. 1998;45:1973–1980. [PubMed] [Google Scholar]
- 12.Cho KR, Vogelstein B. Genetic alterations in the adenoma-carcinoma sequence. Cancer. 1992;70:1727–1731. [DOI] [PubMed] [Google Scholar]
- 13.Wilentz RE, Hruban RH. Pathology of cancer of the pancreas. Surg Oncol Clin North Am. 1998;7:43–65. [PubMed] [Google Scholar]
- 14.Wilentz RE, Albores-Saaverdra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327. [DOI] [PubMed] [Google Scholar]
- 15.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. [DOI] [PubMed] [Google Scholar]
- 16.Moriya T, Kimura W, Sakurai F, et al. Minute invasive ductal carcinoma of the residual pancreas after distal pancreatectomy for intraductal papillary mucinous tumors. Int J Gastrointest Cancer. 2002;31:191–197. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7:12–18. [DOI] [PubMed] [Google Scholar]
- 18.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: Part 2. Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Azar C, Van de Stadt J, Rickaert F, et al. Intraductal papillary mucinous tumors of the pancreas: clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai E, Ueki T, Chijiwa K, et al. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called “mucinous ductal ectasia”: histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19:576–589. [DOI] [PubMed] [Google Scholar]
- 23.House GM, Guo M, Iacobuzio-Donahue C, et al. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. [DOI] [PubMed] [Google Scholar]